Abstract
Objective:
Antioxidant therapy is a promising treatment option for non-alcoholic fatty liver disease (NAFLD) after failure of lifestyle modification. We aimed to explore the efficacy of combined vitamin E and C therapy compared to no treatment for NAFLD.
Methods:
A literature search was performed in Ovid Embase, Ovid Medline, PubMed, Cochrane Library, Scopus, and Web of Science from inception to 28th April 2020. A systematic review and meta-analysis were conducted on randomized controlled trials that assessed vitamin E and C co-treatment in NAFLD. Quality of evidence was appraised using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach. Assessed outcomes were changes in imaging findings, histological features, and serum transaminases. Subgroup analyses that compared adult versus children were further explored.
Results:
Four studies (n=260) satisfied our eligibility criteria. Vitamin co-treatment did not improve ultrasonographic liver brightness, histological parameters of hepatocyte injury (steatosis, lobular inflammation, and ballooning), fibrosis grading (standardized mean difference [SMD ]: 0.02, 95% CI: -0.40 to 0.45, I2=13%), serum aspartate transaminase (mean difference [MD]: -0.05, 95% CI: -2.59 to 2.50, I2=0%), and serum alanine transaminase (MD: 2.82, 95% CI: -2.11 to 7.76, I2=57%). Subgroup stratifications illustrated similar findings.
Conclusion:
Vitamin co-treatment may have limited efficacy in NAFLD. However, we have little confidence in our effect estimates due to bias and other major constraints.
Key Words: Antioxidant, liver histology, liver brightness, serum transaminase
Introduction
Non-alcoholic fatty liver disease (NAFLD) is characterized by the asymptomatic accumulation of lipids in hepatic parenchyma, despite an absence of significant alcohol consumption or other secondary causes (Amanullah et al., 2019; Chalasani et al., 2018). It involves a wide histological spectrum from non-alcoholic fatty liver (NAFL), a clinically benign steatosis without hepatocyte injury, to non-alcoholic steatohepatitis (NASH), indicated by steatosis and injury with or without fibrosis (Chalasani et al., 2018). The global prevalence of NAFLD is estimated as 25% (Angulo, 2002; Buzzetti et al., 2016), with a striking 75% prevalence in obese individuals, and presumably an even higher figure in Type 2 diabetes mellitus (T2DM) patients (Masarone et al., 2014; Masarone et al., 2017; Masarone et al., 2018). Therefore, it is rapidly becoming the top cause for cirrhosis, leading to liver failure and/or hepatocellular carcinoma (HCC) needing transplantation (Li et al., 2018).
According to the multiple-hit hypothesis, insulin resistance (IR) is the pivotal factor in NAFLD pathogenesis (Buzzetti et al., 2016; Tilg and Moschen, 2010). Its promotion of increased hepatic lipid influx perpetuates the overproduction of reactive oxygen species (ROS), resulting in hepatocyte dysfunction, upregulation of inflammatory cytokines, and activation of hepatic stellate cells, which ultimately drives hepatic necroinflammation and fibrosis (Buzzetti et al., 2016; Masarone et al., 2018). Therefore, antioxidant therapy is considered a promising option to limit oxidative stress that drives disease progression (Masarone et al., 2018).
The most potent antioxidant found in nature, Vitamin E (α-tocopherol), is a lipid soluble micronutrient derived from vegetables, nuts, oils, and fish (El Hadi et al., 2018). Previous qualitative and quantitative syntheses have shown that vitamin E improves serum transaminases and liver histology in NAFLD, including NASH (Amanullah et al., 2019; Ji et al., 2014; Sato et al., 2015; Xu et al., 2015). Its use in clinical practice is recommended in non-diabetics with biopsy-proven NASH and fibrosis, as the first-line pharmacotherapy if lifestyle modification fails (Chalasani et al., 2018; Chalasani et al., 2012).
To optimize clinical outcomes, multiple studies have also investigated the co-treatment of vitamin E with other drugs (Dufour et al., 2006; Ersoz et al., 2005; Foster et al., 2011; Kawanaka et al., 2013; Perumpail et al., 2018). The rationale for its combination with vitamin C (ascorbic acid) emerged from evidence that vitamin C synergistically augments the regeneration of its oxidized form, enhancing the overall antioxidative capacity (Chan, 1993; Niki, 1987). Additionally, a cross-sectional study demonstrates a potential role of vitamin C in NAFLD, whereby an inverse association between dietary intake and disease incidence has been observed in adults (Wei et al., 2016). To this end, it still remains unclear whether the addition of vitamin C would significantly boost clinical outcomes. Therefore, we aim to delineate the benefits of vitamin E and C co-treatment in NAFLD with robust appraisal of evidence quality.
Materials and Methods
Eligibility Criteria
The inclusion criteria are as follows; (1) Population: participants of any sex, ethnicity, and age with NAFLD (NAFL or NASH) diagnosed by histology, imaging, or serum transaminases; (2) Interventions: oral vitamin E and C co-treatment for more than 1 month at any dose and frequency; (3) Comparison: not receiving vitamin therapy; (4) co-intervention, such as lifestyle modification or drugs, was allowed if they were administered to both intervention and control arms; (5) Outcomes: changes in liver histology, imaging and serum transaminases; (6) Type of design: experimental studies that were either a randomized controlled trial (RCT) or controlled clinical trial (CCT). The exclusion criteria: (1) participants with other co-existing liver diseases, namely viral hepatitis, alcoholic liver disease, autoimmune hepatitis, Wilson’s disease, etc.; (2) participants with possible causes of secondary NAFLD, such as bariatric surgery and total parental nutrition.
Information source and search strategy
A literature search for randomized controlled trials (RCTs) was conducted on Ovid Embase, Ovid Medline, PubMed, Cochrane Library, Scopus, and Web of Science from inception to 28th April 2020. The following keywords and MeSH terms were used: (“vitamin E” or “tocopherol” or “alpha tocopherol”) AND (“vitamin C” or “ascorbic acid” or “ascorb*”) AND (“nonalcoholic fatty liver disease” or “nonalcoholic fatty liver” or “non-alcoholic fatty liver” or “nonalcoholic steatohepatitis” or “non-alcoholic steatohepatitis” or “nonalcoholic steatosis” or “non-alcoholic liver steatosis” or “non-alcoholic steatosis” or “non-alcoholic hepatic steatosis” or “nonalcoholic hepatic steatosis” or “nonalcoholic liver steatosis”). PP and CK performed the searches independently, and duplicates were excluded. PC and AS resolved disagreements by discussion. A cross-reference check was done to identify additional trials. An example of full electronic search can be found in Supplementary Table 1.
Selection of study
Two authors (PP and CK) independently screened titles, abstracts, and outcomes of interest. CK retrieved full-text articles of potentially eligible studies and excluded studies with reasons, including duplicate records based on review of titles. Any inconsistency and disagreement in the process were adjudicated by the third and fourth reviewers (PC and AS).
Data synthesis
Our primary outcome was improvement in steatosis, as measured by liver brightness on ultrasonography. Our secondary outcomes were changes in histological features (steatosis, inflammation, ballooning, fibrosis) and serum transaminases (AST: aspartate transaminase; ALT: alanine transaminase).
Two authors (PP and CK) performed the meta-analysis in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline (Liberati et al., 2009). Disagreements were resolved by discussion with the third and fourth authors (PC and AS). Furthermore, the quality of evidence was evaluated using the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach (Schünemann H, 2013). The level of quality starts from “high” to “moderate” onto “low” then “very low”. Each comparison was downgraded one level due to serious (or two levels if very serious) risk of bias, inconsistency, indirectness, or imprecision. The summary of findings table was created using GRADEpro GDT software (Evidence Prime, 2015).
Data extraction
Two review authors (PP and CK) independently extracted details of trial characteristics (author, year, country, trial design, duration), participants (numbers, mean age, gender proportion, method of NAFLD diagnosis, randomization and blinding, drop-out numbers), treatment characteristics (intervention, control, dosage, frequency), and outcome assessments (histological parameters, imaging findings, liver transaminases). Contact with research authors for missing data was not attempted.
Mean difference (MD) and standard deviations (SD) were used to analyse continuous data. When studies utilized distinctive measurements to assess an outcome, for instance different histological scoring systems, the standardized mean difference (SMD) and SD were used. All pooling was based on post-treatment values as opposed to changes from the baseline. When necessary, we extracted values from figures shown in the reports. With regards to differences in NAFLD pathophysiology in various age groups, a subgroup analysis of adult and children were explored. When studies reported medians and ranges, Wan et al.’s methods were used to approximate mean and SD, whereby a sensitivity analysis was conducted to verify these statistical assumptions (Wan et al., 2014). When two studies were an extension of one another, a sensitivity analysis that excluded the version with higher risk of bias was performed.
Data analysis
RevMan software (Review Manager Version 5.3; Cochrane Library Software, Oxford, England) was used for meta-synthesis. The heterogeneity of studies was performed using chi-square test and I2 statistics (0%, 25%, 50%, or 75% for no, low, moderate, and high heterogeneity, respectively). The fixed effect model was used when no heterogeneity (I2 index <50% or P-value >0.10) was observed. The random-effects model was used in studies with heterogeneity of the studies (p-value < 0.10) and moderate I2 index (50% and over). The authors performed a statistical power for meta-analysis using Cohen’s d effect size. 80% statistical power was considered sufficient. Authors also intended to evaluate publication bias via funnel plot assessments and Egger’s regression, given the known limitations of these tests.
Results
Study selection
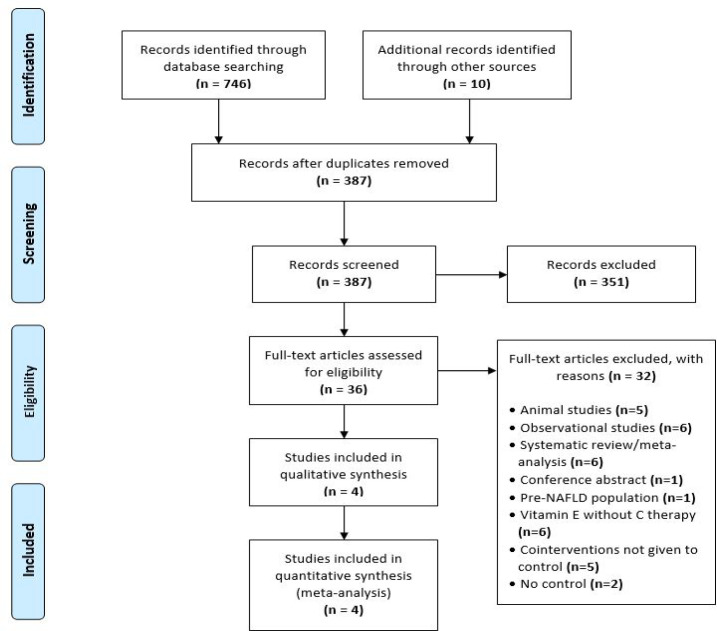
A total of 746 articles was retrieved from the initial search, and 10 additional studies were identified from the cross-reference check (Figure 1). After the preliminary screening, 36 articles were subjected to full review. Four RCTs (n=260) satisfied the inclusion criteria for qualitative and quantitative analyses (Hadzi-Petrushev et al., 2018; Harrison et al., 2003; Nobili et al., 2006; Nobili et al., 2008). Common reasons for exclusion were vitamin E treatment without vitamin C, additional co-interventions not given to control, lack of a placebo arm, or wrong study designs (Ersoz et al., 2005; Kawanaka et al., 2013; Lavine et al., 2011; Murer et al., 2014; Sanyal et al., 2010).
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Review and Meta-Analyses) Flow Diagram
Study characteristics
As shown in Table 1, the four selected trials were published from 2003 to 2018, and were conducted in the United States (Harrison et al., 2003), Italy (Nobili et al., 2006; Nobili et al., 2008), and Macedonia (Hadzi-Petrushev et al., 2018). Two trials were double-blinded (Harrison et al., 2003; Nobili et al., 2006), while Nobili et al., (2008) and Hadzi-Petrushev et al., (2018) were open-label. Notably, Nobili et al., (2008) was a 12-months extension of Nobili et al., (2006) with additional histological assessments and a smaller sample size. Both trials were conducted in children age ranged 5.7-18.8 years (Nobili et al., 2006; Nobili et al., 2008), while others were performed in adults ages 38-70 years (Hadzi-Petrushev et al., 2018; Harrison et al., 2003). Follow-up duration ranged from 3 months to 2 years. Harrison et al., (2003), Nobili et al., (2006), and Nobili et al., (2008) diagnosed NAFLD by biopsy, while Hadzi-Petrushev et al., (2018) by ultrasonography. All four trials had co-intervention administered to both groups, which were either lifestyle modifications (hypocaloric diet plan and/or exercise regimen) (Harrison et al., 2003; Nobili et al., 2006; Nobili et al., 2008) and/or atorvastatin (Hadzi-Petrushev et al., 2018). Hadzi-Petrushev et al., (2018) had a three-armed design, in which two groups were NAFLD patients prescribed with atorvastatin and vitamins or atorvastatin alone, while the third arm were healthy patients given no treatment. Therefore, only data from the two NAFLD groups were used in pooling for analysis.
Table 1.
Characteristics of Included Studies
| Study | Study design | Duration | Intervention Number |
Control number |
NAFLD diagnosis | Age (years) | Gender M (%) | Intervention Description | Control Description | Outcomes | Conflicts of interest |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Harrison et al. 2003, USA | Double blinded | 6 months | 23 | 22 | Biopsy | 50-53 | 44.5 | Vitamin E (1000 IU/day), Vitamin C (1000 mg/day), exercise | Placebo with exercise | Liver histology, alanine transaminase, body mass index | None declared |
| Nobili et al. 2006, Italy | Double blinded | 12 months | 45 | 43 | Biopsy | 9-15.5 | 31.80% | Vitamin E (600 IU/day), Vitamin C (500mg/day), diet, exercise |
Placebo with diet, exercise | Liver brightness, serum transaminase, glucose homeostasis, lipid profiles | None declared |
| Nobili et al. 2008, Italy | Double blinded, then open label | 24 months | 25 | 28 | Biopsy | 5.7-18.8 | 69.80% | Vitamin E (600 IU/day), Vitamin C (500mg/day), diet, exercise | Placebo with diet, exercise | Liver histology, serum transaminase, glucose homeostasis, lipid profiles | None declared |
| Hadzi-Petrushev et al. 2018, Macedonia | Three arms, open label | 3 months | Arm 1 (NAFLD):20 Arm 2 (NAFLD):20 |
Arm 3 (healthy):34 | Ultrasonography | NAFLD: 39-51 Healthy: 38-49 |
100% | Arm 1: Vitamin E (400 IU/day), Vitamin C (1000mg/day), Atorvastatin (20 mg/day) Arm 2: Atorvastatin (20 mg/day) |
Arm 3: Nothing | Serum transaminases, lipid profiles, antioxidant enzymes, inflammatory marker | None declared |
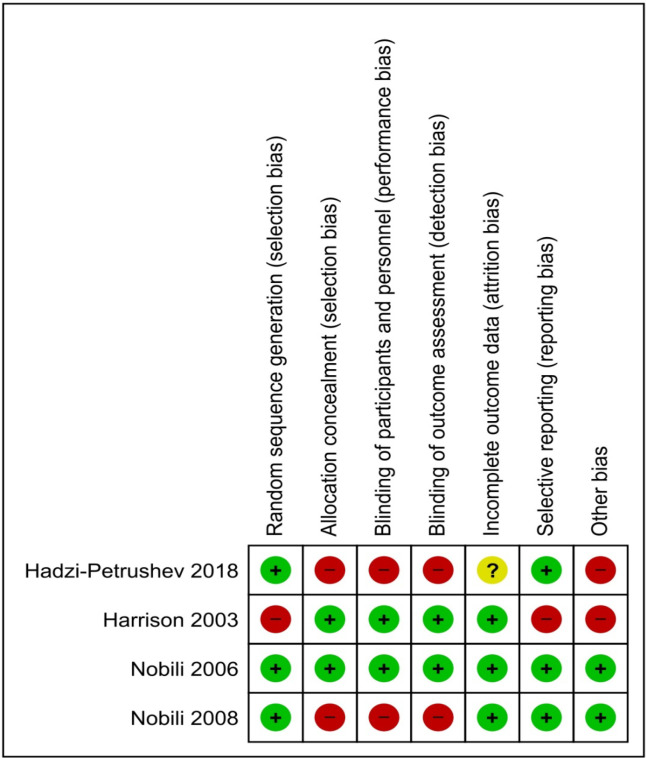
Risk of bias within studies
As shown in Figure 2, research groups in Harrison et al., (2003) lacked homogeneity because the intervention had a significantly greater baseline body mass index (BMI) and diabetic subjects than the control (14 versus 5 patients, respectively). Selective reporting bias was also suspected because the histological grading of steatosis was not addressed, while hepatocyte degeneration/necrosis and lobular inflammation scores were reported as a combined value, though they were assessed independently (Harrison et al., 2003). Hadzi-Petrushev et al., (2018) did not provide numbers and reasons for dropouts and demonstrated gender bias due to exclusive recruitment of male subjects. On the other hand, Nobili et al., (2008) did not blind participants and researchers. Overall, Hadzi-Petrushev et al., (2018), Harrison et al., (2003), and Nobili et al., (2008) portray high risk for bias, while Nobili et al., (2006) demonstrates low risk. Further assessments of publication bias were not conducted, as there were insufficient number of trials.
Figure 2.
Risk of Bias Summary
Effects of vitamin E and C on steatosis assessed via ultrasonography
Qualitative analysis suggests vitamin co-treatment did not markedly reduce steatosis, as measured via ultrasonography (Hadzi-Petrushev et al., 2018; Nobili et al., 2006). Nobili et al., (2006) has reflected a similar outcome between the two groups, whereby vitamin co-treatment led to improvement in 33 subjects, 3 disappearances, and 5 unchanged findings. Their placebo group demonstrated comparable results, as 37 showed reduction, 3 disappearances, and 7 unaltered readings (Nobili et al., 2006). Likewise, Hadzi-Petrushev et al., (2018) has illustrated no improvement in liver fat after treatment in each group. The quality of evidence was downgraded to very low due to serious risk of bias and indirectness (Table 2).
Table 2.
Subgroup and Sensitivity Analyses of Fibrosis and Serum Transaminases
| Analysis | Fibrosis | AST | ALT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Trials (n) | SMD (95% CI) | I2 | Trials (n) | MD (95% CI) | I2 | Trials (n) | MD (95% CI) | I2 | |
| Subgroup analysis | |||||||||
| Adult | 1 | 0.23 (-0.36, 0.81) | NA | 1 | 1.97 (-3.58, 7.52) | NA | 2 | 1.11 (-4.62, 6.83) | 0 |
| Children | 1 | -0.18 (-0.72, 0.36) | NA | 2 | -0.59 (-3.45, 2.27) | 0 | 2 | 3.73 (-4.83, 12.29) | 0.85 |
| Sensitivity analysis | |||||||||
| Trials that explicitly reported means and SDs | 1 | 0.23 (-0.36, 0.81) | NA | 1 | 0.09 (-3.39, 3.57) | NA | 2 | -0.44 (-4.52, 3.63) | 0 |
| Trials without duplicated studies (Nobili et al., 2008) | 0 | NA | NA | 2 | 0.62 (-2.33, 3.57) | 0 | 3 | 0.05 (-3.29, 3.39) | 0 |
SMD, standardized mean difference; MD, mean difference; SD, standard deviation; AST, aspartate transaminase; ALT, alanine transaminase
Effects of vitamin E and C on histological parameters
Nobili et al., (2008) reports significant alleviation in steatosis, lobular inflammation, ballooning, and NAFLD Activity Score (NAS) from the baseline after 2 years of treatment. However, consistent with Harrison et al. (2003), no major improvement in histology was observed when intervention was compared to control. With notable indirectness, among other limitations, the quality of evidence was downgraded to very low (Table 2).
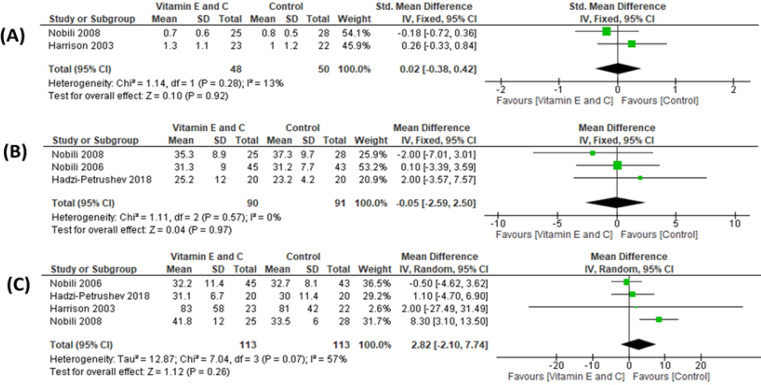
No major improvement in fibrosis grades were observed after vitamin co-treatment in quantitative analysis (Figure 3A) (2 studies, n=98; SMD: 0.02, 95% CI: -0.38 to 0.42, I2=13%) (Harrison et al., 2003; Nobili et al., 2008). Although Harrison et al., (2003) found that diabetics, with higher baseline fibrosis, demonstrated significant improvement, the post-hoc analysis was only done in a limited portion of participants (5 in placebo versus 14 in intervention). The subgroup and sensitivity analyses reveal similar findings (Table 3). Due to high risk of bias, including publication bias and serious imprecision, the quality of evidence for were regarded as very low (Table 2).
Figure 3.
Forest Plot of Comparison: Vitamin E and C co-treatment compared to control in non-alcoholic fatty liver disease (NAFLD). Outcomes: (A) Fibrosis grading on liver biopsy; (B) Serum aspartate transaminase (AST); (C) Serum alanine transaminase (ALT).
Table 3.
Summary of Findings with Appraisal of Evidence Quality According to the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) approach
| Outcomes | Number of participants (studies) |
Certainty of the evidence (GRADE) |
Anticipated absolute effects | |
|---|---|---|---|---|
| Risk with | Risk difference with Vitamin co-treatment | |||
| Liver Brightness (Steatosis) assessed with: Ultrasonography follow up: range 3 months to 12 months |
128 (2 RCTs) (Hadzi-Petrushev et al., 2018; Nobili et al., 2006) |
VERY LOW a,b,c | not pooled | not pooled |
| Hepatocyte injury (steatosis, inflammation, ballooning) assessed with: Histological grading follow up: range 6 months to 2 years |
98 (2 RCTs) (Harrison et al., 2003; Nobili et al., 2008) |
VERY LOW c,d,e,f,g | not pooled | not pooled |
| Fibrosis assessed with: Histological grading Scale from: Grade 1 to Grade 4 follow up: range 6 months to 2 years |
98 (2 RCTs) (Harrison et al., 2003; Nobili et al., 2008) |
VERY LOW c,d,f,g | - | SMD 0.01 higher (0.39 lower to 0.41 higher) |
| Aspartate aminotransferase (AST) assessed with: Serum levels follow up: range 3 months to 2 years |
181 (3 RCTs) (Hadzi-Petrushev et al., 2018; Nobili et al., 2006; Nobili et al., 2008) |
VERY LOW c,f,h,i | The mean aspartate aminotransferase (AST) ranged from 23.2 to 37.25 IU/L | MD 0.05 IU/L lower (2.6 lower to 2.49 higher) |
| Alanine aminotransferase (ALT) assessed with: Serum levels follow up: range 3 months to 2 years |
226 (4 RCTs) (Hadzi-Petrushev et al., 2018; Harrison et al., 2003; Nobili et al., 2006; Nobili et al., 2008) |
VERY LOW c,f,i,j,k | The mean alanine aminotransferase (ALT) ranged from 30.3 to 81 IU/L | MD 2.81 IU/L higher (2.1 lower to 7.71 higher) |
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI); CI, Confidence interval; SMD, Standardized mean difference; MD, Mean difference; GRADE Working Group grades of evidence; High certainty: We are very confident that the true effect lies close to that of the estimate of the effect; Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different; Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect; Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect; Explanations: a. Downgraded one level for high risk of bias (selection, performance, detection, and gender biases in Hadzi-Petrushev et al., 2018; b. Downgraded one level for serious indirectness (liver brightness was used as surrogate marker for liver steatosis); c. Downgraded one level for suspected publication bias (small number of small trials); d. Downgraded one level for high risk of bias (selection, performance, and detection biases in Nobili et al., 2008, and selection bias in Harrison et al., 2003; e. Downgraded one level for serious indirectness (Harrison et al., 2003 did not report data on steatosis, while inflammation and hepatocyte degeneration/necrosis scores were combined); f. Downgraded one level for serious imprecision (sample size less than 400 participants); g. Downgraded one level for serious imprecision (CI includes no difference and appreciable harm); h. Downgraded two levels for high risk of bias (selection, performance, and detection biases in Nobili et al., 2008, selection, performance, detection, and gender biases in Hadzi-Petrushev et al., 2018; i. Downgraded one level for serious indirectness (co-intervention in Hadzi-Petrushev et al., 2018 was atorvastatin, as opposed to other trials which administered lifestyle modification); j. Downgraded two levels for high risk of bias (selection, performance, and detection biases in Nobili et al., 2008, selection, performance, and detection, and gender biases in Hadzi-Petrushev et al., 2018, and selection bias in Harrison et al., 2003; k. Downgraded one level for serious heterogeneity (I2 > 50%)
Effects of vitamin E and C on serum transaminases
Individual studies generally showed improvements in ALT and AST from the baseline (Hadzi-Petrushev et al., 2018; Harrison et al., 2003; Nobili et al., 2006; Nobili et al., 2008). However, pooled results reflected no differences between the two arms (Figure 3B, AST: 3 studies, n=181, MD: -0.05, 95% CI: -2.59 to 2.50, I2=0% and Figure 3C, ALT: 4 studies, n=226, MD: 2.82, 95% CI: -2.11 to 7.76, I2=57%) (Hadzi-Petrushev et al., 2018; Harrison et al., 2003; Nobili et al., 2006; Nobili et al., 2008). The subgroup and sensitivity analyses demonstrates similar effects (Table 3). Both outcomes were regarded as very low-quality evidence (Table 2).
Discussion
As a major health issue, NAFLD is becoming the top cause for cirrhosis and related life-threatening complications (Li et al., 2018). While lifestyle modification remains the cornerstone for management, difficulty in compliance precludes achievement of patients’ long-term goals (Katsagoni et al., 2017). Therefore, there is a need to evaluate potential pharmacological regimens to optimize clinical outcomes. Oxidative stress plays an essential role in pathogenesis and progression of NAFLD; thus, vitamin E use had been extensively scrutinized and translated into practice (Masarone et al., 2018). However, its co-treatment with vitamin C has received less attention, and we hypothesized this combination would further improve outcomes due to their synergistic relationship (Chan, 1993). While no systematic reviews or meta-analyses has been conducted to this end, we aimed to explore the efficacy and to appraise acquired evidence for adopting such a combinative regimen.
Our qualitative result shows vitamin E and C co-treatment did not improve liver brightness, a surrogate marker for steatosis on ultrasonography, and histological features of steatosis, inflammation, and ballooning. Fibrosis gradings and liver enzyme levels were not significantly altered for both adults and children.
While we observed no benefits of combined vitamins in NAFLD, still the findings remain questionable. Since included studies employed co-intervention as lifestyle modification and/or atorvastatin, the underlying benefits of these treatments likely overpowered the true efficacy of vitamins. This might explain individual studies showing major improvements from the baseline, while demonstrating insignificant changes in between-group comparisons (Harrison et al., 2003; Nobili et al., 2006). Therefore, we can only assert that antioxidant therapy with lifestyle modification and/or atorvastatin is no better than the latter alone in improving NAFLD outcomes. This notion is consistent with previous vitamin E research that shows greater efficacy achieved with lifestyle adjustments than antioxidant therapy (Wang et al., 2008). In the absence of trials without co-interventions, the efficacy of vitamin E and C cannot be fairly characterized.
Nevertheless, we strongly urge cautious interpretation of our results due to major limitations. First and foremost, all outcomes were downgraded to very low quality, reflecting minimal confidence in the effect estimates. Apart from high risk of bias, evidence suffered from serious imprecision due to small sample sizes and/or wide confidence intervals that included appreciable harm. Indirectness in comparison was due to variability in demographic characteristics, vitamins dosages, and trial durations. None of the studies prescribed the recommended vitamin E dose of 800 IU/day. We also had a strong suspicion of publication bias because only a limited number of small trials exists on this topic (Guyatt et al., 2011).
Secondly, because histological gradings were not reported in the text or tables in Harrison et al., (2003) and Nobili et al., (2008), data had to be extracted from given figures, and may have compromised precision of the analyses. Additionally, the use of Wan et al.,’s method (2014) to handle missing means and SDs was a mere approximation with questionable accuracy. Although sensitivity analyses were performed to validate this assumption, exclusion of certain trials emphasized the existing small study effects, which further reduced confidence. Pooling of interdependent trials, Nobili et al., (2006) and Nobili et al., (2008), contributed to bias and overestimation of the effect sizes, especially when they were the only trials that provided paediatric data to the analyses. Lastly, there is insufficient evidence to ascertain the safety profile of this combination, specifically in NAFLD patients, as further investigations await.
We recognize the limited benefit of solely inhibiting oxidative stress in NAFLD. With regards to the multiple-hit hypothesis, future efforts could examine novel cocktail regimens that simultaneously target several pathogenic hits, such as insulin resistance and adipokine imbalance in adjunct to oxidative stress, to deliver patients with significant outcomes (Polyzos et al., 2018).
Although our review is the first to appraise quality of evidence, we believe effect estimates from previous vitamin E qualitative and quantitative syntheses may also demonstrate limited confidences, because existing RCTs are mostly underpowered and show methodological shortcomings (Aller et al., 2015; Amanullah et al., 2019; Dufour et al., 2006; Ersoz et al., 2005; Ji et al., 2014; Kawanaka et al., 2013; Sato et al., 2015; Wang et al., 2008; Xu et al., 2015). Thus, re-evaluation of vitamin E usage will be warranted as new evidences from high-quality RCTs emerge.
Combined vitamin E and C therapy has limited efficacy in improving NAFLD histology, imaging, and liver enzymes. However, we have very little confidence in our effect estimates given the limitations of included studies. A strong conclusion that either supports or discourages the use of these combinative regimens cannot be drawn. Nevertheless, this review should emphasize the dire need for additional research in this area.
Author Contribution Statement
All authors conceptualized and designed the study. C.K. and P.P. conducted the literature search, extracted data, appraised the quality of evidence, and drafted the manuscript. All authors critically reviewed and edited the manuscript. All authors approved the final version of the manuscript, including the authorship list.
Acknowledgements
General: The research was supported by the Faculty of Medicine, Srinakharinwirot University, Thailand.
Ethical Declaration
No patients or participants were involved in this study.
Data Availability
Not applicable.
Study Registration
The protocol was registered in PROSPERO at: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020188432
Conflict of Interest
None declared.
References
- Aller R, Izaola O, Gómez S, et al. Effect of silymarin plus vitamin E in patients with non-alcoholic fatty liver disease A randomized clinical pilot study. Eur Rev Med Pharmacol Sci. 2015;19:3118–24. [PubMed] [Google Scholar]
- Amanullah I, Khan YH, Anwar I, et al. Effect of vitamin E in non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomised controlled trials. Postgrad Med J. 2019;95:601–11. doi: 10.1136/postgradmedj-2018-136364. [DOI] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD) Metab Clin Exp. 2016;65:1038–48. doi: 10.1016/j.metabol.2015.12.012. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology . 2012;55(6):2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- Chan AC. Partners in defense, vitamin E and vitamin C. Can J Physiol Pharmacol. 1993;71:725–31. doi: 10.1139/y93-109. [DOI] [PubMed] [Google Scholar]
- Dufour JF, Oneta CM, Gonvers JJ, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–43. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- El Hadi H, Vettor R, Rossato M. Vitamin E as a treatment for nonalcoholic fatty liver disease: Reality or Myth? Antioxidants. 2018;7:12. doi: 10.3390/antiox7010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersoz G, Gunsar F, Karasu Z, et al. Management of fatty liver disease with vitamin E and C compared to ursodeoxycholic acid treatment. Turk J Gastroenterol. 2005;16:124–8. [PubMed] [Google Scholar]
- Foster T, Budoff MJ, Saab S, et al. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: the St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106:71–7. doi: 10.1038/ajg.2010.299. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5 Rating the quality of evidence--publication bias. J Clin Epidemiol. 2011;64:1277–82. doi: 10.1016/j.jclinepi.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Hadzi-Petrushev N, Dimovska K, Jankulovski N, Mitrov D, Mladenov M. Supplementation with alpha-tocopherol and ascorbic acid to nonalcoholic fatty Liver disease’s statin therapy in men. Adv Pharmacol Sci. 2018;2018:4673061. doi: 10.1155/2018/4673061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–90. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- Ji H-F, Sun Y, Shen L. Effect of vitamin E supplementation on aminotransferase levels in patients with NAFLD, NASH, and CHC: Results from a meta-analysis. Nutrition. 2014;30:986–91. doi: 10.1016/j.nut.2014.01.016. [DOI] [PubMed] [Google Scholar]
- Katsagoni , CN , Georgoulis M, Papatheodoridis GV, Panagiotakos DB, Kontogianni MD. Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: A meta-analysis. Metab Clin Exp. 2017;68:119–32. doi: 10.1016/j.metabol.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Kawanaka M, Nishino K, Nakamura J, et al. Treatment of nonalcoholic steatohepatitis with vitamins E and C: a pilot study. Hepat Med. 2013;5:11–6. doi: 10.2147/HMER.S41258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Zhang C, Zhan Y-T. Nonalcoholic fatty liver disease cirrhosis: A Review of Its Epidemiology, Risk Factors, Clinical Presentation, Diagnosis, Management, and Prognosis. Can J Gastroenterol Hepatol. 2018;2018:2784537. doi: 10.1155/2018/2784537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: epidemiology and natural history. Rev Recent Clin Trials. 2014;9:126–33. doi: 10.2174/1574887109666141216111143. [DOI] [PubMed] [Google Scholar]
- Masarone M, Rosato V, Aglitti A, et al. Liver biopsy in type 2 diabetes mellitus: Steatohepatitis represents the sole feature of liver damage. PLoS One. 2017;12:e0178473. doi: 10.1371/journal.pone.0178473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masarone M, Rosato V, Dallio M, et al. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. oxid. Med Cell Longev. 2018;2018:9547613. doi: 10.1155/2018/9547613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murer SB, Aeberli I, Braegger CP, et al. Antioxidant supplements reduced oxidative stress and stabilized liver function tests but did not reduce inflammation in a randomized controlled trial in obese children and adolescents. J Nutr. 2014;144:193–201. doi: 10.3945/jn.113.185561. [DOI] [PubMed] [Google Scholar]
- Niki E. Interaction of ascorbate and alpha-tocopherol. Ann N Y Acad Sci U S A. 1987;498:186–99. doi: 10.1111/j.1749-6632.1987.tb23761.x. [DOI] [PubMed] [Google Scholar]
- Nobili V, Manco M, Devito R, et al. Effect of vitamin E on aminotransferase levels and insulin resistance in children with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2006;24:1553–61. doi: 10.1111/j.1365-2036.2006.03161.x. [DOI] [PubMed] [Google Scholar]
- Nobili V, Manco M, Devito R, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48:119–28. doi: 10.1002/hep.22336. [DOI] [PubMed] [Google Scholar]
- Perumpail BJ, Li AA, John N, et al. The role of vitamin E in the treatment of NAFLD. Diseases. 2018;6:86. doi: 10.3390/diseases6040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyzos SA, Kountouras J, Anastasiadis S, Doulberis M, Katsinelos P. Nonalcoholic fatty liver disease: Is it time for combination treatment and a diabetes-like approach? Hepatology. 2018;68:389. doi: 10.1002/hep.29897. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Chalasani KV, Kowdley A, et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Gosho M, Yamamoto T, et al. Vitamin E has a beneficial effect on nonalcoholic fatty liver disease: a meta-analysis of randomized controlled trials. Nutrition. 2015;31:923–30. doi: 10.1016/j.nut.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836–46. doi: 10.1002/hep.24001. [DOI] [PubMed] [Google Scholar]
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CL, Liang L, Fu JF, et al. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14:1598–602. doi: 10.3748/wjg.14.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Lei G-H, Fu L, et al. Association between dietary Vvitamin C intake and non-alcoholic fatty liver disease: A Cross-Sectional Study among Middle-Aged and Older Adults. PLoS One. 2016;11:e0147985–e. doi: 10.1371/journal.pone.0147985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Tao A, Zhang S, Deng Y, Chen G. Association between vitamin E and non-alcoholic steatohepatitis: A meta-analysis. Int J Clin Exp Med. 2015;8:3924–34. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.