Abstract
Background:
The global incidence of oral squamous cell carcinoma (OSCC) is on the rise with no improvement seen in survival rates. Tobacco consumption varies depending on geographic location, ethnicity and culture. The present case-controlled study aimed to determine the relative risk of OSCC for different tobacco consumption patterns in a selected Sri Lankan population.
Methods:
One hundred and five patients with histopathologically confirmed OSCC attending the National Cancer Institute (Apeksha Hospital) of Sri Lanka and 210 age and gender-matched controls from the community responded to an interviewer-administered questionnaire regarding their smoking and betel-quid chewing (with/ without smokeless tobacco) habits were included in the study. The odds ratios (OR) and 95% confidence intervals (CI) were calculated. p<0.05 was considered as statistically significant.
Results:
The overall risk of OSCC increased 2.93-fold for smokers. Those smoking two packets of cigarettes or more per day (OR=5.56; 95% CI-2.822-10.984; p=0.000) had more than double the risk of OSCC than those smoking 1-2 packets per day. Smoking for more than 20 years had a 3.4-fold risk of OSCC. Consumption of betel quid containing tobacco (smokeless tobacco) had a 4.26-fold higher risk for OSCC (OR=4.26; 95% CI-2.21-8.21; p=0.000), and the risk increased when all four ingredients (betel leaf, slaked lime, areca nut, and tobacco) were consumed together (OR=4.26; 95% CI-2.34-7.74; p=0.000). The combined effect from concurrent smoking and betel chewing emerged as the highest risk for OSCC (OR=15.34) which significantly exceeded the risks evident for the two habits practised in isolation from each other.
Conclusions:
Use of smokeless tobacco, consumption of all four ingredients together, duration of smoking, the number of cigarettes smoked per day and combined consumption of betel quid and smoking are significant risk factors in the development of OSCC among Sri Lankans.
Key Words: Risk, smoking, tobacco, Sri Lanka, oral squamous cell carcinoma
Introduction
The Global Cancer Observatory of the World Health Organization (WHO) reported 377,713 new cases of cancers involving the lip and oral cavity resulting in 177,757 deaths in the year 2020 (Globocan, 2020). Oral Squamous Cell Carcinoma (OSCC), commonly referred to as “oral cancer” is the commonest malignancy among middle-aged and older men in Sri Lanka and South Asia. It accounts for 9.3% mortality among all cancers in both genders (Jayasinghe et al., 2016). The high prevalence of oral cancer in South Central Asia is believed to be the result of greater exposure to risk factors (WHO, 2021).
The association between tobacco consumption and oral cancer is well recognised. Tobacco consumption in Sri Lanka has several different forms. It includes both smoked tobacco in the form of cigarettes, cigars and beedi (a cheap mini-cigar) and smokeless tobacco use through chewing, snuffing, and dipping dissolvable tobacco (Amarasinghe et al., 2018). Smokeless tobacco consumption in Sri Lanka is commonly integrated with betel chewing. In Sri Lanka, a single pack contains 12 cigarettes while a pack of beedi contains 20 individual mini-cigars. WHO data identified 29.4% of males and 0.1% of females in Sri Lanka as smokers (Amarasinghe et al., 2018b). A Global Youth Tobacco Survey conducted in Sri Lanka in 2015 revealed that 3.2% of males and 0.2% of females between the ages of 13-15 years had smoked tobacco at some point during the past 30 days giving an overall prevalence of 1.7%. Moreover, 2.4% of the students comprising of 4.2% of males and 0.5% of females of this age group reportedly consumed smokeless tobacco (WHO, 2016).
While it is assumed that the consumption of tobacco is probably responsible for the high prevalence of OSCC, research in Sri Lanka had been focusing more on its role in the development of oral potentially malignant disorders (OPMDs). Amarasinghe (2010 and 2018) reported community-based case-control studies conducted in rural Sri Lanka to identify risk factors and developed a risk index that could help to screen high-risk populations for OPMDs (Amarasinghe et al., 2010; Amarasinghe et al., 2018a). These investigations used only clinical criteria to define OPMDs and there was no histopathological confirmation for the “cases”. A matched case-controlled study using specific histopathological criteria to define the “cases” with a positive diagnosis for OSCC would be a more robust investigation of the claimed potential link between patterns of tobacco consumption and OSCC. Such investigations may yield data that could be used effectively in developing targeted preventive strategies to reduce the OSCC burden. The present matched case-controlled study aimed to investigate the tobacco consumption patterns and their association with the prevalence of OSCC in Sri Lanka.
Materials and Methods
A matched case-control study was performed to investigate the association of smoking and consumption of smokeless tobacco with OSCC among Sri Lankan adults. Appropriate approvals were obtained from the Ethics Review Committee of the Faculty of Medical Sciences, University of Sri Jayewardenepura (Ref 29/16) and National Cancer Institute (Apeksha Hospital), Maharagama, Sri Lanka.
Definition of cases and controls
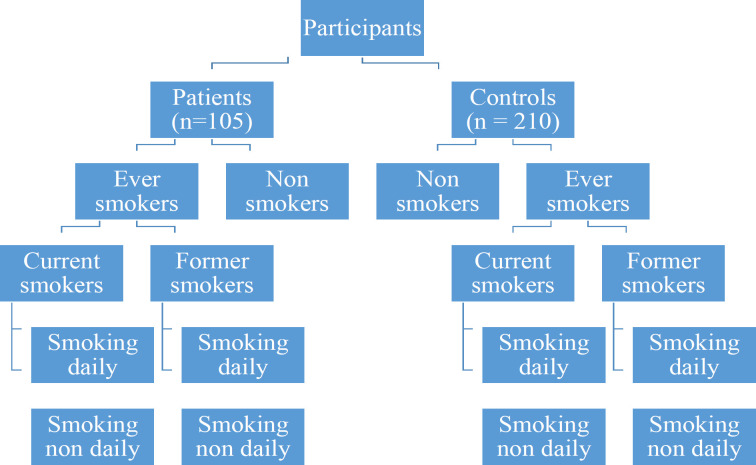
The sample size was calculated, based on the literature relating to case-control studies in two independent groups (Daly and Bourke, 2000). The study sample consisted of 105 patients diagnosed with histopathologically confirmed OSCC treated at the National Cancer Institute (patient group) The cases were selected while the patients were awaiting for the routine clinic or while receiving treatments at the ward. Two hundred and ten age (± 5 years from the age of each case) and gender-matched controls (control group) were selected from the community using open advertisement over twelve months. After obtaining the informed written consent, an interviewer- administered questionnaire was used for both patients and controls to collect data on tobacco consumption. Both cases and controls were selected randomly in order to reduce the confounding errors. All interviews were conducted by the primary investigator and trained five pre-intern medical officers who completed pre-survey calibration to minimize inter-observer variability. Information collected included type, quantity, pattern and frequency of smoking and smokeless tobacco use in association with betel chewing. The data collected using interviewer- administered questionnaire belongs to previous food and consumer habits of the individual participant before developing the disease. Therefore individual participant’s recall bias was expected during data collection. To minimize potential errors due to recall bias on various consumer habits, only the most widely consumed type/habits were considered in the study. The smokers in both the patient group and the control group were further categorised based on the definitions by the Centers for Disease Control and Prevention (CDC), Atlanta, United States. (Schoenborn et al., 2013). The classification is presented in Figure 1.
Figure 1.
Classification of Tobacco Smokers According to the Centers for Disease Control and Prevention (CDC), Atlanta, United States. (Ever smokers – Person who had smoked tobacco products in their lifetime; Never smokers - Persons who had not smoked a tobacco product (cigarette, cigar, beedi, pipe) in their lifetime; Current smokers - Ever smokers who were currently smoking tobacco products; Former smoker - Ever smokers who were not smoking tobacco products during the last 30 days; Daily smokers - Ever smokers who were smoking tobacco products daily; Non-daily smokers - Ever smokers who were smoking tobacco products on a non-daily basis)
Collected data were tabulated and analysed using Statistical Package for the Social Sciences (SPSS) version 16. The odds ratios (OR) with 95% confidence intervals (CI) were calculated to assess the risk factors. Statistical significance for associations was calculated using the chi-square test, with statistical significance set at p<0.05.
Results
The patient group (n=105) included 80 males and 25 females with histopathologically confirmed OSCC. Their age ranged from 35-85 years with a mean age of 60 ± 15.5 years. The mean age of the male patients was 61 ± 16 years while for female patients it was 55 ± 14 years. The control group (n=210, giving a case: control ratio of 1:2) had 160 males and 50 females within an age range of 40-82 years (mean 61 ± 12) with the males having a mean age of 61± 13 years and females 60 ± 12 years.
Smoking and OSCC
Table 1 shows the association between tobacco smoking and OSCC. In this study, 72.3% (76/105) of patients and 47.1% (99/210) of controls were smokers. ‘Ever smokers’ had a significantly higher risk of being diagnosed with an OSCC than ‘never smokers’ (OR=2.93; 95% CI-1.77-4.87; p=< 0.001). “Current daily-smokers” accounted for 30.3% (23/76) in the patients’ group and 28.3% (28/99) among controls while 26.3% (20/76) of patients and 17.2% (17/99) of controls were current ‘non-daily smokers’. Current daily smokers (OR=3.14; 95% CI-11.583-6.247; p=0.001) had a higher risk of being diagnosed with OSCC than former daily smokers (OR=1.66; 95% CI-0.812-3.512; p= 0.158). The current non-daily smokers had a higher risk (OR=4.503; 95% CI-2.096-9.676; p=< 0.001) compared to former non-daily smokers (OR=3.445; 95% CI-1.616-7.324; p=0.001). A gender difference in OSCC risk was evident with female smokers (OR=6.09; 95% CI-1.41-26.17; p=0.008) having a higher risk than males (OR=4.18; 95% CI-2.05-8.51; p=0.000). Cigarette smoking was more prevalent [40.9% (43/105)] in the OSCC patient group and showed a higher risk associated with OSCC (OR=3.35; 95% CI-1.88-5.99; p=0.000) when compared to beedi smoking. A dose-dependent OSCC risk increase was observed for smoking. Those smoking over two packs per day showed a significantly higher risk (OR=5.56; 95% CI-2.82-10.98; p=<0.001). Also evident was a time-dependent OSCC risk escalation, with smoking for more than 20 years showing the highest risk (OR=3.41; 95% CI-1.82-6.36; p=<0.001).
Table 1.
Association between Smoking and OSCC
| Smoking category | Patients | Controls | Odds Ratio (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Risk association among ever smokers | ||||||
| Ever smokers (Total) | 76 | 72.30 | 99 | 47.10 | 2.938 (1.771-4.876) | p = < 0.001 |
| Never smoked (Total) | 29 | 27.60 | 111 | 52.80 | 1 | |
| Breakdown of the ever smoker | ||||||
| Current daily smokers | 23 | 30.26 | 28 | 28.28 | 3.14 (1.583-6.247) | p=0.001 |
| Current non-daily smokers | 20 | 26.31 | 17 | 17.17 | 4.50 (2.096-9.676) | p = < 0.001 |
| Former daily smokers | 15 | 19.73 | 34 | 34.34 | 1.66 (0.812-3.512) | p =0.158 |
| Former non-daily smokers | 18 | 23.68 | 20 | 20.20 | 3.44 (1.616-7.324) | p= 0.001 |
| Gender-based risk association among ever smokers | ||||||
| Male ever smokers | 69 | 86.30 | 96 | 60.00 | 4.18 (2.05 – 8.51) | p = 0.000 |
| Never smoked | 11 | 13.80 | 64 | 10.00 | 1 | |
| Female ever smokers | 7 | 28.00 | 3 | 6.00 | 6.09 (1.41 – 26.17) | p = 0.008 |
| Never smoked | 18 | 72.00 | 47 | 94.00 | 1 | |
| Type of smoking among ever smokers | ||||||
| Cigarette smokers | 43 | 40.90 | 49 | 23.30 | 3.35 (1.88 – 5.99) | p = 0.000 |
| Never smoked (Total) | 29 | 27.60 | 111 | 52.80 | 1 | |
| Beedi smokers | 28 | 26.60 | 44 | 20.90 | 2.43 (1.30-4.55) | p= 0.005 |
| Never smoked (Total) | 29 | 27.60 | 111 | 52.80 | 1 | |
| Quantity of smoking | ||||||
| Less than 1 pack per day | 21 | 20.00 | 42 | 20.00 | 1.94 (0.985- 3.719) | p = 0.054 |
| 1-2 packs per day | 23 | 21.90 | 35 | 16.66 | 2.51 (1.292-4.897) | p = 0.006 |
| More than 2 packs per day | 32 | 30.47 | 22 | 10.47 | 5.56 (2.822-10.984) | p = <0.001 |
| Never smoked | 29 | 27.61 | 111 | 52.85 | 1 | |
| Duration of smoking | ||||||
| < 10 years of smoking | 21 | 20.00 | 35 | 16.66 | 2.29 (1.166-4.524) | p = 0.015 |
| 10-20 years of smoking | 22 | 20.95 | 27 | 12.85 | 3.12 (155-6.25) | p = 0.001 |
| >20 years of smoking | 33 | 31.42 | 37 | 17.61 | 3.41 (1.832- 6.361) | P = <0.001 |
| Not smoked | 29 | 27.61 | 111 | 52.85 | 1 | |
The study showed two peaks of age groups (30-49 and 70-89 years) associated with risk for OSCC among the ever smokers (Table 2). The highest risk was in the 80-89 years (OR=4.81; 95% CI-1.02-22.57; p=0.039) while a lower risk was seen in 50-59 (OR=2.13; 95% CI-0.584-7.776) and 60-69 (OR=1.92; 95% CI-0.666-5.553) age groups.
Table 2.
Association between the Age of the Smokers and OSCC
| Category | Patients – ever smokers. | Controls – ever smokers | Odd Ratio (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Age (years) | 30-39 | 9 | 11.84 | 11 | 11.11 | 4.09 (0.70-23.66) | p= 0.102 |
| 40-49 | 16 | 21.05 | 16 | 16.16 | 4.14 (1.41-12.17) | p= 0.008 | |
| 50-59 | 14 | 18.42 | 23 | 23.23 | 2.13 (0.584-7.776) | p = 0.247 | |
| 60-69 | 14 | 18.42 | 26 | 26.26 | 1.92 (0.666-5.553) | p=0.223 | |
| 70-79 | 13 | 17.10 | 14 | 14.14 | 3.09 (0.94-10.11) | p= 0.057 | |
| 80-89 | 10 | 13.15 | 9 | 9.09 | 4.81 (1.027-22.57) | p=0.039 | |
Smokeless tobacco consumption (betel chewing) and OSCC
Table 3 shows the effects of betel quid consumption and the combined effect of smoking and smokeless tobacco on OSCC risk. Over three quarters [78.0% (82/105)] of the OSCC patients’ group chewed betel quid with nearly a third [31.4 % (33/105)] using four ingredients in the quid; betel, areca nut, slaked-lime and tobacco. Similar to smoking, a dose-dependent association was observed for betel quid chewing: two quids per day showed a 5.38-fold risk of being diagnosed with OSCC. Furthermore, those who habitually kept the betel quid in their mouth during sleep had a 3.21-fold higher risk. More than half of the patients [54.2% (57/105)] used tobacco in both smoked (cigarettes or beedi) and smokeless (with betel quid chewing) forms. The combined use of smoking and betel quid had the greatest risk, a massive 15.34-fold risk (95% CI-5.27-44.67; p=0.000) than those who did not practice either of the two habits.
Table 3.
Association between Betel Chewing and OSCC
| Cases | Controls | Odd ratio (95% CI) | p value | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | ||||
| Betel chewing | Betel only | 9 | 8.57 | 13 | 6.19 | 3.13 (1.19-8.19) | p=0.016 |
| Betel leaf, slaked-lime and areca nut | 22 | 20.95 | 34 | 16.19 | 2.92 (1.45-5.89) | p=0.002 | |
| Betel leaf, slaked-lime, and tobacco | 18 | 17.14 | 24 | 11.42 | 3.39 (1.58-7.25) | p=0.001 | |
| Betel leaf, slaked-lime, areca nut and tobacco | 33 | 31.42 | 35 | 16.66 | 4.26 (2.21-8.21) | ||
| Never | p=0.000 | ||||||
| 23 | 104 | 1 | |||||
| Consumption of betel quid per day | 1 pack | 30 | 36.58 | 47 | 45.63 | 2.96 (1.56-5.64) | p=0.001 |
| 1-2 quid | 30 | 36.58 | 37 | 35.92 | 3.77 (1.95-7.29) | p=0.000 | |
| >2 quid | 22 | 26.82 | 19 | 18.44 | 5.38 (2.51-11.53) | p=0.000 | |
| Overnight keeping the mixture in the mouth | 46 | 43.80 | 41 | 19.50 | 3.21 (1.92-5.37) | p=0.000 | |
| Combined use of smoking and betel quid | |||||||
| Combined use of smoking and betel chewing | 57 | 54.20 | 65 | 30.90 | 15.34 (5.27-44.67) | p=0.000 | |
| Not smoking and betel chewing | 4 | 0.01 | 70 | 33.30 | 1 | ||
Discussion
There is a scarcity of case-controlled studies reporting OSCC risk factors in Sri Lanka. Despite the limitation of recall bias, the present study with case: control ratio of 1:2 provides stronger insights into the risk association between tobacco consumption and OSCC which is of value in cancer prevention strategy formulations.
In the present study, a significantly high proportion of the study population admitted to have the habit of smoking (Table 1). Cigarette smoking is identified as a principal risk factor for the development of OSCC and combined with advanced age, the risk of cancer development is amplified (Wünsch-Filho, 2002). Cigarette smoke contains many carcinogenic compounds. These mainly include benzene (Centers for Disease Control Prevention, 2010) and hydrogen cyanide (Morgan et al., 2017). In the present study, being an ‘ever smoker’ had a 2.93-fold increased risk of developing OSCC. Similar risk associations (OR=2.2 and OR=2.5) were reported from Papua New Guinea and a hospital-based case-control study conducted in India (Thomas et al., 2007); (Gowda et al., 2020). A meta-analysis based on published case-control studies reported tobacco smoking and oral cancer risk varying between 1.36 to 20.8 (Sadri and Mahjub, 2007).
A study from two regional cancer hospitals in South India reported an OR of 1.76 for former smokers and 2.90 for current smokers (Znaor et al., 2003). Interestingly, among “ever smokers” the current non-daily smokers in the present study had the highest risk (OR=4.5). On the contrary, a study from Papua New Guinea has shown non-daily smokers having a lower risk (OR=0.89; 95% CI-0.34-2.32) compared to current daily smokers (OR=2.50; 95% CI-1.35-4.61) (Thomas et al., 2007). Furthermore, a study of Chinese males showed no risk difference among former smokers (OR=1.47; 95% CI-1.23-2.56) and current smokers (OR=1.45; 95% CI-0.145-4.19) (Wang et al., 2015). Our observed high risk of OSCC in current non-daily smokers could be the result of a cumulative effect of their smoking rather than the frequency. Intermittent smoking has been shown to cause more damaging effects than continuous smoking because they tend not to consider themselves “smokers” and consequently, can remain unidentified (Schane et al., 2010). In the present study, the current smokers showed a greater risk compared to former smokers. In a Brazilian study which also showed current smokers (OR=4.45; 95% CI-2.79-7.07) have a greater risk than former smokers (OR=1.38; 95% CI-0.88-2.16) aligning in conformity with the present study’s findings (Andrade et al., 2015).
Oral cancer is the commonest malignancy among Sri Lankan males (NCCP, 2015). Interestingly, among “ever smokers” in the present study, females had a higher risk of OSCC than males (Table 1), aligning with the findings of an Indian study (Gowda et al., 2020). Females reportedly are at an increased risk of lung, oral and oropharyngeal cancer compared to males who had similar cigarette smoking exposure levels (Risch et al., 1993; Neugut and Jacobson, 2006). It could be due to the induction of Aldo-keto reductases, enzymes linked to polycyclic aromatic hydrocarbon induced genotoxicity, in the oral mucosa of women more than in men (Boyle et al., 2010). However, the exact underlying mechanisms for this observed gender-dependent difference remains poorly understood.
In the present study, the highest risk for OSCC was in the 80-89 age group (OR=4.81) with two slightly lower peaks in 30-39 (OR=4.09) and 40-49 (OR=4.14) age groups. Two Indian studies reported more younger OSCC patients than older patients (Sherin et al., 2008); (Tandon et al., 2017). The higher risk among youngsters could be due to the increasing trend in smoking among the younger population as shown by the Global Youth Tobacco Survey in Sri Lanka (WHO, 2016). Reportedly, most cigarette users (65.6%; males 66.6% and females 49.4%) had their first smoking experience when aged between 13 to 15 years. Furthermore, the WHO report highlights a similar pattern for beedi smoking (total of 63.7%). Perhaps this explains the present study’s OSCC peak in younger age groups since they could be getting exposed to carcinogenic substances from ages as young as 13-15 years and presenting later with cancers around 30-49 years of age. However, a study conducted in a Chinese population reported the highest OR (3.98) among the 50-59 years of age (Wang et al., 2015). In the present study, we observed a drop in OSCC risk in middle age, potentially the result of lesser indulgence in tobacco use due to a more responsible lifestyle and employment careers. However, retirement pushes many into some social isolation and a sedentary lifestyle. Some individuals may resort to smoking and betel chewing to compensate resulting in a higher OSCC risk downstream.
The present study showed cigarette smokers having a greater risk than beedi smokers. Studies from India had shown conflicting results with some reporting higher risks with cigarette smoking while others showing the opposite. Globally, 96% of smokers use cigarettes and in Sri Lanka too, the vast majority are cigarette smokers (Alcohol & Drug Information Centre, 2020). This is despite the average price of a pack of beedi (around LKR 160.00 - during the study period) being considerably cheaper compared to the average price of a pack of cigarettes (LKR 1,300.00 - during the study period).
The current study showed a dose-dependent risk increment in OSCC where the risk increases from 1.94 to 5.56 when consuming 1 pack per day to more than 2 packs per day. These findings corroborate the results of two other studies reported from Italy and Spain (Tenore et al., 2020) (Moreno-Lopez et al., 2000).
Consumption of smokeless tobacco (chewing betel quid containing tobacco, lime, and areca nut) has shown a strong association with oral cancer. In Sri Lanka, 15.8% use smokeless tobacco products which include 8.6% of youth (Non Communicable Disease Risk Factor Survey Sri Lanka, 2015). Betel leaf has a strong association with Sri Lankan culture. Offering betel leaves to adults and clergy is considered a mark of respect and is a frequent occurrence in many cultural and religious functions. Smokeless tobacco use along with betel quid is highly prevalent, more so in rural Sri Lanka and among people over 65 years of age. For many elderly people, betel chewing is an activity to mitigate loneliness and boredom and many rural folks keep the quid in the mouth for longer periods. Chewing tobacco mixture with slaked-lime and keeping the mixture in the mouth overnight had been previously reported as a risk factor for OSCC (Jasotharan et al., 2014).
The composition of betel-quid may vary from one country to another and also within different communities in a country. Betel chewing without tobacco was reported to have a 2.1 fold risk for OSCC which increased to 8.7 when tobacco was added (Znaor et al., 2003). In the present study, consumption of betel leaf, slaked lime, and areca nut showed an OR of 2.92, and the addition of tobacco to these three ingredients increased the risk to 4.26-fold. Furthermore, chewing more than two quids per day had an OR of 5.3 for OSCC. Betel and areca nut chewing is considered the 4th most commonly used addictive substance after tobacco, alcohol, and caffeine (Healthline Media, 2020).
Betel chewing with tobacco and slaked-lime may have a stronger effect than smoking, presumably due to the direct contact of carcinogens with the oral epithelium. However, the etiologic role of these chemicals is not well understood. Regular betel and areca nut chewing may damage the oral epithelial cell lining by inducing chronic irritation and inflammation (Evaluation of Carcinogenic Risks to Humans, 2004). Areca nut contains arecoline which is known to have carcinogenic properties (Shah et al., 2012). Betel quid chewing predisposes to oral Candida colonization, thus altering the composition of the oral microbiome (Hernandez et al., 2017).
In the present study, the combined effect from concurrent smoking and betel chewing emerged as the highest risk for OSCC (OR=15.34) which significantly exceeded the risks evident for the two habits practised in isolation from each other. Although two studies from India reported the relative risk to be in the range of 3.8-3.94 another study from Taiwan reported a much greater risk (OR=26.56; 95% CI-14.52-48.58; p<.001) endorsing the potential synergistic effect of combined smoking and smokeless tobacco use as a risk factor for OSCC (Subapriya et al., 2007; Lin et al., 2011; Gowda et al., 2020).
The present study confirms a strong association between tobacco consumption and OSCC and importantly highlights the cumulative risk of concurrent tobacco smoking and betel chewing practised by many in Sri Lanka. The evident dose and time-dependent cumulative risk for both smoking and smokeless tobacco use further endorse these associations. Furthermore, the prevalence peaks evident in the younger age groups concern and merits serious consideration and further research to investigate the aforementioned associations in greater depth.
The preventive programmes against OSCC in Sri Lanka should address the cultural association of betel chewing in the Sri Lankan way of life. Programmes cognisant of cultural sensitivity and adopting a unified approach to target both smoking and betel quid chewing and paying more attention to deliver and reinforce health messages to the younger population, in particular, would help to sharpen the weapons against the deadly disease of oral cancer.
Author Contribution Statement
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Acknowledgments
Funding Statement
This paper includes the outcome of a subsection of a large study funded by university research grants, University of Sri Jayewardenepura ASP/01/RE/MED/2016/46, ASP/01/RE/MED/2017/62 and a grant from the Centre for Cancer Research (003/2017), University of Sri Jayewardenepura Sri Lanka.
The authors appreciate the efforts of all health professionals and staff who took their time to help us gather information for this case control study. The authors also give special thanks to all the participants in this study.
Ethical Approval
The Ethics Review Committee of the Faculty of Medical Sciences, University of Sri Jayewardenepura (Ref 29/16) and National Cancer Institute (Apeksha Hospital), Maharagama, Sri Lanka approved the study, and all participants signed an informed consent agreement.
Availability of data
All data generated or analysed during this study are included in this published article; the primary data can be obtained from the corresponding authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Alcohol & Drug Information Centre. Alcohol & Drug Information Centre. 2020. [Google Scholar]
- Amarasinghe A, Usgodaarachchi U, Johnson N, et al. High prevalence of lifestyle factors attributable for oral cancer, and of oral potentially malignant disorders in rural Sri Lanka. Asian Pac J Cancer Prev. 2018a;19:2485. doi: 10.22034/APJCP.2018.19.9.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarasinghe H, Johnson N, Lalloo R, et al. Derivation and validation of a risk-factor model for detection of oral potentially malignant disorders in populations with high prevalence. Br J Cancer. 2010;103:303–9. doi: 10.1038/sj.bjc.6605778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amarasinghe H, Ranaweera S, Ranasinghe T, et al. Economic cost of tobacco-related cancers in Sri Lanka. Tob Control. 2018b;27:542–6. doi: 10.1136/tobaccocontrol-2017-053791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade JOM, Santos CAdST, Oliveira MC. Associated factors with oral cancer: a study of case control in a population of the Brazil’s Northeast. Rev Bras Epidemiol. 2015;18:894–905. doi: 10.1590/1980-5497201500040017. [DOI] [PubMed] [Google Scholar]
- Boyle JO, Gümüş ZH, Kacker A, et al. Effects of cigarette smoke on the human oral mucosal transcriptome. Cancer Prev Res (Phila) 2010;3:266–78. doi: 10.1158/1940-6207.CAPR-09-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control Prevention. How tobacco smoke causes disease: The biology and behavioral basis for smoking-attributable disease: A report of the surgeon general. 2010 [PubMed] [Google Scholar]
- Daly L, Bourke GJ. Interpretation and Uses of Medical Statistics. Fifth Edition. John Wiley & Sons; 2000. [Google Scholar]
- Evaluation of Carcinogenic Risks to Humans. Betel-quid and areca-nut chewing and some areca-nut derived nitrosamines. IARC Monogr Eval Carcinog Risks Hum. 2004;85:1–334. [PMC free article] [PubMed] [Google Scholar]
- Globocan. Cancer incidence and mortality statistics worldwide and by region. 2020. [Google Scholar]
- Gowda KL, Vijay C, Lokesh V, et al. A Hospital Based Case Control Study on Oral Cancer: KMIO (Regional Cancer Center) Experience. J Med Sci Clin Res. 2020;8:1022–31. [Google Scholar]
- Healthline Media. Healthline. 2020. [Google Scholar]
- Hernandez BY, Zhu X, Goodman MT, et al. Betel nut chewing, oral premalignant lesions, and the oral microbiome. PLoS One. 2017;12:e0172196. doi: 10.1371/journal.pone.0172196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasotharan V, Beumy Saluja N, Fathima Nahthiya F, et al. Descriptive study on socio-demographic and risk factors associated with the oral cancers, Batticaloa district. J Blood Disorders Transf. 2014;5:2. [Google Scholar]
- Jayasinghe R, Sherminie L, Amarasinghe H, et al. Level of awareness of oral cancer and oral potentially malignant disorders among medical and dental undergraduates. Ceylon Med J. 2016:61. doi: 10.4038/cmj.v61i2.8289. [DOI] [PubMed] [Google Scholar]
- Lin W-J, Jiang R-S, Wu S-H, et al. Smoking, alcohol, and betel quid and oral cancer: a prospective cohort study. J Oncol. 2011:2011. doi: 10.1155/2011/525976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Lopez L, Esparza-Gomez G, Gonzalez-Navarro A, et al. Risk of oral cancer associated with tobacco smoking, alcohol consumption and oral hygiene: a case-control study in Madrid, Spain. Oral Oncol. 2000;36:170–4. doi: 10.1016/s1368-8375(99)00084-6. [DOI] [PubMed] [Google Scholar]
- Morgan JC, Byron MJ, Baig SA, et al. How people think about the chemicals in cigarette smoke: a systematic review. J Behav Med. 2017;40:553–64. doi: 10.1007/s10865-017-9823-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCP. National Cancer Control Programme. 2015 [Google Scholar]
- Neugut AI, Jacobson JS. Women and lung cancer: gender equality at a crossroad? JAMA. 2006;296:218–9. doi: 10.1001/jama.296.2.218. [DOI] [PubMed] [Google Scholar]
- Non Communicable Disease Risk Factor Survey Sri Lanka. Non Communicable Disease Risk Factor Survey Sri Lanka. 2015. [Google Scholar]
- Risch HA, Howe GR, Jain M, et al. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol. 1993;138:281–93. doi: 10.1093/oxfordjournals.aje.a116857. [DOI] [PubMed] [Google Scholar]
- Sadri G, Mahjub H. Tobacco smoking and oral cancer: a meta-analysis. J Res Health Sci. 2007;7:18–23. [PubMed] [Google Scholar]
- Schane RE, Ling PM, Glantz SA. Health effects of light and intermittent smoking: a review. Circulation. 2010;121:1518–22. doi: 10.1161/CIRCULATIONAHA.109.904235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah G, Chaturvedi P, Vaishampayan S. Arecanut as an emerging etiology of oral cancers in India. Indian J Med Paediatr Oncol. 2012;33:71. doi: 10.4103/0971-5851.99726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherin N, Simi T, Shameena P, et al. Changing trends in oral cancer. Indian J Cancer. 2008;45 doi: 10.4103/0019-509x.44063. [DOI] [PubMed] [Google Scholar]
- Subapriya R, Thangavelu A, Mathavan B, et al. Assessment of risk factors for oral squamous cell carcinoma in Chidambaram, Southern India: a case–control study. Eur J Cancer Prev. 2007;16:251–6. doi: 10.1097/01.cej.0000228402.53106.9e. [DOI] [PubMed] [Google Scholar]
- Tandon P, Dadhich A, Saluja H, et al. The prevalence of squamous cell carcinoma in different sites of oral cavity at our Rural Health Care Centre in Loni, Maharashtra–a retrospective 10-year study. Contemp Oncol. 2017;21:178. doi: 10.5114/wo.2017.68628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenore G, Nuvoli A, Mohsen A, et al. Tobacco, alcohol and family history of cancer as risk factors of oral squamous cell carcinoma: Case-Control Retrospective Study. Appl Sci. 2020;10:3896. [Google Scholar]
- Thomas SJ, Bain CJ, Battistutta D, et al. Betel quid not containing tobacco and oral cancer: A report on a case–control study in Papua New Guinea and a meta-analysis of current evidence. Int J Cancer. 2007;120:1318–23. doi: 10.1002/ijc.22304. [DOI] [PubMed] [Google Scholar]
- Wang X, Xu J, Wang L, et al. The role of cigarette smoking and alcohol consumption in the differentiation of oral squamous cell carcinoma for the males in China. J Cancer Res Ther. 2015;11:141. doi: 10.4103/0973-1482.137981. [DOI] [PubMed] [Google Scholar]
- WHO. Oral cancer. 2021. [Google Scholar]
- Wünsch-Filho V. The epidemiology of oral and pharynx cancer in Brazil. Oral Oncol. 2002;38:737–46. doi: 10.1016/s1368-8375(02)00066-0. [DOI] [PubMed] [Google Scholar]
- Znaor A, Brennan P, Gajalakshmi V, et al. Independent and combined effects of tobacco smoking, chewing and alcohol drinking on the risk of oral, pharyngeal and esophageal cancers in Indian men. Int J Cancer. 2003;105:681–6. doi: 10.1002/ijc.11114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article; the primary data can be obtained from the corresponding authors.