Abstract
Background:
Patients undergoing surgical treatment for solid tumors are at risk for development of secondary lymphedema due to intraoperative lymphatic vessel injury. The damaged lymphatic vessels fail to adequately regenerate and lymphatic obstruction leads to fluid and protein accumulation in the interstitial space and chronic lymphedema develops as a result. There are currently no effective pharmacological agents that reduce the risk of developing lymphedema or treat pre-existing lymphedema, and management is largely palliative. The present study investigated the efficacy of various 9-cis retinoic acid (9-cis RA) dosing strategies in reducing postsurgical lymphedema by utilizing a well-established mouse tail lymphedema model.
Methods and Results:
Short-duration treatment with 9-cis RA did not demonstrate a significant reduction in postoperative tail volume, nor an improvement in lymphatic clearance. However, long-term treatment with 9-cis RA resulted in decreased overall tail volume, dermal thickness, and epidermal thickness, with an associated increase in functional lymphatic clearance and lymphatic vessel density, assessed by LYVE-1 immunostaining, compared with control. These effects were seen at the site of lymphatic injury, with no significant changes observed in uninjured sites such as ear skin and the diaphragm.
Conclusions:
Given the reported results indicating that 9-cis RA is a potent promoter of lymphangiogenesis and improved lymphatic clearance at sites of lymphatic injury, investigation of postoperative 9-cis RA administration to patients at high risk of developing lymphedema may demonstrate positive efficacy and reduced rates of postsurgical lymphedema.
Keywords: lymphedema, post-surgical lymphedema, lymphangiogenesis, 9-cis retinoic acid, mouse tail lymphedema
Introduction
Lymphedema is a debilitating and disfiguring disease resulting from insufficient lymphatic drainage and subsequent accumulation of lymphatic fluid. Patients commonly present with a combination of swelling and firmness of the affected region, causing symptoms of heaviness, pain, and recurrent infections. Histologic changes seen in lymphedematous tissues include dilated lymphatic vessels, inflammatory cell infiltration, increased skin and subcutaneous tissue thickness, and increased collagen deposition with fibrosis.1–3
Etiologies of this progressive disease are broad and include congenital, surgical, radiation, or infection factors.4,5 Despite efforts to create varied approaches for the treatment and prevention of lymphedema, the incidence of this disease has been on the rise due to an increase in cancer survivorship and average life expectancy.
Patients with solid tumors such as breast cancer, gynecological or urological malignancies, melanoma, and sarcoma are at significant risk for cancer-associated lymphedema.6 As part of the surgical treatment for these cancers, lymphatic channels may be damaged, and these drainage pathways must spontaneously regenerate to restore the lymphatic circulation.
In ∼30% of patients, adequate lymphatic regeneration does not occur, which results in progressive accumulation of fluid and proteins in the interstitial space.7 This lymphatic obstruction is further associated with inflammation, adipose cell proliferation, and eventual tissue fibrosis.7,8 The chronic nature of the disease can cause subsequent disfigurement, loss of mobility and function, and increased risk of infection and serve as a precursor to malignant transformation.
There are no effective drug therapies approved to mitigate the risk of or treat established lymphedema. The mainstay of clinical disease management is focused on relieving symptoms through modalities such as massage, manual lymphatic drainage, exercise, pneumatic pumps, and compression bandage therapy. The long-term compliance with these treatments is poor because they are time-consuming, disruptive to activities of daily living, and costly.9,10 Efforts have been made to minimize the incidence of secondary lymphedema, such as the development of algorithms to minimize the morbidity of sentinel lymph node biopsy11 as well as the refinement of radiotherapy to minimize risk to lymph node basins.
Nevertheless, lymphedema remains a significant and growing burden, fueled in part by the increased rates of cancer survivorship.12 For patients who have reached end-stage lymphedema, characterized by extensive adipose deposition, fibrosis, and recurrent episodes of cellulitis, treatment options are limited and even less effective. Thus, the best approach to have an impact on this disease is to identify patients at risk early after lymphatic injury and mitigation develop strategies based on our understanding of lymphedema pathophysiology.
9-cis RA is an endogenous retinoic acid isoform that has been studied in various disease processes and has shown therapeutic potential in conditions ranging from skin diseases to solid organ tumors.13,14 Topical 9-cis RA (alitretinoin) is approved by the U.S. Food and Drug Administration (FDA) for human use for Kaposi's sarcoma-associated skin lesions and is available as an oral suspension in the United Kingdom for treatment of refractory eczema. Studies have shown this drug to be generally well tolerated, with a benign side effect profile, even with high-dose systemic administration.15
In previous studies, we demonstrated that 9-cis retinoic acid (9-cis RA) is a potent promoter of lymphangiogenesis that stimulates lymphatic endothelial cell (LEC) proliferation, migration, and tubule formation.16 Using preclinical murine lymphedema models, we have shown that treatment with RA significantly accelerates lymphatic regeneration following injury, restores functional lymphatic drainage, and prevents lymphedema development.17 Other studies have demonstrated the role of retinoic acid exposure in increasing lymphatic progenitor formation and upregulating LYVE-1 and VEGFR-3 in embryonic tissue.18
Additionally, retinoic acid has been shown to be necessary for neural crest differentiation into LECs, and blockade of retinoic acid signaling results in abnormal lymphatic vessel development.19 These findings suggest that 9-cis RA would be an excellent drug candidate to be repurposed for prevention of secondary cancer-associated lymphedema. To advance this goal, we investigated the effect of modifying the treatment duration. Previously, 9-cis RA was administered to experimental animals through daily intraperitoneal injection for 6 weeks.16
While treatment-related morbidity was acceptable, we sought to determine whether shorter durations of therapy were sufficient to determine the minimal effective dose. In addition, we wished to determine if 9-cis RA induced lymphangiogenesis specifically at the site of lymphatic injury or whether there were off-target effects on quiescent LECs at distant anatomic sites.
Methods
Animals
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 8th edition, 2011) and the Animal Welfare Act. Our protocol was approved by the University of Southern California Institutional Animal Care and Use Committee. Fifty adult BALB/c mice, 12–14 weeks old and weighing 21–30 g, were purchased from Jackson Laboratories (Jackson, Bar Harbor, ME). Animals were maintained in a temperature- and light-controlled environment with ad libitum access to a normal chow diet and water.
Mouse tail secondary lymphedema model
We modified a well-described lymphedema tail model. Mice were anesthetized using 2.5% isoflurane, and a 5-mm-wide circumferential skin excision was made 20 mm from the base of the tail. Underlying lymphatic vessels were visualized using isosulfan blue and ligated under a dissecting surgical microscope.7,20,21 Daily bacitracin antibiotic ointment was applied to the surgical site to prevent wound infection and desiccation.
Experimental design
Animals were randomly assigned to one of five groups following surgery. Mice received daily intraperitoneal injections comprising 100 μL of vehicle solution (90 μL of sunflower seed oil/10 μL of 100% ethanol) or 0.08 mg/kg 9-cis RA dissolved in 100 μL of vehicle solution.22 The treatments administered to each group were as follows: control group: vehicle for 45 days following surgery; 7-day group: 9-cis RA for 7 days; 14-day group: 9-cis RA for 14 days; 45-day group: 9-cis RA for 45 days; and delay group: 9-cis RA for 7 days beginning 1 week after the surgery.
The treatment durations were chosen based on the findings of our previous experiment, which demonstrated the greatest increase in swelling during the first postoperative week, and a plateau in percent change of tail volume following 14 days of treatment.16 We thus hypothesized that treatment throughout the first week would be sufficient to blunt the initial spike in tail volume increase, preventing postsurgical lymphedema. The lack of significant change in tail volume following 14 days of treatment in this experiment led us to believe that 14 days may be a sufficient treatment period to prevent postsurgical lymphedema. Animals that developed surgical site infections or tail necrosis were sacrificed and excluded from the analysis.
Animals were photographed every 7 days for a 6-week duration, and the tail diameter was measured using ImageJ (National Institutes of Health, Bethesda, MD). Measurements were taken at 5-mm intervals from the distal-most edge of the wound for a total of seven measurements per animal per time point. Total distal tail volume was calculated using the truncated cone formula.17,23 Percent change in distal tail volume was recorded, analyzed, and plotted using GraphPad Prism 7 (GraphPad Software, Inc.).
Indocyanine green lymphangiography
On day 45, animals were anesthetized using 2.5% isoflurane, and 5 μL of 2.5 mg/mL indocyanine green (ICG) was injected into the distal end of each animal's tail 15 minutes before imaging. The SPY Elite Fluorescence Imaging System (Novadaq Technologies, BC, Canada) was used to capture fluorescent images and heat maps of the tails at 0, 1, 2, 3, 6, 12, 24, and 48 hours following ICG injection.
Capture conditions were kept constant throughout the experiment. The fluorescence intensity of the tails at each time point was quantified using a previously described method for measuring cell fluorescence using ImageJ.24,25 Values were recorded and plotted using GraphPad Prism 7. Percent change in fluorescence intensity from peak fluorescence uptake at 6 hours was used to measure ICG clearance.
Tissue processing and staining
Tail specimens were collected on postoperative day 47. Specimens were harvested to include 1 cm proximal and 1 cm distal to the wound. Tails were inked to enable proper orientation, fixed in 10% neutral buffered formalin overnight, decalcified in 10% EDTA (pH: 7.3) for 6 days at 4°C, embedded in paraffin, and sectioned at 3–5 μm, then stained with hematoxylin and eosin (H&E) or through immunohistochemical techniques.
Sections were deparaffinized and underwent antigen retrieval with sodium citrate buffer, incubated overnight with the primary antibody (1:200) at 4°C, washed, and incubated with a suitable secondary antibody (1:200), then stained with ImmPACT DAB Substrate (Vector Laboratories, Inc., Burlingame, CA) and counterstained with Delafield hematoxylin (Sigma-Aldrich) diluted 1:4. Podoplanin (clone 8.1.1, sc-53533; Santa Cruz Biotechnology, Inc., Dallas, TX) was used to immunohistochemically mark lymphatics in paraffin-embedded tail sections.
Bilateral animal ears were harvested, removed of all hair using depilatory cream, and fixed in 4% paraformaldehyde. The ears were split between the inner and outer layers under a dissecting microscope and the cartilage removed before immunofluorescence staining. The whole mounted specimens were subsequently stained with anti-LYVE-1 (rabbit anti-mouse; AngioBio Co.) and anti-CD-31 (rat anti-mouse; BD Biosciences) antibodies after washing with PBS, permeabilized with 0.5% Triton X-100 (Sigma-Aldrich), and incubated with the primary antibody (1:500) overnight. Green secondary antibodies (1:200 donkey anti-rabbit Alexa Fluor 488; Thermo Fisher Scientific, Canoga Park, CA) were used to stain for LYVE-1.
Slides were counterstained with hematoxylin and mounted. All images were obtained with a Keyence BZ-X700 microscope (Itasca, IL), and epidermal and dermal thicknesses were measured using ImageJ. The thickest part of the epidermis and dermis was measured in five high-power fields per section, as previously described. On immunohistochemistry slides, podoplanin-stained vessels in the dermis and subcutis were quantified using ImageJ.
Two high-power fields per section were randomly selected and analyzed by a blinded reviewer. The reviewer scored the number of podoplanin-stained vessels and the area of the dermis and subcutaneous tissue in each image. Lymphatic vessel density was calculated from the absolute lymphatic number normalized to the dermal and subcutaneous area measured (lymphatic vessels/mm2).24
For immunofluorescence-stained slides, LYVE-1-stained vessels were quantified with ImageJ to assess lymphatic density, lymphatic vessel width, interlymphatic vessel distance, branching points, loops, and blind-ended sacs.
India ink staining of diaphragms
On postoperative day 47, each animal was intraperitoneally injected with 5cc of India ink, which was diluted twofold with distilled water. Ten minutes after India ink injection, the animals were euthanized, and the diaphragms were harvested in their entirety. The specimens were then rinsed under running tap water for 1 minute and mounted on the stage of a dissecting microscope equipped with a digital camera. The whole-mount specimen, excluding the central tendon, was analyzed for passive lymphatic absorption of India ink utilizing the percent stained area on ImageJ.
Statistics
All statistical analyses were performed with GraphPad Prism 7. Analysis of variance and unpaired Student's t-tests were used to compare treatments against controls at each measurement interval.
Results
Effect of early withdrawal or delayed initiation of 9-cis RA on mouse tail lymphedema
Previously, we demonstrated that daily systemic administration of 9-cis RA (0.08 mg/kg) for 42 days significantly prevents development of lymphedema after lymphatic disruption. Although 9-cis RA is a safe FDA-approved drug, long-term systemic therapy can have unintended side effects such as alopecia, exfoliative dermatitis, and hyperlipidemia. In addition, like other retinoids, 9-cis RA is teratogenic.15
We therefore wished to determine if a limited window of administration was sufficient for 9-cis RA to mitigate lymphedema after surgical injury. We were also interested in whether delayed administration of 9-cis RA could be effective as this may be an important issue in clinical translation (i.e., if a patient undergoes lymphadenectomy and is not started on 9-cis RA immediately postop, would 9-cis RA be effective if started weeks later?).
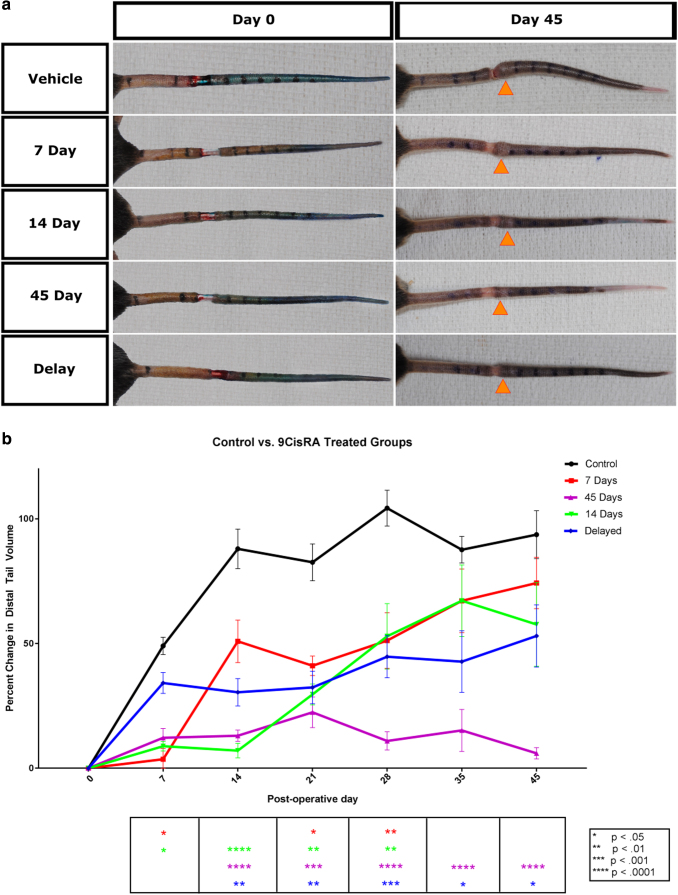
To address these questions, we induced lymphedema using an established mouse tail model and randomly assigned samples to the following treatment groups: control; 7 days; 14 days; 7-day delay; and 45 days. The percent changes in tail volumes from presurgery were recorded until day 42 for all groups. Animals treated with daily 9-cis RA treatment had significantly lower tail volumes than controls (Fig. 1a). Tail volumes were highly significant beginning the second week of the treatment and showed a significant difference throughout the entire duration of the experiment.
FIG. 1.
Effect of 9-cis retinoic acid on lymphedema. (a) Representative photos of mouse tails on the day of surgical excision of lymphatics and on the final day of the experiment. Orange arrows indicate the location of the largest tail circumference on day 45 in each group. (b) Graphical representation of percent change in tail volume from baseline volume as a function of time. Graph compares the increase in tail volume of the control group with delay, 7-, 14-, and 45-day groups. Values are reported as mean ± standard error.
Animals treated for 7 and 14 days showed early differences from the control group; however, tail volumes approached levels similar to the untreated control group past the 4-week time point. Interestingly, the 7-day delay group showed a statistically significant decrease in tail volumes from day 14 onward (Fig. 1b and Tables 1 and 2).
Table 1.
Comparisons of Mean Values Between the Control Group and 9-cis Retinoic Acid-Treated Groups
| 9-cis Retinoic acid |
|||||
|---|---|---|---|---|---|
| Control | 7 Days | 14 Days | 45 Days | Delay | |
| Percent increase in tail volume (at day 42) | 93.67% | 74.27% | 57.65% | 59.93% | 53.04% |
| Dermal thickness (μm) | 679.5 | 616.6 | 352.4 | 525.2 | 502 |
| Epidermal thickness (μm) | 153.9 | 109.2 | 52.07 | 83.14 | 73.51 |
| ICG clearance (at 48 hours) | −39.68% | −41.91% | −76.69% | −74.72% | −76.6% |
| Tail lymphatic density (vessels/μm2) | 2.977 × 10−5 | 4.954 × 10−5 | 7.821 × 10−5 | 8.754 × 10−5 | 5.814 × 10−5 |
ICG, indocyanine green.
Table 2.
Summary of Statistical Analysis Results Comparing the Control Group with 9-cis Retinoic Acid-Treated Groups
| 9-cis Retinoic acid |
||||
|---|---|---|---|---|
| 7 Days | 14 Days | 45 Days | Delay | |
| Percent increase in tail volume (at day 42) | — | ↓ | ↓↓↓ | ↓↓ |
| Dermal thickness (μm) | — | ↓↓↓ | ↓↓ | ↓↓ |
| Epidermal thickness (μm) | ↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ |
| ICG clearance (at 48 hours) | — | ↑↑↑ | ↑↑↑ | ↑↑↑ |
| Tail lymphatic density (vessels/μm2) | — | ↑ | ↑↑ | — |
↑/↓: Trending toward significance.
↑↑/↓↓: Significant at a p-value of at least 0.05.
↑↑↑/↓↓↓: Significant at a p-value of at least 0.01.
—: No statistical difference versus the control group.
Note: Direction of the arrow signifies whether the observed mean is greater than or less than the control mean.
Lymphatic clearance
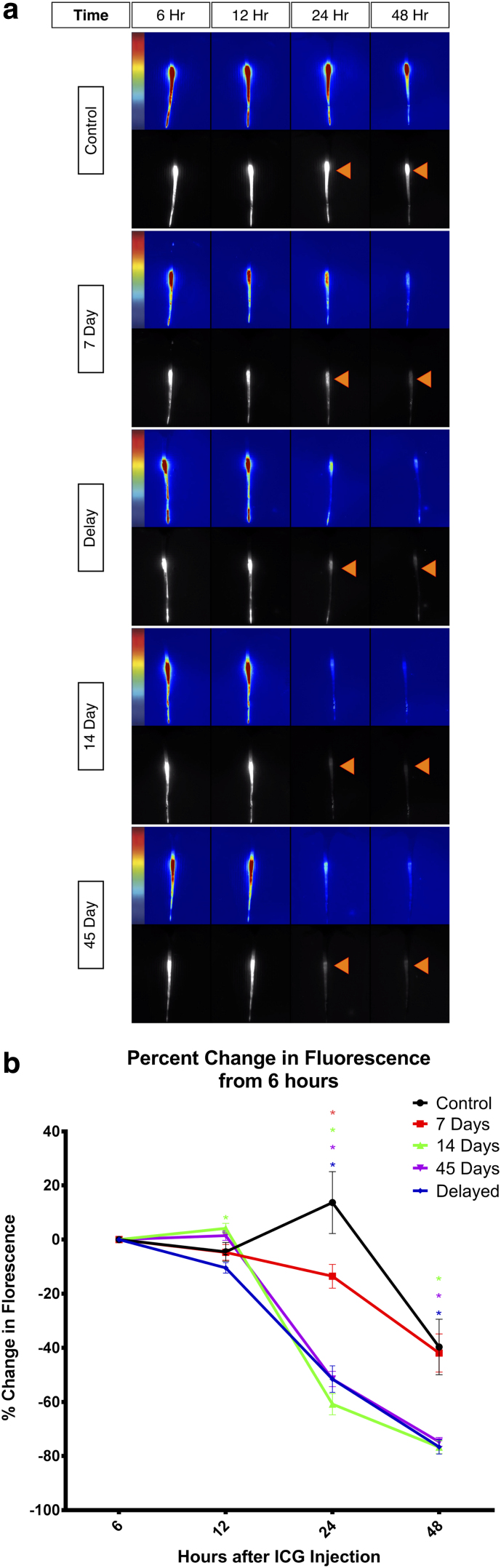
To compare functional lymphatic clearance at the wound site, the change in fluorescence intensity following ICG injection into the distal edge of the tail was quantified. ICG lymphangiography was performed on all animals on day 45 of the experiment. ICG drains from the distal tail proximally, resulting in a washout of contrast and decrease in fluorescence intensity of the tail over time (Fig. 2a). In all groups, ICG fluorescence peaked 6 hours postinjection and plateaued for 12 hours, showing a subsequent divergence beginning at the 24th hour.
FIG. 2.
ICG lymphangiography. (a) Lymphatic clearance of each group at 6, 12, 24, and 48 hours after injection of ICG, measured using ICG lymphangiography. Orange arrows at 24- and 48-hour time points signify washout in treatment groups compared with the relatively small change in fluorescence intensity in the control group. (b) Graphical representation of ICG clearance in each group represented by the percent change in fluorescence. Values are reported as mean ± standard error. ICG, indocyanine green.
All groups showed a significant decrease in the percent change of fluorescence intensity compared with controls at 24 hours. Forty-eight hours after the injection, the 14- and 42-day and delay groups showed a significant decrease in fluorescence intensity; however, the 7-day group was no longer significant compared with the control at this time point (Fig. 2b).
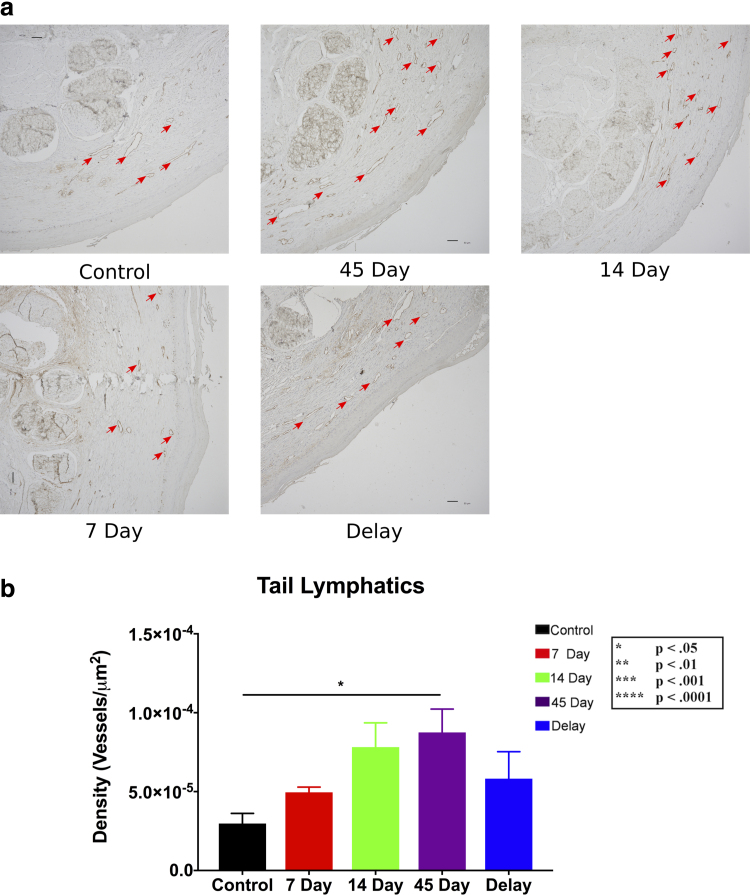
To assess histologic changes after various treatment durations with 9-cis RA, tails were harvested at the endpoint of the experiment. Immunohistochemistry analysis demonstrated an upward trend in the lymphatic density with longer durations of therapy; however, only the 45-day treatment group showed a significant increase compared with controls (p < 0.05) (Fig. 3a, b).
FIG. 3.
Lymphatic vessel density. (a) Representative cross-sectional 10 × immunohistochemistry images of podoplanin+ vessels (stained brown), comparing the vessel density of the control group with 9-cis RA-treated groups. Scale bars: 50 μm. (b) Quantification of lymphatic vessel density for each group. A statistically significant difference was observed between the 45-day group and the control group (p < 0.05), and the 14-day group was trending toward significance (p = 0.0901). Values are reported as mean ± standard error.
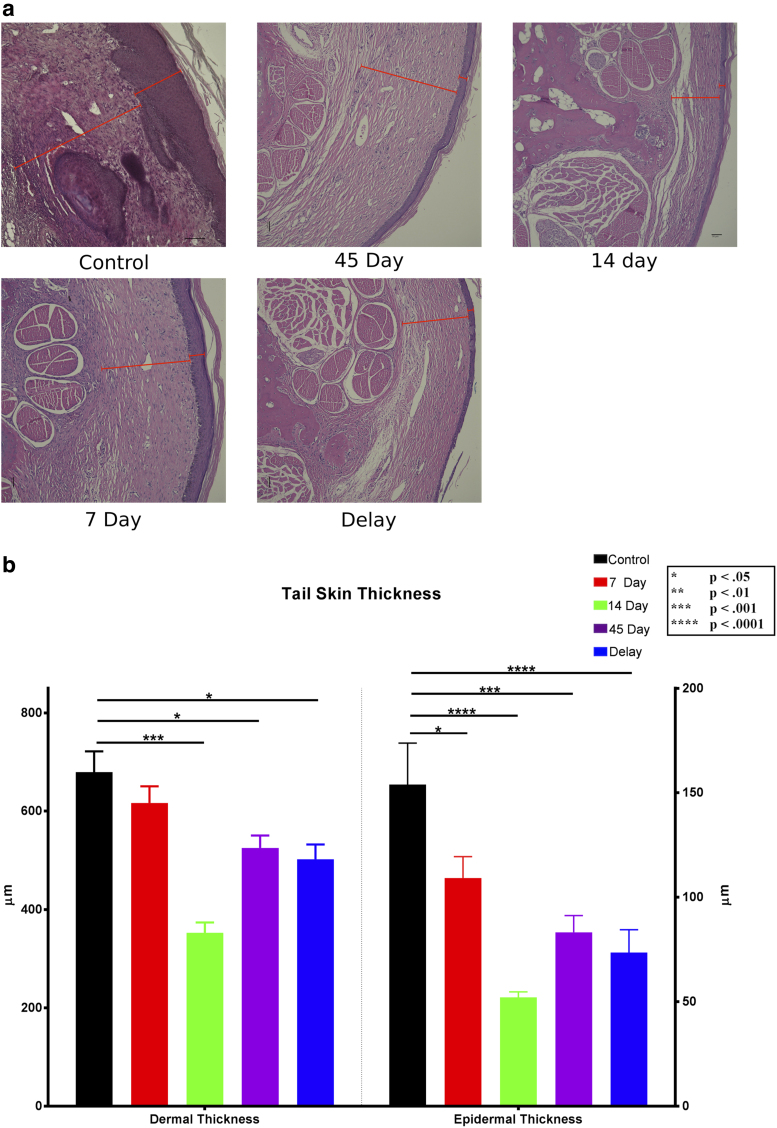
H&E staining of tail tissue was used to further analyze the effect of 9-cis RA on the histologic manifestations of lymphedema. Dermal thickness was assessed by measuring the distance between the epidermal–dermal junction and the subcutaneous layer. Like the lymphatic density analysis data, animals with prolonged 9-cis RA treatment demonstrated a thinner dermal layer compared with the control (Fig. 4a).
FIG. 4.
Dermal and epidermal thicknesses. (a) Representative 10 × cross-sectional images of H&E-stained tails. Red indicators represent the thickness of the dermis (inner lines) and epidermis (outer lines). Scale bar in the 14-day group: 50 μm. Scale bar in the control group: 100 μm. (b) Quantification of dermal and epidermal thicknesses from H&E-stained slides. The control group had a significantly thicker epidermis than the 7-, 14-, and 45-day and delay groups (p = 0.0411, 0.0001, 0.0001, and 0.0001, respectively). The dermis in control tails was also significantly thicker than the 14- and 45-day and delay groups (p = 0.0001, 0.0357, and 0.0141, respectively). H&E, hematoxylin and eosin.
Dermal thickness differed significantly between the control group (679.5 ± 42.12 μm) and the 14-day (352.4 ± 21.29 μm, p < 0.0001), 45-day (525.2 ± 25.09 μm, p < 0.05), and delay (502 ± 30.17 μm, p < 0.05) groups (Fig. 4b). Similarly, epidermal thickness was quantified by measuring the distance between the outer edge of the epidermis and the epidermal–dermal junction. The mean epidermal tail thickness of control animals (153.9 ± 19.83 μm) was significantly greater than all 9-cis RA-treated groups, including the 7-day group (109.2 ± 10.21 μm, p < 0.05), 14-day group (52.07 ± 2.616 μm, p < 0.0001), 45-day group (83.14 ± 8.099 μm, p < 0.001), and delay group (73.51 ± 10.94 μm, p < 0.0001) (Fig. 4b).
Effect of 9-cis RA on lymphatics in uninjured tissues
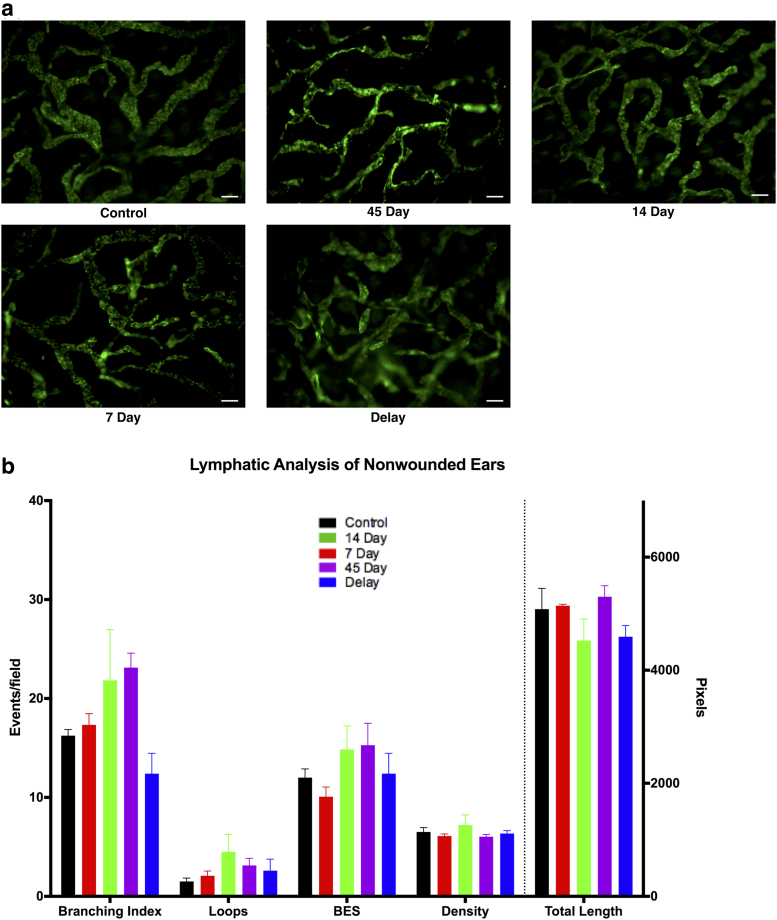
To determine whether 9-cis RA affects lymphatic vessels at sites of uninjured tissue far from the tail injury, whole-mount ear preparations were stained for LYVE-1 (immunofluorescence) and quantified (Fig. 5a). Lymphatic vessel analysis of uninjured ears revealed no significant difference in the number of branch points, blind-ended sacs, loops, total length, or density of lymphatic vessels between control animals and those treated with 9-cis RA for any duration (p = 0.05) (Fig. 5b).
FIG. 5.
Lymphatic analysis of distant uninjured site. (a) Representative images of LYVE-1-stained uninjured ears from mice. Scale bars: 100 μm. (b) Quantification of the number of branches, loops, blind-ended sacs, total vessel length, and vessel density. No significant differences were found between the control and treatment groups.
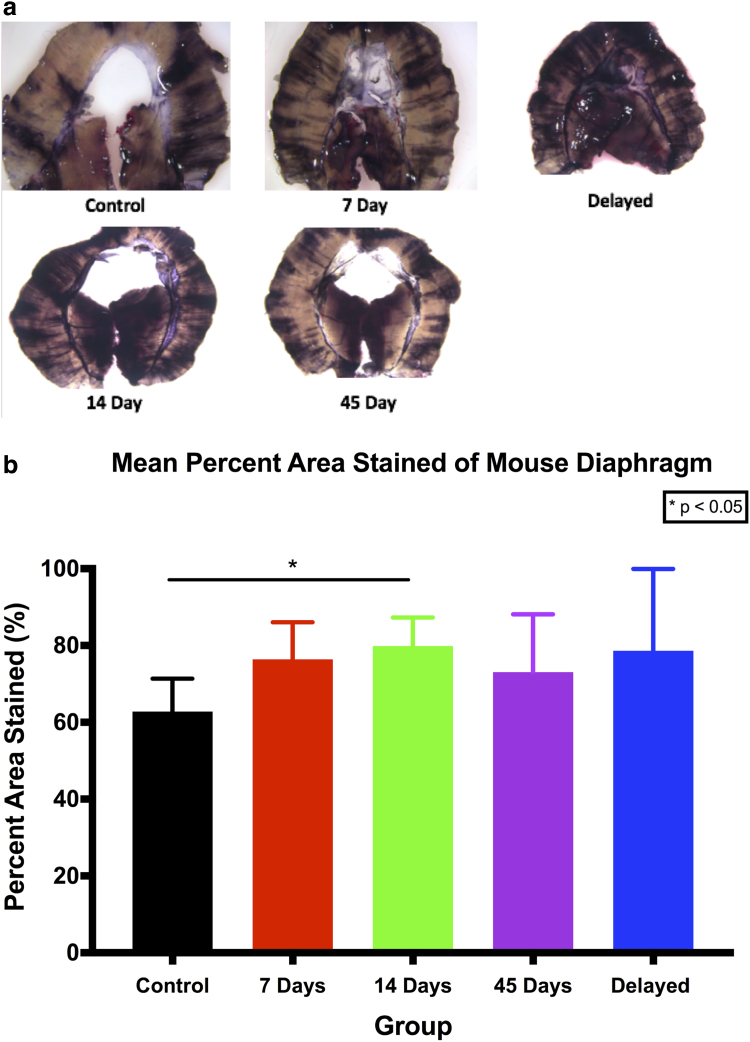
To further analyze the effect of 9-cis RA on the lymphatics of uninjured distant tissue, we utilized the semiquantitative India ink assay of mouse diaphragms. The unique purpose of the diaphragm is to drain excess intraperitoneal fluid back to the lymphatic system. As such, passive lymphatic uptake of India ink in the peritoneal cavity into the diaphragmatic lymphatics is demonstrative of lymphatic function, with a heavier ink load seen in diaphragmatic tissues with more robust lymphatic networks (Fig. 6a).
FIG. 6.
Lymphatic analysis of India ink-stained mouse diaphragms. (a) Representative photos of mouse diaphragms stained with India ink. (b) Graphical representation of the mean percent area stained with India ink. The only statistically significant difference found was between the control group and 14-day group (p = 0.0182). *p < 0.05.
Quantification of the percent stained area of diaphragms showed that there were essentially no differences, except in the 14-day treatment group compared with controls (p < 0.05) (Fig. 6b).
Discussion
Results from the current study significantly improve our understanding of how 9-cis RA stimulates lymphatic regeneration, improves lymphatic clearance, and prevents clinical lymphedema. Short-duration treatment with 9-cis RA for 7 days had no significant effect on tail lymphedema, and the 14-day treatment showed more of a response than the 7-day group, but less than the 45-day group. The 14-day group demonstrated improvement in lymphatic function, demonstrated by improved ICG clearance, but did not show a significant reduction in tail volume (Tables 1 and 2).
The association of other risk factors of lymphedema, including infection, radiation, and obesity, suggests that lymphedema is a multifactorial disease whereby lymphatic disruption is necessary, but not sufficient for development of chronic lymphedema. Given the association of inflammation and lymphedema, we studied the effects of 9-cis RA on uninjured tissue distant from the experimental wound site.
Histologic analysis demonstrated that sites without lymphatic injury or inflammation (i.e., ear and diaphragm) did not show an appreciable difference in lymphatic density upon 9-cis RA therapy compared with controls. This is an important finding, suggesting that the inflammatory process associated with tissue injury sets the stage for 9-cis RA-induced lymphatic regeneration. While the mechanism of enhanced activity at injury sites compared with uninjured tissue has yet to be elucidated, from a clinical translational standpoint, this is a favorable property of 9-cis RA.
Interestingly, the delay group that received 7 days of 9-cis RA 1 week after lymphatic injury showed a significant improvement in lymphedema with associated decreased tail volume, decreased dermal thickness, and improved lymphatic clearance. Overall, the 7-day delay treatment group showed a clinical and histologic response similar to the treated animals in the 14-day group (Tables 1 and 2).
We hypothesize that the improved lymphangiogenic effects seen with the delayed short-duration 7-day therapy with 9-cis RA were attributable to the intricate interaction of 9-cis RA with a more robust inflammatory cell infiltrate during days 7–14. Lymphangiogenesis serves as an instigating factor of chronic inflammation as well as downstream sequelae of inflammation.
As a consequence, injured soft tissues with disrupted lymphatic beds are prone to a vicious cycle of an upregulated immune response and activation of the indirect lymphangiogenic pathway. As such, our data reflect the importance of the timing of the 9-cis RA therapy as being tantamount to the duration of treatment in prevention of clinical lymphedema.
In summary, we have added to our understanding of the optimal 9-cis RA treatment regimen for stimulating lymphatic regeneration, improving lymphatic function, and ultimately preventing clinical lymphedema using a well-established tail lymphedema model. Given the increasing incidence of lymphedema in an era where primary treatment is mainly limited to palliative therapy without an effective cure, further studies in the development of 9-cis RA as a preventive measure for secondary lymphedema are crucial.
In this light, our findings from this study provide strong support that intermediate duration therapy (14 days) with 9-cis RA is sufficient for improving lymphatic function and preventing clinical lymphedema, with the potential to limit side effects of systemic therapy. The ability to translate these findings to human patients at risk for development of lymphedema will help facilitate finding a preventive solution to this morbid disease.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The work in this study was funded by grants from the National Institutes of Health (K08HL132110 and R01HL157626 to A.K.W.).
References
- 1. Mihara M, Hara H, Hayashi Y, et al. Pathological steps of cancer-related lymphedema: Histological changes in the collecting lymphatic vessels after lymphadenectomy. PLoS One 2012; 7:e41126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Donnell TF Jr., Rasmussen JC, Sevick-Muraca EM.. New diagnostic modalities in the evaluation of lymphedema. J Vasc Surg Venous Lymphat Disord 2017; 5:261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Witte CL, Witte MH. Disorders of lymph flow. Acad Radiol 1995; 2:324–334. [DOI] [PubMed] [Google Scholar]
- 4. Allen RJ Jr., Cheng MH. Lymphedema surgery: Patient selection and an overview of surgical techniques. J Surg Oncol 2016; 113:923–931. [DOI] [PubMed] [Google Scholar]
- 5. Ly CL, Kataru RP, Mehrara BJ. Inflammatory manifestations of lymphedema. Int J Mol Sci 2017; 18:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cormier JN, Askew RL, Mungovan KS, Xing Y, Ross MI, Armer JM. Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010; 116:5138–5149. [DOI] [PubMed] [Google Scholar]
- 7. Brorson H. Adipose tissue in lymphedema: The ignorance of adipose tissue in lymphedema. Lymphology 2004; 37:175–177. [PubMed] [Google Scholar]
- 8. Dayan JH, Ly CL, Kataru RP, Mehrara BJ. Lymphedema: Pathogenesis and novel therapies. Annu Rev Med 2018; 69:263–276. [DOI] [PubMed] [Google Scholar]
- 9. Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: A 2-year follow-up study. J Clin Oncol 2009; 27:2007–2014. [DOI] [PubMed] [Google Scholar]
- 10. Kayıran O, De La Cruz C, Tane K, Soran A. Lymphedema: From diagnosis to treatment. Turk J Surg 2017; 33:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dayan JH, Dayan E, Smith ML. Reverse lymphatic mapping: A new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015; 135:277–285. [DOI] [PubMed] [Google Scholar]
- 12. Rockson SG. Diagnosis and management of lymphatic vascular disease. J Am Coll Cardiol 2008; 52:799–806. [DOI] [PubMed] [Google Scholar]
- 13. Schindler M, Drozdenko G, Kuhl AA, Worm M. Immunomodulation in patients with chronic hand eczema treated with oral alitretinoin. Int Arch Allergy Immunol 2014; 165:18–26. [DOI] [PubMed] [Google Scholar]
- 14. Szabo DR, Baghy K, Szabo PM, et al. Antitumoral effects of 9-cis retinoic acid in adrenocortical cancer. Cell Mol Life Sci 2014; 71:917–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hofmann WK, Kell WJ, Fenaux P, et al. Oral 9-cis retinoic acid (Alitretinoin) in the treatment of myelodysplastic syndromes: Results from a pilot study. Leukemia 2000; 14:1583–1588. [DOI] [PubMed] [Google Scholar]
- 16. Bramos A, Perrault D, Yang S, Jung E, Hong YK, Wong AK. Prevention of postsurgical lymphedema by 9-cis retinoic acid. Ann Surg 2016; 264:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Choi I, Lee S, Kyoung Chung H, et al. 9-cis retinoic acid promotes lymphangiogenesis and enhances lymphatic vessel regeneration: Therapeutic implications of 9-cis retinoic acid for secondary lymphedema. Circulation 2012; 125:872–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marino D, Dabouras V, Brändli AW, Detmar M. A role for all-trans-retinoic acid in the early steps of lymphatic vasculature development. J Vasc Res 2011; 48:236–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burger NB, Stuurman KE, Kok E, et al. Involvement of neurons and retinoic acid in lymphatic development: New insights in increased nuchal translucency. Prenat Diagn 2014; 34:1312–1319. [DOI] [PubMed] [Google Scholar]
- 20. Hinrichs CS, Watroba NL, Rezaishiraz H, et al. Lymphedema secondary to postmastectomy radiation: Incidence and risk factors. Ann Surg Oncol 2004; 11:573–580. [DOI] [PubMed] [Google Scholar]
- 21. Warren AG, Brorson H, Borud LJ, Slavin SA. Lymphedema: A comprehensive review. Ann Plast Surg 2007; 59:464–472. [DOI] [PubMed] [Google Scholar]
- 22. Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001; 92:1368–1377. [DOI] [PubMed] [Google Scholar]
- 23. Sitzia J. Volume measurement in lymphoedema treatment: Examination of formulae. Eur J Cancer Care (Engl) 1995; 4:11–16. [DOI] [PubMed] [Google Scholar]
- 24. Rossi A, Sozio F, Sestini P, et al. Lymphatic and blood vessels in scleroderma skin, a morphometric analysis. Human Pathology 2010; 41:366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Szuba A, Shin WS, Strauss HW, Rockson S. The third circulation: Radionuclide lymphoscintigraphy in the evaluation of lymphedema. J Nucl Med 2003; 44:43–57. [PubMed] [Google Scholar]