Abstract
Background
Prenatal exposure to polycyclic aromatic hydrocarbons (PAH) may increase risk of pediatric asthma, but existing human studies are limited.
Objectives
We estimated associations between gestational PAHs and pediatric asthma in a diverse US sample and evaluated effect modification by child sex, maternal asthma, and prenatal vitamin D status.
Methods
We pooled two prospective pregnancy cohorts in the ECHO PATHWAYS Consortium, CANDLE and TIDES, for an analytic sample of N=1296 mother-child dyads. Mono-hydroxylated PAH metabolites (OH-PAHs) were measured in mid-pregnancy urine. Mothers completed the International Study on Allergies and Asthma in Childhood survey at child age 4–6 years. Poisson regression with robust standard errors was used to estimate relative risk of current wheeze, current asthma, ever asthma, and strict asthma associated with each metabolite, adjusted for potential confounders. We used interaction models to assess effect modification. We explored associations between OH-PAH mixtures and outcomes using logistic regression weighted quantile sum augmented by a permutation test to control Type 1 errors.
Results
The sociodemographically diverse sample spanned five cities. Mean (SD) child age at assessment was 4.4 (0.4) years. While there was little evidence that either individual OH-PAHs or mixtures were associated with outcomes, we observed effect modification by child sex for most pairs of OH-PAHs and outcomes, with adverse associations specific to females. For example, a 2-fold increase in 2-hydroxy-phenanthrene was associated with current asthma in females but not males (RRfemale = 1.29 [95% CI: 1.09, 1.52], RRmale = 0.95 [95% CI: 0.79, 1.13]; pinteraction = 0.004). There was no consistent evidence of modification by vitamin D status or maternal asthma.
Discussion
This analysis, the largest cohort study of gestational PAH exposure and childhood asthma to date, suggests adverse associations for females only. These preliminary findings are consistent with hypothesized endocrine disruption properties of PAHs, which may lead to sexually dimorphic effects.
Keywords: Pediatric asthma, airway, endocrine disruption, polycyclic aromatic hydrocarbons, mixtures
INTRODUCTION
Childhood asthma and other atopic diseases account for substantial morbidity in the US, disproportionately affecting children from lower income and racial/ethnic minoritized groups in urban areas.1–3 Asthma is a complex disease, with genetic and environmental contributions to disease development, and risk may also be influenced by prenatal exposures, including environmental toxicants.4–6 Polycyclic aromatic hydrocarbons (PAHs) are combustion by-products found in ambient air pollution, with elevated concentrations observed near roadways, industrial point sources involving fossil fuel combustion, and wildfires.7,8 Other common PAH sources include smoking, exposure to environmental tobacco smoke (ETS), and consumption of grilled foods.8,10 Exposure to PAHs is widespread in the general population11 but varies by sociodemographic characteristics, including by race, ethnicity and household income.9,12,13
While the carcinogenic impacts of PAHs are well-described,8,14 recent work suggests noncarcinogenic endpoints of PAH exposure, including pediatric asthma as well as respiratory conditions and morbidity in adults.15–17 PAHs readily cross the placental barrier, and prenatal exposure may affect fetal airway development, possibly mediated through increased inflammation and oxidative stress as well as disruptions to endocrine and immune system development.17–22 Prenatal PAH exposure may also cause epigenetic changes in the fetus, with subsequent developmental effects.23–25 Thus, it has been hypothesized that maternal PAH exposure in the prenatal period could increase risk of offspring airway and allergy conditions in early childhood, but few epidemiological studies incorporating individual level measures of prenatal PAH exposure have been conducted to date, as recently reviewed by Lag, et al.15
Three longitudinal cohort studies have investigated associations between individual prenatal PAH exposure and childhood asthma. The Columbia Center for Children’s Environmental Health study characterized maternal exposure by measuring airborne PAHs in the personal air of pregnant woman and followed children’s airway-related development through early childhood.26–28 Some evidence linking prenatal PAHs to poorer airway health was observed, but the associations were not consistent over time. In addition, the exposure assessment method did not capture exposure due to maternal ingestion (e.g., diet). Two cohort studies in Poland measured PAH exposure using both personal air monitoring and exposure biomarkers (DNA adducts in cord blood or urinary metabolites), providing a broader perspective on maternal PAH exposure; these findings suggested adverse impacts of prenatal PAH exposure on airway and immune system outcomes.29–31 As found in a recent systematic review, the evidence for a causal link between prenatal PAHs and asthma is inconclusive.32
Here, we extend the evidence base relating prenatal PAH exposure to pediatric asthma in several ways. By combining two cohorts participating in the ECHO PATHWAYS Consortium, the Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) study and The Infant Development and the Environment Study (TIDES), we achieved a sociodemographically diverse sample spanning five US metropolitan areas. Concentrations of mono-hydroxylated metabolites of PAHs (OH-PAH) in pregnancy urine were quantified, providing perspective on gestational exposure to PAHs through all routes of exposure.33 We hypothesized that higher exposure to PAHs in utero could increase risk of early childhood asthma, and that associations would vary by metabolite and may be driven by specific mixtures of metabolites. In secondary analyses, we evaluated evidence of mixture effects by using weighted quantile sum (WQS) regression augmented by a permutation test to control Type I error, as people are commonly exposed to PAHs as complex mixtures rather than independent compounds in isolation.11 Based on what is known about possible mechanisms for developmental toxicity of PAHs, we also hypothesized that effects could vary by certain maternal and child factors. Therefore, our secondary research aims also include assessments of effect modification by child sex, maternal asthma, and prenatal vitamin D status.
Methods
Study population
We included mother-child dyads enrolled in two cohorts of the ECHO PATHWAYS Consortium: the Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) study and The Infant Development and the Environment Study (TIDES). The CANDLE study was approved by the University of Tennessee Health Science Center Institutional Review Board (IRB), and TIDES study procedures were approved by IRBs at each study site as well as the TIDES coordinating center. Pregnant women provided informed consent at enrollment. The current analysis was conducted by the ECHO PATHWAYS Consortium and was approved by the University of Washington IRB.
The CANDLE cohort recruited pregnant women (N=1503) from the Memphis, TN, area between 2006–2011.34 Women were eligible if they were between 16 and 40 years old, lived in Shelby County, intended to deliver at one of five Memphis hospitals, and were in the second trimester of a low medical-risk pregnancy. Prenatal study visits included biospecimen collection and maternal surveys, and labor and delivery data were abstracted from the medical record. After birth, follow up with mothers and children occurred at regular intervals by means of clinic, phone and home visits, including a clinic visit at age 4–6 years.
TIDES is a multi-site pregnancy cohort with enrollment sites in San Francisco, CA; Minneapolis, MN; Rochester, NY; and Seattle, WA.35 Women were enrolled (N=900) in their first trimester from obstetric clinics associated with academic medical centers, 2010 through 2012. Inclusion criteria were being at least 18 years of age and intending to deliver at one of the study hospitals. Prenatal study visits occurred in each trimester, including biospecimen collection and maternal surveys. A birth exam and record review were conducted. Mother-child dyads were followed with remote surveys in early childhood followed by a clinic visit at age 4–5 years.
Maternal urinary PAH metabolites
Our primary exposure of interest was exposure to PAHs in pregnancy, measured as mono-hydroxylated PAH metabolites in a spot urine sample collected in mid-pregnancy. The gestational age at time of urine collection ranged from 12.9 to 33.4 weeks, with a median of 21.6 weeks. This distribution was similar between cohorts. Urinary specific gravity (SG) of each sample was determined using a handheld refractometer at the time of collection. Samples were stored at −80 degrees C in study biorepositories until the time of analysis at the Wadsworth Laboratory, New York State Department of Health. Twelve urinary metabolites of seven parent PAHs were quantified in samples: two metabolites of naphthalene (1-hydroxynaphthalene [1-NAP], 2-hydroxynaphthalene [2-NAP]), four of phenanthrene (2-hydroxyphenanthrene [2-PHEN], 3-hydroxyphenanthrene [3-PHEN], 4-hydroxyphenanthrene [4-PHEN], combined 1/9-hydroxyphenanthrene [1/9-PHEN]), two of hydroxychrysene (1-hydroxychrysene [1-CHRY], 6-hydroxychrysene [6-CHRY]), 2/3/9-hydroxyfluorene (2/3/9-FLUO), 1-hydroxypyrene (1-PYR), 3-hydroxybenzo[c]phenanthrene (3-BCP), and 1-hydroxybenzo[a]anthracene (1-BAA).
The laboratory methods have been previously described.36–38 In brief, OH-PAHs were extracted from urine by liquid-liquid extraction followed by LC-MS/MS analysis. Urine samples were fortified with isotopically-labeled internal standard mixture and mixed with 0.5 M ammonium acetate buffer containing 200 units/mL of β-glucuronidase/sulfatase enzyme (MP Biomedicals, LLC, Solon, OH, USA). Samples were incubated overnight at 37°C and then extracted with 7 mL of 80% pentane: 20% toluene (v:v) by shaking on a reciprocating shaker for one hour and centrifuged at 3600 x g for 20 minutes. The supernatant was transferred into a new glass tube for instrumental analysis. OH-PAHs were chromatographically separated using a Waters Acquity I-Class UPLC system (Waters; Milford, MA, USA) connected with an Acquity UPLC BEH C18 column (50 × 2.1 mm, 1.7 μm, Waters; Milford, MA, USA). Finally, identification and quantification of OH-PAHs was performed on an ABSCIEX 5500 triple quadrupole mass spectrometer (Applied Biosystems; Foster City, CA, USA). Quality assurance protocols included analysis of two Standard Reference Materials (SRM 3672, SRM 3673) containing certified values for several OH-PAHs. Recoveries of analytes in reference materials ranged from 79 to 109%. Prior to injection of samples, a 13-point standard calibration curve (0.02 – 200 ng/mL) was constructed. To ensure instrument stability, calibration standards were injected repeatedly throughout the sample run. The limits of detection (LOD) ranged from 0.02 to 0.12 ng/mL.
Samples below the limit of detection (LOD) were imputed as LOD/√2 for the main analyses; a sensitivity analysis using an alternate approach for regression models is described below. OH-PAH concentrations were adjusted for sample specific gravity to account for urinary dilution using the Levine-Fahey equation for use in descriptive summaries and in analyses of mixtures.39 To approximate overall PAH exposure by participant characteristics, in descriptive analyses, we calculated total OH-PAH as the sum of SG-adjusted OH-PAH metabolites. In addition, sums of naphthalene and phenanthrene metabolites (ΣOH-NAP and ΣOH-PHEN) were calculated as the molar sums of 1-NAP and 2-NAP and of 2-PHEN, 3-PHEN, 4-PHEN and 1/9-PHEN, respectively.
Child airway outcomes
At child age 4–6 years, parents completed surveys about general child health and medication use as well as focused questions on airway and allergy conditions, including the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire.40 Four outcomes were defined, based on survey responses 41–42: 1) current wheezing, defined as positive response to both: “Has your child ever had wheezing or whistling in the chest at any time in the past?” and “Has your child ever had wheezing or whistling in the chest in the last 12 months?; 2) ever asthma, defined as positive response to the question “Has your child ever had asthma?”; 3) current asthma, defined as positive responses to 2 of the following 3: current wheeze (as defined above), ever asthma (as defined above), and asthma medication use; and 4) strict asthma, defined as positive response to the question “Has your child ever had asthma?” as well as either current wheeze (as defined above) or asthma medication use. In CANDLE, asthma medication use was defined as an affirmative response to the question “In the past 12 months has your child used any medications for asthma or wheeze?” In TIDES, free-text response reporting any serious health conditions (asthma or asthma symptoms) and medications for those conditions (beta-2-agonist, inhaled or oral corticosteroid) in the prior 24 months were used to define asthma medication use. To maximize the use of available data, participants who were missing data for asthma-specific medication use and did not indicate yes to both ever asthma and current wheeze questions (N=6) were considered not to have current asthma.
Other measures
Covariates were ascertained by maternal report at enrollment (age at enrollment, educational attainment, race, pre-pregnancy body mass index [BMI], household income at enrollment, parity, history of asthma, smoking during pregnancy, and exposure to environmental tobacco smoke during pregnancy and in the postnatal period), medical record abstraction (infant sex, gestational age and birthweight), or bioassays (pregnancy urinary cotinine and plasma vitamin D). Harmonization of data across the two cohorts was completed according to standard procedures for data processing, including algorithmic transformations and unit conversions.43 For example, categorical variables were combined in such a way to preserve the most detail from original data collection instruments, as done in previous PATHWAYS analyses.42,44,45
Urinary cotinine was measured in two pregnancy urine samples for each CANDLE woman (approximately second and third trimesters) and three pregnancy samples for TIDES (one in each trimester). Urine samples from mid-pregnancy in CANDLE were assayed for cotinine using a one-step lateral flow chromatographic immunoassay that identified samples positive for cotinine based on a threshold of 200 ng/mL.46 Late pregnancy CANDLE samples and all TIDES urine samples were analyzed for a measure of continuous cotinine using solid phase extraction to extract cotinine from urine samples and LC-MS/MS to identify and quantify cotinine concentrations.
Vitamin D was measured in second trimester blood samples in the CANDLE cohort by commercial enzymatic immunoassay (IDS, Boldon, Tyne and Wear, UK)47 within three months of sample collection. The minimum detection range of this assay was 2 ng/mL, with interassay variability <6% and precision within 1 SD of mean, using NIST SRM972 as standard. Vitamin D levels were dichotomized at “possible” deficiency, defined as <20 ng/mL,48,49 for assessment of effect modification by prenatal vitamin D.
Statistical analyses
For main analyses, we included mother-child dyads with OH-PAH measurements in mid-pregnancy urine and at least one primary outcome measure at child age between 4 and 6.5 years. We excluded children who were born very preterm (prior to 32 weeks gestation) due to impacts of preterm birth on airway development.50 In sensitivity analyses, we repeated main models with exclusion of children born prior to 37 weeks. We also excluded prenatal smokers, identified by self-report or a measure of pregnancy cotinine from any timepoint in pregnancy exceeding 200 ng/mL,46 to mitigate confounding. Smoking is a strong driver of PAH exposure and is also associated with increased risk of pediatric asthma development, and we could not adjust for this important confounder because we had insufficient data on the extent of smoking, for women who were smokers.52 Therefore, we have estimated associations between PAHs and airway outcomes in a population restricted to nonsmokers and can only generalize study findings to a nonsmoking population. This approach is consistent with other studies of this research question.
We summarized participant characteristics (overall, by cohort, and by quartile of total OH-PAH) using descriptive statistics. To estimate the risk ratio (RR) for each child airway outcome in association with individual OH-PAHs as well as ΣOH-NAP and ΣOH-PHEN, we used Poisson regression with robust standard errors. OH-PAHs were log-transformed, imposing the assumption that risk increases linearly per multiplicative increase on OH-PAHs, and analyzed as raw (not SG-adjusted) concentrations in separate regression models. We repeated main analyses with SG-adjusted OH-PAHs as the primary predictor and observed no meaningful change (results not shown). We included metabolites with at least 70% of values detectable (>LOD) in the overall sample in regression analyses, excluding those with lower detection rates. We adjusted for potential confounders, identified a priori as factors likely to be directly or indirectly associated with prenatal PAH exposure and also predictive of pediatric airway outcomes (see Figure S1 for conceptual model). We also adjusted for precision variables, defined as factors that predict outcome yet are likely not associated with exposure. Staged modeling was used in order to assess the effect of increasing adjustment on effect estimates. In minimal adjustment models, we controlled for child age at assessment (continuous), child sex, study site, batch of OH-PAH analysis, and urinary SG (continuous). Full adjustment models, considered our main models, additionally included maternal age (continuous), education (< high school, high school diploma, graduated college or technical school, and some graduate work or graduate/professional degree), self-identified race (Black and non-Black), pre-pregnancy BMI (continuous), household income (<15k, 15–25k, 25–45k, 45–55k, 55–65k, 65–65k, >=75k), parity (prior birth or not), maternal history of asthma, postnatal smoke exposure (any vs. none), environmental tobacco smoke exposure in pregnancy (continuous cotinine), and season of birth (categorical; winter, spring, summer, and fall). An expanded model additionally included two potential confounders that we did not include in full adjustment model because they could be on the causal pathway: gestational age at birth (continuous) and birthweight (continuous).
We conducted a number of sensitivity analyses to test the robustness of our main findings to aspects of our analytic approach. We repeated primary analyses, using full adjustment models, with the following variations: 1) exclusion of one site at a time and exclusion of each cohort; 2) addition of product terms between site and covariates for which associations may vary by site (child race, maternal education, household income, and season), to assess the possibility of site-specific confounding; 3) modeling of continuous covariates as flexible splines, to test nonlinearities in associations between confounders and outcomes; and 4) multiple imputation of <LOD OH-PAH values using central likelihood multiple imputation (CLMI), a method for imputing nondetectable values when LOD varies by batch.52
Secondary analyses included analyses of OH-PAH metabolites as mixtures using logistic WQS regression, which simultaneously accounts for exposure to multiple chemicals in one regression model, as described previously.53 SG-adjusted OH-PAH metabolites were transformed into deciles, and mixture associations were examined using logistic regression, with separate models to evaluate both positive and negative associations. Only OH-PAH metabolites with at least 70% of samples detectable were included. Model estimates were calculated using 1000 bootstrap iterations. In order to maximize statistical power, we did not split the sample into training and validation datasets. Because standard errors estimated using WQS regression using a full sample, without splitting, are likely biased in an anti-conservative direction,54 we implemented a permutation test to estimate accurate p-values for WQS logistic regression. See Supplemental Material for details of the statistical approach, which is similar to the permutation test for linear WQS regression we reported previously.55,56
For WQS regression analyses in which the 95% CIs did not include the null, we applied the permutation test to estimate a p-value (ppermutation), which we considered a more accurate measure of precision than the 95% CIs.55 Note that the permutation test only provides p-values, not 95% CIs, and does not affect the point estimate (i.e., the WQS coefficient).
In secondary analyses we also tested effect modification by child sex, maternal history of asthma, and (in CANDLE only) prenatal vitamin D in separate models using product term models. Strata-specific effect estimates and effect modification p-values were derived from product term models. In post hoc analyses, we additionally conducted WQS regression in strata of child sex. Full adjustment models were used in all secondary analyses.
Finally, we explored whether the exposure-response relationships deviated from linearity by using generalized linear models (GAMs) with adjustment for all covariates in the full models. These analyses were conducted for all models in which linear regression suggested a significant association between OH-PAH and an outcome. We inspected exposure-response curves and associated 95% CIs to determine whether there is statistical evidence of deviations from linearity.
All analyses were conducted in R 3.6 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Characteristics of the study population
Of N=2403 pregnant mothers ever enrolled in the CANDLE and TIDES cohorts, N=1692 attended an age 4–6 year visit with their child and completed a survey on respiratory health that included the standardized ISAAC questions. Of these 1692 dyads, 396 were excluded from this analysis because the child was born before 32 weeks (N=31), the child was over 6.5 years at the time of the visit (N=56), no pregnancy OH-PAH data were available (N=247), and/or on account of evidence that the mother smoked in pregnancy (N=149). N=1296 mother-child dyads met the inclusion criteria; the characteristics of these mothers and children are summarized in Table 1. The study sample was sociodemographically diverse, with 26% of mothers reporting at least some graduate education at enrollment and 41% a high school education or less. One third of mothers had a household income over $75,000 (33%) while 31% reported $25,000 or less. Self-reported maternal race was 44% Black and 47% White, and 4% of the mothers identified as Latino/Hispanic. 16% of mothers reported having asthma, and the mean (SD) pre-pregnancy BMI was 27.4 (7.4) kg/m2. The children in the analytic population were 52% female, and 16% were exposed to tobacco smoke postnatally. Mean (SD) of child age at time of assessment was 4.38 (0.38) years.
Table 1.
Descriptive characteristics of mother-child dyads in pooled CANDLE and TIDES study population*
| Combined analytic population (N=1296) | By quartile of total OH-PAH** | ||||
|---|---|---|---|---|---|
| Quartile 1 (N=324) | Quartile 2 (N=324) | Quartile 3 (N=324) | Quartile 4 (N=324) | ||
| Maternal Variables | |||||
| Age, n (%) | |||||
| <20 years | 91 (7) | 6 (2) | 22 (7) | 31 (10) | 32 (10) |
| 20–<30 years | 588 (46) | 104 (33) | 152 (47) | 161 (50) | 171 (54) |
| 30–<40 years | 579 (45) | 197 (62) | 144 (45) | 124 (39) | 114 (36) |
| 40+ | 20 (2) | 10 (3) | 3 (1) | 5 (2) | 2 (1) |
| Missing | 18 | 7 | 3 | 3 | 5 |
| Education, n (%) | |||||
| Less than high school | 89 (7) | 11 (3) | 20 (6) | 17 (5) | 41 (13) |
| High school completion | 438 (34) | 41 (13) | 106 (33) | 146 (45) | 145 (45) |
| Graduated college or technical school | 425 (33) | 118 (37) | 114 (35) | 103 (32) | 90 (28) |
| Some graduate work or graduate/professional degree | 342 (26) | 153 (47) | 84 (26) | 58 (18) | 47 (15) |
| Missing | 2 | 1 | 0 | 0 | 1 |
| Race, n (%) | |||||
| White | 606 (47) | 230 (71) | 164 (51) | 132 (41) | 80 (25) |
| Black or African American | 566 (44) | 44 (14) | 128 (40) | 174 (54) | 220 (68) |
| Asian | 39 (3) | 19 (6) | 10 (3) | 4 (1) | 6 (2) |
| Native Hawaiian or other Pacific Islander | 2 (0) | 1 (0) | 0 (0) | 0 (0) | 1 (0) |
| American Indian or Alaska Native | 3 (0) | 0 (0) | 2 (1) | 1 (0) | 0 (0) |
| Other | 50 (4) | 17 (5) | 14 (4) | 7 (2) | 12 (4) |
| Multiple | 29 (2) | 13 (4) | 6 (2) | 6 (2) | 4 (1) |
| Missing | 1 | 0 | 0 | 0 | 1 |
| Ethnicity, n (%) | |||||
| Not Hispanic or Latino | 1237 (96) | 307 (95) | 310 (97) | 309 (96) | 311 (97) |
| Hispanic or Latino | 50 (4) | 16 (5) | 10 (3) | 13 (4) | 11 (3) |
| Missing | 9 | 0 | 4 | 2 | 2 |
| Prior live births, median (IQR) | 0.7 (1.13) | 0.28 (0.74) | 0.63 (0.93) | 0.87 (1.24) | 1 (1.37) |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Maternal asthma, n (%) | |||||
| No | 1075 (84) | 266 (83) | 287 (89) | 263 (82) | 259 (81) |
| Yes | 207 (16) | 53 (17) | 35 (11) | 57 (18) | 62 (19) |
| Missing | 14 | 5 | 2 | 4 | 3 |
| Pre-pregnancy BMI, mean ± SD | 27.35 ± 7.37 | 25 ± 5.23 | 26.83 ± 7.33 | 28.37 ± 8.12 | 29.19 ± 7.79 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Child Variables | |||||
| Infant sex, (%) | |||||
| Male | 626 (48) | 149 (46) | 159 (49) | 147 (45) | 171 (53) |
| Female | 670 (52) | 175 (54) | 165 (51) | 177 (55) | 153 (47) |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Preterm birth (< 37 weeks), n (%) | |||||
| No | 1179 (91) | 299 (92) | 293 (90) | 293 (90) | 294 (91) |
| Yes | 117 (9) | 25 (8) | 31 (10) | 31 (10) | 30 (9) |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Birth weight (grams), mean ± SD | 3310 ± 522 | 3400 ± 505 | 3310 ± 542 | 3300 ± 505 | 3230 ± 526 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Child age (years) at outcome assessment | |||||
| Mean ± SD | 4.38 ± 0.38 | 4.43 ± 0.35 | 4.37 ± 0.38 | 4.35 ± 0.37 | 4.36 ± 0.41 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| Postnatal secondhand smoke | |||||
| No | 1087 (84) | 307 (95) | 267 (83) | 269 (83) | 244 (76) |
| Yes | 206 (16) | 17 (5) | 56 (17) | 55 (17) | 78 (24) |
| Missing | 3 | 0 | 1 | 0 | 2 |
| Income at enrollment, n (%) | |||||
| <$15k | 236 (19) | 31 (10) | 50 (16) | 66 (21) | 89 (30) |
| $15k – $25k | 150 (12) | 16 (5) | 42 (13) | 42 (14) | 50 (17) |
| $25k – $45k | 191 (15) | 28 (9) | 55 (18) | 55 (18) | 53 (18) |
| $45k – $55k | 99 (8) | 23 (7) | 21 (7) | 23 (7) | 32 (11) |
| $55k – $65k | 71 (6) | 23 (7) | 14 (4) | 21 (7) | 13 (4) |
| $65k – $75k | 84 (7) | 28 (9) | 25 (8) | 18 (6) | 13 (4) |
| >=$75k | 410 (33) | 167 (53) | 107 (34) | 85 (27) | 51 (17) |
| Missing | 55 | 8 | 10 | 14 | 23 |
Characteristics are summarized for the entire analytic population, as well as by four quartiles of total PAH exposure, calculated as the sum of all SG-corrected concentrations of PAH metabolites. All characteristics were reported by mothers, except infant sex, birthweight, and preterm status, which were ascertained from birth medical records. Abbreviations: SD – standard deviation; BMI – body mass index; OH-PAH – mono-hydroxylated-polycyclic aromatic hydrocarbon.
Concentrations of total urinary OH-PAHs: quartile 1 (0–4.1), quartile 2 (4.2–7.0), quartile 3 (7.1–11.4) and quartile 4 (11.5–2433). All concentrations are in ng/mL.
The study population included dyads from five study sites: one CANDLE site (Memphis, n=829) and four TIDES sites (Seattle, n=96; San Francisco, n=131; Minneapolis, n=140; Rochester, NY, n=100). Table S1 summarizes characteristics of participants by cohort. A higher percentage of participants in CANDLE identified as being Black and reported a lower household income at enrollment, compared to TIDES. Child age at time of assessment were similar between cohorts (mean [SD] = 4.30 [0.38] and 4.51 [0.33] in CANDLE and TIDES, respectively.)
Pregnancy OH-PAH measurements
Urinary concentrations of OH-PAHs measured in mid-pregnancy varied strongly by metabolite (Table S2). Nondetectable levels occurred for all metabolites, and the % <LOD in the overall sample ranged from 0.31% for 2-NAP to 99.77% for 1-BAA. The % detectable for 1-PYR in the pooled sample was 69.4%; because this is just below the threshold specified a priori for inclusion in epidemiological models (70%), we included it in regression analyses. Concentrations of metabolites exhibited moderate-to-strong pairwise correlations (Table S3). The most strongly correlated pairs were observed among phenanthrene metabolites (2-PHEN, 3-PHEN and 4-PHEN) and between 2/3/9-FLUO and both 2-PHEN and 3-PHEN.
Women in the CANDLE cohort (Memphis) were more likely to have total OH-PAH levels above the median in this study population (Table 1). In the pooled sample, women with higher levels of total OH-PAH in pregnancy urine were on average younger, of lower education at enrollment, in a household with lower income, and more likely to have had prior births compared to those in lower quartiles of OH-PAH. Children exposed to higher PAH in utero were more likely to be exposed to ETS in early childhood.
The distributions of OH-PAHs did not vary appreciably between strata of effect modifiers: infant sex (Table S4), maternal asthma (Table S5), and vitamin D (Table S6).
Airway outcomes
The respiratory survey yielded four outcome metrics related to asthma and wheeze. The overall prevalence of current asthma, current wheeze, ever asthma and strict asthma was 11.9%, 15.5%, 11.4% and 9.2%, respectively (Table 2). We observed strong site-specific variations in outcome prevalence, with the highest rates in Memphis and Rochester.
Table 2:
Reported asthma and wheeze outcomes at age 4–6 years in CANDLE and TIDES cohorts*
| Outcomes | Combined analytic population (N=1296) | CANDLE (n=829) | TIDES Sites | |||
|---|---|---|---|---|---|---|
| Seattle (n=96) | San Francisco (n=131) | Minneapolis (n=140) | Rochester NY (n=100) | |||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Current asthma 1 | ||||||
| No | 1142 (88.1) | 696 (84.0) | 95 (99.0) | 128 (97.7) | 132 (94.3) | 91 (91.0) |
| Yes | 154 (11.9) | 133 (16.0) | 1 (1.0) | 3 (2.3) | 8 (5.7) | 9 (9.0) |
| Current wheeze 2 | ||||||
| No | 1092 (84.5) | 669 (80.9) | 93 (96.9) | 121 (93.1) | 124 (88.6) | 85 (85.9) |
| Yes | 200 (15.5) | 158 (19.1) | 3 (3.1) | 9 (6.9) | 16 (11.4) | 14 (14.1) |
| Ever asthma 3 | ||||||
| No | 1145 (88.6) | 709 (85.7) | 93 (97.9) | 126 (96.2) | 132 (94.3) | 85 (85.0) |
| Yes | 148 (11.4) | 118 (14.3) | 2 (2.1) | 5 (3.8) | 8 (5.7) | 15 (15.0) |
| Strict asthma 4 | ||||||
| No | 1160 (90.8) | 721 (88.1) | 93 (98.9) | 127 (97.7) | 132 (94.3) | 87 (90.6) |
| Yes | 118 (9.2) | 97 (11.9) | 1 (1.1) | 3 (2.3) | 8 (5.7) | 9 (9.4) |
The prevalences of four airway outcomes are summarized, for the overall analytic population as well as by five study sites. Outcomes are determined based on parent report on the International Study of Asthma and Allergies in Childhood (ISAAC) questionnaire at the time of child age 4–6 years old.
Current asthma is defined as positive responses to 2 of the following 3, (1) current wheeze (as defined below), (2) ever asthma (as defined below), and (3) medication use. No missing values
Current wheeze is defined as positive response to questions: “Has your child ever had wheezing or whistling in the chest at any time in the past?” and “Has your child ever had wheezing or whistling in the chest in the last 12 months?” N=4 missing.
Ever asthma is defined as positive response to the question “Has your child ever had asthma?” N=3 missing.
Strict asthma defined as positive response to the question “Has your child ever had asthma?” and “Wheeze in the past 12 months” or to the questions about use of asthma medications. N=18 missing.
Associations between individual OH-PAH metabolites and airway outcomes
Several metabolites were associated with increased risk of reported asthma and wheeze outcomes in minimally adjusted models (Figure 1). With additional adjustment for potential confounders in the full adjustment models, these associations were attenuated and lost precision. The 95% confidence intervals for associations of individual OH-PAHs with every outcome measure overlapped the null in full adjustment models. Marginally significant increased risks of current asthma and current wheeze in association with higher levels of some metabolites persisted in full adjustment models. Specifically, a two-fold increase in 1-NAP was associated with elevated risk of current asthma (RR=1.07; 95% CI: 0.99, 1.15). Likewise, 1/9-PHEN was associated with increased risk of current asthma (RR=1.09; 95% CI: 0.99, 1.19) and current wheeze (RR=1.06; 95% CI: 0.98, 1.14), though confidence intervals spanned the null. Associations between OH-PAHs and both ever asthma and strict asthma were generally consistent with null effects. The fully adjusted association between 2/3/9-FLUO and ever asthma suggested protective effects of 2/3/9-FLUO (RR=0.88; 95% CI: 0.74, 1.04), though confidence overlapped the null and nearly 30% of samples had nondetectable values of this metabolite.
Figure 1: Associations between prenatal OH-PAH metabolites and asthma outcomes at 4–6 years.
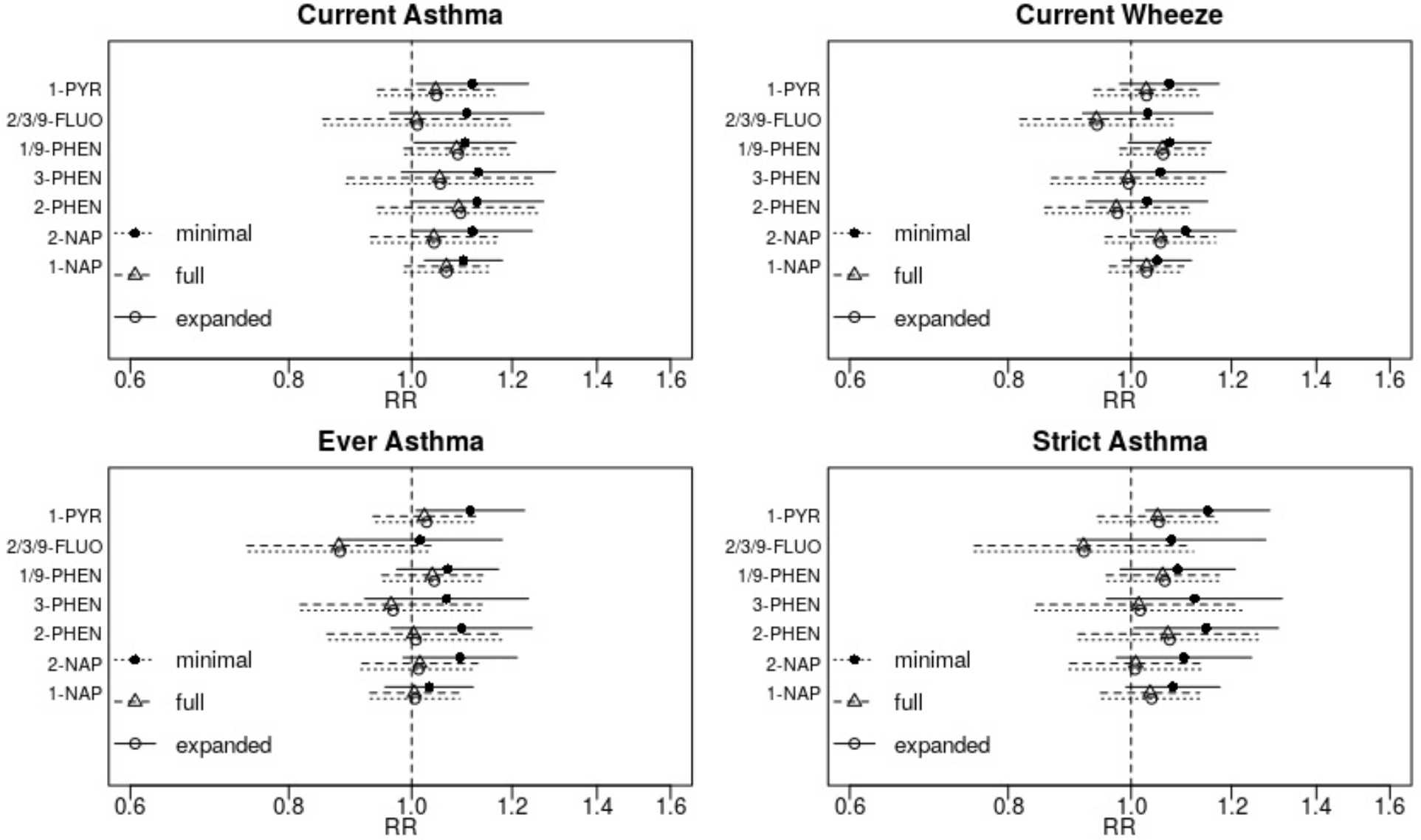
Adjusted relative risks (RR) and 95% confidence intervals were scaled to a two-fold increase in individual OH-PAH metabolite. Associations were estimated using Poisson regression with robust standard errors and adjusted as follows: (minimal model) child age at assessment, child sex, study site, batch of OH-PAH analysis, and urinary specific gravity; (full model) all covariates in minimal model plus maternal age, education, race, pre-pregnancy BMI, household income, parity, maternal history of asthma, enrollment year, postnatal smoke exposure, and season of birth; (expanded) all covariates in full model plus gestational age at birth and birthweight.
Abbreviations: OH-PAH – mono-hydroxylated-polycyclic aromatic hydrocarbon; 1-PYR - 1-hydroxypyrene; 2/3/9 -FLUO – combined 2- and 3- and 9-hydroxyfluorene; 1/9-PHEN – combined 1- and 9-hydroxyphenanthrene; 3-PHEN - 3-hydroxyphenanthrene; 2-PHEN - 2-hydroxyphenanthrene; 2-NAP - 2-hydroxynaphthalene; 1-NAP - 1-hydroxynaphthalene.
Associations did not change meaningfully with additional control for gestational age at birth and birthweight, potential confounders that could also act as mediators (expanded adjustment model; Figure 1). Similar findings were observed when children born prior to 37 weeks gestation were excluded from analysis (data not shown). Results also did not change with addition of product terms between site and key covariates (child race, maternal education, household income, and season) or when continuous covariates were modeled as splines (data not shown). Imputation of nondetectable OH-PAH values using CLMI also did not change findings (data not show).
We repeated all analyses with exclusion of one site at a time as well as limited to each cohort, to test the robustness of results to study sample (Figure S1). When the analysis was restricted to CANDLE only, findings were similar to the pooled two-cohort analysis. When conducted in TIDES alone, all confidence intervals become substantially wider, likely due to reduced statistical power. The suggestive associations between 1/9-PHEN and current asthma observed in the pooled sample were strengthened and more precise (RR=1.62 per two-fold increase in exposure; 95% CI: 1.10, 2.39). In addition, some associations not observed in the pooled sample appeared in TIDES-only analysis, including significant associations between 1/9-PHEN and both ever asthma and strict asthma, as well as 3-PHEN and current asthma and strict asthma. Leaving out one TIDES site at a time from the pooled two cohort sample did not meaningfully impact results (Figure S1).
Analysis of PAH mixtures
In secondary analyses, we used WQS regression to determine whether mixtures of metabolites were associated with outcomes. No mixtures were associated with increased risk of any outcomes in the overall population (Table 3). A mixture dominated by 2/3/9-FLUO (61% weight in the mixture) was associated with decreased risk of ever asthma in full sample analyses (adjusted OR=0.91 [95% CI: 0.83, 1.00] per one-unit increase in the WQS index) but application of the permutation test indicated that this association was imprecise (ppermutation = 0.27).
Table 3:
Associations between OH-PAH mixtures and airway outcomes, estimated by logistic weighted quantile sum (WQS) regression*
| Outcome | Direction | OR (95% CI) | ppermutation |
|---|---|---|---|
| Current wheeze | Negative | 0.96 (0.88, 1.04) | NA |
| Positive | 1.04 (0.94, 1.14) | NA | |
| Current asthma | Negative | 1.00 (0.90, 1.11) | NA |
| Positive | 1.09 (0.98, 1.22) | NA | |
| Ever asthma | Negative | 0.91 (0.83, 1.00) | 0.27 |
| Positive | 0.98 (0.88, 1.10) | NA | |
| Strict asthma | Negative | 0.96 (0.86, 1.07) | NA |
| Positive | 1.06 (0.94, 1.19) | NA |
Logistic regression weighted quantile sum regression was performed using SG-adjusted OH-PAH metabolites categorized into deciles. Effect estimates represent the increased odds of reported outcome, scaled to a one-unit increase in WQS index and adjusted for all covariates in the full adjustment models: child age at assessment (continuous), child sex, study site, batch of OH-PAH analysis, and urinary specific gravity (continuous), maternal age (continuous), education (< high school, high school diploma, graduated college or technical school, and some graduate work or graduate/professional degree), race (Black and non-Black), pre-pregnancy BMI (continuous), household income (<15k, 15–25k, 25–45k, 45–55k, 55–65k, 65–65k, >=75k), parity (prior birth or not), maternal history of asthma, enrollment year, postnatal smoke exposure (any vs. none), and season of birth (categorical; winter, spring, summer, and fall). 95% CIs were estimated using the full analytic sample, without splitting into training and validation datasets. A permutation test p-value (ppermutation) was estimated for any model in which the full-sample 95% CIs did not include the null.
Abbreviations: CI – confidence interval; BMI – body mass index; OH-PAH – mono-hydroxylated-polycyclic aromatic hydrocarbon; WQS – weighted quantile sum.
Effect modification by sex, maternal asthma and vitamin D
In interaction models that include all covariates in main adjustment models, associations between most OH-PAH metabolites and asthma outcomes (ever asthma, current asthma and strict asthma) differed significantly between males and females (Figure 2). In all cases, stronger adverse associations were observed in females. For current asthma, there was no evidence of associations for males but fairly consistent adverse associations for females across all metabolites, though some female-specific associations lacked precision. For 2-PHEN, for example, RRmale = 0.95 (95%CI: 0.79, 1.13) and RRfemale = 1.29 (95%CI: 1.09, 1.52); pinteraction = 0.004.
Figure 2: Assessment of effect modification by child sex.
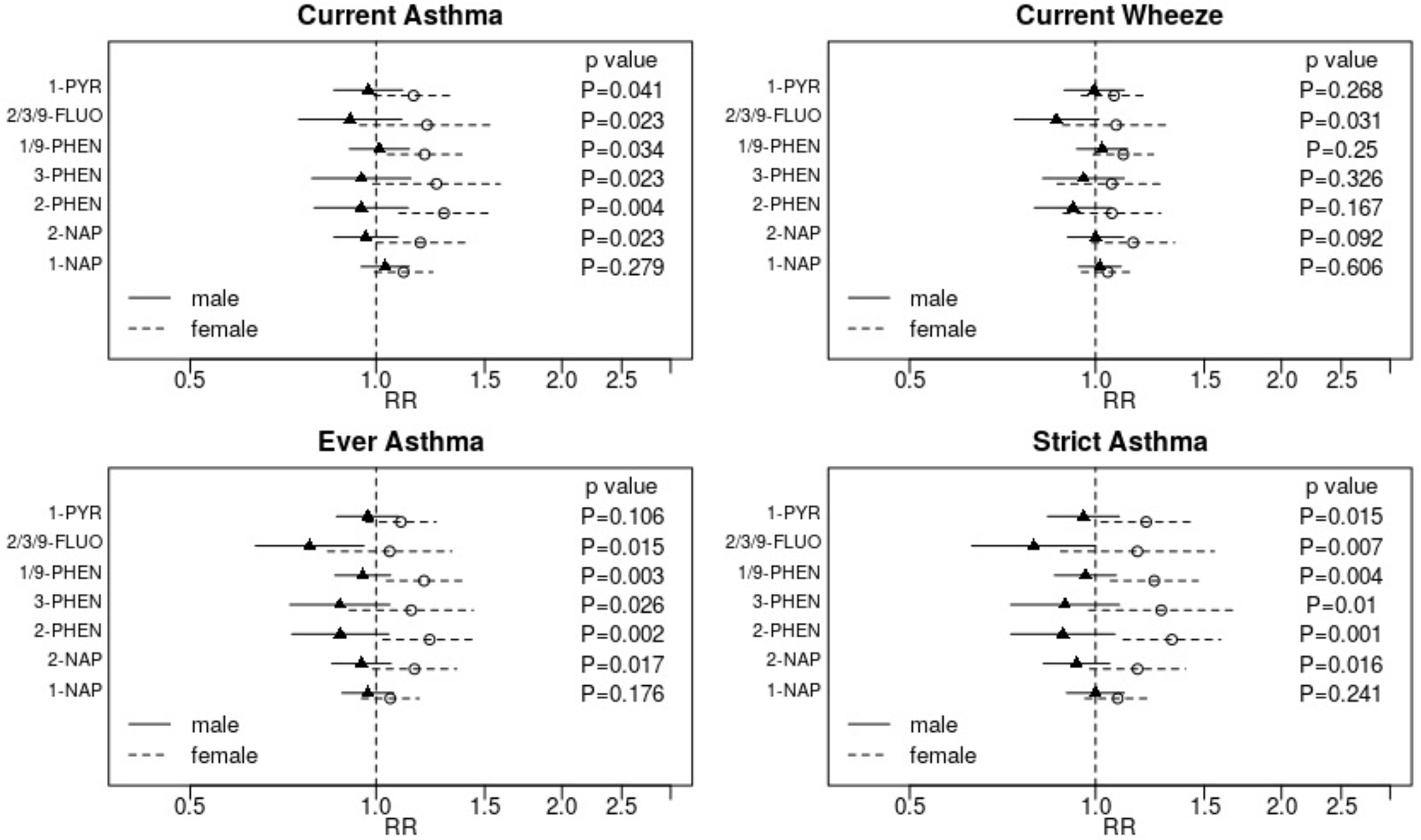
Adjusted relative risks (RR) and 95% confidence intervals for the association between each outcome and a two-fold increase in individual OH-PAH metabolite are displayed. Strata specific results were derived from interaction models. Displayed p-values are for the interaction term. Associations are adjusted for child age at assessment, child sex, study site, batch of OH-PAH analysis, urinary specific gravity, maternal age, education, race, pre-pregnancy BMI, household income, parity, maternal history of asthma, enrollment year, postnatal smoke exposure, and season of birth.
Abbreviations: OH-PAH – mono-hydroxylated-polycyclic aromatic hydrocarbon; 1-PYR - 1-hydroxypyrene; 2/3/9 -FLUO – combined 2- and 3- and 9-hydroxyfluorene; 1/9-PHEN – combined 1- and 9-hydroxyphenanthrene; 3-PHEN - 3-hydroxyphenanthrene; 2-PHEN - 2-hydroxyphenanthrene; 2-NAP - 2-hydroxynaphthalene; 1-NAP - 1-hydroxynaphthalene.
The sex-specific patterns observed for ever-asthma and strict asthma were similar to those for current asthma, though the collective evidence for reduced risk of these outcomes in males with higher concentrations of OH-PAH metabolites was slightly stronger for these outcomes. The suggestion of protective associations was most striking for 2/3/9-FLUO, a metabolite with a large proportion of nondetectable values in this sample. For ever asthma, RRmale = 0.78 (95% CI: 0.64, 0.96) and RRfemale = 1.05 (95% CI: 0.83, 1.33) for a two-fold increase in 2/3/9-FLUO; pinteraction = 0.015. For strict asthma, RRmale = 0.79 (95% CI: 0.64, 0.99) and RRfemale = 1.17 (95% CI: 0.88, 1.56); pinteraction = 0.007. Consistent with analyses of current asthma, some metabolites were associated with higher risk of ever asthma and strict asthma in females (e.g., RRfemale = 1.29 [95% CI: 1.09, 1.59] and RRfemale = 1.20 [95% CI: 1.04, 1.38] for 2-PHEN and 1/9-PHEN, respectively, and ever asthma). The assessment of whether associations between OH-PAH and current wheeze are modified by sex was largely null, though higher 2/3/9-FLUO was associated with marginally reduced risk of wheeze in males: RRmale = 0.86 (95% CI: 0.74, 1.01) and RRfemale = 1.08 (95% CI: 0.89, 1.32); pinteraction = 0.031.
In post hoc analyses, we conducted sex-specific WQS regression to explore whether OH-PAH mixtures were associated with airway outcomes in strata of child sex. In females, a OH-PAH mixture dominated by 2-PHEN (weight = 0.40) and 1/9-PHEN (0.25) was suggestively associated with increased risk of strict asthma (OR=1.17 [95%CI: 0.98, 1.41] per one unit increase in WQS index, adjusted for all covariates in the full model). In male children, a mixture dominated by 2/3/9-FLUO (weight = 0.53) and 1/9-PHEN (weight = 0.22) was associated with decreased risk of ever asthma (OR=0.86 [95%CI: 0.74, 0.99] per one unit increase in WQS index, in the full adjustment model), but the permutation test showed that this association was not significant at a level of alpha<0.05 (ppermutation = 0.26). We also explored whether any of the sex-specific associations exhibited evidence of nonlinearity but saw no deviations from linearity in GAM plots (results not shown).
Associations between OH-PAH and outcomes also varied by maternal history of asthma, with suggestions of protective associations in children of mothers with a family history of asthma and adverse associations without maternal history asthma (Figure 3). For all outcomes, increased risks with higher 1/9-PHEN were evident for children of mothers with no asthma history, and tests of interaction indicated a significant difference by maternal asthma history. Evidence of effect modification was strongest for “ever asthma.” Both 2/3/9-FLUO and 3-PHEN were associated with lower risk of ever asthma for children born to mothers with a history of asthma (pinteraction = 0.018 and 0.009, respectively). Similar patterns were observed for current wheeze and strict asthma, though the statistical evidence of effect modification was weaker.
Figure 3: Assessment of effect modification by maternal asthma.
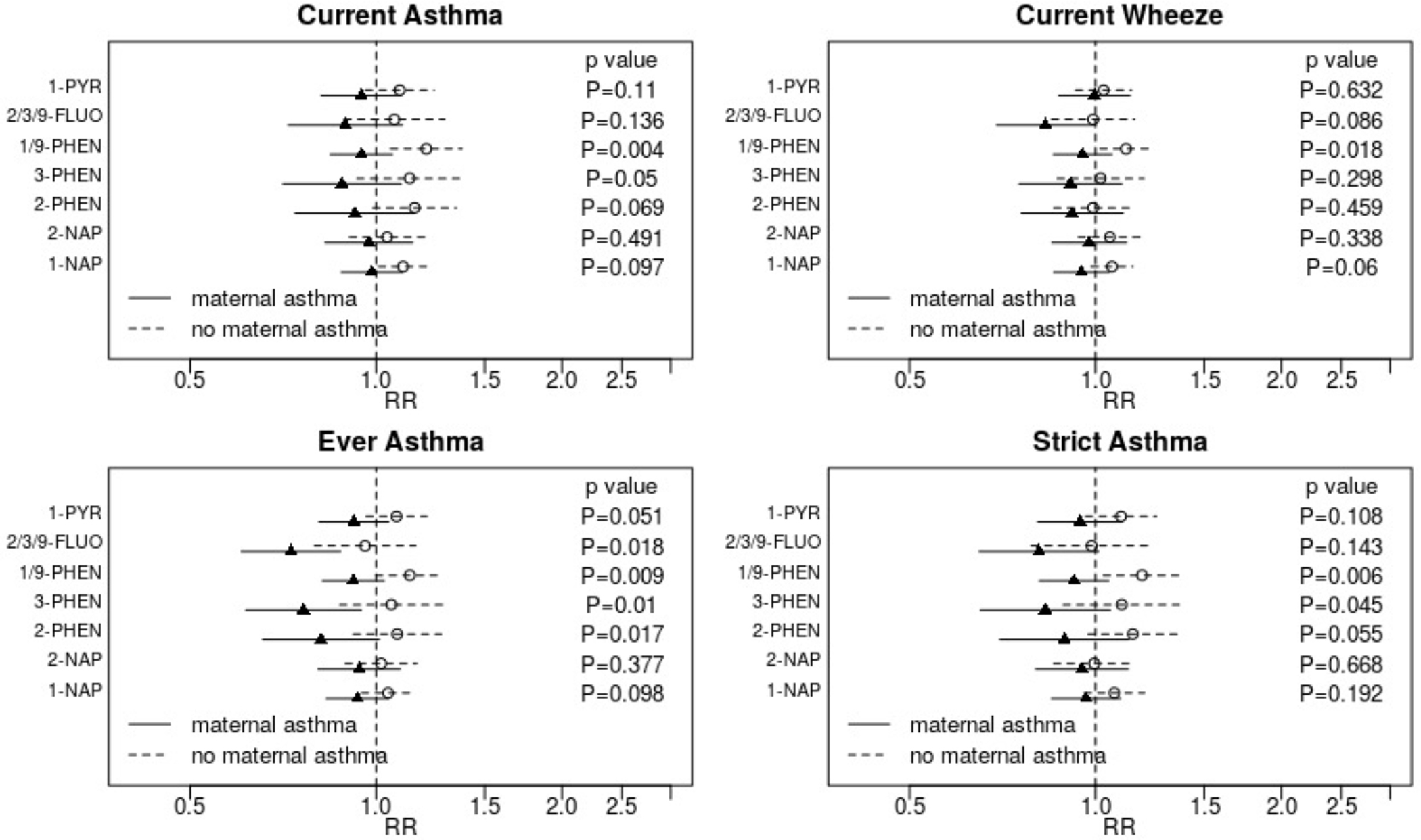
Adjusted relative risks (RR) and 95% confidence intervals for the association between each outcome and a two-fold increase in individual OH-PAH metabolite are displayed. Strata specific results were derived from interaction models. Displayed p-values are for the interaction term. Associations are adjusted for child age at assessment, child sex, study site, batch of OH-PAH analysis, urinary specific gravity, maternal age, education, race, pre-pregnancy, household income, parity, maternal history of asthma, enrollment year, postnatal smoke exposure, and season of birth.
Abbreviations: OH-PAH – mono-hydroxylated-polycyclic aromatic hydrocarbon; 1-PYR - 1-hydroxypyrene; 2/3/9 -FLUO – combined 2- and 3- and 9-hydroxyfluorene; 1/9-PHEN – combined 1- and 9-hydroxyphenanthrene; 3-PHEN - 3-hydroxyphenanthrene; 2-PHEN - 2-hydroxyphenanthrene; 2-NAP - 2-hydroxynaphthalene; 1-NAP - 1-hydroxynaphthalene.
There was no evidence that associations between OH-PAHs and outcomes varied by prenatal vitamin D status, as evaluated in the CANDLE cohort only (Figure 4).
Figure 4: Assessment of effect modification by prenatal vitamin D status.
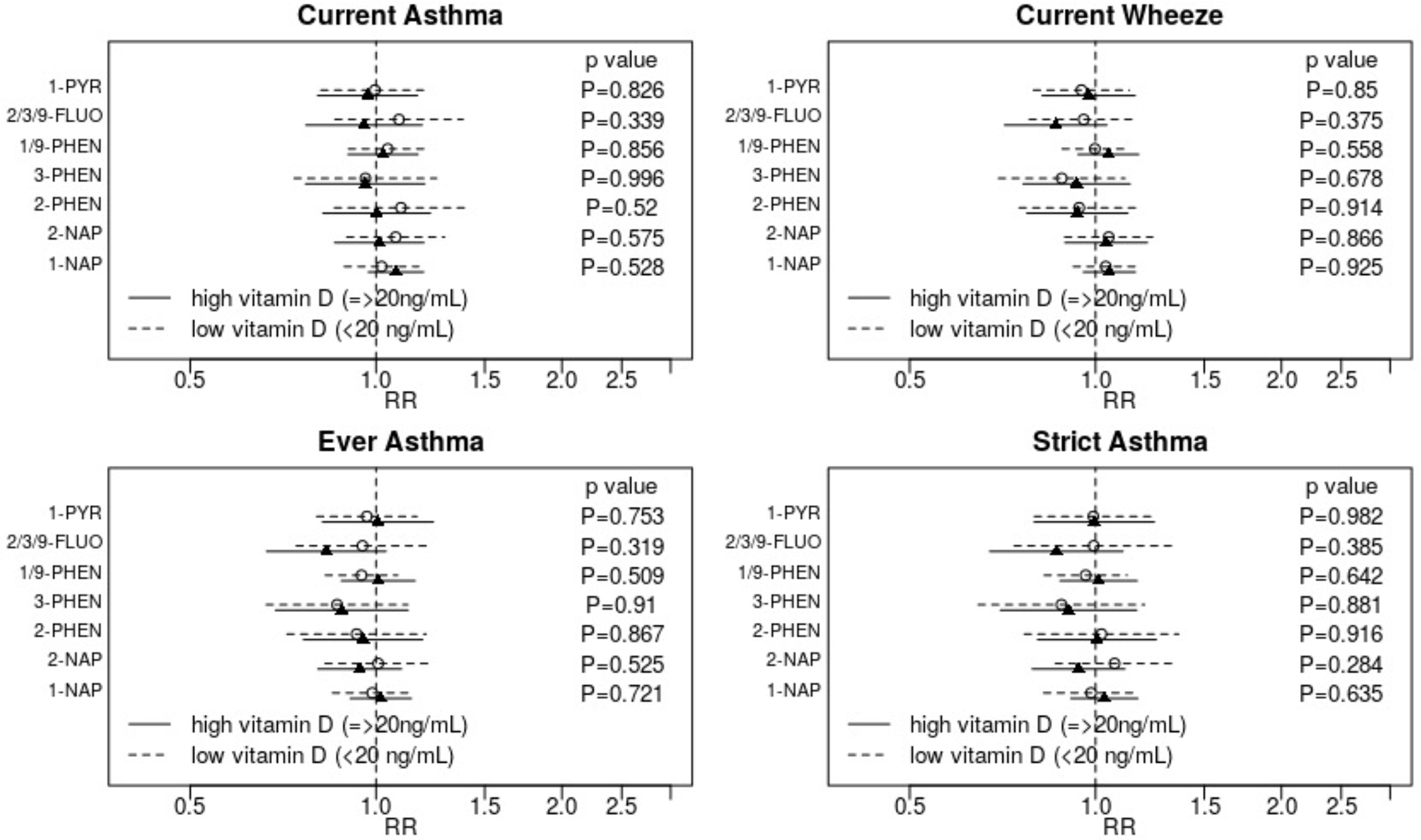
Adjusted relative risks (RR) and 95% confidence intervals for the association between each outcome and a two-fold increase in individual OH-PAH metabolite are displayed. Strata specific results were derived from interaction models. Displayed p-values are for the interaction term. Associations are adjusted for child age at assessment, child sex, study site, batch of OH-PAH analysis, urinary specific gravity, maternal age, education, race, pre-pregnancy BMI, household income, parity, maternal history of asthma, enrollment year, postnatal smoke exposure, vitamin D, and season of birth.
Abbreviations: OH-PAH – mono-hydroxylated-polycyclic aromatic hydrocarbon; 1-PYR - 1-hydroxypyrene; 2/3/9 -FLUO – combined 2- and 3- and 9-hydroxyfluorene; 1/9-PHEN – combined 1- and 9-hydroxyphenanthrene; 3-PHEN - 3-hydroxyphenanthrene; 2-PHEN - 2-hydroxyphenanthrene; 2-NAP - 2-hydroxynaphthalene; 1-NAP - 1-hydroxynaphthalene.
DISCUSSION
We conducted a pooled multi-cohort study of gestational PAH exposure and early childhood wheeze and asthma outcomes in a diverse, well-characterized study population of nonsmoking mothers. In the overall sample, we observed little support for the hypothesis that higher PAH exposure in mid-pregnancy increases risk of asthma or wheeze outcomes at age 4–6 years. However, notable sex-specific associations between individual metabolites and airway outcomes were evident: adverse associations were found in females, but not males. We explored possible modification by maternal history of asthma and found very limited evidence of modification, and we observed no evidence of modification by vitamin D. To our knowledge, this is the largest cohort study of these associations to date and the first data suggesting that adverse effects of gestational PAH exposure on airway health may be specific to female children.
These findings add to a mixed body of evidence supporting the hypothesis that prenatal PAH exposure may increase risk of asthma in childhood. Previously, only three cohort studies have investigated prenatal PAH exposure and pediatric asthma and wheeze development, and these have been conducted in relatively small and homogeneous study populations. The Columbia Center for Children’s Environmental Health estimated associations between prenatal PAH, measured using personal air monitoring, and various airway-related outcomes across early childhood in an urban, low-income New York City population226–28. They found that total PAH concentrations in maternal personal air was associated with higher risk of asthma at 12–24 months in the overall study population (n=303) and that associations were stronger with co-exposure to environmental tobacco smoke (ETS).26 In the older children (5–6 years), adverse associations were only apparent in those exposed to prenatal ETS27 and in nonatopic children.28 This research question has also been explored in a longitudinal cohort study based in Krakow, Poland.29–30 Of 339 children, those classified as relatively high prenatal exposure (based on PAH DNA-adducts in cord blood) experienced a higher number of wheezing days and other respiratory symptoms at age two, but associations were not evident in the third and fourth years of life.29–30 Additional analyses using personal air monitoring for PAHs indicated that PAH exposure was linked to poorer airway outcomes as well as elevated exhaled nitric oxide, a marker of eosinophilic airway inflammation.57 Finally, another longitudinal cohort study in Poland investigated relationships between prenatal PAHs, quantified as 1-PYR in maternal urine, in a sample of 455 mother-child pairs.31 To our knowledge, ours is the largest and most diverse study of pregnancy PAHs and child airway to date. Our null findings in the overall population stand in contrast to those of other studies, but it is difficult to compare across study populations given differences in the way PAH exposure is quantified. In addition, between-study inconsistencies could reflect differences in population characteristics that affect susceptibility to toxic effects of PAHs.
Evidence connecting prenatal exposure to PAH to airway health may also be drawn from several investigations of postnatal PAH exposure and pediatric airway health. These analyses suggest that higher childhood PAH exposure is associated with increased risk of asthma,58–60 wheeze,58,60 airway symptoms,61 IgE,59,62 lung function decrements,63–69 and elevated levels of oxidative stress biomarkers59,62 in a wide variety of study populations. Extrapolation of findings from studies of postnatal exposure to health effects of in utero exposure is challenged by the different routes of offspring exposure (i.e., primarily inhalation and ingestion for postnatal exposure vs. transplacental for gestational exposure). In addition, gestational exposure may exert toxicity through effects on the placenta, including epigenetic modifications.23–25 Despite these differences, both prenatal and postnatal PAH exposure could increase levels of inflammatory biomarkers and oxidative stress, causing adverse developmental impacts on a fetus or a child. This suggests a possible unifying mechanism by which both prenatal and postnatal exposures can increase risk of childhood asthma.70 More epidemiological evidence is needed to confirm how PAH exposure, whether prenatal or postnatal, affects child development.
Our study extends limited existing research on potential effect modifiers of associations between PAH exposure and airway development. With a relatively large sample size, we were better powered than previous cohort studies to explore effect modification. One notable finding was significant effect modification by child sex, with adverse effects observed only in female children. Few studies have explored sex-specific effects of prenatal or childhood exposure to PAH on asthma and related conditions. A cross-sectional analysis of PAH exposure and lung function in young adults in Sweden uncovered no evidence that associations varied by sex.63 Similarly, no differences by child sex were observed in a study of PAHs in ambient air and pediatric lung function.58 Liu et al. used NHANES data from a US population to explore associations between PAHs, including 10 OH-PAH analytes, and childhood asthma, wheezing and coughing, assessed concurrently with exposure.58 They observed that 2-PHEN was associated with higher odds of diagnosed asthma in males, while higher exposure to 4-PHEN was linked to wheezing in females. No tests of interaction were conducted, however, and a very large number of comparisons were made without accounting for multiple comparisons.
Our finding that associations varied significantly by child sex may be explained by suspected endocrine disrupting properties of PAHs, as observed in animal and in vitro experiments.71 Proposed mechanisms for these endocrine-disrupting effects involve established estrogenic properties of PAHs.71–75 Estrogen and other sex hormones may play a key role in asthma development, potentially explaining the strong sex differences in asthma prevalence as well as variations in asthma risk based on stage of pubertal development.76–78 There is evidence that exposure to maternal hormones during fetal development, specifically, affects airway and immune system development, and that sexes may be differentially affected by hormone exposure.79 Notably, sexually dimorphic associations have been observed between other endocrine disrupting compounds and child asthma.80–82 Our findings lend support to the possibility that prenatal PAH exposure may increase risk of asthma through increased estrogenic activity, differentially affecting females; however, more research is needed to confirm this link.
We also tested the hypothesis that prenatal vitamin D status may modify any true adverse effects of prenatal PAH exposures. Evidence from human, animal and in vitro research indicates that sufficient maternal vitamin D in key windows of pregnancy may protect against development of asthma and recurring wheeze in offspring, though some controversy in the literature persists.83–85,86 Hypothesized mechanisms for such protective effects involve vitamin D’s anti-inflammatory properties,83 which could buffer against oxidative stress caused by prenatal PAH exposure. Han et al.67 conducted a cross-sectional analysis using data from the 2007–2012 National Health and Nutrition Examination Survey and found that higher childhood exposure to PAHs was linked to poorer lung function, but only for children with vitamin D deficiency. Similar findings have been reported in analyses of air pollution exposure, though exposure assessment was not specific to PAH compounds.87–88 By contrast, we did not observe any evidence to support modification by prenatal vitamin D in the CANDLE cohort. Previous research in CANDLE showed that the associations between vitamin D and early childhood asthma depends strongly on maternal race.89–90 With adequate sample size, future studies may be able to disentangle three-way interactions between vitamin D, prenatal PAH and maternal race in association with pediatric airway development.
Our study has a number of strengths. We conducted a prospective cohort study with a study population that spanned several US cities, with more socioeconomic and racial diversity than other cohort studies investigating this research topic. The study sample was larger than previous studies, supporting analyses of effect modification. Urinary OH-PAH metabolites were used to estimate PAH exposure across all possible routes of exposure, not limited to inhalation of PAHs in outdoor or indoor air.91–94 Because both PAH exposure and airway outcomes vary strongly by maternal characteristics, lifestyle and health behaviors, and family demographics,9,64 we adjusted for a wide variety of potential confounders, mitigating the potential for residual or unmeasured confounding. To the best of our knowledge, our investigation of effect modification by maternal vitamin D is unique among investigations of PAH exposure during pregnancy and child health outcomes. Finally, to the best of our knowledge, we are the first to apply methods to analyze several PAH metabolites concurrently, as mixtures, which better represents real-life exposure scenarios.11,95 We describe a new permutation text extension to WQS regression for logistic models, which addresses known limitations of WQS regression and builds on our previous development of a permutation test for linear WQS regression.55,56
There are several limitations to note. We analyzed only a single urine sample from each pregnancy to estimate PAH exposure. PAHs have short half-lives in humans, on the order of hours for some OH-PAHs.75 Analyses of OH-PAHs in samples collected repeatedly over time showed low to moderate intraclass correlation coefficients (ICCs) for various metabolites, indicating a moderate to high degree of within-individual temporal variability and the possibility that a single measure of OH-PAH in pregnancy may not be an accurate proxy for average pregnancy-wide exposure.12,37 Another study reported only modest variability and concluded that a single assessment of OH-PAH may be an adequate measure of average pregnancy exposure.9 Null findings in our paper and others could result from measurement of gestational PAH outside a true critical window of exposure or from nondifferential exposure measurement error associated with relying on a single timepoint of PAH exposure, which likely biases observed associations toward the null. Asthma and wheeze outcomes were reported by mothers and may be affected by outcome misclassification on account of inaccurate reporting or limited access to health care. Environmental tobacco smoke (ETS) is a major contributor to PAH exposure as well as a risk factor for the airway outcomes analyzed, and can therefore confound observed associations. We controlled for ETS exposure in the prenatal period with urinary cotinine and in the postnatal period with maternal report of household smokers, but these measures may not accurately capture all ETS exposure, potentially leading to residual confounding.96
An additional limitation is that every metabolite had some nondetectable values, the highest being approximately 30% <LOD for both 2/3/9-FLUO and 1-PYR. Further, the proportions of nondetectable values varied by site and cohort. Imputing <LOD values as LOD/√2 can bias observed associations, especially when the proportion <LOD is 20% or higher. To minimize the potential for bias, we included metabolites with higher proportions of nondetectable values but interpreted associations with these metabolites with caution. We also conducted a sensitivity analysis in which main analyses were repeated with imputation of <LOD values using CLMI, a method for multiply imputing nondetectable values when LODs vary by batch. Further, while we adjusted for a large number of potential confounders, some of them could have been mismeasured, leading to residual confounding. Other important confounders, such as prenatal diet, have not been measured. Finally, we excluded smokers in this analysis to minimize the confounding effect of smoking frequency and intensity on measured associations. As a result, our observed results are only generalizable to a nonsmoking population.
In conclusion, we report novel evidence of sex-specific effects of gestational PAH exposure, with adverse associations evident only for female children. These preliminary findings are consistent with previous in vitro and animal model experiments suggesting that PAHs may have endocrine disrupting properties, potentially affecting fetal airway and immune system development in a sexually dimorphic manner. Given the observational nature of this study and the limitations discussed above, replication in other studies is needed.
Supplementary Material
Acknowledgments
ECHO PATHWAYS is funded by NIH (UG3/UH3OD023271, P30ES007033, and P30ES005022). The Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) study was funded by the Urban Child Institute. The TIDES study was funded by NIH R01ES016863 and P30ES005022 and National Institute of Environmental Health Sciences (NIEHS) Intramural Funding (ZIA10331): Reproductive outcomes and oxidative stress in TIDES (ROOST). Dr. Kannan analyzed OH-PAH metabolites in TIDES with support from the New York University ECHO Cohort Center (NIH UG3/UH3OD023305 [PI: Leonardo Trasande]). Dr. Marnie Hazlehurst was supported in part by the UW NIEHS sponsored Biostatistics, Epidemiologic and Bioinformatic Training in Environmental Health (BEBTEH) Training Grant: NIEHS T32ES015459. We are grateful for the participation of families enrolled in the CANDLE and TIDES cohorts, as well as the dedication of research staff and investigators. This manuscript has been reviewed by PATHWAYS for scientific content and consistency of data interpretation with previous PATHWAYS publications. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Data sharing statement
The data utilized for this study are not publicly available but de-identified data may be available on request, subject to approval by the internal review board and under a formal data use agreement. Contact the corresponding author for more information.
References
- 1.Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health. 2005;26:89–113. doi: 10.1146/annurev.publhealth.26.021304.144528. [DOI] [PubMed] [Google Scholar]
- 2.Louisias M, Phipatanakul W. Managing Asthma in Low-Income, Underrepresented Minority, and Other Disadvantaged Pediatric Populations: Closing the Gap. Curr Allergy Asthma Rep. 2017. Sep 15;17(10):68. doi: 10.1007/s11882-017-0734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaffney AW, Himmelstein DU, Christiani DC, Woolhandler S. Socioeconomic Inequality in Respiratory Health in the US From 1959 to 2018. JAMA Intern Med. 2021. Jul 1;181(7):968–976. doi: 10.1001/jamainternmed.2021.2441. Erratum in: JAMA Intern Med. 2021 Jul 1;181(7):1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao J, Wheeler AJ, Zosky GR, Johnston FH. Long-term impacts of prenatal and infant exposure to fine particulate matter on wheezing and asthma: A systematic review and meta-analysis. Environ Epidemiol. 2019. Apr 12;3(2):e042. doi: 10.1097/EE9.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hehua Z, Qing C, Shanyan G, Qijun W, Yuhong Z. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: A systematic review. Environ Res. 2017. Nov;159:519–530. doi: 10.1016/j.envres.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 6.Li MC, Chen CH, Guo YL. Phthalate esters and childhood asthma: A systematic review and congener-specific meta-analysis. Environ Pollut. 2017. Oct;229:655–660. doi: 10.1016/j.envpol.2017.06.083. Epub 2017 Jul 7. [DOI] [PubMed] [Google Scholar]
- 7.Boström CE, Gerde P, Hanberg A, Jernström B, Johansson C, Kyrklund T, Rannug A, Törnqvist M, Victorin K, Westerholm R. Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect. 2002. Jun;110 Suppl 3(Suppl 3):451–88. doi: 10.1289/ehp.110-1241197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some non-heterocyclic polycyclic aromatic hydrocarbons and some related exposures. IARC Monogr Eval Carcinog Risks Hum. 2010;92:1–853. [PMC free article] [PubMed] [Google Scholar]
- 9.Dobraca D, Lum R, Sjödin A, Calafat AM, Laurent CA, Kushi LH, Windham GC. Urinary biomarkers of polycyclic aromatic hydrocarbons in pre- and peri-pubertal girls in Northern California: Predictors of exposure and temporal variability. Environ Res. 2018. Aug;165:46–54. doi: 10.1016/j.envres.2017.11.011. Epub 2018 Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martorell A, Nieto M, Nadal G, Perello RM, Marce JL, Domingo. Human exposure to polycyclic aromatic hydrocarbons (PAHs) using data from a duplicate diet study in Catalonia, Spain Food Chem. Toxicol, 50 (2012), pp. 4103–4108 [DOI] [PubMed] [Google Scholar]
- 11.CDC. Fourth Report on Human Exposure to Environmental Chemicals, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA: (2019). https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2019-508.pdf [Google Scholar]
- 12.Cathey A, Ferguson KK, McElrath TF, Cantonwine DE, Pace G, Alshawabkeh A, Cordero JF, Meeker JD. Distribution and predictors of urinary polycyclic aromatic hydrocarbon metabolites in two pregnancy cohort studies. Environ Pollut. 2018. Jan;232:556–562. doi: 10.1016/j.envpol.2017.09.087. Epub 2017 Oct 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, Patterson DG Jr. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ Res. 2008. Jul;107(3):320–31. doi: 10.1016/j.envres.2008.01.013. Epub 2008 Mar 3. [DOI] [PubMed] [Google Scholar]
- 14.USEPA. Toxicological Review of Benzo[a]pyrene Executive Summary [CASRN 50–32-8]. Integrated Risk Information System, National Center for Environmental Assessment, Office of Research and Development, U.S. Environmental Protection Agency, Washington, DC: (2017). [Google Scholar]
- 15.Låg M, Øvrevik J, Refsnes M, Holme JA. Potential role of polycyclic aromatic hydrocarbons in air pollution-induced non-malignant respiratory diseases. Respir Res. 2020. Nov 13;21(1):299. doi: 10.1186/s12931-020-01563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramesh A, Harris KJ, Archibong AE (2017). Reproductive toxicity of polycyclic aromatic hydrocarbons. In Reproductive and Developmental Toxicology (Gupta RC, Ed.), 2nd ed., pp. 745–763. Academic Press, Cambridge. [Google Scholar]
- 17.Zhang Y, Dong S, Wang H, Tao S, Kiyama R. Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ Pollut. 2016. Jun;213:809–824. doi: 10.1016/j.envpol.2016.03.050.. Epub 2016 Mar 31. [DOI] [PubMed] [Google Scholar]
- 18.Hýžd’alová M, Pivnicka J, Zapletal O, Vázquez-Gómez G, Matthews J, Neca J, Pencíková K, Machala M, Vondrácek J. Aryl Hydrocarbon Receptor-Dependent Metabolism Plays a Significant Role in Estrogen-Like Effects of Polycyclic Aromatic Hydrocarbons on Cell Proliferation. Toxicol Sci. 2018. Oct 1;165(2):447–461. doi: 10.1093/toxsci/kfy153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darbre PD. Overview of air pollution and endocrine disorders. Int J Gen Med. 2018. May 23;11:191–207. doi: 10.2147/IJGM.S102230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward EC, Murray MJ, Lauer LD, House RV, Irons R, Dean JH. Immunosuppression following 7,12-dimethylbenz[a]anthracene exposure in B6C3F1 mice. Effects on humoral immunity and host resistance. Toxicol Appl Pharmacol. 1984; 75:299–308. [DOI] [PubMed] [Google Scholar]
- 21.Laupeze B, Amiot L, Sparfel L, Le Ferrec E, Fauchet R, Fardel O. Polycyclic aromatic hydrocarbons affect functional differentiation and maturation of human monocyte-derived dendritic cells. J Immunol. 2002; 168:2652–2658. ] [DOI] [PubMed] [Google Scholar]
- 22.Van Grevenynghe J, Rion S, Le Ferrec E, Le Vee M, Amiot L, Fauchet R, Fardel O. Polycyclic aromatic hydrocarbons inhibit differentiation of human monocytes into macrophages. J Immunol. 2003; 170:2374–2381. [DOI] [PubMed] [Google Scholar]
- 23.Perera F, Tang WY, Herbstman J, Tang D, Levin L, Miller R, Ho SM. Relation of DNA methylation of 5’-CpG island of ACSL3 to transplacental exposure to airborne polycyclic aromatic hydrocarbons and childhood asthma. PLoS One. 2009;4(2):e4488. doi: 10.1371/journal.pone.0004488. Epub 2009 Feb 16. Erratum in: PLoS One. 2009;4(8). doi: 10.1371/annotation/6a678269–9623-4a13–8b19–4e9431ff3cb6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herbstman JB, Tang D, Zhu D, Qu L, Sj.din A, Li Z, Camann D, Perera FP. Prenatal exposure to polycyclic aromatic hydrocarbons, benzo[a]pyrene–DNA adducts, and genomic DNA methylation in cord blood. Environ Health Perspect. 2012; 120:733–738. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perera F. Molecular epidemiology, prenatal exposure and prevention of cancer. Environmental Health. 2011; 10(Suppl 1):S5. ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller RL, Garfinkel R, Horton M, Camann D, Perera FP, Whyatt RM, Kinney PL. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest. 2004. Oct;126(4):1071–8. doi: 10.1378/chest.126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosa MJ, Jung KH, Perzanowski MS, Kelvin EA, Darling KW, Camann DE, Chillrud SN, Whyatt RM, Kinney PL, Perera FP, Miller RL. Prenatal exposure to polycyclic aromatic hydrocarbons, environmental tobacco smoke and asthma. Respir Med. 2011. Jun;105(6):869–76. doi: 10.1016/j.rmed.2010.11.022. Epub 2010 Dec 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung KH, Perzanowski M, Rundle A, Moors K, Yan B, Chillrud SN, Whyatt R, Camann D, Perera FP, Miller RL. Polycyclic aromatic hydrocarbon exposure, obesity and childhood asthma in an urban cohort. Environ Res. 2014. Jan;128:35–41. doi: 10.1016/j.envres.2013.12.002. Epub 2013 Dec 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jedrychowski W, Galas A, Pac A, Flak E, Camman D, Rauh V, Perera F. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. Eur J Epidemiol. 2005;20(9):775–82. doi: 10.1007/s10654-005-1048-1. [DOI] [PubMed] [Google Scholar]
- 30.Jedrychowski WA, Perera FP, Maugeri U, Mrozek-Budzyn D, Mroz E, Klimaszewska-Rembiasz M, Flak E, Edwards S, Spengler J, Jacek R, Sowa A. Intrauterine exposure to polycyclic aromatic hydrocarbons, fine particulate matter and early wheeze. Prospective birth cohort study in 4-year olds. Pediatr Allergy Immunol. 2010. Jun;21(4 Pt 2):e723–32. doi: 10.1111/j.1399-3038.2010.01034.x. Epub 2010 Apr 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jerzynska J, Podlecka D, Polanska K, Hanke W, Stelmach I, Stelmach W. Prenatal and postnatal exposure to polycyclic aromatic hydrocarbons and allergy symptoms in city children. Allergol Immunopathol (Madr). 2017. Jan-Feb;45(1):18–24. doi: 10.1016/j.aller.2016.07.006. Epub 2016 Oct 25. [DOI] [PubMed] [Google Scholar]
- 32.Hehua Z, Qing C, Shanyan G, Qijun W, Yuhong Z. The impact of prenatal exposure to air pollution on childhood wheezing and asthma: A systematic review. Environ Res. 2017. Nov;159:519–530. doi: 10.1016/j.envres.2017.08.038. Epub 2017 Sep 8. [DOI] [PubMed] [Google Scholar]
- 33.Brandt HC, Watson WP. Monitoring human occupational and environmental exposures to polycyclic aromatic compounds. Ann Occup Hyg. 2003. Jul;47(5):349–78. doi: 10.1093/annhyg/meg052. [DOI] [PubMed] [Google Scholar]
- 34.LeWinn KZ, Bush NR, Batra A, Tylavsky F, Rehkopf D, 2020. Identification of Modifiable Social and Behavioral Factors Associated With Childhood Cognitive Performance. JAMA pediatrics 174, 1063–1072. 10.1001/jamapediatrics.2020.2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett ES, Sathyanarayana S, Janssen S, Redmon JB, Nguyen RHN, Kobrosly R, et al. 2014. Environmental health attitudes and behaviors: Findings from a large pregnancy cohort study. European Journal of Obstetrics and Gynecology and Reproductive Biology 176:119–125; doi: 10.1016/j.ejogrb.2014.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H, Wang L, Liu C, Stryker Z, Loganathan BG, Kannan K. Phthalate Metabolites, Hydroxy-Polycyclic Aromatic Hydrocarbons, and Bisphenol Analogues in Bovine Urine Collected from China, India, and the United States. Environ Sci Technol. 2019. Oct 1;53(19):11524–11531. doi: 10.1021/acs.est.9b04178. Epub 2019 Sep 12. [DOI] [PubMed] [Google Scholar]
- 37.Zhu H, Martinez-Moral MP, Kannan K. Variability in urinary biomarkers of human exposure to polycyclic aromatic hydrocarbons and its association with oxidative stress. Environ Int 2021. Nov;156:106720. doi: 10.1016/j.envint.2021.106720. Epub 2021 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo Y, Senthilkumar K, Alomirah H, Moon HB, Minh TB, Mohd MA, Nakata H, Kannan K. Concentrations and profiles of urinary polycyclic aromatic hydrocarbon metabolites (OH-PAHs) in several Asian countries. Environ Sci Technol. 2013. Mar 19;47(6):2932–8. doi: 10.1021/es3052262. Epub 2013 Feb 27. [DOI] [PubMed] [Google Scholar]
- 39.Boeniger MF, Lowry LK, Rosenberg J. 1993. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J 54:615–627; doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 40.Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, Mitchell EA, Pearce N, Sibbald B, Stewart AW, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995. Mar;8(3):483–91. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 41.Rosa MJ, Hartman TJ, Adgent M, Gardner K, Gebretsadik T, Moore PE, Davis RL, LeWinn KZ, Bush NR, Tylavsky F, Wright RJ, Carroll KN. Prenatal polyunsaturated fatty acids and child asthma: Effect modification by maternal asthma and child sex. J Allergy Clin Immunol. 2020. Mar;145(3):800–807.e4. doi: 10.1016/j.jaci.2019.10.039. Epub 2019 Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adgent MA, Carroll KN, Hazlehurst MF, Loftus CT, Szpiro AA, Karr CJ, Barrett ES, LeWinn KZ, Bush NR, Tylavsky FA, Kannan K, Sathyanarayana S. A combined cohort analysis of prenatal exposure to phthalate mixtures and childhood asthma. Environ Int 2020. Oct;143:105970. doi: 10.1016/j.envint.2020.105970. Epub 2020 Jul 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quraishi SM, Hazlehurst MF, Loftus CT, Nguyen RHN, Barrett ES, Kaufman JD, Bush NR, Karr CJ, LeWinn KZ, Sathyanarayana S, Tylavsky FA, Szpiro AA, Enquobahrie DA. Association of prenatal exposure to ambient air pollution with adverse birth outcomes and effect modification by socioeconomic factors. Environ Res. 2022. Sep;212(Pt E):113571. doi: 10.1016/j.envres.2022.113571. Epub 2022 May 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni Y, Loftus CT, Szpiro AA, Young MT, Hazlehurst MF, Murphy LE, Tylavsky FA, Mason WA, LeWinn KZ, Sathyanarayana S, Barrett ES, Bush NR, Karr CJ. Associations of Pre- and Postnatal Air Pollution Exposures with Child Behavioral Problems and Cognitive Performance: A U.S. Multi-Cohort Study. Environ Health Perspect. 2022. Jun;130(6):67008. doi: 10.1289/EHP10248. Epub 2022 Jun 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hazlehurst MF, Carroll KN, Loftus CT, Szpiro AA, Moore PE, Kaufman JD, Kirwa K, LeWinn KZ, Bush NR, Sathyanarayana S, Tylavsky FA, Barrett ES, Nguyen RHN, Karr CJ. Maternal exposure to PM2.5 during pregnancy and asthma risk in early childhood: consideration of phases of fetal lung development. Environ Epidemiol. 2021. Apr;5(2):e130. doi: 10.1097/ee9.0000000000000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schick SF, Blount BC, Jacob P Rd, Saliba NA, Bernert JT, El Hellani A, Jatlow P, Pappas RS, Wang L, Foulds J, Ghosh A, Hecht SS, Gomez JC, Martin JR, Mesaros C, Srivastava S, St Helen G, Tarran R, Lorkiewicz PK, Blair IA, Kimmel HL, Doerschuk CM, Benowitz NL, Bhatnagar A. Biomarkers of exposure to new and emerging tobacco delivery products. Am J Physiol Lung Cell Mol Physiol. 2017. Sep 1;313(3):L425–L452. doi: 10.1152/ajplung.00343.2016. Epub 2017 May 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tylavsky FA, Kocak M, Murphy LE, Graff JC, Palmer FB, Völgyi E, Diaz-Thomas AM, Ferry RJ Jr. Gestational Vitamin 25(OH)D Status as a Risk Factor for Receptive Language Development: A 24-Month, Longitudinal, Observational Study. Nutrients. 2015. Dec 2;7(12):9918–30. doi: 10.3390/nu7125499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holick MF. Vitamin D deficiency. N Engl J Med. 2007. Jul 19;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 49.Amrein K, Scherkl M, Hoffmann M, Neuwersch-Sommeregger S, Köstenberger M, Tmava Berisha A, Martucci G, Pilz S, Malle O. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020. Nov;74(11):1498–1513. doi: 10.1038/s41430-020-0558-y. Epub 2020 Jan 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.He H, Butz A, Keet CA, Minkovitz CS, Hong X, Caruso DM, Pearson C, Cohen RT, Wills-Karp M, Zuckerman BS, Hughes ME, Wang X. Preterm Birth with Childhood Asthma: The Role of Degree of Prematurity and Asthma Definitions. Am J Respir Crit Care Med. 2015. Aug 15;192(4):520–3. doi: 10.1164/rccm.201503-0522LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neuman Å, Hohmann C, Orsini N, Pershagen G, Eller E, Kjaer HF, Gehring U, Granell R, Henderson J, Heinrich J, Lau S, Nieuwenhuijsen M, Sunyer J, Tischer C, Torrent M, Wahn U, Wijga AH, Wickman M, Keil T, Bergström A; ENRIECO Consortium. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med. 2012. Nov 15;186(10):1037–43. doi: 10.1164/rccm.201203-0501OC. Epub 2012 Sep 5. [DOI] [PubMed] [Google Scholar]
- 52.Boss J, Mukherjee B, Ferguson KK, Aker A, Alshawabkeh AN, Cordero JF, Meeker JD, Kim S. Estimating Outcome-Exposure Associations when Exposure Biomarker Detection Limits vary Across Batches. Epidemiology. 2019. Sep;30(5):746–755. doi: 10.1097/EDE.0000000000001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. 2015. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat 20:100–120; doi: 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borovicka T, Jr MJ, Kordik P, Jirina M. 2012. Selecting Representative Data Sets. Advances in Data Mining Knowledge Discovery and Applications; doi: 10.5772/50787. [DOI] [Google Scholar]
- 55.Loftus CT, Bush NR, Day DB, Ni Y, Tylavsky FA, Karr CJ, Kannan K, Barrett ES, Szpiro AA, Sathyanarayana S, LeWinn KZ. Exposure to prenatal phthalate mixtures and neurodevelopment in the Conditions Affecting Neurocognitive Development and Learning in Early childhood (CANDLE) study. Environ Int. 2021. May;150:106409. doi: 10.1016/j.envint.2021.106409. Epub 2021 Feb 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Day DB, Collett BR, Barrett ES, Bush NR, Swan SH, Nguyen RHN, Szpiro AA, Sathyanarayana S. Phthalate mixtures in pregnancy, autistic traits, and adverse childhood behavioral outcomes. Environ Int. 2021. Feb;147:106330. doi: 10.1016/j.envint.2020.106330. Epub 2021 Jan 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jedrychowski W, Maugeri U, Mroz E, Flak E, Rembiasz M, Jacek R, Sowa A. Fractional exhaled nitric oxide in healthy non-asthmatic 7-year olds and prenatal exposure to polycyclic aromatic hydrocarbons: nested regression analysis. Pediatr Pulmonol. 2012. Nov;47(11):1131–9. doi: 10.1002/ppul.22570. Epub 2012 May 15. [DOI] [PubMed] [Google Scholar]
- 58.Liu H, Xu C, Jiang ZY, Gu A. Association of polycyclic aromatic hydrocarbons and asthma among children 6–19 years: NHANES 2001–2008 and NHANES 2011–2012 . Respir Med 2016. Jan;110:20–7. doi: 10.1016/j.rmed.2015.11.003. Epub 2015 Nov 10. [DOI] [PubMed] [Google Scholar]
- 59.Wang IJ, Karmaus WJ, Yang CC. Polycyclic aromatic hydrocarbons exposure, oxidative stress, and asthma in children. Int Arch Occup Environ Health. 2017. Apr;90(3):297–303. doi: 10.1007/s00420-017-1198-y. Epub 2017 Feb 7. [DOI] [PubMed] [Google Scholar]
- 60.Jung KH, Yan B, Moors K, Chillrud SN, Perzanowski MS, Whyatt RM, Hoepner L, Goldstein I, Zhang B, Camann D, Kinney PL, Perera FP, Miller RL. Repeated exposure to polycyclic aromatic hydrocarbons and asthma: effect of seroatopy. Ann Allergy Asthma Immunol. 2012. Oct;109(4):249–54. doi: 10.1016/j.anai.2012.07.019. Epub 2012 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gale SL, Noth EM, Mann J, Balmes J, Hammond SK, Tager IB. Polycyclic aromatic hydrocarbon exposure and wheeze in a cohort of children with asthma in Fresno, CA. J Expo Sci Environ Epidemiol 2012. Jul;22(4):386–92. doi: 10.1038/jes.2012.29. Epub 2012 May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Daghri NM, Alokail MS, Abd-Alrahman SH, Draz HM, Yakout SM, Clerici M. Polycyclic aromatic hydrocarbon exposure and pediatric asthma in children: a case-control study. Environ Health. 2013. Jan 3;12:1. doi: 10.1186/1476-069X-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alhamdow A, Zettergren A, Kull I, Hallberg J, Andersson N, Ekström S, Berglund M, Wheelock CE, Essig YJ, Krais AM, Georgelis A, Lindh CH, Melén E, Bergström A. Low-level exposure to polycyclic aromatic hydrocarbons is associated with reduced lung function among Swedish young adults. Environ Res. 2021. Jun;197:111169. doi: 10.1016/j.envres.2021.111169. Epub 2021 Apr 20. [DOI] [PubMed] [Google Scholar]
- 64.Cao L, Zhou Y, Tan A, Shi T, Zhu C, Xiao L, Zhang Z, Yang S, Mu G, Wang X, Wang D, Ma J, Chen W. Oxidative damage mediates the association between polycyclic aromatic hydrocarbon exposure and lung function. Environ Health. 2020. Jul 2;19(1):75. doi: 10.1186/s12940-020-00621-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou Y, Sun H, Xie J, Song Y, Liu Y, Huang X, Zhou T, Rong Y, Wu T, Yuan J, Chen W. Urinary Polycyclic Aromatic Hydrocarbon Metabolites and Altered Lung Function in Wuhan, China. Am J Respir Crit Care Med. 2016. Apr 15;193(8):835–46. doi: 10.1164/rccm.201412-2279OC. [DOI] [PubMed] [Google Scholar]
- 66.Barraza-Villarreal A, Escamilla-Nuñez MC, Schilmann A, Hernandez-Cadena L, Li Z, Romanoff L, Sjödin A, Del Río-Navarro BE, Díaz-Sanchez D, Díaz-Barriga F, Sly P, Romieu I. Lung function, airway inflammation, and polycyclic aromatic hydrocarbons exposure in mexican schoolchildren: a pilot study. J Occup Environ Med. 2014. Apr;56(4):415–9. doi: 10.1097/JOM.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Han YY, Rosser F, Forno E, Celedón JC. Exposure to polycyclic aromatic hydrocarbons, vitamin D, and lung function in children with asthma. Pediatr Pulmonol. 2018. Oct;53(10):1362–1368. doi: 10.1002/ppul.24084. Epub 2018 Jun 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Padula AM, Balmes JR, Eisen EA, Mann J, Noth EM, Lurmann FW, Pratt B, Tager IB, Nadeau K, Hammond SK. Ambient polycyclic aromatic hydrocarbons and pulmonary function in children. J Expo Sci Environ Epidemiol. 2015. May;25(3):295–302. doi: 10.1038/jes.2014.42. Epub 2014 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.and lung function tests and cardiovascular indices among children with asthma living near an industrial complex and petroleum refineries. Environ Res. 2014. Jul;132:38–45. doi: 10.1016/j.envres.2014.03.030. Epub 2014 Apr 16. [DOI] [PubMed] [Google Scholar]
- 70.Lebold KM, Jacoby DB, Drake MG. Inflammatory mechanisms linking maternal and childhood asthma. J Leukoc Biol. 2020. Jul;108(1):113–121. doi: 10.1002/JLB.3MR1219-338R. Epub 2020 Feb 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Y, Dong S, Wang H, Tao S, Kiyama R. Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environ Pollut. 2016. Jun;213:809–824. doi: 10.1016/j.envpol.2016.03.050. Epub 2016 Mar 31. [DOI] [PubMed] [Google Scholar]
- 72.Sievers CK, Shanle EK, Bradfield CA, Xu W. Differential action of monohydroxylated polycyclic aromatic hydrocarbons with estrogen receptors α and β. Toxicol Sci. 2013. Apr;132(2):359–67. doi: 10.1093/toxsci/kfs287. Epub 2012 Sep 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schultz TW, Sinks GD. Xenoestrogenic gene expression: structural features of active polycyclic aromatic hydrocarbons. Environ Toxicol Chem. 2002. Apr;21(4):783–6. [PubMed] [Google Scholar]
- 74.Wenger D, Gerecke AC, Heeb NV, Schmid P, Hueglin C, Naegeli H, Zenobi R. In vitro estrogenicity of ambient particulate matter: contribution of hydroxylated polycyclic aromatic hydrocarbons. J Appl Toxicol. 2009. Apr;29(3):223–32. doi: 10.1002/jat.1400. [DOI] [PubMed] [Google Scholar]
- 75.Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, Webster TF, Sjödin A. Excretion profiles and half-lives of ten urinary polycyclic aromatic hydrocarbon metabolites after dietary exposure. Chem Res Toxicol. 2012. Jul 16;25(7):1452–61. doi: 10.1021/tx300108e. Epub 2012 Jun 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fuseini H, Newcomb DC. Mechanisms Driving Gender Differences in Asthma. Curr Allergy Asthma Rep. 2017. Mar;17(3):19. doi: 10.1007/s11882-017-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chowdhury NU, Guntur VP, Newcomb DC, Wechsler ME. Sex and gender in asthma. Eur Respir Rev. 2021. Nov 17;30(162):210067. doi: 10.1183/16000617.0067-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paaso EM, Jaakkola MS, Lajunen TK, Hugg TT, Jaakkola JJ. The importance of family history in asthma during the first 27 years of life. Am J Respir Crit Care Med. 2013. Sep 1;188(5):624–6. doi: 10.1164/rccm.201212-2236LE. [DOI] [PubMed] [Google Scholar]
- 79.Chalubinski M, Kowalski ML. Endocrine disrupters--potential modulators of the immune system and allergic response. Allergy. 2006. Nov;61(11):1326–35. doi: 10.1111/j.1398-9995.2006.01135.x. [DOI] [PubMed] [Google Scholar]
- 80.Quirós-Alcalá L, Hansel NN, McCormack M, Calafat AM, Ye X, Peng RD, Matsui EC. Exposure to bisphenols and asthma morbidity among low-income urban children with asthma. J Allergy Clin Immunol. 2021. Feb;147(2):577–586.e7. doi: 10.1016/j.jaci.2020.05.031. Epub 2020 Jul 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kiook B, Jong-Tae P, Kyeongmin K. Association of Urinary Bisphenols Concentration with Asthma in Korean Adolescents: Data from the Third Korean National Environmental Health Survey. Toxics. 2021. Nov 4;9(11):291. doi: 10.3390/toxics9110291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Buckley JP, Quirós-Alcalá L, Teitelbaum SL, Calafat AM, Wolff MS, Engel SM. Associations of prenatal environmental phenol and phthalate biomarkers with respiratory and allergic diseases among children aged 6 and 7 years. Environ Int 2018. Jun;115:79–88. doi: 10.1016/j.envint.2018.03.016. Epub 2018 Mar 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pfeffer PE, Hawrylowicz CM. Vitamin D in Asthma: Mechanisms of Action and Considerations for Clinical Trials. Chest. 2018. May;153(5):1229–1239. doi: 10.1016/j.chest.2017.09.005. Epub 2017 Sep 18. [DOI] [PubMed] [Google Scholar]
- 84.Feng H, Xun P, Pike K, Wills AK, Chawes BL, Bisgaard H, Cai W, Wan Y, He K. In utero exposure to 25-hydroxyvitamin D and risk of childhood asthma, wheeze, and respiratory tract infections: A meta-analysis of birth cohort studies. J Allergy Clin Immunol. 2017. May;139(5):1508–1517. doi: 10.1016/j.jaci.2016.06.065. Epub 2016 Sep 14. [DOI] [PubMed] [Google Scholar]
- 85.Lu M, Litonjua AA, O’Connor GT, Zeiger RS, Bacharier L, Schatz M, Carey VJ, Weiss ST, Mirzakhani H. Effect of early and late prenatal vitamin D and maternal asthma status on offspring asthma or recurrent wheeze. J Allergy Clin Immunol. 2021. Apr;147(4):1234–1241.e3. doi: 10.1016/j.jaci.2020.06.041. Epub 2020 Aug 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pacheco-González RM, García-Marcos L, Morales E. Prenatal vitamin D status and respiratory and allergic outcomes in childhood: A meta-analysis of observational studies. Pediatr Allergy Immunol. 2018. May;29(3):243–253. doi: 10.1111/pai.12876. Epub 2018 Mar 8. [DOI] [PubMed] [Google Scholar]
- 87.Bolcas PE, Brandt EB, Zhang Z, Biagini Myers JM, Ruff BP, Khurana Hershey GK. Vitamin D supplementation attenuates asthma development following traffic-related particulate matter exposure. J Allergy Clin Immunol. 2019. Jan;143(1):386–394.e3. doi: 10.1016/j.jaci.2018.04.042. Epub 2018 Jun 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bose S, Diette GB, Woo H, Koehler K, Romero K, Rule AM, Detrick B, Brigham E, McCormack MC, Hansel NN. Vitamin D Status Modifies the Response to Indoor Particulate Matter in Obese Urban Children with Asthma. J Allergy Clin Immunol Pract. 2019. Jul-Aug;7(6):1815–1822.e2. doi: 10.1016/j.jaip.2019.01.051. Epub 2019 Feb 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adams SN, Adgent MA, Gebretsadik T, Hartman TJ, Vereen S, Ortiz C, Tylavsky FA, Carroll KN. Prenatal vitamin D levels and child wheeze and asthma. J Matern Fetal Neonatal Med. 2021. Feb;34(3):323–331. doi: 10.1080/14767058.2019.1607286. Epub 2019 May 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vereen S, Kocak M, Potukuchi PK, Hartman TJ, Tylavsky F, Carroll KN. The association of maternal prenatal vitamin D levels and child current wheeze. Ann Allergy Asthma Immunol. 2018. Jan;120(1):98–99. doi: 10.1016/j.anai.2017.10.005. Epub 2017 Nov 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aquilina NJ, Delgado-Saborit JM, Meddings C, Baker S, Harrison RM, Jacob P 3rd, Wilson M, Yu L, Duan M, Benowitz NL. Environmental and biological monitoring of exposures to PAHs and ETS in the general population. Environ Int 2010. Oct;36(7):763–71. doi: 10.1016/j.envint.2010.05.015. Epub 2010 Jun 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li Z, Mulholland JA, Romanoff LC, Pittman EN, Trinidad DA, Lewin MD, Sjödin A. Assessment of non-occupational exposure to polycyclic aromatic hydrocarbons through personal air sampling and urinary biomonitoring. J Environ Monit. 2010. May;12(5):1110–18. doi: 10.1039/c000689k. [DOI] [PubMed] [Google Scholar]
- 93.Nethery E, Wheeler AJ, Fisher M, Sjödin A, Li Z, Romanoff LC, Foster W, Arbuckle TE. Urinary polycyclic aromatic hydrocarbons as a biomarker of exposure to PAHs in air: a pilot study among pregnant women. J Expo Sci Environ Epidemiol. 2012. Jan-Feb;22(1):70–81. doi: 10.1038/jes.2011.32. Epub 2011 Sep 14. [DOI] [PubMed] [Google Scholar]
- 94.Scherer G, Frank S, Riedel K, Meger-Kossien I, Renner T. Biomonitoring of exposure to polycyclic aromatic hydrocarbons of nonoccupationally exposed persons. Cancer Epidemiol Biomarkers Prev. 2000. Apr;9(4):373–80. [PubMed] [Google Scholar]
- 95.Kim KH, Jahan SA, Kabir E, Brown RJ. A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int 2013. Oct;60:71–80. doi: 10.1016/j.envint.2013.07.019. Epub 2013 Sep 6. [DOI] [PubMed] [Google Scholar]
- 96.Pickett KE, Kasza K, Biesecker G, Wright RJ, Wakschlag LS. Women who remember, women who do not: a methodological study of maternal recall of smoking in pregnancy. Nicotine Tob Res. 2009. Oct;11(10):1166–74. doi: 10.1093/ntr/ntp117. Epub 2009 Jul 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data utilized for this study are not publicly available but de-identified data may be available on request, subject to approval by the internal review board and under a formal data use agreement. Contact the corresponding author for more information.


