Abstract
Copper and its alloys are known as antimicrobial agents that can be used in public places; however, pure copper has a low wear resistance and tends to lose its gloss relatively fast and stainless steel is still more desirable because of its mechanical properties and stable appearance. In this research, German silver coatings, a copper-nickel alloy, are studied as a superior alternative for pure copper coatings. German silver coating on mild steel substrates and stainless steel with two different surface roughnesses was prepared and placed into water bath up to 6 months to investigate the corrosion and exposure effects on the antibacterial behavior. A range of techniques was used to study the microstructure, surface morphology and mechanical properties such as microhardness, coating bonding adhesion, surface roughness and wettability of the coating. Colony count method was used to measure the antibacterial properties, and samples were tested against influenza A virus to evaluate the virucidal activity. The coating thickness was around 130 µm and contained 15% pores and oxides with splats forming inside the coating structure. Inside each splat, columnar grains could be seen with an average of 700 nm width and 4 µm length. The bonding strength of the coating was about 15 MPa, the hardness of coatings was about 180 HV, and the average surface roughness of the as-sprayed samples was about 10 µm. German silver coatings can destroy both Staphylococcus aureus and Escherichia coli by more than 90% after 6 h of exposure time, and it also has a high-level of virucidal activity against influenza A virus after 2 h exposure time. Antibacterial behavior did not show any significant changes after 6 months of immersing samples in water bath. Thus, thermally sprayed German silver coatings exhibited silvery color for a long period of time, while its antimicrobial efficiency was comparable to pure copper coatings.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11666-022-01528-4.
Keywords: antimicrobial coatings, copper-nickel alloy, German silver, thermal spray, wire arc spray
Introduction
Recently, prevalence of healthcare-associated infections (HAIs) due to pathogenic microorganisms (bacteria, viruses and other microorganisms) has become a disastrous complication, leading to a higher mortality rate and economic problems (Ref 1). Every year, hundreds of millions of hospitalized patients suffer from infectious diseases caused by HAIs. Transmission of HAIs occurs via person-to-person contact, touching an infected surface or contaminated medical devices such as door knobs, handles, and stairway railings. (Ref 2). Bacteria and fungi are able to colonize and form biofilms on the surfaces and become resistance to antimicrobial agents, detergents and other types of surfactants (Ref 3). Methicillin-resistant Staphylococcus aureus (MRSA) is a common infection in hospitalized patients which can cause severe diseases. Studies have shown that MRSA may survive on surfaces approximately for 360 days. The standard evaluation for microbial burden after terminal cleaning of hospital surfaces is between 250-500 aerobic colony-forming units (CFU/100m2) (Ref 4, 5).
Among microbes, influenza and the new widespread coronavirus are contagious respiratory illnesses which causes life-threatening complications in elderly and immunosuppressed patients (Ref 6). These viruses may be transferred through contaminated objects and surface particles and can survive on the surfaces for 24-72 h (Ref 7). Moreover, usual surface disinfecting techniques used for hospital equipment are often not efficient enough to destroy the whole microbial population and the surface becomes prone to recontamination (Ref 8).
One strategy to reduce bacterial attachment and the risk of infection is to modify the surface properties to enhance antibacterial efficacy. Therefore, the development of antimicrobial metals has become important. Some metals and their alloys such as silver, copper, nickel, zinc, cobalt, molybdenum, zirconium, and lead were found to have antimicrobial effect against infectious microorganisms. Thus, they can be suitable candidates to use in high-risk environments. Several studies have shown that the antibacterial properties and effectiveness in preventing the biofilm formation of these metals and alloys vary significantly with the bacterial strain (Ref 9). Recent studies have demonstrated significant reduction in microbial burden due to antimicrobial effects of copper compared to the stainless steel, which is commonly used in hospital environment and equipment (Ref 10, 11). Copper and more than 500 types of its alloys are proved to have antibacterial behavior by United States Environmental Protection (EPA) based on the laboratory results and clinical trials. One of the problem in utilizing copper touch surfaces is its corrosion while the surface is exposed to the human sweat, cleaning agents and disinfectant solutions and its deteriorate surface appearance and properties (Ref 12). Hence, copper alloys with a greater corrosion resistance can be a suitable replacement for their mentioned applications (Ref 13). Previous researches have focused on the antibacterial efficiency of copper and its alloys in different conditions and have reposted that the antibacterial property is dependent upon the weight percentage of the copper content such that the copper content should be more than 60%. This in turn affects the physical and mechanical properties of the selected alloy such as corrosion and scratch resistance (Ref 11, 14). The copper antimicrobial activity is owed to the release of copper ions which affect the microbes’ cell membrane integrity, resulting in the generation of oxidative stress and genotoxic that eventually leads to the cells death (Ref 15). This incidence can be described by the amount of available copper ions on the surface of these alloys which is responsible for microbes’ eradication by disrupting the bacterial membrane and decomposition of bacteria DNA.
Copper and some of its alloys were applied in many healthcare settings and public environments as antimicrobial devices and surfaces. They proved to have an effect on the inactivation of COVID-19 and Sars-CoV-2 on touch surfaces (Ref 11). However, stainless steel clean and glossy appearance is always preferred and accustomed over copper and most of its alloys, because they make a modern and stylish environment. One of the copper alloys that has stainless steel like color tint is German silver, a copper-nickel alloy, that has higher corrosion resistance and hardness than pure copper (Ref 8). German silver is a copper alloys containing copper, nickel and zinc and can be considered as an exceptional brass with no silver element in its composition. Copper constitutes 65 percent of this alloy, and the presence of 18 percent nickel and 17 percent zinc is known to improve strength, hardness and corrosion resistance while maintaining great ductility. German silver was developed to resemble silver appearance as a base metal for silver-plated cutlery, coin making and jewelries. Also, because of their great corrosion and electrical resistance, they have industrial usage in marine fittings, plumbing fixtures and heating coils (Ref 16).
In the past few years, researchers have focused on German silver surface properties and its effects on the surface–bacteria interactions. Micro- and nanoscale surface roughness features have shown enhanced antimicrobial behavior by preventing bacterial adhesion (Ref 17). The aim of the current study was to utilize the wire arc thermal spray technique to form an ultrafine microstructure and microscale surface roughness and evaluate the antibacterial and virucidal properties of the German silver alloy.
Materials and Methods
Coatings Preparations
Ultrafine grain structures of German silver coatings were deposited on plain carbon steel sheets using wire arc spraying (Shark 400, GTV, GmbH, Germany). Two German silver wires (C75200, Hangzhou Ualloy Material Co, China) were fed automatically to a wire arc spray gun to meet at an arc point in a gas stream and form a liquid droplet. The molten droplets atomize and are pushed toward a substrate by the gas flow. Several parameters can be set in this method such as deposition rate, spray distance, and spray atmosphere which is usually air but the process can be done in vacuum or reactive or inert atmospheres, atomizing gas which is usually just air but nitrogen or oxygen and fuel mixture can be used instead, atomizing gas pressure and flow rate and post-spraying treatments can affect the coating physical and mechanical properties (Ref 18). The spraying process parameters are shown in Table 1. Prior to the coating, the substrates were cleaned by acetone and then sandblasted by alumina powder particles (Mesh 16) under air pressure of 5 bar.
Table 1.
Wire arc spraying parameters
| Gun | GTV Spark 400 |
| Feed rate | 80 g/min |
| Spray gun tip distance | 100 mm |
| Current | 200 A |
| Voltage | 33 V |
| Coating wire | C75200 (65%Cu, 18%Ni, 17%Zn) |
| Air cap size | 6 mm |
| Spray distance | 200 mm |
| Wire Size | 1.6 mm |
Two groups of German silver coatings were prepared to analyze the effectiveness of surface topography on the antimicrobial activity. In the first group, the sprayed coating was used, while in the second group, the sandpaper polishing was applied to create a smoother surface topography. Also, two further groups of stainless steel 304 sheets were used as reference materials. A rough stainless steel sheet prepared by sandblast machine and a finer sheet were prepared by sandpaper polishing. All samples were cut into a standard dimension for antibacterial and virucidal experiments. Control materials for all the surfaces were used to compare the antibacterial and virucidal performances of these surfaces with a material that is proved to act neutral to bacteria and influenza virus. The abbreviations for the coatings and their controls are listed in Table 2.
Table 2.
Sample names with their abbreviations in the text
| Metal samples | Type of metal |
|---|---|
| RGSC | As-sprayed German Silver Coating |
| PGSC | Polished German Silver Coating |
| SBSS | Sandblasted 304 Stainless Steel |
| PSS | Polished 304 Stainless Steel |
Surface Characterization
To analyze the surface microstructure of the coatings, scanning electron microscopy instrument (SEM, Philips XL30, the Netherlands) equipped with energy-dispersive x-ray spectroscopy (EDS) was utilized. The etching process was performed by exposing the cross-sectional surface of the samples to the etching solution for 5 s. The etchant solution consisted of 1.5 mL HNO3, 0.5 g AgNO3 and 50 mL H2O which can be used for copper and its alloys. The EDS instrument was employed to conduct surface elemental composition. For phase composition characterization, X pert Philips XRD instrument with Cu Kα (λ = 1.542 nm) radiation was used to perform XRD analysis. The XRD patterns were recorded in 20-80º of 2Ө range with 0.05º step size and time of 1 s per step.
Coatings Mechanical Characteristics
To evaluate the coating adhesion, three samples were prepared by wire cut machine with 2.5 cm diameter based on ASTM 6b33 standard. For this experiment, very strong epoxy glue (UHU Plus Endfest 300) was utilized to stick the samples to the tensile pull test machine grips. The samples were put into an oven with 180 centigrade degree for five minutes to achieve the maximum adhesion according to glue manufacturer’s instructions. The coatings of each sample should peel off completely from substrate to ensure that tensile machine calculates the correct coating adhesion.
Contact angle measurements were conducted using dynamic contact angle meter (dataPhysics Instrument GmbH, DCAT11, Germany). The wettability of the coating was measured as the sample was submerged in and pulled out from distilled water (γ = 72.75 mN/m).
A surface roughness measurement was determined using portable surface roughness tester instrument (Mitutoyo Surftest SJ-210, Japan). Results are shown as mean ± SD of the calculations.
Antibacterial Experiments
The coupons (Table 2) were cleaned with acetone and then sterilized by autoclaving at 121ºC for 15 min. The antimicrobial efficiency for each coupon sample was evaluated by colony counting method of two Gram-positive and negative standard opportunistic strains of Staphylococcus aureus (ATCC 6538) and Escherichia coli (ATCC 25,922). The strains were grown overnight in 5 ml Luria–Bertani (LB) medium and incubated in a shaking incubator at 37 °C for 16 h. Then, 50 µl (1.5 × 108 CFU/mL) of each bacterial suspension was inoculated into 5 ml fresh LB broth, which is equivalent to an optical density (OD) of 0.19 at 600 nm for E. coli and 0.1 for S. aureus. The bacterial suspension (5 µl) was disposed on the surface of each coupon sample placed in the sterile Petri dish, and a sterile circular round microscope coverslip was put on bacterial suspension to spread and maximize the contact surface area of bacterial suspension with coupons and incubated at room temperature for 6 h. Following, the coupon was transferred to a sterile falcon tube with 2.5 ml of new LB broth and vortexed for 10 s to wash the bacteria from the surfaces. Afterward, 100 µl of suspensions was put on the surface of LB agar plate and incubated overnight at 37ºC. The numbers of colony-forming units (CFU) are considered as an indication for the viability of bacteria in contact with the sample surface. The stainless steel 304 sheets and circular round coverslip with bacteria were used as control groups. The antibacterial rate was calculated according to the following equation:
In this equation, NR is the mean CFU/mL from test samples after contact time, and N0 is the mean CFU/mL for the control sample. To explore the effect of water corrosion on coupons, they were incubated in water bath for zero, three and six months at room temperature.
Virucidal Activity Evaluation
The RGSC and PGSC samples were used after autoclave sterilization. For each sample, 50 µl of the stock virus (TCID50 = 108) was added into each well of a 24-well plate. After 30, 60 and 120 min of the metal exposure to the virus, 450 μl of the culture medium was added to each well and mixed thoroughly and tenfold serial dilutions were prepared and exposed to the cultured MDCK cells in 96-well plate (100 μl/well) (Ref 19). After 1 h incubation at 37 °C and 5% CO2 incubator, the dilutions were removed and the media containing tosylsulfonyl phenylalanyl chloromethyl ketone (TPCK) (1 µg/ml) was added (100 μl/ well). Following 48 h incubation, the infectious dose of the virus was measured for the control and all other samples by the hemagglutination assay (HA test) and TCID50 method using Karber formula (Ref 20). The virus sample which was not in touch with metallic samples and the cells that were not in contact with any material was considered as positive and negative controls, respectively. The antiviral test was repeated twice.
Statistical Analysis
All the experiments were performed in triplicates, and the statistical analysis was performed using GraphPad Prism 8. Also, antiviral tests results were analyzed by SPSS 19.0 Software (SPSS Inc., Chicago, IL., USA) and Student t test. The data were presented as mean ± SD, and P value less than 0.05 was considered statistically significant.
Results and Discussion
Microstructural Investigation
Microstructure Analysis and Surface Morphological Evaluations
Thickness is one of the most important aspects of the coatings as it can affect the coating resistance to corrosion or scratches and lifespan and it can provide more copper ions. The cross-sectional SEM micrograph of the German silver coating is shown in Fig. 1 which demonstrates a coating thickness of around 130 µm. The coating consisted of splat boundaries and some level of porosities.
Fig. 1.

SEM micrograph from cross section of the wire arc German silver coating. An appropriate adhesion bonding can be seen in this microstructure, and splats are locked onto the substrate
The backscatter image of Fig. 1 was analyzed by EDS to calculate the percentage of elements in the coating and is presented in Table 3. In this research, German silver coating had sufficient amount of copper with 65 percent. Previous studies have demonstrated that for an effective antimicrobial property, copper concentrations in the coating should be greater than 60 percent (Ref 11). Thin oxide layers were formed between splats during the thermal spray process due to the presence of oxygen which adhere to molten nickel, copper and zinc particles. The molten oxide particles solidified before main elements and cause the next layers of molten particles to form disorderly on previous splats. This disorder arrangement will cause a rougher surface and more wettability which results in better antimicrobial behavior.
Table 3.
Percentage of elements in the German silver coating
| Elements | Weight Percent, % | Atomic Percent, % |
|---|---|---|
| Oxygen | 7 | 24 |
| Nickel | 14 | 12 |
| Copper | 63 | 51 |
| Zinc | 16 | 13 |
The surface morphology of the German silver coating is shown in Fig. 2. The average surface roughness, Ra, of substrates after sandblasting and before coating was 10 ± 2 μm. In addition, during the coating process, some molten particles were solidified before hitting the substrate and some splashed particles from the already impacted molten droplets causing a rough surface which is an important aspect in antimicrobial applications (Ref 21). In this figure, lamellae nature of the thermally sprayed coating can be observed clearly. The diameter of these solidified particles and splats appears to be around 90 µm.
Fig. 2.

SEM micrograph from surface of the German silver coatings
Etched cross section images of the German silver coating in different magnifications can be used to investigate the coating microstructure. Figure 3 shows the splats formation inside the coating with an average thickness of around 8 µm. Heat transfers during solidification formed parallel columnar grains inside each splat which were perpendicular to the substrate and surface of other splats. These grains inside the splats can be seen in Fig. 4 and the average grain width found to be around 700 nm. Also, it can be seen that lots of pores were formed between the splats and by comparing the images from backscattered electrons (BSE) and secondary electrons (SE) in SEM, the percentage of these pores and voids were measured as approximately 15%. It is proved that existence of pores and voids inside the coating structure can increase the antimicrobial properties (Ref 19). To sum up, wire arc thermal spray process has resulted in an increase of the grain boundaries, free energy and pores inside the coating structure which can improve the antimicrobial efficiency.
Fig. 3.

SEM image of the etched cross section in 1000X magnification shows splats and their thicknesses
Fig. 4.

SEM image of the etched cross section in 4000X magnification shows grains inside splats and the parallel columnar structure inside them
XRD Analysis
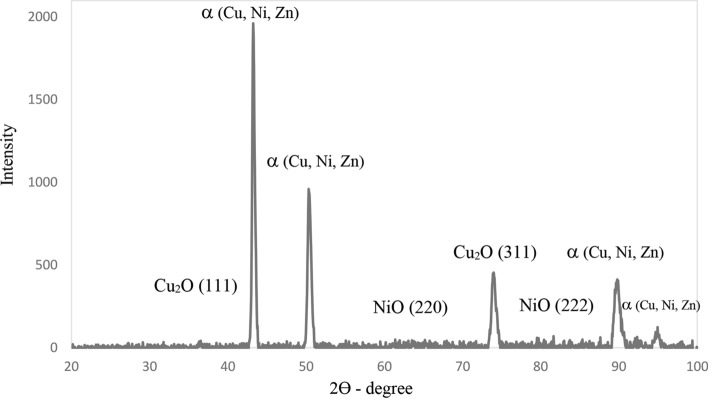
One of the main characteristics of wire arc thermal spray method is oxidation of metallic particles during different stages of coating process which is commonly reported for the copper and its alloys (Ref 21). The in-flight molten particles can interact with the atmosphere surrounding the particles. Moreover, after splat formation, there could be some coatings oxidation due to high temperature of the as-solidified layers. The XRD pattern for the German silver coating is presented in Fig. 5. The main phase composition in the pattern could be α phase, which is tertiary phase, containing copper, zinc and nickel elements. These α phase peaks are close to the pure copper peaks as the main element is α phase in copper. Minor peaks belonging to these element oxides are also detected due to the oxidation of molten metal particles in contact with air during spraying process (Ref 22).
Fig. 5.
X-ray diffraction pattern of German silver coating
Vickers Microhardness and Adhesion Analysis
The adhesion of coating to the substrate is an important factor in practical quality of thermally sprayed coatings, which has an effect on the device lifespan. The average bonding strength of German silver coatings was around 15 ± 3 MPa.
Due to the limited thickness of the coating, microhardness test methods from the surface can be affected by the substrate and indicate the hardness of the substrate material. As a solution, microhardness test was taken from the cross section of the coatings to provide more accurate results. The microhardness analysis of the coatings showed about 180 ± 25 Vickers which was an average from 10 Vickers microhardness test taken from cross section of the coating.
Hydrophobicity and Surface Roughness Measurements
The values of the advancing and receding contact angle in distilled water for the two samples of German silver coatings (RGSC and PGSC) with different surface roughnesses are presented in Table 4. A rougher surface can provide more contact area and thus more metallic ions can be released. In addition, more contacts with the antimicrobial surface can be achieved. The characteristics of materials surfaces such as surface tension energy, surface roughness, and chemical composition have important roles in hydrophilicity of the surface (Ref 23). Surface tension energy determines the shape of the droplets, and liquids contract their surface area to retain the lowest surface-free energy. It can be seen in everyday life that bubbles and small droplets are usually spherical which has the minimum surface for a fixed volume. Previous studies (Ref 24) have shown that the surface free energy of the solid substrate has a strong role on determining the contact angle and wettability of each surface. Surface roughness and the quantification of its impact on contact angle measurement were always controversial but microscopic experiments illustrated that a rough surface has higher contact angle than a polished surface with the same chemical composition. It has been reported that the surface roughness generates contact angle hysteresis by creating barriers on the surfaces in microscopic scale, and these barriers can pin the motion of the contact line and affect the contact angle in macroscopic scale (Ref 24). A Hydrophilic antimicrobial surface can absorb more microbes, and therefore, it increases the mortality rate of microbes (Ref 23).
Table 4.
Contact angle and roughness of the German coating samples
| Sample | Contact Angle | Average Roughness Ra, µm | |
|---|---|---|---|
| θA ± SD | θR ± SD | ||
| RGSC | 96 ± 2.5 | 46 ± 6 | 10 ± 1 |
| PGSC | 93 ± 3.5 | 62 ± 2 | 1 ± 0.5 |
Antimicrobial Evaluations
Antibacterial Activity of German Silver Coating on Staphylococcus aureus
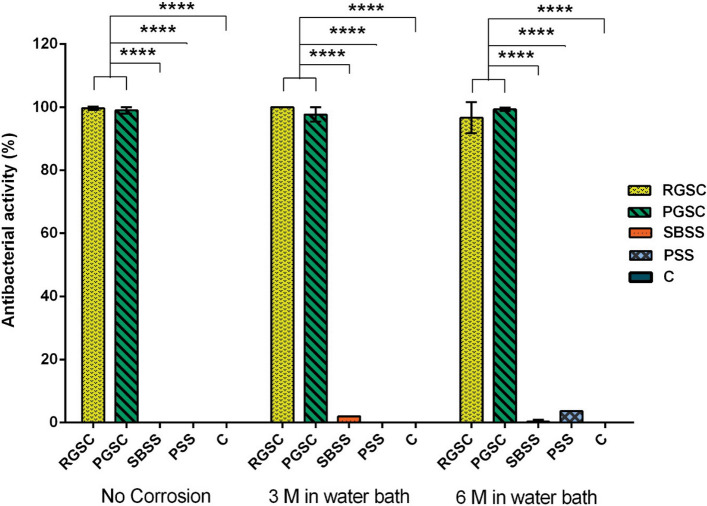
The antibacterial activity of the German silver-coated samples was evaluated by manual counting method following 6 h of incubation. The CFU plates are presented as supplementary data S.1 (A-E), S.2 (A-E), S.3 (A-E). Figure 6 shows the results for antibacterial contact-killing properties of the coated compared to non-coated coupons and control against Staphylococcus aureus. Both coatings (rough and polished) showed significant antibacterial activity near 99% compared to the control. Our results are consistent with previous report by Sundberg et al. (Ref 25) that although stainless steel has many applications in the hospital environments, it does not show appropriate antibacterial properties and as shown in our study, only polished stainless steel samples (PSS) were able to reduce the CFU of S. aureus to less than 10% compared to the negative control.
Fig. 6.
Antibacterial activity of German silver coatings and stainless steel on Staphylococcus aureus at different exposure times in water bath. M (Month). Error bars represent standard deviations of three replicate experiments. RGSC (As-sprayed German Silver Coating) PGSC (Polished German Silver Coating), SBSS (Sandblasted 304 Stainless Steel), PSS (Polished 304 Stainless Steel), C (Coverslip)
Effect of Incubation Time in Water Bath
The effect surface corrosion on the antibacterial behavior of coupons was evaluated. The results showed that only the antibacterial effect of the coupons stored for 6 months in water bath reduced slightly. This could be due to the formation of sediments on the metal surface which could disrupt the bacterial contact with the surfaces of the coatings. The statistical analysis demonstrated that the differences between two coating samples of RGSC and PGSC were not significant. On the other hand, the antibacterial activity of the German silver-coated samples compared to the stainless steel and control was statistically significant and this indicates a profound antibacterial activity of the coated samples. In addition, the results from this study have shown that there was not a significant difference between the two samples of the stainless steel (SBSS and PSS) (Fig. 6).
As it is shown in Fig. 6, the RGSC and PGSC with grain size of approximately 700 nm have shown almost equal antibacterial property. However, as expected, the rough-coated sample (RGSC) has slightly better antibacterial activity, which demonstrates that the structure (contact surface) and the material of the coatings are the factors affecting the bacteria. The concentration of copper ions and the high density of defects are important factors. Studies have shown that the crystalline defects such as increased grain boundaries and dislocations in ultrafine structure typically improve the antibacterial behavior (Ref 26). Ibrahim et al. have found that increasing the number of the crystalline defects in the coating (RGSC and PGSC) increases the penetration of the chemical elements into the coating. Oxygen is the most critical element that is always in contact with the coatings. Increasing the oxygen penetration paths and the presence of rapid diffusion paths in the coatings leads to increasing the oxidation rate, and therefore, the release of metallic ions will also escalate. In addition, due to the negative charge of bacterial membrane and the positive charge of the released metallic ions, higher concentration of metallic ions leads to higher bacterial absorption which causes more bacterial cell destruction and eventually bacterial cell lysis. Thus, producing an ultrafine structure increases the number of crystalline defects and eventually improves the antimicrobial function of the coatings (Ref 27). Moreover, micropores and cracks in the wire arc sprayed structure can contribute in improvement of antibacterial properties of coatings.
In addition, the polished stainless steel sample exhibited a slight antibacterial activity against Staphylococcus aureus in three repeats which was not considered statistically significant, while in most repeats, it had no antimicrobial effects as it was expected.
Antibacterial Activity of German Silver Coating on Escherichia coli
Antibacterial activity of the coatings against Escherichia coli was higher than Staphylococcus aureus at different times of incubation in water bath. Similar results were recently reported by Montero et al. (Ref 10) showed that Copper Armour™ coated has more than 99.9% antibacterial activity against five bacterial pathogens including E. coli and S. aureus. Coatings play a significant role in reducing bacterial numbers, and there was a statistically significant difference between coated samples, stainless steel and controls. These results that are shown in Fig. 7 indicate the potent anti-E. coli behavior of the German silver coatings compared to the control samples (Supplementary Fig. 5, 6).
Fig. 7.
Antibacterial activity of German silver coatings and stainless steel on Escherichia coli at different exposure times in water bath. M (Month). Error bars represent standard deviations of three replicate experiments. RGSC (As-sprayed German Silver Coating), PGSC (Polished German Silver Coating), SBSS (Sandblasted 304 Stainless Steel), PSS (Polished 304 Stainless Steel), C (Coverslip)
Comparison of the antibacterial behavior of coatings against both Gram-positive and Gram-negative bacteria shows that the antimicrobial activity of coatings especially rough coatings (RGSC) was more effective against E. coli than S. aureus which may be related to the differences between their cell wall composition. The Gram-positive bacteria cell wall is composed of thicker peptidoglycan layer compared to the Gram-negative bacteria that provides better protection for these bacteria, to become resistant against antibacterial agents. Thus, it is beneficial to Gram-positive bacteria by providing opportunity for bacteria to adapt itself and become resistant to the antibacterial substances (Ref 28).
Inactivation of Influenza A Virus on German Silver Versus Stainless Steel
It has been shown in previous studies that copper surfaces can inactivate infectivity of influenza A virus (Ref 29). Sundberg et al. observed virucidal activity of copper using cold spray coating that inactivated approximately 9 8% of viruses in 120 min (Ref 25). Another study also compared virucidal activity of solid state of copper and silver particles against influenza A virus. They showed the most reduction in influenza A virus infectivity (3.7 log10 TCID50) by solid-state cuprous oxide (Cu2O) after 30 min exposure on a glass loaded with copper compounds, while solid-state cupric oxide (CuO) and silver sulfide (Ag2S) showed less activity (Ref 30).
In the present study, we confirmed the previous investigations by reduction of influenza A virus infection using two types of thermal-sprayed German silver coatings: polished coating and rough coating. Our results demonstrated the virucidal activity of both coatings, especially the rough coating against influenza A virus as follows: The virus infectivity which was determined by 50% infectious dose of cell culture (TCID50) showed decrements after 30, 60 and 120 min exposure to the coatings (polished and rough) compared to the polished and sandblasted stainless steel as controls. As shown in Fig. 8, the most inactivation efficacy of both coatings on the virus was obtained in 2 h.
Fig. 8.
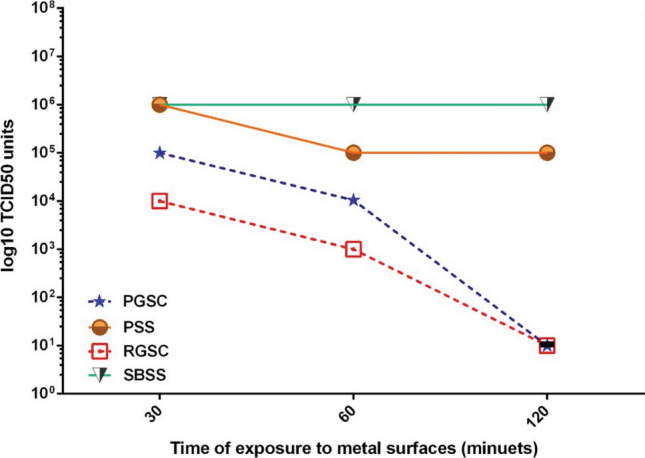
Inactivation of influenza A virus on PGSC and RGSC coatings compared to the controls (SBSS and PSS). The virus infectious doses were evaluated by TCID50 assay. RGSC (As-sprayed German Silver Coating), PGSC (Polished German Silver Coating), SBSS (Sandblasted 304 Stainless Steel), PSS (Polished 304 Stainless Steel). The results are the average of two experiments
The infectious dose reductions after the respected time exposures to the copper metals especially the rough coating compared to the controls (stainless steel) indicate the advantages of porous rough coating (Table 5). In this design, the contact surface of the coating is increased due to the surface porosity that provides more surfaces to contact with virus as previously shown (Ref 31).
Table 5.
Infectious dose reduction after exposure to the metal
| Contact Time (Minutes) | Polished German Silver vs. Polished stainless steel (Log10TCID50 reduction) | Rough German silver vs. Sandblasted steel (Log10TCID50 reduction) |
|---|---|---|
| 1 ± 0.35 | 30 | 2.38 ± 0.18 |
| 1.25 ± 0.35 | 60 | 2.63 ± 0.18 |
| 3.75 ± 1.06 | 120 | 4.88 ± 0.18 |
Our results showed that influenza A virus particles were significantly inactivated after two hours exposure to the German silver surfaces which is consistent with previous studies results (Ref 25, 32). There is another study that showed German silver surfaces inactivate influenza A virus after 6 h (Ref 29). Furthermore, another study by Warnes et al. revealed that surfaces with over 60% copper concentration perform similar to our findings of 62% copper coupon (Ref 33).
Warnes et al. showed that copper disrupts the activity of the virus by destroying the protein shell of the virus (capsid) that provides access to the viral genome for the copper ion and inactivates the gene encoding VPg protein (viral protein genome-linked) ((Ref 33). Our results confirmed the efficient use of the copper in high-risk places such as hospitals and public transports may help to prevent transmission of the contaminations.
Conclusion
In wire arc sprayed coatings, solidification behavior of individual splats has an important role on microstructure development of the coatings and its physical and mechanical properties. In German silver coatings, copper, zinc and nickel were incorporated to form glossy silver-like composition with high antimicrobial efficiency. These elements formed thin layers of oxides during solidification which decreased the viability rate of microbes on the coatings. The effect of copper and its alloys as antimicrobial touch surfaces and coatings has been documented by several researchers, and novel research studies has been focused on the copper effect on influenza virus and coronavirus. Common public places touch surfaces such as stainless steel and plastic show microbial recontamination, and respiratory RNA human viruses remain viable on these surfaces for up to 72 h. However, copper surfaces eradicate COVID-19 viruses in less than 4 h and SARS-CoV-1 viruses in less than 8 h (Ref 11). The copper surfaces can be easily oxidized and loss its appearances in the atmosphere, and its low hardness could be a major drawback for using copper in under wear operation conditions. Results of this study show that the antibacterial efficiency of German silver against Gram-positive S. aureus and Gram-negative E. coli bacteria was more than 90% within 6 h, and their virucidal activity was significantly effective within 2 h for influenza A virus. The coated samples could maintain their antimicrobial property and appearances after exposure to the aqueous environment. Reduction in the bacterial growth numbers on plates of rough German silver coating indicates a better behavior of this type of coating compared to stainless steel which is widely used in medical devices. This coating can be a good alternative for many materials and surfaces in medical equipment surfaces and for preventing and controlling hospital hygiene against a wide range of bacterial contamination. German silver coating is resistance to wear and scratches and could be a good candidate for using in aqueous environments.
The presence of numerous imperfect bonds due to the presence of copper ions in the German silver coating leads to increase in the reactions between the surface and the wet environments in contact with the surface. Due to close surface tension of water and Staphylococcus aureus and Escherichia coli bacteria and due to the fact that bacteria are always in contact with the surfaces exposed to large quantity of water, the above hypothesis is acceptable and efficient in antibacterial behavior of German silver coating.
Supplementary Information
Below is the link to the electronic supplementary material.
Images of bacteria growth on culture media (LB agar) of Staphylococcus aureus from samples that were not incubated in water bath. (A) RGSC (B) PGSC (C) SBSS (D) Control (E) PSS. (TIF 7177 kb)
Images of bacteria growth on culture media (LB agar) of Staphylococcus aureus of samples that were inside water bath for 3 months. (A) RGSC (B) PGSC (C) SBSS (D) Control (E) PSS. (TIF 6880 kb)
Images of bacteria growth on culture media (LB agar) of Staphylococcus aureus of samples that were inside water bath for 6 months. (A) RGSC (B) PGSC (C) SBSS (D) Control (E) PSS. (TIF 7645 kb)
Images of bacteria growth on culture media (LB agar) of Escherichia coli of samples that were not incubated in water bath. (A) RGSC (B) PGSC (C) SBSS (D) Control (E) PSS. (TIF 6908 kb)
Images of bacteria growth on culture media (LB agar) of Escherichia coli of samples that were inside water bath for 3 months. (A) RGSC (B) PGSC (C) SBSS (D) Control (E) PSS. (TIF 6375 kb)
Images of bacteria growth on culture media (LB agar) of Escherichia coli of samples that were in water bath for 6 months. (A) RGSC (B) PGSC (C) SBSS (D) Control (E) PSS. (TIF 6516 kb)
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.F.A. Orrett and M. Land, Methicillin-Resistant Staphylococcus Aureus Prevalence: Current Susceptibility Patterns in Trinidad, BMC Infect. Dis., 2006, 6, p 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.F. Murphy, A. Tchetchik, and I. Furxhi, Reduction of Health Care-Associated Infections (Hais) with Antimicrobial Inorganic Nanoparticles Incorporated in Medical Textiles: An Economic Assessment, Nanomaterials, 2020, 10(5). [DOI] [PMC free article] [PubMed]
- 3.T.R. Garrett, M. Bhakoo and Z. Zhang, Bacterial Adhesion and Biofilms on Surfaces, Prog. Nat. Sci., 2008, 18(9), p 1049-1056. [Google Scholar]
- 4.C.D. Salgado, K.A. Sepkowitz, J.F. John, J.R. Cantey, H.H. Attaway, K.D. Freeman, P.A. Sharpe, H.T. Michels and M.G. Schmidt, Copper Surfaces Reduce the Rate of Healthcare-Acquired Infections in the Intensive Care Unit, Infect. Control Hosp. Epidemiol., 2013, 34(5), p 479-486. [DOI] [PubMed] [Google Scholar]
- 5.M.G. Schmidt, H.H. Attaway, P.A. Sharpe, J. John, K.A. Sepkowitz, A. Morgan, S.E. Fairey, S. Singh, L.L. Steed, J.R. Cantey, K.D. Freeman, H.T. Michels and C.D. Salgado, Sustained Reduction of Microbial Burden on Common Hospital Surfaces through Introduction of Copper, J. Clin. Microbiol., 2012, 50(7), p 2217-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.M.O. Meara, A.O. Brien, E. Feely and M. Conlon, Influenza A Outbreak in a Community Hospital, Ir. Med. J., 2006, 99(6), p 175-177. [PubMed] [Google Scholar]
- 7.R.G. Faix, Survival of Cytomegalovirus on Environmental Surfaces, J. Pediatr., 1985, 106(4), p 649-652. [DOI] [PubMed] [Google Scholar]
- 8.D. Nurhayani and A.A. Korda, The Effect of Nickel Addition on Antimicrobial, Physical, and Mechanical Properties of Copper-Nickel Alloy against Suspensions of Escherichia Coli, AIP Conf. Proc., 2015, 1677, p 1-5. [Google Scholar]
- 9.M. Yasuyuki, K. Kunihiro, S. Kurissery, N. Kanavillil, Y. Sato and Y. Kikuchi, Antibacterial Properties of Nine Pure Metals: A Laboratory Study Using Staphylococcus Aureus and Escherichia Coli, Biofouling, 2010, 26(7), p 851-858. [DOI] [PubMed] [Google Scholar]
- 10.D.A. Montero, C. Arellano, M. Pardo, R. Vera, R. Gálvez, M. Cifuentes, M.A. Berasain, M. Gómez, C. Ramírez, and R.M. Vidal, Antimicrobial Properties of a Novel Copper-Based Composite Coating with Potential for Use in Healthcare Facilities, Antimicrob. Resist. Infect. Control, Antimicrobial Resistance & Infection Control, 2019, 8(1), p 1-10. [DOI] [PMC free article] [PubMed]
- 11.J. Mostaghimi, L. Pershin, H. Salimijazi, M. Nejad, and M. Ringuette, Thermal Spray Copper Alloy Coatings as Potent Biocidal and Virucidal Surfaces, J. Therm. Spray Technol., Springer US, 2021, 30(1-2), p 25-39, doi:10.1007/s11666-021-01161-7. [DOI] [PMC free article] [PubMed]
- 12.M. Walkowicz, P. Osuch, B. Smyrak, T. Knych, E. Rudnik, Ł Cieniek, A. Różańska, A. Chmielarczyk, D. Romaniszyn and M. Bulanda, Impact of Oxidation of Copper and Its Alloys in Laboratory-Simulated Conditions on Their Antimicrobial Efficiency, Corros. Sci., 2018, 140(May), p 321-332. [Google Scholar]
- 13.Antimicrobial , Benefits , of , Copper , Alloy , Touch , Surfaces ,, 2013, 49(1).
- 14.S. Rai, B.E. Hirsch, H.H. Attaway, R. Nadan, S. Fairey, J. Hardy, G. Miller, D. Armellino, W.R. Moran, P. Sharpe, A. Estelle, J.H. Michel, H.T. Michels and M.G. Schmidt, Evaluation of the Antimicrobial Properties of Copper Surfaces in an Outpatient Infectious Disease Practice, Infect. Control Hosp. Epidemiol., 2012, 33(2), p 200-201. [DOI] [PubMed] [Google Scholar]
- 15.M. Vincent, R.E. Duval, P. Hartemann and M. Engels-Deutsch, Contact Killing and Antimicrobial Properties of Copper, J. Appl. Microbiol., 2018, 124(5), p 1032-1046. [DOI] [PubMed] [Google Scholar]
- 16.I. Milošev and T. Kosec, Study of Cu-18Ni-20Zn Nickel Silver and Other Cu-Based Alloys in Artificial Sweat and Physiological Solution, Electrochim. Acta, 2007, 52(24), p 6799-6810. [Google Scholar]
- 17.S. Wu, B. Zhang, Y. Liu, X. Suo, and H. Li, Influence of Surface Topography on Bacterial Adhesion: A Review (Review), Biointerphases, 2018, 13(6), p 060801. [DOI] [PubMed]
- 18.L. Pawlowski, “The Science and Engineering of Thermal Spray Coatings: Second Edition,” The Science and Engineering of Thermal Spray Coatings: Second Edition, 2008.
- 19.S.L. Warnes, Z.R. Little and C.W. Keevil, Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials, MBio, 2015, 6(6), p 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.KARBER and G., 50% End Point Calculation, Arch. fur Exp. Pathol. und Pharmakologie, 1931, 162, p 480-483, http://ci.nii.ac.jp/naid/10024181638/en/. Accessed 18 February 2022.
- 21.O. Sharifahmadian, H.R. Salimijazi, M.H. Fathi, J. Mostaghimi and L. Pershin, Study of the Antibacterial Behavior of Wire Arc Sprayed Copper Coatings, J. Therm. Spray Technol., 2013, 22(2-3), p 371-379. [Google Scholar]
- 22.N. Lebrun and P. Perrot, Copper – Nickel – Zinc, 1879, p 338-354.
- 23.G. Bracco and B. Holst, “Surface Science Techniques,” Springer Series in Surface Sciences, 2013.
- 24.O. Sharifahmadian, H.R. Salimijazi, M.H. Fathi, J. Mostaghimi, and L. Pershin, Relationship between Surface Properties and Antibacterial Behavior of Wire Arc Spray Copper Coatings, Surf. Coatings Technol., Elsevier B.V., 2013, 233, p 74-79, doi:10.1016/j.surfcoat.2013.01.060.
- 25.S. K and C. V, Effectiveness of Nanomaterial Copper Cold Spray Surfaces on Inactivation of Influenza A Virus, J. Biotechnol. Biomater., 2015, 05(04).
- 26.S. Deshpande, S. Sampath and H. Zhang, Mechanisms of Oxidation and Its Role in Microstructural Evolution of Metallic Thermal Spray Coatings - Case Study for Ni-Al, Surf. Coatings Technol., 2006, 200(18–19), p 5395-5406. [Google Scholar]
- 27.M. Ibrahim, M.A. Samad, K. Al-Athel, A.F. Arif, and N. Olalekan, Evaluation of Tribological Properties of Thermally Sprayed Copper and Copper Alloy Coatings, Arab. J. Sci. Eng., Springer Berlin Heidelberg, 2018, 43(9), p 4899-4910, doi:10.1007/s13369-018-3222-2.
- 28.M. Rajagopal and S. Walker, Envelope Structures of Gram-Positive Bacteria., Curr. Top. Microbiol. Immunol., 2017, 404, p 1-44. [DOI] [PMC free article] [PubMed]
- 29.J.O. Noyce, H. Michels and C.W. Keevil, Inactivation of Influenza A Virus on Copper versus Stainless Steel Surfaces, Appl. Environ. Microbiol., 2007, 73(8), p 2748-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.M. Minoshima, Y. Lu, T. Kimura, R. Nakano, H. Ishiguro, Y. Kubota, K. Hashimoto and K. Sunada, Comparison of the Antiviral Effect of Solid-State Copper and Silver Compounds, J. Hazard. Mater., 2016, 312, p 1-7. 10.1016/j.jhazmat.2016.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.V.K. Champagne and D.J. Helfritch, A Demonstration of the Antimicrobial Effectiveness of Various Copper Surfaces, J. Biol. Eng., Journal of Biological Engineering, 2013, 7(1), p 1, doi:10.1186/1754-1611-7-8. [DOI] [PMC free article] [PubMed]
- 32.S.L. Warnes, E.N. Summersgill and C.W. Keevil, Inactivation of Murine Norovirus on a Range of Copper Alloy Surfaces Is Accompanied by Loss of Capsid Integrity, Appl. Environ. Microbiol., 2015, 81(3), p 1085-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.S.L. Warnes and C.W. Keevil, Inactivation of Norovirus on Dry Copper Alloy Surfaces, PLoS One, 2013, 8(9). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Images of bacteria growth on culture media (LB agar) of Staphylococcus aureus from samples that were not incubated in water bath. (A) RGSC (B) PGSC (C) SBSS (D) Control (E) PSS. (TIF 7177 kb)
Images of bacteria growth on culture media (LB agar) of Staphylococcus aureus of samples that were inside water bath for 3 months. (A) RGSC (B) PGSC (C) SBSS (D) Control (E) PSS. (TIF 6880 kb)
Images of bacteria growth on culture media (LB agar) of Staphylococcus aureus of samples that were inside water bath for 6 months. (A) RGSC (B) PGSC (C) SBSS (D) Control (E) PSS. (TIF 7645 kb)
Images of bacteria growth on culture media (LB agar) of Escherichia coli of samples that were not incubated in water bath. (A) RGSC (B) PGSC (C) SBSS (D) Control (E) PSS. (TIF 6908 kb)
Images of bacteria growth on culture media (LB agar) of Escherichia coli of samples that were inside water bath for 3 months. (A) RGSC (B) PGSC (C) SBSS (D) Control (E) PSS. (TIF 6375 kb)
Images of bacteria growth on culture media (LB agar) of Escherichia coli of samples that were in water bath for 6 months. (A) RGSC (B) PGSC (C) SBSS (D) Control (E) PSS. (TIF 6516 kb)