Abstract
Diabetes is an untreatable metabolic disorder characterized by alteration in blood sugar homeostasis, with submucosal insulin therapy being the primary treatment option. This route of drug administration is attributed to low patient comfort due to the risk of pain, distress, and local inflammation/infections. Nanoparticles have indeed been suggested as insulin carriers to allow the drug to be administered via less invasive routes other than injection, such as orally or nasally. The organic-based nanoparticles can be derived from various organic materials (for instance, polysaccharides, lipids, and so on) and thus are prevalently used to enhance the physical and chemical consistency of loaded bioactive compounds (drug) and thus their bioavailability. This review presents various forms of organic nanoparticles (for example, chitosan, dextron, gums, nanoemulsion, alginate, and so on) for enhanced hypoglycemic drug delivery relative to traditional therapies.
Keywords: Diabetes mellitus, Treatment, Organic nanoparticles, Nanoemulsions, Drug delivery systems
INTRODUCTION
Diabetes is a chronic metabolic syndrome characterized by hyperglycemia that has spread widely worldwide (Konda et al., 2020; Mukhtar et al., 2020). Diabetes can result in several health problems such as loss of limbs, vision loss, renal failure, and cardiovascular problems. Since the most recent International Diabetes Federation (IDF) data, nearly 463 million individuals globally are presently being suffered by the diabetes. Diabetes will affect 578 million individuals by 2030 if adequate steps do not restrict the diabetic pandemic (Reddi Nagesh et al., 2020; Sirdah and Reading, 2020).
By 2045, the figure will have risen to 700 million (Verma et al., 2021). The World Health Organization stated that there are 1.5 million deaths annually worldwide, making one of the leading causes of mortality in developed nations due to various factors (for example, stress, minimal physical exercise, unhealthy diet, overweight, genetic inheritance, age, as well as inflammatory processes), which can be believed to be due to an increase the percentage of adults over the age of 65 as well as a sedentary lifestyle (Cho et al., 2018; Sirdah and Reading, 2020). Diabetes is classified into two types based on pathology: type 1 diabetes (5%) (T1D), as well as type 2 diabetes (95%) (T2D) (Eberle and Stichling, 2021).
T1D pathology is insulin insufficiency caused by apoptotic cell death and a low level of insulin-secreting pancreatic β-cells damaged by the autoimmune system’s T cells and B cells. As of now, there is no cure for T1D (Law et al., 2018). T2D is a highly complicated metabolic disease due to insulin resistance in the liver and muscles and increased hepatic glucose output attributed to abnormally high glucagon levels (Amalan et al., 2016; Heindel et al., 2017). Unfortunately, there seems to be no remedy for T2D. The primary therapeutic approaches for T2D include sufficient physical activity, appropriately limited carbohydrates, and adherence to a lengthy medication regimen, including the ingestion of synthetic antidiabetics and insulin administration (da Silva Rosa et al., 2020). Most diabetes treatments, on the other hand, enhance excess weight, gastro-intestinal disturbances, diarrhea, kidney problems, hypersensitivity, and there is a reasonable and fair risk of low blood sugar when used to accomplish tight blood sugar control (Emerenziani et al., 2019).
A further significant impediment seems to be drug resistance. For instance, Sulfonylureas lose efficiency after 6 years of therapy, about 44% of people with diabetes (Bowman et al., 2018). Thus, discover a new anti-diabetic agent with a drug delivery system with increased protection but fewer adverse effects. Traditional drug delivery systems continue to have many constraints, such as inappropriate and ineffective drug concentration, minimal effectiveness, and limited selectivity for the destination, resulting in severe side effects in all other organ systems (Amalan and Vijayakumar, 2015; Verma et al., 2021). Comparable constraints exist while using organic ingredients of nutritional and functional involvement in the management of diabetes. Loading insulin, as well as other drugs into nanoparticles has recently proposed as a much more suitable, non-invasive, as well as safe and secure approach via substitute route of administration (Wang et al., 2021).
Nanoparticles provide particular challenges for biological cells because of their increased surface area, surface chemistry, and reactivity. As a result, it is important to construct these particles and their nanomaterials in a way that makes them noncytotoxic and biocompatible. In recent years, interest has grown in how nanomaterials affect the body. Nanoparticles are intriguing and prospective medication delivery systems because of their distinctive physicochemical characteristics. However, depending on the size, shape, charge, and surface chemistry of the particle, interactions between these particles and blood constituents and cells can have a variety of effects (Souto et al., 2019). Protein binding occurs as soon as a particle enters the systemic circulation, and this further determines the destiny of nanoparticles inside the body. To improve drug delivery to the target tissues, an ideal nanoparticle should be compatible with blood, stable in the physiological milieu, and able to circulate. There is a need for characterization of nanotechnology constructs and production of nanomaterials.
A number of nanoparticle-based delivery systems have been proposed in order to solve enzymatic hydrolysis in the abdomen and hence enhance permeability through gastrointestinal system and hence strengthen following oral uptake and circulation throughout the body (Amalan et al., 2015; Viswanathan et al., 2017). This review discusses the trends and perspectives of organic mediated nanoparticles for diabetes treatment. Since, the nanoparticles may enhance the paracellular absorption of anti-diabetic agents.
NANOTECHNOLOGY IN MEDICATIONS
The phrase “Nanotech” refers to manipulating molecular, atomic, and supramolecular levels at which distinctive quantum theory effects occur (Madkour, 2019). Hence, lowering at least one dimension just at nano-sized (1-100 nm) particles entails the configuration, fabrication, characterization, and application of different nano-sized components in different potential areas, resulting in innovative technological progress (Akhavan et al., 2018). Nanoparticles have such several superior properties compared to parent structures because nano-materials seem to be more completely reliant on shape, size, and interactions that are easily accessible (Kinnear et al., 2017; Natesan and Kim, 2021). The application of nanomaterials and nanodevices in health and pharmaceutics has paved the way for developing unique nanoscience and nanomedicine areas (Nasrollahzadeh et al., 2019). Nanotechnology progressions in treatments can be categorized as follows.
DRUG ADMINISTRATION/THERAPEUTICS:
The production of novel nanoparticle delivery systems intends to reach controlled and targeted pharmacological drug release and bio-distribution. Nanoparticles are also used in drug design to improve absorption rate (Nasrollahzadeh et al., 2019). For instance, numerous drugs are moderately soluble in water. At the same time, some are rapidly absorbed but then eliminated from the body as squandering even before bioactive components attain their optical accumulation, rendering therapies inefficient (Zhou et al., 2016). Moreover, nanotechnology has sparked interest owing to its capacity to produce particles that are fascinated to specific cell types. Several nanoparticles have distinctive characteristics that enable them to be used effectively in therapy (Chenthamara et al., 2019).
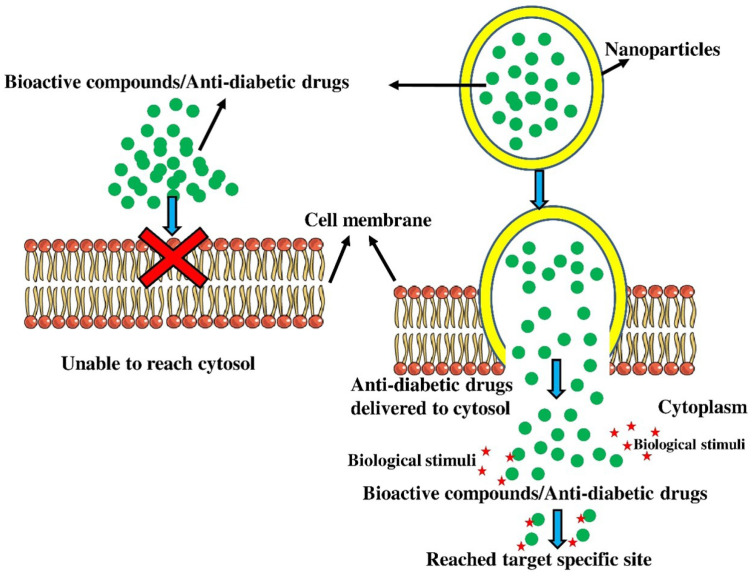
The NPs approach a cell membrane; they interact to plasma membrane as well as outer membrane proteins and pass through the membrane, primarily via endocytosis, direct diffusion, phagocytosis, adhesive interactions, and micropinocytosis. Endocytosis involves NPs being engulfed in membrane vesicles, finally budding as well as squeezing off just to establish endocytic vacuoles, which will then be transported to specialized cytoplasmic trafficking chamber. Endocytosis is divided into various types depending on the cell type as well as the peptides, fats, and some other substances associated with the process. Phagocytosis, macropinocytosis, clathrin-mediated endocytosis, clathrin or caveolae-independent endocytosis, and caveolin-mediated endocytosis are the major endocytosis processes. Pinocytotic processes seem to be more prevalent in several types of cells than phagocytosis, which also occurs primarily in competent phagocytes. Nanoparticles can penetrate the cell through phagocytosis, adhesive interactions, endocytosis, micropinocytosis, and direct diffusion (Fig. 1). Behzadi et al. (2017) also demonstrated that too many nano-sized components can pass cell membrane via passive mechanisms including diffusion as well as adhesive relations, at which heat blood capillaries waves along with line tension contribute significantly in influencing nanoparticle entrance into the cells. The physicochemical characteristics of nanoparticles, including size, composition, shape, and surface charge influence how they are internalized by cells. The drugs are then delivered and controlled by nanoparticles via biological stimuli (Matrix metalloproteinases: MMPs) as well as light activation.
Fig. 1.
Nanoparticles based anti-diabetic drug delivery system. The nanoparticles move through the membrane largely by endocytosis, direct diffusion, phagocytosis, adhesion contacts, and micropinocytosis. They interact with the plasma membrane as well as outer membrane proteins.
DIABETIC TREATMENT WITH NANO-DRUG DELIVERY SYSTEMS
Because of their distinctive in-vivo properties, such as good design flexibility, nanoparticle-based drug delivery systems emerged as a potential framework for improving the oral bioavailability of organic drugs (Purohit et al., 2017). The organic drugs can be loaded with nanoparticles to improve their consistency in the gastrointestinal tract. Furthermore, nanoparticles can help organic drugs cross the mucosal barrier and epithelial cell layer, increasing their oral bioavailability in the bloodstream (Brown et al., 2020; Amalan et al., 2021). Numerous endeavors have been undertaken to develop oral peptide drugs NPs for diabetes mellitus treatment. Several natural product based nanoparticles have been investigated as conventional dosage forms for the treatment of diabetes (Ramakrishnan and Vijayakumar, 2017; Durán-Lobato et al., 2020).
ORGANIC NANOPARTICLES
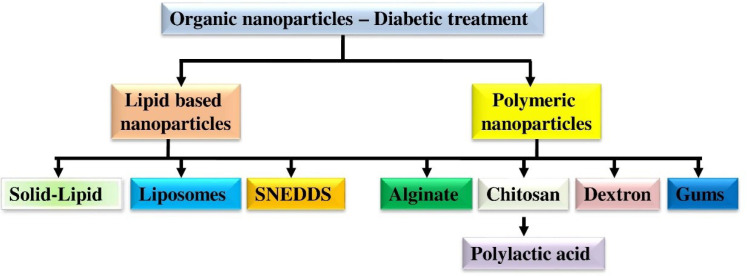
Natural organic polymers-based nanoparticles are low in the production process and cost and abundant in nature. The key benefits of natural organic polymers over inorganic polymers are non-toxicity and biocompatibility (Mansoori et al., 2020). Naturally available polymers such as chitosan, gum rosin, sodium alginate, dextran, and gum arabica have been established to deliver anti-diabetic drugs (Fig. 2) (Nie et al., 2020).
Fig. 2.
Various form of organic nanoparticles for diabetic treatment. Natural organic polymers key benefits over inorganic alternatives are their non-toxicity and biocompatibility. Anti-diabetic medications have been successfully delivered using naturally occurring polymers such alginate, dextran, chitosan, gum arabica, gum rosin, sodium, and gum rosin.
CHITOSAN MEDIATED NANOPARTICLES
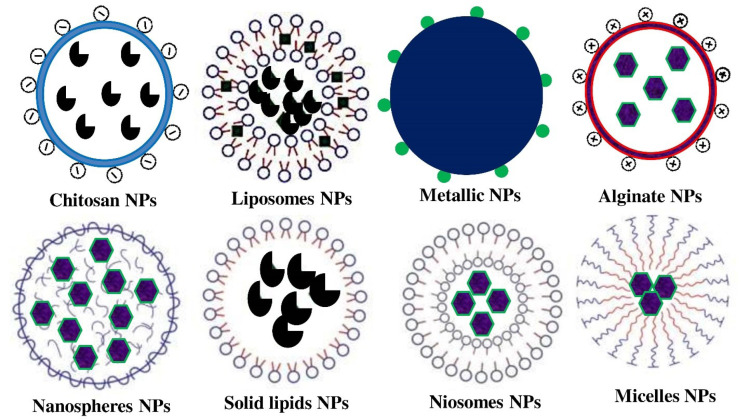
Chitosan, a type of polycationic carbohydrate biopolymer, is created through chitin alkaline deacetylation, obtained from microbial cell walls (fungi), insect cuticles, and crustacean exoskeletons (Philibert et al., 2017; Amalan et al., 2022). Because of its ease of surface functionalization, ability to mix with diverse polymeric materials, non-immunogenicity, non-toxicity, as well as significant suitability with tissues and cells, chitosan is perhaps the most widely used in the shipment of antidiabetic drugs/bioactive (Fig. 3) compounds (Table 1, 2) (Allawadhi et al., 2021). Chitosan-based nanoparticles encapsulating ferulic acid and curcumin were developed through a bottom-up ionic gelation technique. Chitosan’s strong positive charge makes it perfect for antidiabetic drug delivery since it improves adherence to oppositely charged membranes surfaces and enhances cell absorption (Vijayakumar and Subramanian, 2010a; Wu et al., 2020). Furthermore, the chitosan OH and NH2 active groups are susceptible to chemical responses. The inhibitory action of α-glucosidase decreases in the order of catechin-g-chitosan>catechin>acarbose>chitosan, and the α-amylase inhibitory effect decreases in the order of acarbose>catechin-g-chitosan>catechin>chitosan.
Fig. 3.
Pictorial representation of nanoparticles used to deliver the anti-diabetic drugs to treat the diabetes. Chitosan’s high positive charge makes it ideal for delivering anti-diabetic drugs because it increases cell absorption and promotes adhesion to surfaces with opposing charges on membranes.
Table 1.
Organic nanoparticles based bioactive compound treatment of diabetes
| Antidiabetic organic compound | Drug delivery system | Size (nm) | Concentration (mg kg of body weight) | Activity/mechanism | References |
|---|---|---|---|---|---|
| Ferulic acid | Chitosan NPs | 119.5 | 10 | Increasing Antihyperlipidemic, bioavalability, and glucose reducing effects, hepatic glycogen, and curcumin deposition in the liver | Maity et al., 2017; Mukhopadhyay et al., 2018; Nie et al., 2020 |
| Naringenin, Quercetin, and Curcumin | Chitosan-alginate nanoparticles | 50-216.44 | 50-100 | Increasing glucose reducing effects, control hepatic and pancreatic abnormalities | Maity et al., 2017; Mukhopadhyay et al., 2018 |
| Better gourd seed oil, berberine, sage essential oil, and α-tocopherol | Nanoemulsion | 85-143.2 | 25-100 | Increasing glucose reducing effects, liver function maintenance, pancreatic tissue regeneration, free radicals scavenging effects, and bioavailability | Nait Bachir et al., 2019; Xu et al., 2019 |
| Curcumin | PEG-PLA nanoparticles | 117 | 20 | Increasing glucose reducing effects, anti-inflammatory effects and plasma insulin content | El-Naggar et al., 2019; Nie et al., 2020 |
| Glycyrrhizin and thymoquinone | Chitosan and rosin gum nanoparticles and nanocapsules | 70-181 | 20-90 | Increasing glucose reducing effects and antihyperlipidamic effect | Rani et al., 2018 |
| Resveratrol, polypeptide-k, curcumin, cinnamic (trans) acid | SNEDDS | 40-800 | 28-336 | Increasing glucose reducing, antihyperlipidemic effects, and bioavailability | Rani et al., 2018; Nie et al., 2020 |
| Berberine, resveratrol, and myricitrin, | SLNs | 50-150 | 1-100 | Free radicals scavenging, Increasing glucose reducing effects, decrease insulin tolerance, increase islet activity, increase bioavailability | Ahangarpour et al., 2018; Mohseni et al., 2019 |
| Ursolic acid, berberine, gymnemic acids, and betulin | Nanosuspensions | 20-400 | 73-246 | Increasing glucose reducing, antihyperlipidemic effects, decrease insulin tolerance, and increase bioavailability | Singh et al., 2019; Nie et al., 2020 |
| Betanin | Liposomes | 40 | 20 | Increasing glucose reducing, antihyperlipidemic effects, decrease insulin tolerance, stop renal, pancreas, and liver damages | Amjadi et al., 2019 |
SNEDDS, self-nanoemulsifying drug delivery systems; SLNs, solid lipid nanoparticles; PLA, poly lactic acid; PEG, poly ethylene glycol; PLGA, poly lactic-co-glycolic acid.
Table 2.
Commercially available anti-diabetic drugs loaded nanoparticles to treat diabetes mellitus (Souto et al., 2019)
| Anti-diabetic drug | NPs used for drug delivery | Mechanisms involved in treatment | Mode of administration |
|---|---|---|---|
| Insulin | Alginate NPs | Reduction in BSL: longtime hypoglycemic effect | Oral |
| Chitosan NPs | Reduction in BSL: Bio-distribution- kidney, both intestine, and urinary bladder | ||
| Dextran NPs | Reduction in BSL: longtime hypoglycemic effect | ||
| PAA NPs | Effective on Caco2 cells | ||
| PLA NPs | Reduction in BSL: longtime hypoglycemic effect. Distributed at heart, kidney, lungs, spleen, and liver | ||
| PLGA NPs | Reduction in BSL: longtime hypoglycemic effect | ||
| CPP NPs | Reduction in BSL | ||
| Metformin calcein insulin: GLP-1 | Liposomes NPs | Hypoglycemic effect | Oral |
| Gliclazide, insulin metformin, Metformin hydrochloride, repaglinide pioglitazone | Niosomes NPs | Enhanced bioavailability for insulin | Oral |
| Pancreatic insulin (Bovine and human): Calcitonin | Dendrimers NPs | Improved glucoregulatory activity | Subcutaneous |
| Porcine and human insulin (lyophilized) | Micelles NPs | Enhanced bioavailability for insulin | Oral |
One of the biologically useful flavonoids in the human diet is catechin, which is mostly found in red wine, broad beans, black grapes, apricots and tea. Catechin has been shown to possess a number of biological qualities, including antioxidant, antimutagenic, anticarcinogenic, antidiabetic, anti-inflammatory, and antibacterial effects. Antioxidant and anti-diabetic properties of chitosan were improved by grafting catechin onto it using Vc and H2O2 as redox initiators in acetic acid solution. Numerous instrumental techniques were used to describe the produced catechingrafted chitosan (catechin-g-chitosan) to ensure conjugation. Additionally, catechin-g-anti-inflammatory chitosan’s and anti-diabetic properties were identified (Zhu and Zhang, 2014).
Even though it has favorable biocompatibility, it is very seldom used for oral administration of drugs since chitosan can act as a carrier and prone to leaking from the encapsulated bioactive compounds due to its ease of dissolution in acidic environments (Muzzarelli et al., 2015; Natesan and Kim, 2022).
ALGINATE AND CHITOSAN MEDIATED NANOPARTICLES
Alginate is also another natural organic polymer desired after chitosan. Alginate seems to be a water-soluble anion exchange copolymer widely distributed in brown algae cell walls (Abedini et al., 2018). Alginate’s broad pharmaceutical suitability stems from its distinctive tendency to construct hydrogel in the liquid phase or at minimal pH in the occurrence of Ca2+ ions (Abasalizadeh et al., 2020). Electrostatic attraction among oppositely charged groups allows alginate and chitosan to establish polyelectrolyte structures (Fig. 3). The low pH dissolution rate of the alginate channel reduces the high pH solubilization of chitosan, which is far poor dissolvable at elevated pH and stabilizes the alginate (Wasupalli and Verma, 2018).
The alginate and chitosan blend helps protect the encapsulated drug and is efficiently and slowly released compared to chitosan and alginate alone (Table 1, 2). For example, the in vitro study revealed that the chitosan and alginate blend considerably increased the releasing period of curcumin around 40 min under fluid (gastric) stimulation and decreased curcumin deficit by 20% (Nalini et al., 2019). Furthermore, the chitosan and alginate blend prepared curcumin nanoemulsion had around a 30% greater glucose reduction effect than chitosan alone (Laha et al., 2019). Maity et al. (2017) developed innovative alginate covered chitosan core-shell nanoparticles carrier system that was efficient in drug administration of naringenin for diabetic prompted rats. Since the major-shell renovation had developed to reduce size of particles as well as ensuring proper preventative measures of encapsulated naringenin from high metabolism through protecting within the core of nanoparticles (Abdullah et al., 2019).
Accordingly, Mukhopadhyay et al. (2018) prepared pH-based polymeric nanoparticles with corona morphological features for embedding with quercetin, using succinyl chitosan as well as alginate. Because of the existence of carboxyl groups on their structure, succinyl chitosan as well as alginate have high pH sensitivity. In both in vitro and in vivo experiments, the circular nanoformulation can accomplish a pH-sensitive controlled delivery of quercetin and exhibit an anomalous trend (Rehman et al., 2020). In STZ-induced diabetic rats, both core-shell nanoparticles had significant hypoglycemic effects and effectively maintained glucose tolerance when compared to organic compounds. Furthermore, neither nanoparticle was toxic in vivo (Mukhopadhyay et al., 2018).
GUMS, GUM AND CHITOSAN MEDIATED NANOPARTICLES
Organic gums such as locust bean, guar, karaya, gellan, acacia, xanthan, and konjac gel gums have been widely used in developing safe drug delivery applications (Tahir et al., 2019). While exposed to water, they established hydrogels and showed excellent stability over a wide pH range. Gum rosin is a naturally occurring anionic polymer obtained from pine plant (Table 1). Rani et al. (2018) used the nano-precipitation technique, also known as a solvent displacement technique, to establish with thymoquinone-loaded gum based rosin nanocapsules (Nie et al., 2020). Thymoquinone nanocomposites encapsulated half of the quantity of administered as organic thymoquinone but outperformed in T2D rats in terms of antihyperglycemic activity (Vijayakumar and Subramanian, 2010b; Rani et al., 2018).
DEXTRAN MEDIATED NANOPARTICLES
Dextran is a polysaccharide with a negative charge easily soluble in water. It is mostly made up of linear -1,6-linked glucopyranose residues with 1.3-branching. Dextran is derived mainly from Lactobacillus and Streptococcus cultures grown in a sucrose-enriched environment (Rosenberg et al., 2011). Drug encapsulation appears to be difficult due to the poor affinity among water-soluble polymeric (matrix) and lipophilic bioactive compounds. Berberine-loaded O-hexadecyl-dextran nanoparticles were effective as berberine to avoid raised glucose-stimulated cell damage, mitochondrial damage, and reduction of apoptosis in in vitro experiments on hepatocytes (Mortazavi et al., 2020). Despite being extremely degradable, the constraint of organic polymers is related to batch-to-batch significant variation since they are typically retrieved from distinct species, provinces, and climates, making them less appealing than synthetic polymers, which are more adaptable and efficacious (Vijayakumar, 2014; Choudhary et al., 2020).
ADVANCEMENTS IN NANOPARTICLES BASED DRUG DELIVERY SYSTEM
Nanoemulsion based drug delivery system
Organic nanoemulsions are colloidal carriers that include an oily phase, an emulsifier, and an aqueous phase. It is still debatable in nanoemulsions with structured size values and a maximum limit fixed at 100, 200, and 500 nm (Shaker et al., 2019). As the size of the oil phase declines, the energy content needed to break them down raises, indicating that a large amount of energy is required to create nanoemulsions (Sivakumar et al., 2014; Ramakrishnan et al., 2016). Nanoemulsions are drug delivery systems capable of entrapping both hydrophobic and hydrophilic substances. Momordica charantia seed oil was emulsified with a minimal surfactant to oil (0.65) ratio using a 2 stage homogenization method for nanoemulsions preparation (Ramya et al., 2015; Rahman et al., 2020). M. charantia seed oil based nanoemulsions substantially decreased hyperglycemia and oxidative stress in T2D rats induced with alloxan. About 0.5% (w/v) of M. charantia seed oil based nanoemulsions demonstrated significant therapeutic efficiency as compared to similar doses of M. charantia seed oil traditional emulsion, as well as 1% (w/v) M. charantia seed oil nanoemulsion, potentially owing to enhanced bioavailability (Xu et al., 2019). The nanoemulsions (Table 1) increased the absorption of berberine in vivo by about 212.02% to controls and lowered diabetic mice’s blood sugar levels by threefold (Xu et al., 2019).
Furthermore, the therapeutic efficacy of berberine nanoemulsion on diabetes was superior to metformin. A creative delivery mechanism, cyclodextrin-mediated nanosponges, comprises excitable cyclodextrins linked in a tri-dimensional network. They have the potential to outperform organic cyclodextrins (Xu et al., 2019). Their outstanding traits are due to their sponge-like and nanoporous structure, which would be advantageous for the encapsulation of complex hydrophilic and lipophilic bioactive compounds (Mohammadi et al., 2020). To improve the biocompatibility as well as consistency of herb essential oil. Nait Bachir et al. (2019) synthesised 2 engineered nanoemulsions. The first one was stabilized by organic α-cyclodextrin using a physical techniques, as well as the 2nd through α-cyclodextrin nanosponges through C10H6(CO2H)2 as both a cross-linking compound as well as a poly-condensation technique (Nait Bachir et al., 2019)
The in vivo anti-diabetic activity of nanoemulsion stabilized with cyclodextrin nanosponges outperformed that of nanoemulsion stabilized through natural and organic cyclodextrin and free herb essential oil (Nait Bachir et al., 2019). Hatanaka et al. (2010) used a micro fluidizer to prepared through three nanoemulsion of tocopherol at various (10%, 30%, and 50%) concentrations (Hatanaka et al., 2010). In streptozotocin stimulated diabetic rats, 10% tocopherol blended nanoemulsion had a 2.6-fold enhanced bioavailability in vivo and a more substantial antioxidant properties influence on the several organ systems, particularly the liver when compared with the control (oil and -tocopherol). Nevertheless, whenever the α-tocopherol substance of the nanoemulsion had been 30% or greater, extreme droplet agglomeration took place during prolonged storage (Hatanaka et al., 2010).
The main limitation that prevents nanoemulsion from being widely used is its stability. Nanoemulsions are both thermodynamically as well as catalytically volatile systems. In other words, if nanoemulsions are given enough time, phase transition will occur (Damarla et al., 2018). Flocculation maturation is the primary mechanism of nanoemulsion destabilization (Singh et al., 2017). This was revealed that just by determining the size, emulsifier, and oil densities during storage as well as application, the consistency of nanoemulsion might be retained against environmental parameters such as pH and temperature (McClements, 2018).
Self-nanoemulsifying drug delivery systems (SNEDDS)
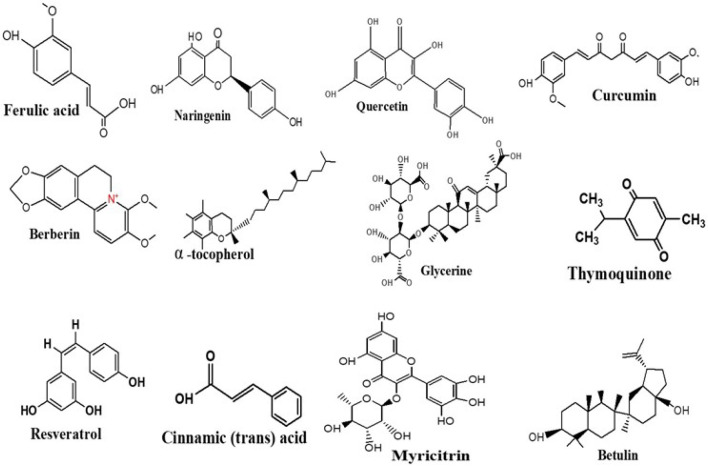
SNEDDS, unlike nanoemulsions, do not contain any liquids, making them more physically and chemically reliable, enabling them to be stored for a lengthy period (Khan et al., 2012). Furthermore, the free energy needed to design SNEDDS seems to be extremely low, as well as the formulation is thermally dynamic. Anhydrous isotropic blends of surfactant, oil, co-surfactant, and drug are called SNEDDS (Sailor, 2021). In vivo, these processes are dissolved by gastrointestinal fluids. Afterward, with mild agitation offered by the gastrointestinal motility of the stomach and intestine, they can establish perfect oil-in-water nanoemulsions with an enormous surface area for improved drug absorption (Rostamabadi et al., 2019). Thus, among the most important aspects of SNEDDS seems to be the alteration whenever bodily secretions neutralize the system following administration (Čerpnjak et al., 2013). Thus, it is important to determine effective self-emulsification areas and select the best emulsifier, co-surfactant, and oil densities to prepare stable SNEDDS. Garg et al. (2019) created SNEDDS of peptide chain k and curcumin to improve antidiabetic efficiency in diabetic rats induced by STZ (Table 1, Fig. 4). A pseudo-ternary stage model was created using oil, surfactant, and co-surfactant (Garg et al., 2019).
Fig. 4.
Structure of anti-diabetic bioactive compounds used with nanoparticles based drug delivery process. The unique attributes of nanoparticles are a result of their nanoporous, sponge-like structure, which is beneficial for encapsulating sophisticated hydrophilic and lipophilic bioactive chemicals.
The liquid formulation had been optimized using the Box–Behnken layout depending on outcomes of drug loading percentage, zeta potential, polydispersity index, and average droplet size (Thapa et al., 2018). The absence of phase transition and drug precipitation under conditions of pH, dispersion, and temperature fluctuations recommended that the optimized formulation was stable (Sipponen et al., 2020). The proportion of emulsifying agents is an important metric for determining emulsification effectiveness. In vitro, the ideal methodology resveratrol SNEDDS self-emulsified in 27 ± 0.8 s without precipitation (Abou Assi et al., 2020). A 10 mg/kg resveratrol nanocomposite had substantial hypolipidemic and hypoglycemic impacts on Streptozotocin and glucose-induced-diabetic mice, comparable to a higher dosage (20 mg/kg) of pure resveratrol (Balata et al., 2016).
In another investigation, the roughly comparable in-vivo bioavailability of trans-cinnamic acid (TCA) based SNEDDS was nearly 246% compared to TCA dispersion, denoting that SNEDDS possess such an exceptional ability to improve bioavailability (Wang et al., 2015). These observations can be determined by various mechanisms, including minimized intra-enterocyte biochemical activity via cytochrome P450, reduced P-gp electron transport activity, and circumvented hepatocellular first-pass metabolic activity via lymphatic assimilation. Trans-cinnamic acid improved its anti-diabetic effectiveness in alloxan-stimulated diabetic rats, which can be equivalent to metformin (Wang et al., 2015). SNEDDS have several advantages, nevertheless, they often have had some drawbacks. Characterization and conceptualization concerns include the significant relation between in vitro and in vivo experiments, the use of a substantial quantity of surfactant, bioactive compound in vivo, lipid oxidation possibilities, and the uncertainty of reduced encapsulation, and so on (Chatterjee et al., 2016). To compensate for their inadequacies, researchers are now focusing on emerging applications of SNEDDS, including self-double emulsions, solid SNEDDS, sustained release SNEDDS, completely saturated SNEDDS, and focused SNEDDS (Rehman et al., 2017). The histopathological, biochemical, and hematological results of streptozotocin-stimulated diabetic rats demonstrated that polypeptide-k packed SNEDDS had a greater anti-diabetic efficiency than the naïve form.
CONCLUSION
The bioactive compounds derived from biological sources are promising anti-diabetic agents because they are abundant, have substantial therapeutic benefits, and have few adverse effects. In general, bioactive bounds have 4 low blood sugar mechanisms: they reduce carbohydrate breakdown as well as glucose absorption, they promote glucose metabolism in the body, they enhance insulin action as well as sensitivity, and they have free radical scavenging and anti-inflammatory properties. Nevertheless, traditional orally ingested of anti-diabetic phytoconstituents has some flaws. Nanoparticle drug delivery processes for bioactive compounds to cure T2D preserve the benefits of orally administered and resolve the disadvantages of previous drug delivery. This review discussed organic bioactive compound coated oral nanoparticles drug delivery systems for T2D therapy, including organic polymeric nanoparticles, lipid-based nanostructures, vesicles structures, and nanoemulsions. According to research, oral nanoparticle drug delivery processes for T2D cure do have the following benefits:
1) Encapsulating drugs in organic nanoparticles delivery methods can enhance their stability and defend them from enzyme-mediated degradation in the gastrointestinal system. 2) Nano delivery methods act just at the bio-molecular range to enhance cells drug absorption or perhaps to restrict drug efflux processes such as the P-glycoprotein pump, improving the pharmacodynamic and pharmacokinetic nature of anti-diabetic substances. 3) First-pass hepatocellular metabolic activity can drastically reduce oral drug bioavailability. Glymphatic drug transfer is the best approach for enhancing drug delivery by avoiding first-pass metabolic activity. Nano delivery method can transfer drugs into the lymphatic system via M cells and enhance drug release in the circulatory system. 4) Customizing nanocarrier advancements achieves drug release control, demand for these products prevention, and targeted drug delivery system. 2 Bioactive compounds have demonstrated improved antidiabetic efficiency in nanoparticles-based drug delivery processes with improved absorption, significantly reduced toxic effect, target-specific, as well as reduced dosages and medicating regularity due to the benefits listed above.
Nevertheless, there are some gaps in current studies. On the hand, it is important to note that perhaps the large number of studies do not include a control set prescribed with a non-loaded nano carrier, implying that the drug-independent influence of a carrier can also be overlooked. Most substances used throughout the formulation of nano delivery methods are typically derived from inorganic compounds or synthetic materials via a somewhat more complex and time-consuming synthesis method, which might undoubtedly lead to considerable toxicity. Though organic materials are used as nanocarriers, organic bioactive may very well be initiated into the preparation requirements, and protection is of the utmost importance. However, most published literature comes from cellular and animal prototypes rather than a clinical investigation. Because animal toxicological investigation has boundaries, and clinical trials are eventually critical. Just curcumin nanocapsules have undergone clinical trials as an excellent anti-diabetic nano-drug. Despite the reality that only a few extreme side effects have been noted during therapies, prolonged human toxicity testing investigation has still been underwhelming. The FDA recently released guidelines to aid in nanoparticle-based clinical products’ secure progression. Too much clinical study is necessary. Above all, nanoparticle-based drug delivery methods for T2D treatment are quite a viable anti-diabetic drug delivery strategy. One such review could provide scientists with exciting anti-diabetic organic-based drug delivery methods to investigate further pharmacotherapy opportunities.
REFERENCES
- Abasalizadeh F., Moghaddam S. V., Alizadeh E., Kashani E., Fazljou S. M. B., Torbati M., Akbarzadeh A. Alginate-based hydrogels as drug delivery vehicles in cancer treatment and their applications in wound dressing and 3D bioprinting. J. Biol. Eng. 2020;14:8. doi: 10.1186/s13036-020-0227-7.962bc0dc3fc04b448dd430ea0c47e754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullah M. F., Nuge T., Andriyana A., Ang B. C., Muhamad F. Core-shell fibers: design, roles, and controllable release strategies in tissue engineering and drug delivery. Polymers. 2019;11:2008. doi: 10.3390/polym11122008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedini F., Ebrahimi M., Roozbehani A. H., Domb A. J., Hosseinkhani H. Overview on natural hydrophilic polysaccharide polymers in drug delivery. Polym. Adv. Technol. 2018;29:2564–2573. doi: 10.1002/pat.4375. [DOI] [Google Scholar]
- Abou Assi R., Abdulbaqi I., Seok Ming T., Siok Yee C., Wahab H., Asif S. M., Darwis Y. Liquid and solid self-emulsifying drug delivery systems (SEDDs) as carriers for the oral delivery of azithromycin: optimization, in vitro characterization and stability assessment. Pharmaceutics. 2020;12:1052. doi: 10.3390/pharmaceutics12111052.c2ab8bdba0ad49569429a37752c9aa68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahangarpour A., Oroojan A. A., Khorsandi L., Kouchak M., Badavi M. Solid lipid nanoparticles of myricitrin have antioxidant and antidiabetic effects on streptozotocin-nicotinamide-induced diabetic model and myotube cell of male mouse. Oxid. Med. Cell. Longev. 2018;2018:7496936. doi: 10.1155/2018/7496936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhavan S., Assadpour E., Katouzian I., Jafari S. M. Lipid nano scale cargos for the protection and delivery of food bioactive ingredients and nutraceuticals. Trends Food Sci. Technol. 2018;74:132–146. doi: 10.1016/j.tifs.2018.02.001. [DOI] [Google Scholar]
- Allawadhi P., Singh V., Govindaraj K., Khurana I., Sarode L. P., Navik U., Banothu A. K., Weiskirchen R., Bharani K. K., Khurana A. Biomedical applications of polysaccharide nanoparticles for chronic inflammatory disorders: focus on rheumatoid arthritis, diabetes and organ fibrosis. Carbohydr. Polym. 2021;281:118923. doi: 10.1016/j.carbpol.2021.118923. [DOI] [PubMed] [Google Scholar]
- Amalan V., Jose Vinoth Raja A., Karthikeyan B., Vijayakumar N., Kim S. J. Synthesis, characterization of p-coumaric acid nanoparticles and their in vitro antioxidant, anti-inflammatory, antimicrobial and antidiabetic activities. Curr. Pharm. Biotechnol. 2022 doi: 10.2174/1389201023666220822112923. doi: 10.2174/1389201023666220822112923 [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- Amalan V., Jose Vinoth Raja A., Rajeswari R., Jayaprakash R., Vijayakumar N. Antithrombotic, antihemolytic activities and protein conjugation properties of silver nanoparticles synthesized from Turbinaria ornata. Asian J. Chem. 2021;33:1736–1742. doi: 10.14233/ajchem.2021.23237. [DOI] [Google Scholar]
- Amalan V., Vijayakumar N. Antihyperglycemic effect of p-coumaric acid on streptozotocin induced diabetic rats. Indian J. Appl. Res. 2015;5:10–13. [Google Scholar]
- Amalan V., Vijayakumar N., Indumathi D., Ramakrishnan A. Antidiabetic and antihyperlipidemic activity of p-coumaric acid in diabetic rats, role of pancreatic GLUT 2: In vivo approach. Biomed. Pharmacother. 2016;84:230–236. doi: 10.1016/j.biopha.2016.09.039. [DOI] [PubMed] [Google Scholar]
- Amalan V., Vijayakumar N., Ramakrishnan A. p-Coumaric acid regulates blood glucose and antioxidant levels in streptozotocin induced diabetic rats. J. Chem. Pharm. Res. 2015;7:831–839. [Google Scholar]
- Amjadi S., Abbasi M. M., Shokouhi B., Ghorbani M., Hamishehkar H. Enhancement of therapeutic efficacy of betanin for diabetes treatment by liposomal nanocarriers. J. Funct. Foods. 2019;59:119–128. doi: 10.1016/j.jff.2019.05.015. [DOI] [Google Scholar]
- Balata G. F., Essa E. A., Shamardl H. A., Zaidan S. H., Abourehab M. A. Self-emulsifying drug delivery systems as a tool to improve solubility and bioavailability of resveratrol. Drug Des. Devel. Ther. 2016;10:117–128. doi: 10.2147/DDDT.S95905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi S., Serpooshan V., Tao W., Hamaly M. A., Alkawareek M. Y., Dreaden E. C., Brown D., Alkilany A. M., Farokhzad O. C., Mahmoudi M. Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 2017;46:4218–4244. doi: 10.1039/C6CS00636A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman P., Sulen Å., Barbetti F., Beltrand J., Svalastoga P., Codner E., Tessmann E. H., Juliusson P. B., Skrivarhaug T., Pearson E. R., Flanagan S. E., Babiker T., Thomas N. J., Shepherd M. H., Ellard S., Klimes I., Szopa M., Polak M., Iafusco D., Hattersley A. T., Njølstad P. R. Neonatal Diabetes International Collaborative Group, author. Effectiveness and safety of long-term treatment with sulfonylureas in patients with neonatal diabetes due to KCNJ11 mutations: an international cohort study. Lancet Diabetes Endocrinol. 2018;6:637–646. doi: 10.1016/S2213-8587(18)30106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. D., Whitehead K. A., Mitragotri S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020;5:127–148. doi: 10.1038/s41578-019-0156-6. [DOI] [Google Scholar]
- Čerpnjak K., Zvonar A., Gašperlin M., Vrečer F. Lipid-based systems as a promising approach for enhancing the bioavailability of poorly water-soluble drugs. Acta Pharm. 2013;63:427–445. doi: 10.2478/acph-2013-0040. [DOI] [PubMed] [Google Scholar]
- Chatterjee B., Hamed Almurisi S., Ahmed Mahdi Dukhan A., Mandal U. K., Sengupta P. Controversies with self-emulsifying drug delivery system from pharmacokinetic point of view. Drug Deliv. 2016;23:3639–3652. doi: 10.1080/10717544.2016.1214990. [DOI] [PubMed] [Google Scholar]
- Chenthamara D., Subramaniam S., Ramakrishnan S. G., Krishnaswamy S., Essa M. M., Lin F. H., Qoronfleh M. W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019;23:20. doi: 10.1186/s40824-019-0166-x.6f743f16e3d5482889f1bfef93e22203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho N., Shaw J., Karuranga S., Huang Y., da Rocha Fernandes J., Ohlrogge A., Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Choudhary P., Assemany P. P., Naaz F., Bhattacharya A., de Siqueira Castro J., do Couto Couto E., Calijuri M. L., Pant K. K., Malik A. A review of biochemical and thermochemical energy conversion routes of wastewater grown algal biomass. Sci. Total Environ. 2020;726:137961. doi: 10.1016/j.scitotenv.2020.137961. [DOI] [PubMed] [Google Scholar]
- da Silva Rosa S. C., Nayak N., Caymo A. M., Gordon J. W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep. 2020;8:e14607. doi: 10.14814/phy2.14607.c819aac9017e40b28a5fb6fa2a052ea7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damarla K., Rachuri Y., Suresh E., Kumar A. Nanoemulsions with all ionic liquid components as recyclable nanoreactors. Langmuir. 2018;34:10081–10091. doi: 10.1021/acs.langmuir.8b01909. [DOI] [PubMed] [Google Scholar]
- Durán-Lobato M., Niu Z., Alonso M. J. Oral delivery of biologics for precision medicine. Adv. Mater. 2020;32:1901935. doi: 10.1002/adma.201901935. [DOI] [PubMed] [Google Scholar]
- Eberle C., Stichling S. Impact of COVID-19 lockdown on glycemic control in patients with type 1 and type 2 diabetes mellitus: a systematic review. Diabetol. Metab. Syndr. 2021;13:95. doi: 10.1186/s13098-021-00705-9.89355bef5f63430fa6971f9b6d445a21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Naggar M. E., Al-Joufi F., Anwar M., Attia M. F., El-Bana M. A. Curcumin-loaded PLA-PEG copolymer nanoparticles for treatment of liver inflammation in streptozotocin-induced diabetic rats. Colloids Surf. B Biointerfaces. 2019;177:389–398. doi: 10.1016/j.colsurfb.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Emerenziani S., Pier Luca Guarino M., Trillo Asensio L. M., Altomare A., Ribolsi M., Balestrieri P., Cicala M. Role of overweight and obesity in gastrointestinal disease. Nutrients. 2019;12:111. doi: 10.3390/nu12010111.0af20284427f419e83cff8167122f974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg V., Kaur P., Gulati M., Singh S. K., Kumar B., Pandey N. K., Yadav A. K., Kumar R., Kuppusamy G., De A., Puttappa N., Wadhwa S. Coadministration of polypeptide-k and curcumin through solid self-nanoemulsifying drug delivery system for better therapeutic effect against diabetes mellitus: formulation, optimization, biopharmaceutical characterization, and pharmacodynamic assessment. Assay Drug Dev. Technol. 2019;17:201–221. doi: 10.1089/adt.2018.902. [DOI] [PubMed] [Google Scholar]
- Hatanaka J., Chikamori H., Sato H., Uchida S., Debari K., Onoue S., Yamada S. Physicochemical and pharmacological characterization of α-tocopherol-loaded nano-emulsion system. Int. J. Pharm. 2010;396:188–193. doi: 10.1016/j.ijpharm.2010.06.017. [DOI] [PubMed] [Google Scholar]
- Heindel J. J., Blumberg B., Cave M., Machtinger R., Mantovani A., Mendez M. A., Nadal A., Palanza P., Panzica G., Sargis R., Vandenberg L. N., Vom Saal F. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol. 2017;68:3–33. doi: 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. W., Kotta S., Ansari S. H., Sharma R. K., Ali J. Potentials and challenges in self-nanoemulsifying drug delivery systems. Expert Opin. Drug Deliv. 2012;9:1305–1317. doi: 10.1517/17425247.2012.719870. [DOI] [PubMed] [Google Scholar]
- Kinnear C., Moore T. L., Rodriguez-Lorenzo L., Rothen-Rutishauser B., Petri-Fink A. Form follows function: nanoparticle shape and its implications for nanomedicine. Chem. Rev. 2017;117:11476–11521. doi: 10.1021/acs.chemrev.7b00194. [DOI] [PubMed] [Google Scholar]
- Konda P. Y., Poondla V., Jaiswal K. K., Dasari S., Uyyala R., Surtineni V. P., Egi J. Y., Masilamani A. J. A., Bestha L., Konanki S., Muthulingam M., Lingamgunta L. K., Aloor B. P., Tirumalaraju S., Sade A., Ratnam Kamsala V., Nagaraja S., Ramakrishnan R., Natesan V. Pathophysiology of high fat diet induced obesity: impact of probiotic banana juice on obesity associated complications and hepatosteatosis. Sci. Rep. 2020;10:16894. doi: 10.1038/s41598-020-73670-4.bcb99e63b6b4489691f3e6d933cf6b90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laha B., Maiti S., Sen K. K., Jana S. Green Synthesis, Characterization and Applications of Nanoparticles, Micro and Nano Technol. Elsevier; 2019. Chapter 14 - Nanoscale polysaccharide-based particles for the delivery of therapeutic molecules; pp. 347–368. [DOI] [Google Scholar]
- Law B. A., Liao X., Moore K. S., Southard A., Roddy P., Ji R., Szulc Z., Bielawska A., Schulze P. C., Cowart L. A. Lipotoxic very-long-chain ceramides cause mitochondrial dysfunction, oxidative stress, and cell death in cardiomyocytes. FASEB J. 2018;32:1403–1416. doi: 10.1096/fj.201700300R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madkour L. H. Nanoelectronic Materials. Springer; Cham: 2019. Introduction to nanotechnology (NT) and nanomaterials (NMs) pp. 1–47. [DOI] [Google Scholar]
- Maity S., Mukhopadhyay P., Kundu P. P., Chakraborti A. S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals-an in vitro and in vivo approach. Carbohydr. Polym. 2017;170:124–132. doi: 10.1016/j.carbpol.2017.04.066. [DOI] [PubMed] [Google Scholar]
- Mansoori S., Davarnejad R., Matsuura T., Ismail A. F. Membranes based on non-synthetic (natural) polymers for wastewater treatment. Polym. Test. 2020;84:106381. doi: 10.1016/j.polymertesting.2020.106381. [DOI] [Google Scholar]
- McClements D. J. Encapsulation, protection, and delivery of bioactive proteins and peptides using nanoparticle and microparticle systems: a review. Adv. Colloid Interface Sci. 2018;253:1–22. doi: 10.1016/j.cis.2018.02.002. [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Jafari S. M., Hamishehkar H., Ghanbarzadeh B. Phytosterols as the core or stabilizing agent in different nanocarriers. Trends Food Sci. Technol. 2020;101:73–88. doi: 10.1016/j.tifs.2020.05.004. [DOI] [Google Scholar]
- Mohseni R., ArabSadeghabadi Z., Ziamajidi N., Abbasalipourkabir R., RezaeiFarimani A. Oral administration of resveratrol-loaded solid lipid nanoparticle improves insulin resistance through targeting expression of SNARE proteins in adipose and muscle tissue in rats with type 2 diabetes. Nanoscale Res. Lett. 2019;14:227. doi: 10.1186/s11671-019-3042-7.8290e93f1bdc4c018a23493676f4e591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi H., Nikfar B., Esmaeili S. A., Rafieenia F., Saburi E., Chaichian S., Gorji M. A. H., Momtazi-Borojeni A. A. Potential cytotoxic and anti-metastatic effects of berberine on gynaecological cancers with drug-associated resistance. Eur. J. Med. Chem. 2020;187:111951. doi: 10.1016/j.ejmech.2019.111951. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay P., Maity S., Mandal S., Chakraborti A. S., Prajapati A., Kundu P. P. Preparation, characterization and in vivo evaluation of pH sensitive, safe quercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetes treatment. Carbohydr. Polym. 2018;182:42–51. doi: 10.1016/j.carbpol.2017.10.098. [DOI] [PubMed] [Google Scholar]
- Mukhtar Y., Galalain A., Yunusa U. A modern overview on diabetes mellitus: a chronic endocrine disorder. Eur. J. Biol. 2020;5:1–14. doi: 10.47672/ejb.409. [DOI] [Google Scholar]
- Muzzarelli R. A., El Mehtedi M., Bottegoni C., Aquili A., Gigante A. Genipin-crosslinked chitosan gels and scaffolds for tissue engineering and regeneration of cartilage and bone. Mar. Drugs. 2015;13:7314–7338. doi: 10.3390/md13127068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nait Bachir Y., Nait Bachir R., Hadj-Ziane-Zafour A. Nanodispersions stabilized by β-cyclodextrin nanosponges: application for simultaneous enhancement of bioactivity and stability of sage essential oil. Drug Dev. Ind. Pharm. 2019;45:333–347. doi: 10.1080/03639045.2018.1542705. [DOI] [PubMed] [Google Scholar]
- Nalini T., Basha S. K., Sadiq A. M. M., Kumari V. S., Kaviyarasu K. Development and characterization of alginate/chitosan nanoparticulate system for hydrophobic drug encapsulation. J. Drug Deliv. Sci. Technol. 2019;52:65–72. doi: 10.1016/j.jddst.2019.04.002. [DOI] [Google Scholar]
- Nasrollahzadeh M., Sajadi S. M., Sajjadi M., Issaabadi Z. Interface Science and Technology. Vol. 28. Elsevier; 2019. Chapter 4 - Applications of nanotechnology in daily life; pp. 113–143. [DOI] [Google Scholar]
- Natesan V., Kim S. J. Diabetic nephropathy - a review of risk factors, progression, mechanism, and dietary management. Biomol. Ther. (Seoul) 2021;29:365–372. doi: 10.4062/biomolther.2020.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natesan V., Kim S. J. Metabolic bone diseases and new drug developments. Biomol. Ther. (Seoul) 2022;30:309–319. doi: 10.4062/biomolther.2022.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X., Chen Z., Pang L., Wang L., Jiang H., Chen Y., Zhang Z., Fu C., Ren B., Zhang J. Oral Nano drug delivery systems for the treatment of type 2 diabetes mellitus: an available administration strategy for antidiabetic phytocompounds. Int. J. Nanomed. 2020;15:10215–10240. doi: 10.2147/IJN.S285134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert T., Lee B. H., Fabien N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol. 2017;181:1314–1337. doi: 10.1007/s12010-016-2286-2. [DOI] [PubMed] [Google Scholar]
- Purohit R., Mittal A., Dalela S., Warudkar V., Purohit K., Purohit S. Social, environmental and ethical impacts of nanotechnology. Mater. Today Proc. 2017;4:5461–5467. doi: 10.1016/j.matpr.2017.05.058. [DOI] [Google Scholar]
- Rahman H. S., Othman H. H., Hammadi N. I., Yeap S. K., Amin K. M., Samad N. A., Alitheen N. B. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int. J. Nanomed. 2020;15:2439–2483. doi: 10.2147/IJN.S227805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan A., Vijayakumar N. Urea cycle pathway targeted therapeutic action of naringin against ammonium chloride induced hyperammonemic rats. Biomed. Pharmacother. 2017;94:1028–1037. doi: 10.1016/j.biopha.2017.08.028. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan A., Vijayakumar N., Renuka M. Effect of naringin on ammonium chloride-induced hyperammonemic rats: a dose-dependent study. J. Acute Med. 2016;6:55–60. doi: 10.1016/j.jacme.2016.08.001. [DOI] [Google Scholar]
- Ramya A., Vijayakumar N., Renuka M. Antiarthritic effect of aqueous extarct of lawsonia inermis.l - an invitro study. Int. J. Modn. Res. Revs. 2015;3:744–747. [Google Scholar]
- Rani R., Dahiya S., Dhingra D., Dilbaghi N., Kim K. H., Kumar S. Improvement of antihyperglycemic activity of nano-thymoquinone in rat model of type-2 diabetes. Chem. Biol. Interact. 2018;295:119–132. doi: 10.1016/j.cbi.2018.02.006. [DOI] [PubMed] [Google Scholar]
- Reddi Nagesh M., Vijayakumar N., Keserla Bhavani K. A review on diabetes mellitus- an annihilatory metabolic disorder. J. Pharm. Sci. Res. 2020;12:232–235. [Google Scholar]
- Rehman A., Jafari S. M., Tong Q., Riaz T., Assadpour E., Aadil R. M., Niazi S., Khan I. M., Shehzad Q., Ali A., Khan S. Drug nanodelivery systems based on natural polysaccharides against different diseases. Adv. Colloid Interface Sci. 2020;284:102251. doi: 10.1016/j.cis.2020.102251. [DOI] [PubMed] [Google Scholar]
- Rehman F. U., Shah K. U., Shah S. U., Khan I. U., Khan G. M., Khan A. From nanoemulsions to self-nanoemulsions, with recent advances in self-nanoemulsifying drug delivery systems (SNEDDS) Expert Opin. Drug Deliv. 2017;14:1325–1340. doi: 10.1080/17425247.2016.1218462. [DOI] [PubMed] [Google Scholar]
- Rosenberg K. J., Goren T., Crockett R., Spencer N. D. Load-induced transitions in the lubricity of adsorbed poly (l-lysine)-g-dextran as a Function of polysaccharide chain density. ACS Appl. Mater. Interfaces. 2011;3:3020–3025. doi: 10.1021/am200521m. [DOI] [PubMed] [Google Scholar]
- Rostamabadi H., Falsafi S. R., Jafari S. M. Nanoencapsulation of carotenoids within lipid-based nanocarriers. J. Control. Release. 2019;298:38–67. doi: 10.1016/j.jconrel.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Sailor G. U. Nanocarriers: Drug Delivery System. Springer; 2021. Self-nanoemulsifying drug delivery systems (SNEDDS): an innovative approach to improve oral bioavailability; pp. 255–280. [DOI] [Google Scholar]
- Shaker D. S., Ishak R. A., Ghoneim A., Elhuoni M. A. Nanoemulsion: a review on mechanisms for the transdermal delivery of hydrophobic and hydrophilic drugs. Sci. Pharm. 2019;87:17. doi: 10.3390/scipharm87030017. [DOI] [Google Scholar]
- Singh A. K., Pandey H., Ramteke P. W., Mishra S. B. Nano-suspension of ursolic acid for improving oral bioavailability and attenuation of type II diabetes: a histopathological investigation. Biocatal. Agric. Biotechnol. 2019;22:101433. doi: 10.1016/j.bcab.2019.101433. [DOI] [Google Scholar]
- Singh Y., Meher J. G., Raval K., Khan F. A., Chaurasia M., Jain N. K., Chourasia M. K. Nanoemulsion: concepts, development and applications in drug delivery. J. Control. Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- Sipponen M. H., Henn A., Penttilä P., Österberg M. Lignin-fatty acid hybrid nanocapsules for scalable thermal energy storage in phase-change materials. Chem. Eng. J. 2020;393:124711. doi: 10.1016/j.cej.2020.124711. [DOI] [Google Scholar]
- Sirdah M. M., Reading N. S. Genetic predisposition in type 2 diabetes: a promising approach toward a personalized management of diabetes. Clin. Genet. 2020;98:525–547. doi: 10.1111/cge.13772. [DOI] [PubMed] [Google Scholar]
- Sivakumar M., Tang S. Y., Tan K. W. Cavitation technology-a greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason. Sonochem. 2014;21:2069–2083. doi: 10.1016/j.ultsonch.2014.03.025. [DOI] [PubMed] [Google Scholar]
- Souto E. B., Souto S. B., Campos J. R., Severino P., Pashirova T. N., Zakharova L. Y., Silva A. M., Durazzo A., Lucarini M., Izzo A. A., Santini A. Nanoparticle delivery systems in the treatment of diabetes complications. Molecules. 2019;24:4209. doi: 10.3390/molecules24234209.a6668500ba6b43c4a8cdd1a2a444ea98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir H. E., Xiaobo Z., Mahunu G. K., Arslan M., Abdalhai M., Zhihua L. Recent developments in gum edible coating applications for fruits and vegetables preservation: a review. Carbohydr. Polym. 2019;224:115141. doi: 10.1016/j.carbpol.2019.115141. [DOI] [PubMed] [Google Scholar]
- Thapa C., Ahad A., Aqil M., Imam S. S., Sultana Y. Formulation and optimization of nanostructured lipid carriers to enhance oral bioavailability of telmisartan using Box-Behnken design. J. Drug Deliv. Sci. Technol. 2018;44:431–439. doi: 10.1016/j.jddst.2018.02.003. [DOI] [Google Scholar]
- Verma A. K., Goyal Y., Bhatt D., Dev K., Alsahli M. A., Rahmani A. H., Almatroudi A. A compendium of perspectives on diabetes: a challenge for sustainable health in the modern era. Diabetes Metab. Syndr. Obes. Targets Ther. 2021;14:2775–2787. doi: 10.2147/DMSO.S304751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar Hepatoprotective effect of Semecarpus anacardiumin rats: a molecular approach. Int. J. Biol. Sci. 2014;5:175–182. [Google Scholar]
- Vijayakumar N., Subramanian P. Protective effect of Semecarpus Anacardium nut extract against hyperammonemia in rats. J. Herb. Med. Toxicol. 2010a;4:77–82. [Google Scholar]
- Vijayakumar N., Subramanian P. Neuroprotective effect of semecarpus anacardium against hyperammonemia in rats. J. Pharm. Res. 2010b;3:1564–1568. [Google Scholar]
- Viswanathan P., Muralidaran Y., Ragavan G. Nanostructures for Oral Medicine. Elsevier; 2017. Chapter 7 - Challenges in oral drug delivery: a nano-based strategy to overcome; pp. 173–201. [DOI] [Google Scholar]
- Wang H., Li Q., Deng W., Omari-Siaw E., Wang Q., Wang S., Wang S., Cao X., Xu X., Yu J. Self-nanoemulsifying drug delivery system of trans-cinnamic acid: formulation development and pharmacodynamic evaluation in alloxan-induced type 2 diabetic rat model. Drug Dev. Res. 2015;76:82–93. doi: 10.1002/ddr.21244. [DOI] [PubMed] [Google Scholar]
- Wang H., Xu Z., Zhao M., Liu G., Wu J. Advances of hydrogel dressings in diabetic wounds. Biomater. Sci. 2021;9:1530–1546. doi: 10.1039/D0BM01747G. [DOI] [PubMed] [Google Scholar]
- Wasupalli G. K., Verma D. Molecular interactions in self-assembled nano-structures of chitosan-sodium alginate based polyelectrolyte complexes. Int. J. Biol. Macromol. 2018;114:10–17. doi: 10.1016/j.ijbiomac.2018.03.075. [DOI] [PubMed] [Google Scholar]
- Wu D., Zhu L., Li Y., Zhang X., Xu S., Yang G., Delair T. Chitosan-based colloidal polyelectrolyte complexes for drug delivery: a review. Carbohydr. Polym. 2020;238:116126. doi: 10.1016/j.carbpol.2020.116126. [DOI] [PubMed] [Google Scholar]
- Xu H. Y., Liu C. S., Huang C. L., Chen L., Zheng Y. R., Huang S. H., Long X. Y. Nanoemulsion improves hypoglycemic efficacy of berberine by overcoming its gastrointestinal challenge. Colloids Surf. B Biointerfaces. 2019;181:927–934. doi: 10.1016/j.colsurfb.2019.06.006. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Wang J., Gu Z., Wang S., Zhu W., Acen, Soloshonok V. A., Izawa K., Liu H. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II-III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem. Rev. 2016;116:422–518. doi: 10.1021/acs.chemrev.5b00392. [DOI] [PubMed] [Google Scholar]
- Zhu W., Zhang Z. Preparation and characterization of catechin-grafted chitosan with antioxidant and antidiabetic potential. Int. J. Biol. Macromol. 2014;70:150–155. doi: 10.1016/j.ijbiomac.2014.06.047. [DOI] [PubMed] [Google Scholar]