Abstract
The American Diabetes Association (ADA) “Standards of Care in Diabetes” includes the ADA’s current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee, are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA’s clinical practice recommendations and a full list of Professional Practice Committee members, please refer to Introduction and Methodology. Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC.
Introduction
Obesity is a chronic and often progressive disease with numerous medical, physical, and psychosocial complications, including a substantially increased risk for type 2 diabetes (1). There is strong and consistent evidence that obesity management can delay the progression from prediabetes to type 2 diabetes (2–6) and is highly beneficial in treating type 2 diabetes (7–18). In people with type 2 diabetes and overweight or obesity, modest weight loss improves glycemia and reduces the need for glucose-lowering medications (7–9), and larger weight loss substantially reduces A1C and fasting glucose and has been shown to promote sustained diabetes remission through at least 2 years (11,19–23). Several modalities, including intensive behavioral counseling, obesity pharmacotherapy, and bariatric surgery, may aid in achieving and maintaining meaningful weight loss and reducing obesity-associated health risks. Metabolic surgery strongly improves glycemia and often leads to remission of diabetes, improved quality of life, improved cardiovascular outcomes, and reduced mortality. The importance of addressing obesity is further heightened by numerous studies showing that both obesity and diabetes increase the risk for more severe coronavirus disease 2019 (COVID-19) infections (24–27). This section aims to provide evidence-based recommendations for obesity management, including behavioral, pharmacologic, and surgical interventions, in people with type 2 diabetes and in those at risk. This section focuses on obesity management in adults; further discussion on obesity in older individuals and children can be found in Section 13, “Older Adults,” and Section 14, “Children and Adolescents,” respectively.
Assessment
Recommendations
8.1 Use person-centered, nonjudgmental language that fosters collaboration between individuals and health care professionals, including person-first language (e.g., “person with obesity” rather than “obese person”). E
8.2 Measure height and weight and calculate BMI at annual visits or more frequently. Assess weight trajectory to inform treatment considerations. E
8.3 Based on clinical considerations, such as the presence of comorbid heart failure or significant unexplained weight gain or loss, weight may need to be monitored and evaluated more frequently. B If deterioration of medical status is associated with significant weight gain or loss, inpatient evaluation should be considered, especially focused on associations between medication use, food intake, and glycemic status. E
8.4 Accommodations should be made to provide privacy during weighing. E
8.5 Individuals with diabetes and overweight or obesity may benefit from modest or larger magnitudes of weight loss. Relatively small weight loss (approximately 3–7% of baseline weight) improves glycemia and other intermediate cardiovascular risk factors. A Larger, sustained weight losses (>10%) usually confer greater benefits, including disease-modifying effects and possible remission of type 2 diabetes, and may improve long-term cardiovascular outcomes and mortality. B
A person-centered communication style that uses inclusive and nonjudgmental language and active listening to elicit individual preferences and beliefs and assesses potential barriers to care should be used to optimize health outcomes and health-related quality of life. Use person-first language (e.g., “person with obesity” rather than “obese person”) to avoid defining people by their condition (28–30).
Height and weight should be measured to calculate BMI annually or more frequently when appropriate (20). BMI, calculated as weight in kilograms divided by the square of height in meters (kg/m2), is calculated automatically by most electronic medical records. Use BMI to document weight status (overweight: BMI 25–29.9 kg/m2; obesity class I: BMI 30–34.9 kg/m2; obesity class II: BMI 35–39.9 kg/m2; obesity class III: BMI ≥40 kg/m2) but note that misclassification can occur, particularly in very muscular or frail individuals. In some groups, notably Asian and Asian American populations, the BMI cut points to define overweight and obesity are lower than those in other populations due to differences in body composition and cardiometabolic risk (Table 8.1) (30,31). Clinical considerations, such as the presence of comorbid heart failure or unexplained weight change, may warrant more frequent weight measurement and evaluation (32,33). If weighing is questioned or refused, the practitioner should be mindful of possible prior stigmatizing experiences and query for concerns, and the value of weight monitoring should be explained as a part of the medical evaluation process that helps to inform treatment decisions (34,35). Accommodations should be made to ensure privacy during weighing, particularly for those individuals who report or exhibit a high level of weight-related distress or dissatisfaction. Scales should be situated in a private area or room. Weight should be measured and reported nonjudgmentally. Care should be taken to regard a person’s weight (and weight changes) and BMI as sensitive health information. In addition to weight and BMI, assessment of weight distribution (including propensity for central/visceral adipose deposition) and weight gain pattern and trajectory can further inform risk stratification and treatment options (36).
Table 8.1.
Treatment options for overweight and obesity in type 2 diabetes
| BMI category (kg/m2) | |||
|---|---|---|---|
| Treatment | 25.0–26.9 (or 23.0–24.9*) | 27.0–29.9 (or 25.0–27.4*) | ≥30.0 (or ≥27.5*) |
| Nutrition, physical activity, and behavioral counseling | † | † | † |
| Pharmacotherapy | † | † | |
| Metabolic surgery | † | ||
Recommended cut points for Asian American individuals (expert opinion).
Treatment may be indicated for select motivated individuals.
Health care professionals should advise individuals with overweight or obesity and those with increasing weight trajectories that, in general, higher BMIs increase the risk of diabetes, cardiovascular disease, and all-cause mortality, as well as other adverse health and quality of life outcomes. Health care professionals should assess readiness to engage in behavioral changes for weight loss and jointly determine behavioral and weight loss goals and individualized intervention strategies (37). Strategies may include nutrition changes, physical activity, behavioral counseling, pharmacologic therapy, medical devices, and metabolic surgery (Table 8.1). The latter three strategies may be considered for carefully selected individuals as adjuncts to nutrition changes, physical activity, and behavioral counseling.
Among people with type 2 diabetes and overweight or obesity who have inadequate glycemic, blood pressure, and lipid control and/or other obesity-related medical conditions, modest and sustained weight loss improves glycemia, blood pressure, and lipids and may reduce the need for medications (7–9,38). Greater weight loss may produce even greater benefits (21,22).
As little as 3–7% weight loss reduces the risk for diabetes in people at risk and improves glycemia in those with diabetes (2,7,8,39,40). Given the challenge of losing weight and maintaining weight loss, aiming for relatively small and attainable weight loss is often an effective clinical strategy, particularly for individuals who feel overwhelmed by larger weight loss targets. Nevertheless, mounting data from intensive nutrition and behavioral change interventions, pharmacotherapy, and bariatric surgery have shown that more substantial weight loss usually confers still greater benefits on glycemia and possibly disease remission as well as other cardiometabolic and quality-of-life outcomes (6,21–23,41–50).
With the increasing availability of more effective obesity treatments, individuals with diabetes and overweight or obesity should be informed of the potential benefits of both modest and more substantial weight loss and guided in the range of available treatment options, as discussed in the sections below. Shared decision-making should be used when counseling on behavioral changes, intervention choices, and weight management goals.
Nutrition, Physical Activity, and Behavioral Therapy
Recommendations
8.6 Nutrition, physical activity, and behavioral therapy to achieve and maintain ≥5% weight loss are recommended for most people with type 2 diabetes and overweight or obesity. Additional weight loss usually results in further improvements in the management of diabetes and cardiovascular risk. B
8.7 Such interventions should include a high frequency of counseling (≥16 sessions in 6 months) and focus on nutrition changes, physical activity, and behavioral strategies to achieve a 500–750 kcal/day energy deficit. A
8.8 An individual’s preferences, motivation, and life circumstances should be considered, along with medical status, when weight loss interventions are recommended. C
8.9 Behavioral changes that create an energy deficit, regardless of macronutrient composition, will result in weight loss. Nutrition recommendations should be individualized to the person’s preferences and nutritional needs. A
8.10 Evaluate systemic, structural, and socioeconomic factors that may impact nutrition patterns and food choices, such as food insecurity and hunger, access to healthful food options, cultural circumstances, and social determinants of health. C
8.11 For those who achieve weight loss goals, long-term (≥1 year) weight maintenance programs are recommended when available. Such programs should, at minimum, provide monthly contact and support, recommend ongoing monitoring of body weight (weekly or more frequently) and other self-monitoring strategies, and encourage regular physical activity (200–300 min/week). A
8.12 Short-term nutrition intervention using structured, very-low-calorie meals (800–1,000 kcal/day) may be prescribed for carefully selected individuals by trained practitioners in medical settings with close monitoring. Long-term, comprehensive weight maintenance strategies and counseling should be integrated to maintain weight loss. B
8.13 There is no clear evidence that nutrition supplements are effective for weight loss. A
For a more detailed discussion of lifestyle management approaches and recommendations, see Section 5, “Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes.” For a detailed discussion of nutrition interventions, please also refer to “Nutrition Therapy for Adults With Diabetes or Prediabetes: A Consensus Report” (127).
Look AHEAD Trial
Although the Action for Health in Diabetes (Look AHEAD) trial did not show that the intensive lifestyle intervention reduced cardiovascular events in adults with type 2 diabetes and overweight or obesity (39), it did confirm the feasibility of achieving and maintaining long-term weight loss in people with type 2 diabetes. In the intensive lifestyle intervention group, mean weight loss was 4.7% at 8 years (40). Approximately 50% of intensive lifestyle intervention participants lost and maintained ≥5% of their initial body weight, and 27% lost and maintained ≥10% of their initial body weight at 8 years (40). Participants assigned to the intensive lifestyle group required fewer glucose-, blood pressure–, and lipid-lowering medications than those randomly assigned to standard care. Secondary analyses of the Look AHEAD trial and other large cardiovascular outcome studies document additional weight loss benefits in people with type 2 diabetes, including improved mobility, physical and sexual function, and health-related quality of life (32). Moreover, several subgroups had improved cardiovascular outcomes, including those who achieved >10% weight loss (41) and those with moderately or poorly managed diabetes (A1C >6.8%) at baseline (42).
Behavioral Interventions
Significant weight loss can be attained with lifestyle programs that achieve a 500–750 kcal/day energy deficit, which in most cases is approximately 1,200–1,500 kcal/day for women and 1,500–1,800 kcal/day for men, adjusted for the individual’s baseline body weight. Clinical benefits typically begin upon achieving 3–5% weight loss (20,51), and the benefits of weight loss are progressive; more intensive weight loss goals (>5%, >7%, >15%, etc.) may be pursued if needed to achieve further health improvements and/or if the individual is more motivated and more intensive goals can be feasibly and safely attained.
Nutrition interventions may differ by macronutrient goals and food choices as long as they create the necessary energy deficit to promote weight loss (20,52–54). Using meal replacement plans prescribed by trained practitioners, with close monitoring, can be beneficial. Within the intensive lifestyle intervention group of the Look AHEAD trial, for example, the use of a partial meal replacement plan was associated with improvements in nutrition quality and weight loss (51). The nutrition choice should be based on the individual’s health status and preferences, including a determination of food availability and other cultural circumstances that could affect nutrition patterns (55).
Intensive behavioral interventions should include ≥16 sessions during the initial 6 months and focus on nutrition changes, physical activity, and behavioral strategies to achieve an ∼500–750 kcal/day energy deficit. Interventions should be provided by trained interventionists in either individual or group sessions (51). Assessing an individual’s motivation level, life circumstances, and willingness to implement behavioral changes to achieve weight loss should be considered along with medical status when weight loss interventions are recommended and initiated (37,56).
People with type 2 diabetes and overweight or obesity who have lost weight should be offered long-term (≥1 year) comprehensive weight loss maintenance programs that provide at least monthly contact with trained interventionists and focus on ongoing monitoring of body weight (weekly or more frequently) and/or other self-monitoring strategies such as tracking intake, steps, etc.; continued focus on nutrition and behavioral changes; and participation in high levels of physical activity (200–300 min/week) (57). Some commercial and proprietary weight loss programs have shown promising weight loss results. However, most lack evidence of effectiveness, many do not satisfy guideline recommendations, and some promote unscientific and possibly dangerous practices (58,59).
When provided by trained practitioners in medical settings with ongoing monitoring, short-term (generally up to 3 months) intensive nutrition intervention may be prescribed for carefully selected individuals, such as those requiring weight loss before surgery and those needing greater weight loss and glycemic improvements. When integrated with behavioral support and counseling, structured very-low-calorie meals, typically 800–1,000 kcal/day, utilizing high-protein foods and meal replacement products, may increase the pace and/or magnitude of initial weight loss and glycemic improvements compared with standard behavioral interventions (21,22). As weight regain is common, such interventions should include long-term, comprehensive weight maintenance strategies and counseling to maintain weight loss and behavioral changes (60,61).
Despite widespread marketing and exorbitant claims, there is no clear evidence that nutrition supplements (such as herbs and botanicals, high-dose vitamins and minerals, amino acids, enzymes, antioxidants, etc.) are effective for obesity management or weight loss (62–64). Several large systematic reviews show that most trials evaluating nutrition supplements for weight loss are of low quality and at high risk for bias. High-quality published studies show little or no weight loss benefits. In contrast, vitamin/mineral (e.g., iron, vitamin B12, vitamin D) supplementation may be indicated in cases of documented deficiency, and protein supplements may be indicated as adjuncts to medically supervised weight loss therapies.
Health disparities adversely affect people who have systematically experienced greater obstacles to health based on their race or ethnicity, socioeconomic status, gender, disability, or other factors. Overwhelming research shows that these disparities may significantly affect health outcomes, including increasing the risk for obesity, diabetes, and diabetes-related complications. Health care professionals should evaluate systemic, structural, and socioeconomic factors that may impact food choices, access to healthful foods, and nutrition patterns; behavioral patterns, such as neighborhood safety and availability of safe outdoor spaces for physical activity; environmental exposures; access to health care; social contexts; and, ultimately, diabetes risk and outcomes. For a detailed discussion of social determinants of health, refer to “Social Determinants of Health: A Scientific Review” (65).
Pharmacotherapy
Recommendations
8.14 When choosing glucose-lowering medications for people with type 2 diabetes and overweight or obesity, consider the medication’s effect on weight. B
8.15 Whenever possible, minimize medications for comorbid conditions that are associated with weight gain. E
8.16 Obesity pharmacotherapy is effective as an adjunct to nutrition, physical activity, and behavioral counseling for selected people with type 2 diabetes and BMI ≥27 kg/m2. Potential benefits and risks must be considered. A
8.17 If obesity pharmacotherapy is effective (typically defined as ≥5% weight loss after 3 months’ use), further weight loss is likely with continued use. When early response is insufficient (typically <5% weight loss after 3 months’ use) or if there are significant safety or tolerability issues, consider discontinuation of the medication and evaluate alternative medications or treatment approaches. A
Glucose-Lowering Therapy
A meta-analysis of 227 randomized controlled trials of glucose-lowering treatments in type 2 diabetes found that A1C changes were not associated with baseline BMI, indicating that people with obesity can benefit from the same types of treatments for diabetes as normal-weight individuals (66). As numerous effective medications are available when considering medication plans, health care professionals should consider each medication’s effect on weight. Agents associated with varying degrees of weight loss include metformin, α-glucosidase inhibitors, sodium–glucose cotransporter 2 inhibitors, glucagon-like peptide 1 receptor agonists, dual glucagon-like peptide 1/glucose–dependent insulinotropic polypeptide receptor agonist (tirzepatide), and amylin mimetics. Dipeptidyl peptidase 4 inhibitors are weight neutral. In contrast, insulin secretagogues, thiazolidinediones, and insulin are often associated with weight gain (see Section 9, “Pharmacologic Approaches to Glycemic Treatment”).
Concomitant Medications
Health care professionals should carefully review the patient’s concomitant medications and, whenever possible, minimize or provide alternatives for medications that promote weight gain. Examples of medications associated with weight gain include antipsychotics (e.g., clozapine, olanzapine, risperidone), some antidepressants (e.g., tricyclic antidepressants, some selective serotonin reuptake inhibitors, and monoamine oxidase inhibitors), glucocorticoids, injectable progestins, some anticonvulsants (e.g., gabapentin, pregabalin), and possibly sedating antihistamines and anticholinergics (67).
Approved Obesity Pharmacotherapy Options
The U.S. Food and Drug Administration (FDA) has approved medications for both short-term and long-term weight management as adjuncts to nutrition, physical activity and behavioral therapy. Nearly all FDA-approved obesity medications have been shown to improve glycemia in people with type 2 diabetes and delay progression to type 2 diabetes in at-risk individuals (23). Phentermine and other older adrenergic agents are indicated for short-term (≤12 weeks) treatment (68). Five medications are FDA approved for long-term use (>12 weeks) in adults with BMI ≥27 kg/m2 with one or more obesity-associated comorbid conditions (e.g., type 2 diabetes, hypertension, and/or dyslipidemia) who are motivated to lose weight (23). (Refer to Section 14, “Children and Adolescents,” for medications approved for adolescents with obesity.) Medications approved by the FDA for the treatment of obesity, summarized in Table 8.2, include orlistat, phentermine/topiramate ER, naltrexone/bupropion ER, liraglutide 3 mg, and semaglutide 2.4 mg. (In addition, setmelanotide, a melanocortin 4 receptor agonist, is approved for use in cases of rare genetic mutations resulting in severe hyperphagia and extreme obesity, such as leptin receptor deficiency and proopiomelanocortin deficiency.) In principle, medications help improve adherence to nutrition recommendations, in most cases by modulating appetite or satiety. Health care professionals should be knowledgeable about the product label and balance the potential benefits of successful weight loss against the potential risks of the medication for each individual. These medications are contraindicated in individuals who are pregnant or actively trying to conceive and not recommended for use in women who are nursing. Individuals of reproductive potential should receive counseling regarding the use of reliable methods of contraception. Of note, while weight loss medications are often used in people with type 1 diabetes, clinical trial data in this population are limited.
Table 8.2.
Medications approved by the FDA for the treatment of overweight or obesity in adults
| 1-Year (52- or 56-week) mean weight loss (% loss from baseline) | |||||||
|---|---|---|---|---|---|---|---|
| Medication name | Typical adult maintenance dose | Average wholesale price (30-day supply) (128) | National Average Drug Acquisition Cost (30-day supply) (129) | Treatment arms | Weight loss (% loss from baseline) | Common side effects (130–134) | Possible safety concerns/considerations (130–134) |
| Short-term treatment (≤12 weeks) | |||||||
| Sympathomimetic amine anorectic | |||||||
| Phentermine (135) | 8–37.5 mg q.d.* | $5–$56 (37.5 mg dose) | $2–$3 (37.5 mg dose) | 15 mg q.d.† | 6.1 | Dry mouth, insomnia, dizziness, irritability, increased blood pressure, elevated heart rate | • Contraindicated for use in combination with monoamine oxidase inhibitors |
| 7.5 mg q.d.† PBO |
5.5 1.2 |
||||||
| Long-term treatment (>12 weeks) | |||||||
| Lipase inhibitor | |||||||
| Orlistat (4) | 60 mg t.i.d. (OTC) 120 mg t.i.d. (Rx) |
$41−$82 $781−$904 |
NA $722 |
120 mg t.i.d.‡ PBO |
9.6 5.6 |
Abdominal pain, flatulence, fecal urgency | • Potential malabsorption of fat-soluble vitamins (A, D, E, K) and of certain medications (e.g., cyclosporine, thyroid hormone, anticonvulsants, etc.) • Rare cases of severe liver injury reported • Cholelithiasis • Nephrolithiasis |
| Sympathomimetic amine anorectic/antiepileptic combination | |||||||
| Phentermine/topiramate ER (45) | 7.5 mg/46 mg q.d.§ | $223 (7.5 mg/46 mg dose) | $179 (7.5 mg/46 mg dose) | 15 mg/92 mg q.d.∥ | 9.8 | Constipation, paresthesia, insomnia, nasopharyngitis, xerostomia, increased blood pressure | • Contraindicated for use in combination with monoamine oxidase inhibitors • Birth defects • Cognitive impairment • Acute angle-closure glaucoma |
| 7.5 mg/46 mg q.d.∥ PBO |
7.8 1.2 |
||||||
| Opioid antagonist/antidepressant combination | |||||||
| Naltrexone/bupropion ER (16) | 16 mg/180 mg b.i.d. | $750 | $599 | 16 mg/180 mg b.i.d. PBO |
5.0 1.8 |
Constipation, nausea, headache, xerostomia, insomnia, elevated heart rate and blood pressure | • Contraindicated in people with unmanaged hypertension and/or seizure disorders • Contraindicated for use with chronic opioid therapy • Acute angle-closure glaucoma |
| Black box warning: | |||||||
| • Risk of suicidal behavior/ideation in people younger than 24 years old who have depression | |||||||
| Glucagon-like peptide 1 receptor agonist | |||||||
| Liraglutide (17)** | 3 mg q.d. | $1,619 | $1,295 | 3.0 mg q.d. 1.8 mg q.d. PBO |
6.0 4.7 2.0 |
Gastrointestinal side effects (nausea, vomiting, diarrhea, esophageal reflux), injection site reactions, elevated heart rate, hypoglycemia | • Pancreatitis has been reported in clinical trials, but causality has not been established. Discontinue if pancreatitis is suspected. • Use caution in people with kidney disease when initiating or increasing dose due to potential risk of acute kidney injury. • May cause cholelithiasis and gallstone-related complications. |
| Black box warning: | |||||||
| • Risk of thyroid C-cell tumors in rodents; human relevance not determined | |||||||
| Semaglutide (46,47) | 2.4 mg once weekly | $1,619 | $1,295 | 2.4 mg weekly PBO |
9.6 3.4 |
Gastrointestinal side effects (nausea, vomiting, diarrhea, esophageal reflux), injection site reactions, elevated heart rate, hypoglycemia | • Pancreatitis has been reported in clinical trials, but causality has not been established. Discontinue if pancreatitis is suspected. • May cause cholelithiasis and gallstone-related complications. |
| Black box warning: | |||||||
| • Risk of thyroid C-cell tumors in rodents; human relevance not determined | |||||||
All medications are contraindicated in individuals who are or may become pregnant. Individuals of reproductive potential must be counseled regarding the use of reliable methods of contraception. Select safety and side effect information is provided; for a comprehensive discussion of safety considerations, please refer to the prescribing information for each agent. b.i.d., twice daily; ER, extended release; OTC, over the counter; NA, data not available; PBO, placebo; q.d., daily; Rx, prescription; t.i.d., three times daily.
Use lowest effective dose; maximum appropriate dose is 37.5 mg.
Duration of treatment was 28 weeks in a general adult population with obesity.
Enrolled participants had normal (79%) or impaired (21%) glucose tolerance.
Maximum dose, depending on response, is 15 mg/92 mg q.d.
Approximately 68% of enrolled participants had type 2 diabetes or impaired glucose tolerance.
Agent has demonstrated cardiovascular safety in a dedicated cardiovascular outcome trial (47).
Assessing Efficacy and Safety
Upon initiating weight loss medication, assess efficacy and safety at least monthly for the first 3 months and at least quarterly thereafter. Modeling from published clinical trials consistently shows that early responders have improved long-term outcomes (69–71). Unless clinical circumstances (such as poor tolerability) or other considerations (such as financial expense or individual preference) suggest otherwise, those who achieve sufficient early weight loss upon starting a chronic weight loss medication (typically defined as >5% weight loss after 3 months’ use) should continue the medication. When early use appears ineffective (typically <5% weight loss after 3 months’ use), it is unlikely that continued use will improve weight outcomes; as such, it should be recommended to discontinue the medication and consider other treatment options.
Medical Devices for Weight Loss
While gastric banding devices have fallen out of favor in recent years, since 2015, several minimally invasive medical devices have been approved by the FDA for short-term weight loss, including implanted gastric balloons, a vagus nerve stimulator, and gastric aspiration therapy (72). Given the current high cost, limited insurance coverage, and paucity of data in people with diabetes, medical devices for weight loss are rarely utilized at this time, and it remains to be seen how they may be used in the future (73).
An oral hydrogel (Plenity) has recently been approved for long-term use in those with BMI >25 kg/m2 to simulate the space-occupying effect of implantable gastric balloons. Taken with water 30 min before meals, the hydrogel expands to fill a portion of the stomach volume to help decrease food intake during meals. Though average weight loss is relatively small (2–3% greater than placebo), the subgroup of participants with prediabetes or diabetes at baseline had improved weight loss outcomes (8.1% weight loss) compared with the overall treatment (6.4% weight loss) and placebo (4.4% weight loss) groups (74).
Metabolic Surgery
Recommendations
8.18 Metabolic surgery should be a recommended option to treat type 2 diabetes in screened surgical candidates with BMI ≥40 kg/m2 (BMI ≥37.5 kg/m2 in Asian American individuals) and in adults with BMI 35.0–39.9 kg/m2 (32.5–37.4 kg/m2 in Asian American individuals) who do not achieve durable weight loss and improvement in comorbidities (including hyperglycemia) with nonsurgical methods. A
8.19 Metabolic surgery may be considered as an option to treat type 2 diabetes in adults with BMI 30.0–34.9 kg/m2 (27.5–32.4 kg/m2 in Asian American individuals) who do not achieve durable weight loss and improvement in comorbidities (including hyperglycemia) with nonsurgical methods. A
8.20 Metabolic surgery should be performed in high-volume centers with multidisciplinary teams knowledgeable about and experienced in managing obesity, diabetes, and gastrointestinal surgery. E
8.21 People being considered for metabolic surgery should be evaluated for comorbid psychological conditions and social and situational circumstances that have the potential to interfere with surgery outcomes. B
8.22 People who undergo metabolic surgery should receive long-term medical and behavioral support and routine micronutrient, nutritional, and metabolic status monitoring. B
8.23 If postbariatric hypoglycemia is suspected, clinical evaluation should exclude other potential disorders contributing to hypoglycemia, and management includes education, medical nutrition therapy with a dietitian experienced in postbariatric hypoglycemia, and medication treatment, as needed. A Continuous glucose monitoring should be considered as an important adjunct to improve safety by alerting individuals to hypoglycemia, especially for those with severe hypoglycemia or hypoglycemia unawareness. E
8.24 People who undergo metabolic surgery should routinely be evaluated to assess the need for ongoing mental health services to help with the adjustment to medical and psychosocial changes after surgery. C
Surgical procedures for obesity treatment—often referred to interchangeably as bariatric surgery, weight loss surgery, metabolic surgery, or metabolic/bariatric surgery—can promote significant and durable weight loss and improve type 2 diabetes. Given the magnitude and rapidity of improvement of hyperglycemia and glucose homeostasis, these procedures have been suggested as treatments for type 2 diabetes even in the absence of severe obesity and will be referred to here as “metabolic surgery.”
A substantial body of evidence, including data from numerous large cohort studies and randomized controlled (nonblinded) clinical trials, demonstrates that metabolic surgery achieves superior glycemic control and reduction of cardiovascular risk in people with type 2 diabetes and obesity compared with nonsurgical intervention (18). In addition to improving glycemia, metabolic surgery reduces the incidence of microvascular disease (75), improves quality of life (43,76,77), decreases cancer risk, and improves cardiovascular disease risk factors and long-term cardiovascular events (78–89). Cohort studies that match surgical and nonsurgical subjects strongly suggest that metabolic surgery reduces all-cause mortality (90,91).
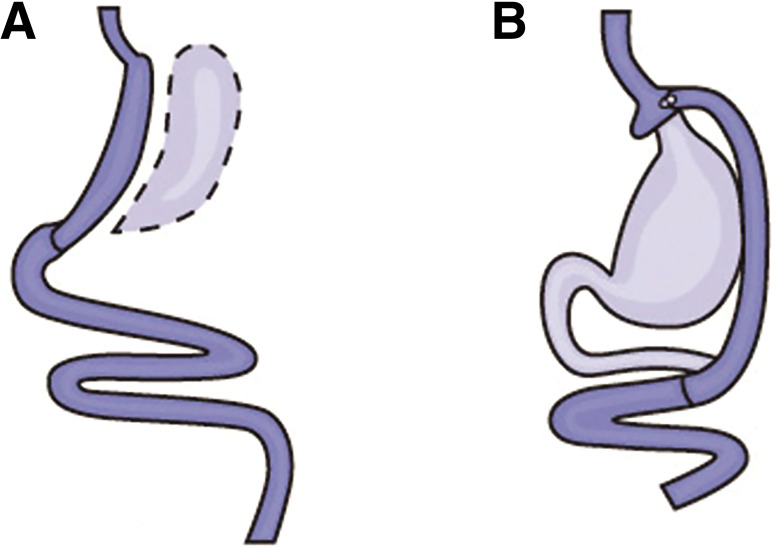
The overwhelming majority of procedures in the U.S. are vertical sleeve gastrectomy (VSG) and Roux-en-Y gastric bypass (RYGB). Both procedures result in an anatomically smaller stomach pouch and often robust changes in enteroendocrine hormones. In VSG, ∼80% of the stomach is removed, leaving behind a long, thin sleeve-shaped pouch. RYGB creates a much smaller stomach pouch (roughly the size of a walnut), which is then attached to the distal small intestine, thereby bypassing the duodenum and jejunum (Fig. 8.1).
Figure 8.1.
A: Vertical sleeve gastrectomy. B: Roux-en-Y gastric bypass surgery. Images reprinted from National Institute of Diabetes and Digestive and Kidney Diseases (92).
Several organizations recommend lowering the BMI criteria for metabolic surgery to 30 kg/m2 (27.5 kg/m2 for Asian American individuals) for people with type 2 diabetes who have not achieved sufficient weight loss and improved comorbidities (including hyperglycemia) with reasonable nonsurgical treatments. Studies have documented diabetes remission after 1–5 years in 30–63% of patients with RYGB (18,93).
Most notably, the Surgical Treatment and Medications Potentially Eradicate Diabetes Efficiently (STAMPEDE) trial, which randomized 150 participants with unmanaged diabetes to receive either metabolic surgery or medical treatment, found that 29% of those treated with RYGB and 23% treated with VSG achieved A1C of 6.0% or lower after 5 years (43). Available data suggest an erosion of diabetes remission over time (44); at least 35–50% of patients who initially achieve remission of diabetes eventually experience recurrence. Still, the median disease-free period among such individuals following RYGB is 8.3 years (94,95), and the majority of those who undergo surgery maintain substantial improvement of glycemia from baseline for at least 5–15 years (43,76,79,80,95–98).
Exceedingly few presurgical predictors of success have been identified, but younger age, shorter duration of diabetes (e.g., <8 years) (70), and lesser severity of diabetes (better glycemic control, nonuse of insulin) are associated with higher rates of diabetes remission (43,79,97,99). Greater baseline visceral fat area may also predict improved postoperative outcomes, especially among Asian American people with type 2 diabetes (100).
Although surgery has been shown to improve the metabolic profiles of people with type 1 diabetes, larger and longer-term studies are needed to determine the role of metabolic surgery in such individuals (101).
Whereas metabolic surgery has greater initial costs than nonsurgical obesity treatments, retrospective analyses and modeling studies suggest that surgery may be cost-effective or even cost-saving for individuals with type 2 diabetes. However, these results largely depend on assumptions about the long-term effectiveness and safety of the procedures (102,103).
Potential Risks and Complications
The safety of metabolic surgery has improved significantly with continued refinement of minimally invasive (laparoscopic) approaches, enhanced training and credentialing, and involvement of multidisciplinary teams. Perioperative mortality rates are typically 0.1–0.5%, similar to those of common abdominal procedures such as cholecystectomy or hysterectomy (104–108). Major complications occur in 2–6% of those undergoing metabolic surgery, which compares favorably with the rates for other commonly performed elective operations (108). Postsurgical recovery times and morbidity have also dramatically declined. Minor complications and need for operative reintervention occur in up to 15% (104–113). Empirical data suggest that the proficiency of the operating surgeon and surgical team is an important factor in determining mortality, complications, reoperations, and readmissions (114). Accordingly, metabolic surgery should be performed in high-volume centers with multidisciplinary teams experienced in managing diabetes, obesity, and gastrointestinal surgery.
Beyond the perioperative period, longer-term risks include vitamin and mineral deficiencies, anemia, osteoporosis, dumping syndrome, and severe hypoglycemia (115). Nutritional and micronutrient deficiencies and related complications occur with a variable frequency depending on the type of procedure and require routine monitoring of micronutrient and nutritional status and lifelong vitamin/nutritional supplementation (115). Dumping syndrome usually occurs shortly (10–30 min) after a meal and may present with diarrhea, nausea, vomiting, palpitations, and fatigue; hypoglycemia is usually not present at the time of symptoms but, in some cases, may develop several hours later.
Postbariatric hypoglycemia (PBH) can occur with RYGB, VSG, and other gastrointestinal procedures and may severely impact quality of life (116–118). PBH is driven in part by altered gastric emptying of ingested nutrients, leading to rapid intestinal glucose absorption and excessive postprandial secretion of glucagon-like peptide 1 and other gastrointestinal peptides. As a result, overstimulation of insulin release and a sharp drop in plasma glucose occur, most commonly 1–3 h after a high-carbohydrate meal. Symptoms range from sweating, tremor, tachycardia, and increased hunger to impaired cognition, loss of consciousness, and seizures. In contrast to dumping syndrome, which often occurs soon after surgery and improves over time, PBH typically presents >1 year post-surgery. Diagnosis is primarily made by a thorough history, detailed records of food intake, physical activity, and symptom patterns, and exclusion of other potential causes (e.g., malnutrition, side effects of medications or supplements, dumping syndrome, and insulinoma). Initial management includes education to facilitate reduced intake of rapidly digested carbohydrates while ensuring adequate intake of protein and healthy fats, and vitamin/nutrient supplements. When available, patients should be offered medical nutrition therapy with a dietitian experienced in PBH and the use of continuous glucose monitoring (ideally real-time continuous glucose monitoring, which can detect dropping glucose levels before severe hypoglycemia occurs), especially for those with hypoglycemia unawareness. Medication treatment, if needed, is primarily aimed at slowing carbohydrate absorption (e.g., acarbose) or reducing glucagon-like peptide 1 and insulin secretion (e.g., diazoxide, octreotide) (119).
People who undergo metabolic surgery may also be at increased risk for substance abuse, worsening or new-onset depression and/or anxiety disorders, and suicidal ideation (115,120–125). Candidates for metabolic surgery should be assessed by a mental health professional with expertise in obesity management prior to consideration for surgery (126). Surgery should be postponed in individuals with alcohol or substance use disorders, severe depression, suicidal ideation, or other significant mental health conditions until these conditions have been sufficiently addressed. Individuals with preoperative or new-onset psychopathology should be assessed regularly following surgery to optimize mental health and postsurgical outcomes.
Footnotes
Disclosure information for each author is available at https://doi.org/10.2337/dc23-SDIS.
Suggested citation: ElSayed NA, Aleppo G, Aroda VR, et al., American Diabetes Association. 8. Obesity and weight management for the prevention and treatment of type 2 diabetes: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1):S128–S139
References
- 1. Narayan KMV, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care 2007;30:1562–1566 [DOI] [PubMed] [Google Scholar]
- 2. Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garvey WT, Ryan DH, Henry R, et al. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care 2014;37:912–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torgerson JS, Hauptman J, Boldrin MN, Sjöström L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 2004;27:155–161 [DOI] [PubMed] [Google Scholar]
- 5. le Roux CW, Astrup A, Fujioka K, et al.; SCALE Obesity Prediabetes NN8022-1839 Study Group . 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017;389:1399–1409 [DOI] [PubMed] [Google Scholar]
- 6. Booth H, Khan O, Prevost T, et al. Incidence of type 2 diabetes after bariatric surgery: population-based matched cohort study. Lancet Diabetes Endocrinol 2014;2:963–968 [DOI] [PubMed] [Google Scholar]
- 7. UKPDS Group . UK Prospective Diabetes Study 7: response of fasting plasma glucose to diet therapy in newly presenting type II diabetic patients. Metabolism 1990;39:905–912 [PubMed] [Google Scholar]
- 8. Goldstein DJ. Beneficial health effects of modest weight loss. Int J Obes Relat Metab Disord 1992;16:397–415 [PubMed] [Google Scholar]
- 9. Pastors JG, Warshaw H, Daly A, Franz M, Kulkarni K. The evidence for the effectiveness of medical nutrition therapy in diabetes management. Diabetes Care 2002;25:608–613 [DOI] [PubMed] [Google Scholar]
- 10. Lim EL, Hollingsworth KG, Aribisala BS, Chen MJ, Mathers JC, Taylor R. Reversal of type 2 diabetes: normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011;54:2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackness C, Karmally W, Febres G, et al. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell function in type 2 diabetic patients. Diabetes 2013;62:3027–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rothberg AE, McEwen LN, Kraftson AT, Fowler CE, Herman WH. Very-low-energy diet for type 2 diabetes: an underutilized therapy? J Diabetes Complications 2014;28:506–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hollander PA, Elbein SC, Hirsch IB, et al. Role of orlistat in the treatment of obese patients with type 2 diabetes. A 1-year randomized double-blind study. Diabetes Care 1998;21:1288–1294 [DOI] [PubMed] [Google Scholar]
- 14. Garvey WT, Ryan DH, Bohannon NJV, et al. Weight-loss therapy in type 2 diabetes: effects of phentermine and topiramate extended release. Diabetes Care 2014;37:3309–3316 [DOI] [PubMed] [Google Scholar]
- 15. O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012;20:1426–1436 [DOI] [PubMed] [Google Scholar]
- 16. Hollander P, Gupta AK, Plodkowski R, et al.; COR-Diabetes Study Group . Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care 2013;36:4022–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Davies MJ, Bergenstal R, Bode B, et al.; NN8022-1922 Study Group . Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA 2015;314:687–699 [DOI] [PubMed] [Google Scholar]
- 18. Rubino F, Nathan DM, Eckel RH, et al.; Delegates of the 2nd Diabetes Surgery Summit . Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Obes Surg 2017;27:2–21 [DOI] [PubMed] [Google Scholar]
- 19. Steven S, Hollingsworth KG, Al-Mrabeh A, et al. Very low-calorie diet and 6 months of weight stability in type 2 diabetes: pathophysiological changes in responders and nonresponders. Diabetes Care 2016;39:808–815 [DOI] [PubMed] [Google Scholar]
- 20. Jensen MD, Ryan DH, Apovian CM, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; Obesity Society . 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol 2014;63(25 Pt B):2985–3023 [DOI] [PubMed] [Google Scholar]
- 21. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet 2018;391:541–551 [DOI] [PubMed] [Google Scholar]
- 22. Lean MEJ, Leslie WS, Barnes AC, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol 2019;7:344–355 [DOI] [PubMed] [Google Scholar]
- 23. Kahan S, Fujioka K. Obesity pharmacotherapy in patients with type 2 diabetes. Diabetes Spectr 2017;30:250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cao P, Song Y, Zhuang Z, et al. Obesity and COVID-19 in adult patients with diabetes. Diabetes 2021;70:1061–1069 [DOI] [PubMed] [Google Scholar]
- 25. Richardson S, Hirsch JS, Narasimhan M, et al.; the Northwell COVID-19 Research Consortium . Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020;323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu Y, Yang J, Shi J, Zhang P, Wang X. Obesity is associated with increased severity of disease in COVID-19 pneumonia: a systematic review and meta-analysis. Eur J Med Res 2020;25:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev 2020;21:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. AMA Manual of Style Committee . AMA Manual of Style: A Guide for Authors and Editors. 11th ed. New York, Oxford University Press, 2020 [Google Scholar]
- 29. American Medical Association . Person-First Language for Obesity H-440.821. Accessed 12 October 2022. Available from https://policysearch.ama-assn.org/policyfinder/detail/obesity?uri=%2FAMADoc%2FHOD.xml-H-440.821.xml
- 30. WHO Expert Consultation . Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163 [DOI] [PubMed] [Google Scholar]
- 31. Araneta MRG, Kanaya A, Hsu WC, et al. Optimum BMI cutpoints to screen Asian Americans for type 2 diabetes. Diabetes Care 2015;38:814–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803 [DOI] [PubMed] [Google Scholar]
- 33. Bosch X, Monclús E, Escoda O, et al. Unintentional weight loss: clinical characteristics and outcomes in a prospective cohort of 2677 patients. PLoS One 2017;12:e0175125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilding JPH. The importance of weight management in type 2 diabetes mellitus. Int J Clin Pract 2014;68:682–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Van Gaal L, Scheen A. Weight management in type 2 diabetes: current and emerging approaches to treatment. Diabetes Care 2015;38:1161–1172 [DOI] [PubMed] [Google Scholar]
- 36. Kushner RF, Batsis JA, Butsch WS, et al. Weight history in clinical practice: the state of the science and future directions. Obesity (Silver Spring) 2020;28:9–17 [DOI] [PubMed] [Google Scholar]
- 37. Warren J, Smalley B, Barefoot N. Higher motivation for weight loss in African American than Caucasian rural patients with hypertension and/or diabetes. Ethn Dis 2016;26:77–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rothberg AE, McEwen LN, Kraftson AT, et al. Impact of weight loss on waist circumference and the components of the metabolic syndrome. BMJ Open Diabetes Res Care 2017;5:e000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wing RR, Bolin P, Brancati FL, et al.; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Look AHEAD Research Group . Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring) 2014;22:5–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gregg EW, Jakicic JM, Blackburn G, et al.; Look AHEAD Research Group . Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol 2016;4:913–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baum A, Scarpa J, Bruzelius E, Tamler R, Basu S, Faghmous J. Targeting weight loss interventions to reduce cardiovascular complications of type 2 diabetes: a machine learning-based post-hoc analysis of heterogeneous treatment effects in the Look AHEAD trial. Lancet Diabetes Endocrinol 2017;5:808–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schauer PR, Bhatt DL, Kirwan JP, et al.; STAMPEDE Investigators . Bariatric surgery versus intensive medical therapy for diabetes—5-year outcomes. N Engl J Med 2017;376:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ikramuddin S, Korner J, Lee WJ, et al. Durability of addition of Roux-en-Y gastric bypass to lifestyle intervention and medical management in achieving primary treatment goals for uncontrolled type 2 diabetes in mild to moderate obesity: a randomized control trial. Diabetes Care 2016;39:1510–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011;377:1341–1352 [DOI] [PubMed] [Google Scholar]
- 46. Davies M, Færch L, Jeppesen OK, et al.; STEP 2 Study Group . Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet 2021;397:971–984 [DOI] [PubMed] [Google Scholar]
- 47. Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wing RR, Bolin P, Brancati FL, et al.; Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 2013;369:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet 2021;398:143–155 [DOI] [PubMed] [Google Scholar]
- 50. Frías JP, Davies MJ, Rosenstock J, et al.; SURPASS-2 Investigators . Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med 2021;385:503–515 [DOI] [PubMed] [Google Scholar]
- 51. Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet 2015;115:1447–1463 [DOI] [PubMed] [Google Scholar]
- 52. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Souza RJ, Bray GA, Carey VJ, et al. Effects of 4 weight-loss diets differing in fat, protein, and carbohydrate on fat mass, lean mass, visceral adipose tissue, and hepatic fat: results from the POUNDS LOST trial. Am J Clin Nutr 2012;95:614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA 2014;312:923–933 [DOI] [PubMed] [Google Scholar]
- 55. Leung CW, Epel ES, Ritchie LD, Crawford PB, Laraia BA. Food insecurity is inversely associated with diet quality of lower-income adults. J Acad Nutr Diet 2014;114:1943–53.e2 [DOI] [PubMed] [Google Scholar]
- 56. Kahan S, Manson JE. Obesity treatment, beyond the guidelines: practical suggestions for clinical practice. JAMA 2019;321:1349–1350 [DOI] [PubMed] [Google Scholar]
- 57. Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW; American College of Sports Medicine . American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009;41:459–471 [DOI] [PubMed] [Google Scholar]
- 58. Gudzune KA, Doshi RS, Mehta AK, et al. Efficacy of commercial weight-loss programs: an updated systematic review. Ann Intern Med 2015;162:501–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bloom B, Mehta AK, Clark JM, Gudzune KA. Guideline-concordant weight-loss programs in an urban area are uncommon and difficult to identify through the internet. Obesity (Silver Spring) 2016;24:583–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity (Silver Spring) 2006;14:1283–1293 [DOI] [PubMed] [Google Scholar]
- 61. Johansson K, Neovius M, Hemmingsson E. Effects of anti-obesity drugs, diet, and exercise on weight-loss maintenance after a very-low-calorie diet or low-calorie diet: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr 2014;99:14–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Batsis JA, Apolzan JW, Bagley PJ, et al. A systematic review of dietary supplements and alternative therapies for weight loss. Obesity (Silver Spring) 2021;29:1102–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bessell E, Maunder A, Lauche R, Adams J, Sainsbury A, Fuller NR. Efficacy of dietary supplements containing isolated organic compounds for weight loss: a systematic review and meta-analysis of randomised placebo-controlled trials. Int J Obes 2021;45:1631–1643 [DOI] [PubMed] [Google Scholar]
- 64. Maunder A, Bessell E, Lauche R, Adams J, Sainsbury A, Fuller NR. Effectiveness of herbal medicines for weight loss: a systematic review and meta-analysis of randomized controlled trials. Diabetes Obes Metab 2020;22:891–903 [DOI] [PubMed] [Google Scholar]
- 65. Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 2020;44:258–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cai X, Yang W, Gao X, Zhou L, Han X, Ji L. Baseline body mass index and the efficacy of hypoglycemic treatment in type 2 diabetes: a meta-analysis. PLoS One 2016;11:e0166625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Domecq JP, Prutsky G, Leppin A, et al. Clinical review: drugs commonly associated with weight change: a systematic review and meta-analysis. J Clin Endocrinol Metab 2015;100:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Drugs.com . Phentermine [FDA prescribing information]. Accessed 17 October 2022. Available from https://www.drugs.com/pro/phentermine.html
- 69. Apovian CM, Aronne LJ, Bessesen DH, et al.; Endocrine Society . Pharmacological management of obesity: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2015;100:342–362 [DOI] [PubMed] [Google Scholar]
- 70. Fujioka K, O’Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring) 2016;24:2278–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fujioka K, Plodkowski R, O’Neil PM, Gilder K, Walsh B, Greenway FL. The relationship between early weight loss and weight loss at 1 year with naltrexone ER/bupropion ER combination therapy. Int J Obes 2016;40:1369–1375 [DOI] [PubMed] [Google Scholar]
- 72. Sullivan S. Endoscopic medical devices for primary obesity treatment in patients with diabetes. Diabetes Spectr 2017;30:258–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kahan S, Saunders KH, Kaplan LM. Combining obesity pharmacotherapy with endoscopic bariatric and metabolic therapies. Techniques and Innovations in Gastrointestinal Endoscopy. 2020;22:154–158 [Google Scholar]
- 74. Greenway FL, Aronne LJ, Raben A, et al. A randomized, double-blind, placebo-controlled study of Gelesis100: a novel nonsystemic oral hydrogel for weight loss. Obesity (Silver Spring) 2019;27:205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. O’Brien R, Johnson E, Haneuse S, et al. Microvascular outcomes in patients with diabetes after bariatric surgery versus usual care: a matched cohort study. Ann Intern Med 2018;169:300–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet 2015;386:964–973 [DOI] [PubMed] [Google Scholar]
- 77. Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg 2014;149:716–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sjöström L, Lindroos AK, Peltonen M, et al.; Swedish Obese Subjects Study Scientific Group . Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 79. Sjöström L, Peltonen M, Jacobson P, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014;311:2297–2304 [DOI] [PubMed] [Google Scholar]
- 80. Adams TD, Davidson LE, Litwin SE, et al. Health benefits of gastric bypass surgery after 6 years. JAMA 2012;308:1122–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sjöström L, Narbro K, Sjöström CD, et al.; Swedish Obese Subjects Study . Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 82. Sjöström L, Gummesson A, Sjöström CD, et al.; Swedish Obese Subjects Study . Effects of bariatric surgery on cancer incidence in obese patients in Sweden (Swedish Obese Subjects Study): a prospective, controlled intervention trial. Lancet Oncol 2009;10:653–662 [DOI] [PubMed] [Google Scholar]
- 83. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012;307:56–65 [DOI] [PubMed] [Google Scholar]
- 84. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med 2007;357:753–761 [DOI] [PubMed] [Google Scholar]
- 85. Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA 2015;313:62–70 [DOI] [PubMed] [Google Scholar]
- 86. Adams TD, Arterburn DE, Nathan DM, Eckel RH. Clinical outcomes of metabolic surgery: microvascular and macrovascular complications. Diabetes Care 2016;39:912–923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sheng B, Truong K, Spitler H, Zhang L, Tong X, Chen L. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg 2017;27:2724–2732 [DOI] [PubMed] [Google Scholar]
- 88. Fisher DP, Johnson E, Haneuse S, et al. Association between bariatric surgery and macrovascular disease outcomes in patients with type 2 diabetes and severe obesity. JAMA 2018;320:1570–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Billeter AT, Scheurlen KM, Probst P, et al. Meta-analysis of metabolic surgery versus medical treatment for microvascular complications in patients with type 2 diabetes mellitus. Br J Surg 2018;105:168–181 [DOI] [PubMed] [Google Scholar]
- 90. Aminian A, Zajichek A, Arterburn DE, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA 2019;322:1271–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Syn NL, Cummings DE, Wang LZ, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174,772 participants. Lancet 2021;397:1830–1841 [DOI] [PubMed] [Google Scholar]
- 92. National Institute of Diabetes and Digestive and Kidney Diseases . Diabetes for Health Professionals. Accessed 17 October 2022. Available from https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes
- 93. Isaman DJM, Rothberg AE, Herman WH. Reconciliation of type 2 diabetes remission rates in studies of Roux-en-Y gastric bypass. Diabetes Care 2016;39:2247–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sjöholm K, Pajunen P, Jacobson P, et al. Incidence and remission of type 2 diabetes in relation to degree of obesity at baseline and 2 year weight change: the Swedish Obese Subjects (SOS) study. Diabetologia 2015;58:1448–1453 [DOI] [PubMed] [Google Scholar]
- 95. Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg 2013;23:93–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Cohen RV, Pinheiro JC, Schiavon CA, Salles JE, Wajchenberg BL, Cummings DE. Effects of gastric bypass surgery in patients with type 2 diabetes and only mild obesity. Diabetes Care 2012;35:1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Brethauer SA, Aminian A, Romero-Talamás H, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg 2013;258:628–636; discussion 636–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hsu CC, Almulaifi A, Chen JC, et al. Effect of bariatric surgery vs medical treatment on type 2 diabetes in patients with body mass index lower than 35: five-year outcomes. JAMA Surg 2015;150:1117–1124 [DOI] [PubMed] [Google Scholar]
- 99. Hariri K, Guevara D, Jayaram A, Kini SU, Herron DM, Fernandez-Ranvier G. Preoperative insulin therapy as a marker for type 2 diabetes remission in obese patients after bariatric surgery. Surg Obes Relat Dis 2018;14:332–337 [DOI] [PubMed] [Google Scholar]
- 100. Yu H, Di J, Bao Y, et al. Visceral fat area as a new predictor of short-term diabetes remission after Roux-en-Y gastric bypass surgery in Chinese patients with a body mass index less than 35 kg/m2. Surg Obes Relat Dis 2015;11:6–11 [DOI] [PubMed] [Google Scholar]
- 101. Kirwan JP, Aminian A, Kashyap SR, Burguera B, Brethauer SA, Schauer PR. Bariatric surgery in obese patients with type 1 diabetes. Diabetes Care 2016;39:941–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rubin JK, Hinrichs-Krapels S, Hesketh R, Martin A, Herman WH, Rubino F. Identifying barriers to appropriate use of metabolic/bariatric surgery for type 2 diabetes treatment: policy lab results. Diabetes Care 2016;39:954–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Fouse T, Schauer P. The socioeconomic impact of morbid obesity and factors affecting access to obesity surgery. Surg Clin North Am 2016;96:669–679 [DOI] [PubMed] [Google Scholar]
- 104. Flum DR, Belle SH, King WC, et al.; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium . Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med 2009;361:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Courcoulas AP, Christian NJ, Belle SH, et al.; Longitudinal Assessment of Bariatric Surgery (LABS) Consortium . Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013;310:2416–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Arterburn DE, Courcoulas AP. Bariatric surgery for obesity and metabolic conditions in adults. BMJ 2014;349:g3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Young MT, Gebhart A, Phelan MJ, Nguyen NT. Use and outcomes of laparoscopic sleeve gastrectomy vs laparoscopic gastric bypass: analysis of the American College of Surgeons NSQIP. J Am Coll Surg 2015;220:880–885 [DOI] [PubMed] [Google Scholar]
- 108. Aminian A, Brethauer SA, Kirwan JP, Kashyap SR, Burguera B, Schauer PR. How safe is metabolic/diabetes surgery? Diabetes Obes Metab 2015;17:198–201 [DOI] [PubMed] [Google Scholar]
- 109. Birkmeyer NJO, Dimick JB, Share D, et al.; Michigan Bariatric Surgery Collaborative . Hospital complication rates with bariatric surgery in Michigan. JAMA 2010;304:435–442 [DOI] [PubMed] [Google Scholar]
- 110. Altieri MS, Yang J, Telem DA, et al. Lap band outcomes from 19,221 patients across centers and over a decade within the state of New York. Surg Endosc 2016;30:1725–1732 [DOI] [PubMed] [Google Scholar]
- 111. Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg 2011;254:410–420; discussion 420–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nguyen NT, Slone JA, Nguyen XMT, Hartman JS, Hoyt DB. A prospective randomized trial of laparoscopic gastric bypass versus laparoscopic adjustable gastric banding for the treatment of morbid obesity: outcomes, quality of life, and costs. Ann Surg 2009;250:631–641 [DOI] [PubMed] [Google Scholar]
- 113. Courcoulas AP, King WC, Belle SH, et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) study. JAMA Surg 2018;153:427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Birkmeyer JD, Finks JF, O’Reilly A, et al.; Michigan Bariatric Surgery Collaborative . Surgical skill and complication rates after bariatric surgery. N Engl J Med 2013;369:1434–1442 [DOI] [PubMed] [Google Scholar]
- 115. Mechanick JI, Apovian C, Brethauer S, et al. Clinical practice guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures—2019 update: cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, the Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists—executive summary. Endocr Pract 2019;25:1346–1359 [DOI] [PubMed] [Google Scholar]
- 116. Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med 2005;353:249–254 [DOI] [PubMed] [Google Scholar]
- 117. Sheehan A, Patti ME. Hypoglycemia after upper gastrointestinal surgery: clinical approach to assessment, diagnosis, and treatment. Diabetes Metab Syndr Obes 2020;13:4469–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lee D, Dreyfuss JM, Sheehan A, Puleio A, Mulla CM, Patti ME. Glycemic patterns are distinct in post-bariatric hypoglycemia after gastric bypass (PBH-RYGB). J Clin Endocrinol Metab 2021;106:2291–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia after gastric bypass surgery: current concepts and controversies. J Clin Endocrinol Metab 2018;103:2815–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Conason A, Teixeira J, Hsu CH, Puma L, Knafo D, Geliebter A. Substance use following bariatric weight loss surgery. JAMA Surg 2013;148:145–150 [DOI] [PubMed] [Google Scholar]
- 121. Bhatti JA, Nathens AB, Thiruchelvam D, Grantcharov T, Goldstein BI, Redelmeier DA. Self-harm emergencies after bariatric surgery: a population-based cohort study. JAMA Surg 2016;151:226–232 [DOI] [PubMed] [Google Scholar]
- 122. Peterhänsel C, Petroff D, Klinitzke G, Kersting A, Wagner B. Risk of completed suicide after bariatric surgery: a systematic review. Obes Rev 2013;14:369–382 [DOI] [PubMed] [Google Scholar]
- 123. Jakobsen GS, Småstuen MC, Sandbu R, et al. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA 2018;319:291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. King WC, Chen JY, Mitchell JE, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA 2012;307:2516–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Young-Hyman D, Peyrot M. Psychosocial Care for People with Diabetes. 1st ed. Alexandria, VA, American Diabetes Association, 2012 [Google Scholar]
- 126. Greenberg I, Sogg S, M Perna F. Behavioral and psychological care in weight loss surgery: best practice update. Obesity (Silver Spring) 2009;17:880–884 [DOI] [PubMed] [Google Scholar]
- 127. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. IBM . Micromedex Red Book. Accessed 9 November 2022. Available from https://www.ibm.com/products/micromedex-red-book
- 129. Data.Medicaid.gov . NADAC (National Average Drug Acquisition Cost). Accessed 4 October 2022. Available from https://data.medicaid.gov/dataset/dfa2ab14-06c2-457a-9e36-5cb6d80f8d93
- 130. U.S. National Library of Medicine . Phentermine–phentermine hydrochloride capsule. Accessed 17 October 2022. Available from https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=737eef3b-9a6b-4ab3-a25c-49d84d2a0197
- 131. Nalpropion Pharmaceuticals . Contrave (naltrexone HCl/bupropion HCl) extended-release tablets. Accessed 17 October 2022. Available at https://contrave.com
- 132. CHEPLAPHARM and H2-Pharma . Xenical (orlistat). Accessed 17 October 2022. Available from https://xenical.com
- 133. Vivus . Qsymia (phentermine and topiramate extended-release) capsules. Accessed 17 October 2022. Available from https://qsymia.com
- 134. Novo Nordisk . Saxenda (liraglutide injection 3 mg). Accessed 17 October 2022. Available from https://www.saxenda.com
- 135. Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013;21:2163–2171 [DOI] [PubMed] [Google Scholar]