Abstract
The American Diabetes Association (ADA) “Standards of Care in Diabetes” includes the ADA’s current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee, are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA’s clinical practice recommendations and a full list of Professional Practice Committee members, please refer to Introduction and Methodology. Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC.
Assessment of Glycemic Control
Glycemic control is assessed by the A1C measurement, continuous glucose monitoring (CGM) using time in range (TIR) and/or glucose management indicator (GMI), and blood glucose monitoring (BGM). A1C is the metric used to date in clinical trials demonstrating the benefits of improved glycemic control. Individual glucose monitoring (discussed in detail in Section 7, “Diabetes Technology”) is a useful tool for diabetes self-management, which includes meals, physical activity, and medication adjustment, particularly in individuals taking insulin. CGM serves an increasingly important role in the management of the effectiveness and safety of treatment in many people with type 1 diabetes and in selected people with type 2 diabetes. Individuals on a variety of insulin treatment plans can benefit from CGM with improved glucose control, decreased hypoglycemia, and enhanced self-efficacy (Section 7, “Diabetes Technology”) (1).
Glycemic Assessment
Recommendations
6.1 Assess glycemic status (A1C or other glycemic measurement such as time in range or glucose management indicator) at least two times a year in patients who are meeting treatment goals (and who have stable glycemic control). E
6.2 Assess glycemic status at least quarterly and as needed in patients whose therapy has recently changed and/or who are not meeting glycemic goals. E
A1C reflects average glycemia over approximately 3 months. The performance of the test is generally excellent for National Glycohemoglobin Standardization Program (NGSP)-certified assays (ngsp.org). The test is the primary tool for assessing glycemic control and has a strong predictive value for diabetes complications (2–4). Thus, A1C testing should be performed routinely in all people with diabetes at initial assessment and as part of continuing care. Measurement approximately every 3 months determines whether patients’ glycemic targets have been reached and maintained. A 14-day CGM assessment of TIR and GMI can serve as a surrogate for A1C for use in clinical management (5–9). The frequency of A1C testing should depend on the clinical situation, the treatment plan, and the clinician’s judgment. The use of point-of-care A1C testing or CGM-derived TIR and GMI may provide an opportunity for more timely treatment changes during encounters between patients and health care professionals. People with type 2 diabetes with stable glycemia well within target may do well with A1C testing or other glucose assessment only twice per year. Unstable or intensively managed patients or people not at goal with treatment adjustments may require testing more frequently (every 3 months with interim assessments as needed for safety) (10). CGM parameters can be tracked in the clinic or via telehealth to optimize diabetes management.
A1C Limitations
The A1C test is an indirect measure of average glycemia and, as such, is subject to limitations. As with any laboratory test, there is variability in the measurement of A1C. Although A1C variability is lower on an intraindividual basis than that of blood glucose measurements, clinicians should exercise judgment when using A1C as the sole basis for assessing glycemic control, particularly if the result is close to the threshold that might prompt a change in medication therapy. For example, conditions that affect red blood cell turnover (hemolytic and other anemias, glucose-6-phosphate dehydrogenase deficiency, recent blood transfusion, use of drugs that stimulate erythropoiesis, end-stage kidney disease, and pregnancy) may result in discrepancies between the A1C result and the patient’s true mean glycemia (11). Hemoglobin variants must be considered, particularly when the A1C result does not correlate with the patient’s CGM or BGM levels. However, most assays in use in the U.S. are accurate in individuals who are heterozygous for the most common variants (ngsp.org/interf.asp). Other measures of average glycemia such as fructosamine and 1,5-anhydroglucitol are available, but their translation into average glucose levels and their prognostic significance are not as clear as for A1C and CGM. Though some variability in the relationship between average glucose levels and A1C exists among different individuals, in general the association between mean glucose and A1C within an individual correlates over time (12).
A1C does not provide a measure of glycemic variability or hypoglycemia. For patients prone to glycemic variability, especially people with type 1 diabetes or type 2 diabetes with severe insulin deficiency, glycemic control is best evaluated by the combination of results from BGM/CGM and A1C. Discordant results between BGM/CGM and A1C can be the result of the conditions outlined above or glycemic variability, with BGM missing the extremes.
Correlation Between BGM and A1C
Table 6.1 shows the correlation between A1C levels and mean glucose levels based on the international A1C-Derived Average Glucose (ADAG) study, which assessed the correlation between A1C and frequent BGM and CGM in 507 adults (83% non-Hispanic White) with type 1, type 2, and no diabetes (13), and an empirical study of the average blood glucose levels at premeal, postmeal, and bedtime associated with specified A1C levels using data from the ADAG trial (14). The American Diabetes Association (ADA) and the American Association for Clinical Chemistry have determined that the correlation (r = 0.92) in the ADAG trial is strong enough to justify reporting both the A1C result and the estimated average glucose (eAG) result when a clinician orders the A1C test. Clinicians should note that the mean plasma glucose numbers in Table 6.1 are based on ∼2,700 readings per A1C measurement in the ADAG trial. In a report, mean glucose measured with CGM versus central laboratory–measured A1C in 387 participants in three randomized trials demonstrated that A1C may underestimate or overestimate mean glucose in individuals (12). Thus, as suggested, a patient’s BGM or CGM profile has considerable potential for optimizing their glycemic management (13).
Table 6.1.
Estimated average glucose (eAG)
| A1C (%) | mg/dL* | mmol/L |
|---|---|---|
| 5 | 97 (76–120) | 5.4 (4.2–6.7) |
| 6 | 126 (100–152) | 7.0 (5.5–8.5) |
| 7 | 154 (123–185) | 8.6 (6.8–10.3) |
| 8 | 183 (147–217) | 10.2 (8.1–12.1) |
| 9 | 212 (170–249) | 11.8 (9.4–13.9) |
| 10 | 240 (193–282) | 13.4 (10.7–15.7) |
| 11 | 269 (217–314) | 14.9 (12.0–17.5) |
| 12 | 298 (240–347) | 16.5 (13.3–19.3) |
Data in parentheses are 95% CI. A calculator for converting A1C results into eAG, in either mg/dL or mmol/L, is available at professional.diabetes.org/eAG.
A1C Differences in Ethnic Populations and Children
In the ADAG study, there were no significant differences among racial and ethnic groups in the regression lines between A1C and mean glucose, although the study was underpowered to detect a difference and there was a trend toward a difference between the African and African American and the non-Hispanic White cohorts, with higher A1C values observed in the African and African American cohorts compared with non-Hispanic White cohorts for a given mean glucose. Other studies have also demonstrated higher A1C levels in African American participants than in White participants at a given mean glucose concentration (15,16). In contrast, a recent report in Afro-Caribbean individuals found lower A1C relative to glucose values (17). Taken together, A1C and glucose parameters are essential for the optimal assessment of glycemic status.
A1C assays are available that do not demonstrate a statistically significant difference in individuals with hemoglobin variants. Other assays have statistically significant interference, but the difference is not clinically significant. Use of an assay with such statistically significant interference may explain a report that for any level of mean glycemia, African American individuals heterozygous for the common hemoglobin variant HbS had lower A1C by about 0.3 percentage points when compared with those without the trait (18,19). Another genetic variant, X-linked glucose-6-phosphate dehydrogenase G202A, carried by 11% of African American individuals, was associated with a decrease in A1C of about 0.8% in hemizygous men and 0.7% in homozygous women compared with those without the trait (20).
A small study comparing A1C to CGM data in children with type 1 diabetes found a highly statistically significant correlation between A1C and mean blood glucose, although the correlation (r = 0.7) was significantly lower than that in the ADAG trial (21). Whether there are clinically meaningful differences in how A1C relates to average glucose in children or in different ethnicities is an area for further study (15,22,23). Until further evidence is available, it seems prudent to establish A1C goals in these populations with consideration of individualized CGM, BGM, and A1C results. Limitations in perfect alignment between glycemic measurements do not interfere with the usefulness of BGM/CGM for insulin dose adjustments.
Glucose Assessment by Continuous Glucose Monitoring
Recommendations
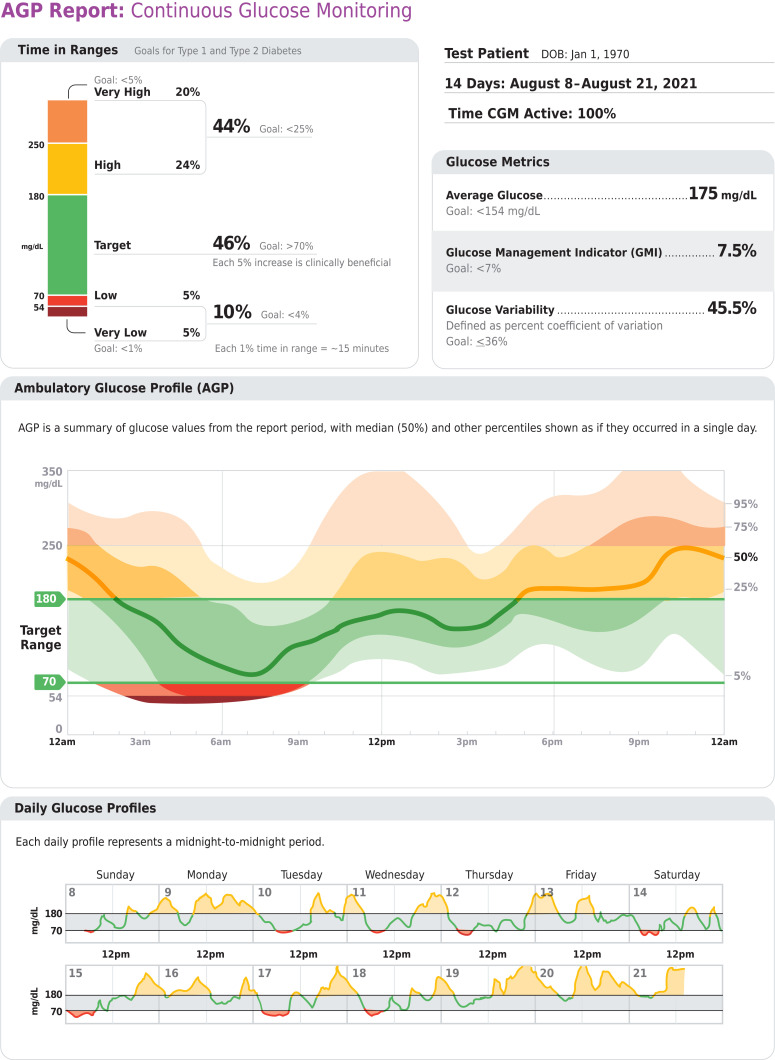
6.3 Standardized, single-page glucose reports from continuous glucose monitoring (CGM) devices with visual cues, such as the ambulatory glucose profile, should be considered as a standard summary for all CGM devices. E
6.4 Time in range is associated with the risk of microvascular complications and can be used for assessment of glycemic control. Additionally, time below range and time above range are useful parameters for the evaluation of the treatment plan (Table 6.2). C
Table 6.2.
Standardized CGM metrics for clinical care
| 1. Number of days CGM device is worn (recommend 14 days) | |
| 2. Percentage of time CGM device is active (recommend 70% of data from 14 days) | |
| 3. Mean glucose | |
| 4. Glucose management indicator | |
| 5. Glycemic variability (%CV) target ≤36%* | |
| 6. TAR: % of readings and time >250 mg/dL (>13.9 mmol/L) | Level 2 hyperglycemia |
| 7. TAR: % of readings and time 181–250 mg/dL (10.1–13.9 mmol/L) | Level 1 hyperglycemia |
| 8. TIR: % of readings and time 70–180 mg/dL (3.9–10.0 mmol/L) | In range |
| 9. TBR: % of readings and time 54–69 mg/dL (3.0–3.8 mmol/L) | Level 1 hypoglycemia |
| 10. TBR: % of readings and time <54 mg/dL (<3.0 mmol/L) | Level 2 hypoglycemia |
CGM, continuous glucose monitoring; CV, coefficient of variation; TAR, time above range; TBR, time below range; TIR, time in range.
Some studies suggest that lower %CV targets (<33%) provide additional protection against hypoglycemia for those receiving insulin or sulfonylureas. Adapted from Battelino et al. (35).
CGM is rapidly improving diabetes management. As stated in the recommendations, time in range (TIR) is a useful metric of glycemic control and glucose patterns, and it correlates well with A1C in most studies (24–29). New data support the premise that increased TIR correlates with the risk of complications. The studies supporting this assertion are reviewed in more detail in Section 7, “Diabetes Technology”; they include cross-sectional data and cohort studies (30–32) demonstrating TIR as an acceptable end point for clinical trials moving forward and that it can be used for assessment of glycemic control. Additionally, time below range (<70 and <54 mg/dL [3.9 and 3.0 mmol/L]) and time above range (>180 mg/dL [10.0 mmol/L]) are useful parameters for insulin dose adjustments and reevaluation of the treatment plan.
For many people with diabetes, glucose monitoring is key for achieving glycemic targets. Major clinical trials of insulin-treated patients have included BGM as part of multifactorial interventions to demonstrate the benefit of intensive glycemic control on diabetes complications (33). BGM is thus an integral component of effective therapy of patients taking insulin. In recent years, CGM has become a standard method for glucose monitoring for most adults with type 1 diabetes (34). Both approaches to glucose monitoring allow patients to evaluate individual responses to therapy and assess whether glycemic targets are being safely achieved. The international consensus on TIR provides guidance on standardized CGM metrics (Table 6.2) and considerations for clinical interpretation and care (35). To make these metrics more actionable, standardized reports with visual cues, such as the ambulatory glucose profile (Fig 6.1), are recommended (35) and may help the patient and the health care professional better interpret the data to guide treatment decisions (24,27). BGM and CGM can be useful to guide medical nutrition therapy and physical activity, prevent hypoglycemia, and aid medication management. While A1C is currently the primary measure to guide glucose management and a valuable risk marker for developing diabetes complications, the CGM metrics TIR (with time below range and time above range) and GMI provide the insights for a more personalized diabetes management plan. The incorporation of these metrics into clinical practice is in evolution, and remote access to these data can be critical for telehealth. A rapid optimization and harmonization of CGM terminology and remote access is occurring to meet patient and health care professional needs (36–38). The patient’s specific needs and goals should dictate BGM frequency and timing and consideration of CGM use. Please refer to Section 7, “Diabetes Technology,” for a more complete discussion of the use of BGM and CGM.
Figure 6.1.
Key points included in standard ambulatory glucose profile (AGP) report. Reprinted from Holt et al. (34).
With the advent of new technology, CGM has evolved rapidly in both accuracy and affordability. As such, many patients have these data available to assist with self-management and their health care professionals’ assessment of glycemic status. Reports can be generated from CGM that will allow the health care professional and person with diabetes to determine TIR, calculate GMI, and assess hypoglycemia, hyperglycemia, and glycemic variability. As discussed in a recent consensus document, a report formatted as shown in Fig. 6.1 can be generated (35). Published data from two retrospective studies suggest a strong correlation between TIR and A1C, with a goal of 70% TIR aligning with an A1C of ∼7% (8,26). Note the goals of therapy next to each metric in Fig. 6.1 (e.g., low, <4%; very low, <1%) as values to guide changes in therapy.
Glycemic Goals
For glycemic goals in older adults, please refer to Section 13, “Older Adults.” For glycemic goals in children, please refer to Section 14, “Children and Adolescents.” For glycemic goals during pregnancy, please refer to Section 15, “Management of Diabetes in Pregnancy.” Overall, regardless of the population being served, it is critical for the glycemic targets to be woven into the overall person-centered strategy. For example, in a very young child, safety and simplicity may outweigh the need for glycemic stability in the short run. Simplification may decrease parental anxiety and build trust and confidence, which could support further strengthening of glycemic targets and self-efficacy. In healthy older adults, there is no empiric need to loosen control; however, less stringent A1C goals may be appropriate for patients with limited life expectancy or where the harms of treatment are greater than the benefits (39,40).
However, the health care professional needs to work with an individual and should consider adjusting targets for simplifying the treatment plan if this change is needed to improve safety and medication-taking behavior. Setting goals by face-to-face or remote consultations has been shown to be more effective than usual care for glycemic control in type 2 diabetes for fasting plasma glucose and glycated hemoglobin (41).
Recommendations
6.5a An A1C goal for many nonpregnant adults of <7% (53 mmol/mol) without significant hypoglycemia is appropriate. A
6.5b If using ambulatory glucose profile/glucose management indicator to assess glycemia, a parallel goal for many nonpregnant adults is time in range of >70% with time below range <4% and time <54 mg/dL <1%. For those with frailty or at high risk of hypoglycemia, a target of >50% time in range with <1% time below range is recommended. (See Fig. 6.1 and Table 6.2.) B
6.6 On the basis of health care professional judgment and patient preference, achievement of lower A1C levels than the goal of 7% may be acceptable and even beneficial if it can be achieved safely without significant hypoglycemia or other adverse effects of treatment. B
6.7 Less stringent A1C goals (such as <8% [64 mmol/mol]) may be appropriate for patients with limited life expectancy or where the harms of treatment are greater than the benefits. Health care professionals should consider deintensification of therapy if appropriate to reduce the risk of hypoglycemia in patients with inappropriate stringent A1C targets. B
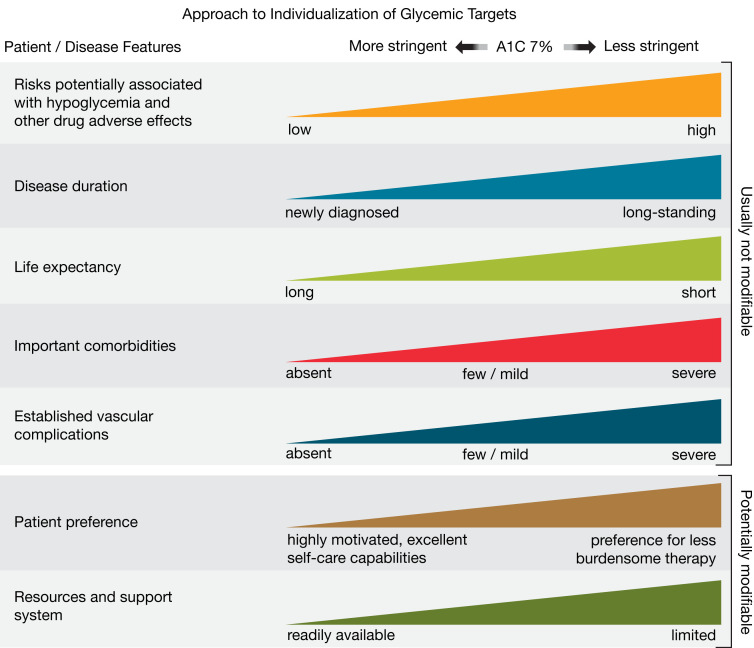
6.8 Reassess glycemic targets based on the individualized criteria in Fig. 6.2. E
6.9 Setting a glycemic goal during consultations is likely to improve patient outcomes. E
Figure 6.2.
Patient and disease factors used to determine optimal glycemic targets. Characteristics and predicaments toward the left justify more stringent efforts to lower A1C; those toward the right suggest less stringent efforts. A1C 7% = 53 mmol/mol. Adapted with permission from Inzucchi et al. (71).
A1C and Microvascular Complications
Hyperglycemia defines diabetes, and glycemic control is fundamental to diabetes management. The Diabetes Control and Complications Trial (DCCT) (33), a prospective randomized controlled trial of intensive (mean A1C about 7% [53 mmol/mol]) versus standard (mean A1C about 9% [75 mmol/mol]) glycemic control in people with type 1 diabetes, showed definitively that better glycemic control is associated with 50–76% reductions in rates of development and progression of microvascular (retinopathy, neuropathy, and diabetic kidney disease) complications. Follow-up of the DCCT cohorts in the Epidemiology of Diabetes Interventions and Complications (EDIC) study (42,43) demonstrated persistence of these microvascular benefits over two decades despite the fact that the glycemic separation between the treatment groups diminished and disappeared during follow-up.
The Kumamoto Study (44) and UK Prospective Diabetes Study (UKPDS) (45,46) confirmed that intensive glycemic control significantly decreased rates of microvascular complications in people with short-duration type 2 diabetes. Long-term follow-up of the UKPDS cohorts showed enduring effects of early glycemic control on most microvascular complications (47).
Therefore, achieving A1C targets of <7% (53 mmol/mol) has been shown to reduce microvascular complications of type 1 and type 2 diabetes when instituted early in the course of disease (2,48). Findings from the DCCT (33) and UKPDS (49) studies demonstrate a curvilinear relationship between A1C and microvascular complications. Such analyses suggest that, on a population level, the greatest number of complications will be averted by taking patients from very poor control to fair/good control. These analyses also suggest that further lowering of A1C from 7 to 6% (53 mmol/mol to 42 mmol/mol) is associated with further reduction in the risk of microvascular complications, although the absolute risk reductions become much smaller. The implication of these findings is that there is no need to deintensify therapy for an individual with an A1C between 6 and 7% in the setting of low hypoglycemia risk with a long life expectancy. There are now newer agents that do not cause hypoglycemia, making it possible to maintain glucose control without the risk of hypoglycemia (see Section 9, “Pharmacologic Approaches to Glycemic Treatment”).
Given the substantially increased risk of hypoglycemia in type 1 diabetes and with polypharmacy in type 2 diabetes, the risks of lower glycemic targets may outweigh the potential benefits on microvascular complications. Three landmark trials (Action to Control Cardiovascular Risk in Diabetes [ACCORD], Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation [ADVANCE], and Veterans Affairs Diabetes Trial [VADT]) were conducted to test the effects of near normalization of blood glucose on cardiovascular outcomes in individuals with long-standing type 2 diabetes and either known cardiovascular disease (CVD) or high cardiovascular risk. These trials showed that lower A1C levels were associated with reduced onset or progression of some microvascular complications (50–52).
The concerning mortality findings in the ACCORD trial discussed below and the relatively intense efforts required to achieve near euglycemia should also be considered when setting glycemic targets for individuals with long-standing diabetes, such as those populations studied in ACCORD, ADVANCE, and VADT. Findings from these studies suggest caution is needed in treating diabetes to near-normal A1C goals in people with long-standing type 2 diabetes with or at significant risk of CVD.
These landmark studies need to be considered with an important caveat; glucagon-like peptide 1 (GLP-1) receptor agonists and sodium–glucose cotransporter 2 (SGLT2) inhibitors were not approved at the time of these trials. As such, these agents with established cardiovascular and renal benefits appear to be safe and beneficial in this group of individuals at high risk for cardiorenal complications. Randomized clinical trials examining these agents for cardiovascular safety were not designed to test higher versus lower A1C; therefore, beyond post hoc analysis of these trials, we do not have evidence that it is the glucose lowering by these agents that confers the CVD and renal benefit (53). As such, based on clinician judgment and patient preferences, select patients, especially those with little comorbidity and a long life expectancy, may benefit from adopting more intensive glycemic targets if they can achieve them safely and without hypoglycemia or significant therapeutic burden.
A1C and Cardiovascular Disease Outcomes
Cardiovascular Disease and Type 1 Diabetes
CVD is a more common cause of death than microvascular complications in populations with diabetes. There is evidence for a cardiovascular benefit of intensive glycemic control after long-term follow-up of cohorts treated early in the course of type 1 diabetes. In the DCCT, there was a trend toward lower risk of CVD events with intensive control. In the 9-year post-DCCT follow-up of the EDIC cohort, participants previously randomized to the intensive arm had a significant 57% reduction in the risk of nonfatal myocardial infarction (MI), stroke, or cardiovascular death compared with those previously randomized to the standard arm (54). The benefit of intensive glycemic control in this cohort with type 1 diabetes has been shown to persist for several decades (55) and to be associated with a modest reduction in all-cause mortality (56).
Cardiovascular Disease and Type 2 Diabetes
In type 2 diabetes, there is evidence that more intensive treatment of glycemia in newly diagnosed patients may reduce long-term CVD rates. In addition, data from the Swedish National Diabetes Registry (56) and the Joint Asia Diabetes Evaluation (JADE) demonstrate greater proportions of people with diabetes being diagnosed at <40 years of age and a demonstrably increased burden of heart disease and years of life lost in people diagnosed at a younger age (57–60). Thus, to prevent both microvascular and macrovascular complications of diabetes, there is a major call to overcome therapeutic inertia and treat to target for an individual patient (60,61). During the UKPDS, there was a 16% reduction in CVD events (combined fatal or nonfatal MI and sudden death) in the intensive glycemic control arm that did not reach statistical significance (P = 0.052), and there was no suggestion of benefit on other CVD outcomes (e.g., stroke). Similar to the DCCT/EDIC, after 10 years of observational follow-up, those originally randomized to intensive glycemic control had significant long-term reductions in MI (15% with sulfonylurea or insulin as initial pharmacotherapy, 33% with metformin as initial pharmacotherapy) and in all-cause mortality (13% and 27%, respectively) (47).
ACCORD, ADVANCE, and VADT suggested no significant reduction in CVD outcomes with intensive glycemic control in participants followed for shorter durations (3.5–5.6 years) and who had more advanced type 2 diabetes and CVD risk than the UKPDS participants. All three trials were conducted in relatively older participants with a longer known duration of diabetes (mean duration 8–11 years) and either CVD or multiple cardiovascular risk factors. The target A1C among intensive-control participants was <6% (42 mmol/mol) in ACCORD, <6.5% (48 mmol/mol) in ADVANCE, and a 1.5% reduction in A1C compared with control participants in VADT, with achieved A1C of 6.4% vs. 7.5% (46 mmol/mol vs. 58 mmol/mol) in ACCORD, 6.5% vs. 7.3% (48 mmol/mol vs. 56 mmol/mol) in ADVANCE, and 6.9% vs. 8.4% (52 mmol/mol vs. 68 mmol/mol) in VADT. Details of these studies are reviewed extensively in the joint ADA position statement “Intensive Glycemic Control and the Prevention of Cardiovascular Events: Implications of the ACCORD, ADVANCE, and VA Diabetes Trials” (61).
The glycemic control comparison in ACCORD was halted early due to an increased mortality rate in the intensive compared with the standard treatment arm (1.41% vs. 1.14% per year; hazard ratio 1.22 [95% CI 1.01–1.46]), with a similar increase in cardiovascular deaths. Analysis of the ACCORD data did not identify a clear explanation for the excess mortality in the intensive treatment arm (62).
Longer-term follow-up has shown no evidence of cardiovascular benefit, or harm, in the ADVANCE trial (63). The end-stage renal disease rate was lower in the intensive treatment group over follow-up. However, 10-year follow-up of the VADT cohort (64) did demonstrate a reduction in the risk of cardiovascular events (52.7 [control group] vs. 44.1 [intervention group] events per 1,000 person-years) with no benefit in cardiovascular or overall mortality. Heterogeneity of mortality effects across studies was noted, which may reflect differences in glycemic targets, therapeutic approaches, and, importantly, population characteristics (65).
Mortality findings in ACCORD (62) and subgroup analyses of VADT (66) suggest that the potential risks of intensive glycemic control may outweigh its benefits in higher-risk individuals. In all three trials, severe hypoglycemia was significantly more likely in participants who were randomly assigned to the intensive glycemic control arm. Individuals with a long duration of diabetes, a known history of hypoglycemia, advanced atherosclerosis, or advanced age/frailty may benefit from less aggressive targets (67,68).
As discussed further below, severe hypoglycemia is a potent marker of high absolute risk of cardiovascular events and mortality (69). Therefore, health care professionals should be vigilant in preventing hypoglycemia and should not aggressively attempt to achieve near-normal A1C levels in people in whom such targets cannot be safely and reasonably achieved. As discussed in Section 9, “Pharmacologic Approaches to Glycemic Treatment,” addition of specific SGLT2 inhibitors or GLP-1 receptor agonists that have demonstrated CVD benefit is recommended in patients with established CVD, chronic kidney disease, and heart failure. As outlined in more detail in Section 9, “Pharmacologic Approaches to Glycemic Treatment,” and Section 10, “Cardiovascular Disease and Risk Management,” the cardiovascular benefits of SGLT2 inhibitors or GLP-1 receptor agonists are not contingent upon A1C lowering; therefore, initiation can be considered in people with type 2 diabetes and CVD independent of the current A1C or A1C goal or metformin therapy. Based on these considerations, the following two strategies are offered (70):
If already on dual therapy or multiple glucose-lowering therapies and not on an SGLT2 inhibitor or GLP-1 receptor agonist, consider switching to one of these agents with proven cardiovascular benefit.
Introduce SGLT2 inhibitors or GLP-1 receptor agonists in people with CVD at A1C goal (independent of metformin) for cardiovascular benefit, independent of baseline A1C or individualized A1C target.
Setting and Modifying A1C Goals
Numerous factors must be considered when setting glycemic targets. The ADA proposes general targets appropriate for many people but emphasizes the importance of individualization based on key patient characteristics. Glycemic targets must be individualized in the context of shared decision-making to address individual needs and preferences and consider characteristics that influence risks and benefits of therapy; this approach may optimize engagement and self-efficacy.
The factors to consider in individualizing goals are depicted in Fig. 6.2. This figure is not designed to be applied rigidly but to be used as a broad construct to guide clinical decision-making (71) and engage people with type 1 and type 2 diabetes in shared decision-making. More aggressive targets may be recommended if they can be achieved safely and with an acceptable burden of therapy and if life expectancy is sufficient to reap the benefits of stringent targets. Less stringent targets (A1C up to 8% [64 mmol/mol]) may be recommended if the patient’s life expectancy is such that the benefits of an intensive goal may not be realized, or if the risks and burdens outweigh the potential benefits. Severe or frequent hypoglycemia is an absolute indication for the modification of treatment plans, including setting higher glycemic goals.
Diabetes is a chronic disease that progresses over decades. Thus, a goal that might be appropriate for an individual early in the course of their diabetes may change over time. Newly diagnosed patients and/or those without comorbidities that limit life expectancy may benefit from intensive control proven to prevent microvascular complications. Both DCCT/EDIC and UKPDS demonstrated metabolic memory, or a legacy effect, in which a finite period of intensive control yielded benefits that extended for decades after that control ended. Thus, a finite period of intensive control to near-normal A1C may yield enduring benefits even if control is subsequently deintensified as patient characteristics change. Over time, comorbidities may emerge, decreasing life expectancy and thereby decreasing the potential to reap benefits from intensive control. Also, with longer disease duration, diabetes may become more difficult to control, with increasing risks and burdens of therapy. Thus, A1C targets should be reevaluated over time to balance the risks and benefits as patient factors change.
Recommended glycemic targets for many nonpregnant adults are shown in Table 6.3. The recommendations include blood glucose levels that appear to correlate with achievement of an A1C of <7% (53 mmol/mol). Pregnancy recommendations are discussed in more detail in Section 15, “Management of Diabetes in Pregnancy.”
Table 6.3.
Summary of glycemic recommendations for many nonpregnant adults with diabetes
| A1C | <7.0% (53 mmol/mol)*# |
| Preprandial capillary plasma glucose | 80–130 mg/dL* (4.4–7.2 mmol/L) |
| Peak postprandial capillary plasma glucose† | <180 mg/dL* (10.0 mmol/L) |
More or less stringent glycemic goals may be appropriate for individual patients.
CGM may be used to assess glycemic target as noted in Recommendation 6.5b and Fig. 6.1. Goals should be individualized based on duration of diabetes, age/life expectancy, comorbid conditions, known CVD or advanced microvascular complications, hypoglycemia unawareness, and individual patient considerations (as per Fig. 6.2).
Postprandial glucose may be targeted if A1C goals are not met despite reaching preprandial glucose goals. Postprandial glucose measurements should be made 1–2 h after the beginning of the meal, generally peak levels in people with diabetes.
The issue of preprandial versus postprandial BGM targets is complex (72,73). Elevated postchallenge (2-h oral glucose tolerance test) glucose values have been associated with increased cardiovascular risk independent of fasting plasma glucose in some epidemiologic studies, whereas intervention trials have not shown postprandial glucose to be a cardiovascular risk factor independent of A1C. In people with diabetes, surrogate measures of vascular pathology, such as endothelial dysfunction, are negatively affected by postprandial hyperglycemia. It is clear that postprandial hyperglycemia, like preprandial hyperglycemia, contributes to elevated A1C levels, with its relative contribution being greater at A1C levels that are closer to 7% (53 mmol/mol). However, outcome studies have shown A1C to be the primary predictor of complications, and landmark trials of glycemic control such as the DCCT and UKPDS relied overwhelmingly on preprandial BGM. Additionally, a randomized controlled trial in patients with known CVD found no CVD benefit of insulin treatment plans targeting postprandial glucose compared with those targeting preprandial glucose (73). Therefore, it is reasonable to check postprandial glucose in individuals who have premeal glucose values within target but A1C values above target. In addition, when intensifying insulin therapy, measuring postprandial plasma glucose 1–2 h after the start of a meal (using BGM or CGM) and using treatments aimed at reducing postprandial plasma glucose values to <180 mg/dL (10.0 mmol/L) may help to lower A1C.
An analysis of data from 470 participants in the ADAG study (237 with type 1 diabetes and 147 with type 2 diabetes) found that the glucose ranges highlighted in Table 6.1 are adequate to meet targets and decrease hypoglycemia (14). These findings support that premeal glucose targets may be relaxed without undermining overall glycemic control as measured by A1C. These data prompted the revision in the ADA-recommended premeal glucose target to 80–130 mg/dL (4.4–7.2 mmol/L) but did not affect the definition of hypoglycemia.
Hypoglycemia
Recommendations
6.10 Occurrence and risk for hypoglycemia should be reviewed at every encounter and investigated as indicated. Awareness of hypoglycemia should be considered using validated tools. C
6.11 Glucose (approximately 15–20 g) is the preferred treatment for the conscious individual with blood glucose <70 mg/dL (3.9 mmol/L), although any form of carbohydrate that contains glucose may be used. Fifteen minutes after treatment, if blood glucose monitoring (BGM) shows continued hypoglycemia, the treatment should be repeated. Once the BGM or glucose pattern is trending up, the individual should consume a meal or snack to prevent recurrence of hypoglycemia. B
6.12 Glucagon should be prescribed for all individuals at increased risk of level 2 or 3 hypoglycemia, so that it is available should it be needed. Caregivers, school personnel, or family members providing support to these individuals should know where it is and when and how to administer it. Glucagon administration is not limited to health care professionals. E
6.13 Hypoglycemia unawareness or one or more episodes of level 3 hypoglycemia should trigger hypoglycemia avoidance education and reevaluation and adjustment of the treatment plan to decrease hypoglycemia. E
6.14 Insulin-treated patients with hypoglycemia unawareness, one level 3 hypoglycemic event, or a pattern of unexplained level 2 hypoglycemia should be advised to raise their glycemic targets to strictly avoid hypoglycemia for at least several weeks in order to partially reverse hypoglycemia unawareness and reduce risk of future episodes. A
6.15 Ongoing assessment of cognitive function is suggested with increased vigilance for hypoglycemia by the clinician, patient, and caregivers if impaired or declining cognition is found. B
Hypoglycemia is the major limiting factor in the glycemic management of type 1 and type 2 diabetes. Recommendations regarding the classification of hypoglycemia are outlined in Table 6.4 (74–83). Level 1 hypoglycemia is defined as a measurable glucose concentration <70 mg/dL (3.9 mmol/L) but ≥54 mg/dL (3.0 mmol/L). A blood glucose concentration of 70 mg/dL (3.9 mmol/L) has been recognized as a threshold for neuroendocrine responses to falling glucose in people without diabetes. Because many people with diabetes demonstrate impaired counterregulatory responses to hypoglycemia and/or experience hypoglycemia unawareness, a measured glucose level <70 mg/dL (3.9 mmol/L) is considered clinically important (independent of the severity of acute hypoglycemic symptoms). Level 2 hypoglycemia (defined as a blood glucose concentration <54 mg/dL [3.0 mmol/L]) is the threshold at which neuroglycopenic symptoms begin to occur and requires immediate action to resolve the hypoglycemic event. If a patient has level 2 hypoglycemia without adrenergic or neuroglycopenic symptoms, they likely have hypoglycemia unawareness (discussed further below). This clinical scenario warrants investigation and review of the treatment plan (75,79). Use Clarke score, Gold score, or Pedersen-Bjergaard score to assess impaired awareness (76). Lastly, level 3 hypoglycemia is defined as a severe event characterized by altered mental and/or physical functioning that requires assistance from another person for recovery.
Table 6.4.
Classification of hypoglycemia
| Glycemic criteria/description | |
|---|---|
| Level 1 | Glucose <70 mg/dL (3.9 mmol/L) and ≥54 mg/dL (3.0 mmol/L) |
| Level 2 | Glucose <54 mg/dL (3.0 mmol/L) |
| Level 3 | A severe event characterized by altered mental and/or physical status requiring assistance for treatment of hypoglycemia |
Reprinted from Agiostratidou et al. (74).
Symptoms of hypoglycemia include, but are not limited to, shakiness, irritability, confusion, tachycardia, and hunger. Hypoglycemia may be inconvenient or frightening to people with diabetes. Level 3 hypoglycemia may be recognized or unrecognized and can progress to loss of consciousness, seizure, coma, or death. Hypoglycemia is reversed by administration of rapid-acting glucose or glucagon. Hypoglycemia can cause acute harm to the person with diabetes or others, especially if it causes falls, motor vehicle accidents, or other injury. Recurrent level 2 hypoglycemia and/or level 3 hypoglycemia is an urgent medical issue and requires intervention with medical treatment plan adjustment, behavioral intervention, and, in some cases, use of technology to assist with hypoglycemia prevention and identification (76,79–82). A large cohort study suggested that among older adults with type 2 diabetes, a history of level 3 hypoglycemia was associated with greater risk of dementia (84). Conversely, in a substudy of the ACCORD trial, cognitive impairment at baseline or decline in cognitive function during the trial was significantly associated with subsequent episodes of level 3 hypoglycemia (85). Evidence from DCCT/EDIC, which involved adolescents and younger adults with type 1 diabetes, found no association between frequency of level 3 hypoglycemia and cognitive decline (86).
Studies of rates of level 3 hypoglycemia that rely on claims data for hospitalization, emergency department visits, and ambulance use substantially underestimate rates of level 3 hypoglycemia (87) yet reveal a high burden of hypoglycemia in adults over 60 years of age in the community (88). African American individuals are at substantially increased risk of level 3 hypoglycemia (88,89). In addition to age and race, other important risk factors found in a community-based epidemiologic cohort of older adults with type 2 diabetes include insulin use, poor or moderate versus good glycemic control, albuminuria, and poor cognitive function (88). Level 3 hypoglycemia was associated with mortality in participants in both the standard and the intensive glycemia arms of the ACCORD trial, but the relationships between hypoglycemia, achieved A1C, and treatment intensity were not straightforward. An association of level 3 hypoglycemia with mortality was also found in the ADVANCE trial (90). An association between self-reported level 3 hypoglycemia and 5-year mortality has also been reported in clinical practice (91). Glucose variability is also associated with an increased risk for hypoglycemia (92).
Young children with type 1 diabetes and the elderly, including those with type 1 and type 2 diabetes (84,93), are noted as particularly vulnerable to hypoglycemia because of their reduced ability to recognize hypoglycemic symptoms and effectively communicate their needs. Individualized glucose targets, patient education, nutrition intervention (e.g., bedtime snack to prevent overnight hypoglycemia when specifically needed to treat low blood glucose), physical activity management, medication adjustment, glucose monitoring, and routine clinical surveillance may improve patient outcomes (94). CGM with automated low glucose suspend and hybrid closed-loop systems have been shown to be effective in reducing hypoglycemia in type 1 diabetes (95). For people with type 1 diabetes with level 3 hypoglycemia and hypoglycemia unawareness that persists despite medical treatment, human islet transplantation may be an option, but the approach remains experimental (96,97).
In 2015, the ADA changed its preprandial glycemic target from 70–130 mg/dL (3.9–7.2 mmol/L) to 80–130 mg/dL (4.4–7.2 mmol/L). This change reflects the results of the ADAG study, which demonstrated that higher glycemic targets corresponded to A1C goals (14). An additional goal of raising the lower range of the glycemic target was to limit overtreatment and provide a safety margin in patients titrating glucose-lowering drugs such as insulin to glycemic targets.
Hypoglycemia Treatment
Health care professionals should continue to counsel patients to treat hypoglycemia with fast-acting carbohydrates at the hypoglycemia alert value of 70 mg/dL (3.9 mmol/L) or less. This should be reviewed at each patient visit. Hypoglycemia treatment requires ingestion of glucose- or carbohydrate-containing foods (98–100). The acute glycemic response correlates better with the glucose content of food than with the carbohydrate content of food. Pure glucose is the preferred treatment, but any form of carbohydrate that contains glucose will raise blood glucose. Added fat may retard and then prolong the acute glycemic response. In type 2 diabetes, ingested protein may increase insulin response without increasing plasma glucose concentrations (101). Therefore, carbohydrate sources high in protein should not be used to treat or prevent hypoglycemia. Ongoing insulin activity or insulin secretagogues may lead to recurrent hypoglycemia unless more food is ingested after recovery. Once the glucose returns to normal, the individual should be counseled to eat a meal or snack to prevent recurrent hypoglycemia.
Glucagon
The use of glucagon is indicated for the treatment of hypoglycemia in people unable or unwilling to consume carbohydrates by mouth. Those in close contact with, or having custodial care of, people with hypoglycemia-prone diabetes (family members, roommates, school personnel, childcare professionals, correctional institution staff, or coworkers) should be instructed on the use of glucagon, including where the glucagon product is kept and when and how to administer it. An individual does not need to be a health care professional to safely administer glucagon. In addition to traditional glucagon injection powder that requires reconstitution prior to injection, intranasal glucagon and ready-to-inject glucagon preparations for subcutaneous injection are available and may be beneficial in view of safety, efficacy, and ease of use. Care should be taken to ensure that glucagon products are not expired (102).
Hypoglycemia Prevention
Hypoglycemia prevention is a critical component of diabetes management. BGM and, for some individuals, CGM are essential tools to assess therapy and detect incipient hypoglycemia. People with diabetes should understand situations that increase their risk of hypoglycemia, such as when fasting for laboratory tests or procedures, when meals are delayed, during and after the consumption of alcohol, during and after intense physical activity, and during sleep. Hypoglycemia may increase the risk of harm to self or others, such as when driving. Teaching people with diabetes to balance insulin use and carbohydrate intake and physical activity are necessary, but these strategies are not always sufficient for prevention (77,103–105). Formal training programs to increase awareness of hypoglycemia and to develop strategies to decrease hypoglycemia have been developed, including the Blood Glucose Awareness Training Program, Dose Adjusted for Normal Eating (DAFNE), and DAFNEplus. Conversely, some individuals with type 1 diabetes or type 2 diabetes and hypoglycemia who have a fear of hyperglycemia are resistant to relaxation of glycemic targets (74–83). Regardless of the factors contributing to hypoglycemia and hypoglycemia unawareness, this represents an urgent medical issue requiring intervention.
In type 1 diabetes and severely insulin-deficient type 2 diabetes, hypoglycemia unawareness (or hypoglycemia-associated autonomic failure) can severely compromise stringent diabetes control and quality of life. This syndrome is characterized by deficient counterregulatory hormone release, especially in older adults, and a diminished autonomic response, which are both risk factors for and caused by hypoglycemia. A corollary to this “vicious cycle” is that several weeks of avoidance of hypoglycemia has been demonstrated to improve counterregulation and hypoglycemia awareness in many people with diabetes (106). Hence, individuals with one or more episodes of clinically significant hypoglycemia may benefit from at least short-term relaxation of glycemic targets and availability of glucagon (107). Any person with recurrent hypoglycemia or hypoglycemia unawareness should have their glucose management treatment plan adjusted.
Use of CGM Technology in Hypoglycemia Prevention
With the advent of sensor-augmented CGM and CGM-assisted pump therapy, there has been a promise of alarm-based prevention of hypoglycemia (108,109). To date, there have been a number of randomized controlled trials in adults with type 1 diabetes and studies in adults and children with type 1 diabetes using real-time CGM (see Section 7, “Diabetes Technology”). These studies had differing A1C at entry and differing primary end points and thus must be interpreted carefully. Real-time CGM studies can be divided into studies with elevated A1C with the primary end point of A1C reduction and studies with A1C near target with the primary end point of reduction in hypoglycemia (98,109–124). In people with type 1 and type 2 diabetes with A1C above target, CGM improved A1C between 0.3 and 0.6%. For studies targeting hypoglycemia, most studies demonstrated a significant reduction in time spent between 54 and 70 mg/dL. A report in people with type 1 diabetes over the age of 60 years revealed a small but statistically significant decrease in hypoglycemia (125). No study to date has reported a decrease in level 3 hypoglycemia. In a single study using intermittently scanned CGM, adults with type 1 diabetes with A1C near goal and impaired awareness of hypoglycemia demonstrated no change in A1C and decreased level 2 hypoglycemia (115). For people with type 2 diabetes, studies examining the impact of CGM on hypoglycemic events are limited; a recent meta-analysis does not reflect a significant impact on hypoglycemic events in type 2 diabetes (126), whereas improvements in A1C were observed in most studies (126–132). Overall, real-time CGM appears to be a useful tool for decreasing time spent in a hypoglycemic range in people with impaired awareness. For people with type 2 diabetes, other strategies to assist them with insulin dosing can improve A1C with minimal hypoglycemia (133,134).
Intercurrent Illness
For further information on management of individuals with hyperglycemia in the hospital, see Section 16, “Diabetes Care in the Hospital.”
Stressful events (e.g., illness, trauma, surgery) may worsen glycemic control and precipitate diabetic ketoacidosis or nonketotic hyperglycemic hyperosmolar state, life-threatening conditions that require immediate medical care to prevent complications and death. Any condition leading to deterioration in glycemic control necessitates more frequent monitoring of blood glucose; ketosis-prone patients also require urine or blood ketone monitoring. If accompanied by ketosis, vomiting, or alteration in the level of consciousness, marked hyperglycemia requires temporary adjustment of the treatment plan and immediate interaction with the diabetes care team. The patient treated with noninsulin therapies or medical nutrition therapy alone may require insulin. Adequate fluid and caloric intake must be ensured. Infection or dehydration are more likely to necessitate hospitalization of individuals with diabetes versus those without diabetes.
A clinician with expertise in diabetes management should treat the hospitalized patient. For further information on the management of diabetic ketoacidosis and the nonketotic hyperglycemic hyperosmolar state, please refer to the ADA consensus report “Hyperglycemic Crises in Adult Patients With Diabetes” (134).
Footnotes
Disclosure information for each author is available at https://doi.org/10.2337/dc23-SDIS.
Suggested citation: ElSayed NA, Aleppo G, Aroda VR, et al., American Diabetes Association. 6. Glycemic targets: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1):S97–S110
References
- 1. Deshmukh H, Wilmot EG, Gregory R, et al. Effect of flash glucose monitoring on glycemic control, hypoglycemia, diabetes-related distress, and resource utilization in the Association of British Clinical Diabetologists (ABCD) nationwide audit. Diabetes Care 2020;43:2153–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Laiteerapong N, Ham SA, Gao Y, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (the Diabetes & Aging Study). Diabetes Care 2019;42:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000;321:405–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Little RR, Rohlfing CL; National Glycohemoglobin Standardization Program (NGSP) Steering Committee . Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem 2011;57:205–214 [DOI] [PubMed] [Google Scholar]
- 5. Valenzano M, Cibrario Bertolotti I, Valenzano A, Grassi G. Time in range-A1c hemoglobin relationship in continuous glucose monitoring of type 1 diabetes: a real-world study. BMJ Open Diabetes Res Care 2021;9:e001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fabris C, Heinemann L, Beck R, Cobelli C, Kovatchev B. Estimation of hemoglobin A1c from continuous glucose monitoring data in individuals with type 1 diabetes: is time in range all we need? Diabetes Technol Ther 2020;22:501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ranjan AG, Rosenlund SV, Hansen TW, Rossing P, Andersen S, Nørgaard K. Improved time in range over 1 year is associated with reduced albuminuria in individuals with sensor-augmented insulin pump-treated type 1 diabetes. Diabetes Care 2020;43:2882–2885 [DOI] [PubMed] [Google Scholar]
- 8. Beck RW, Bergenstal RM, Cheng P, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol 2019;13:614–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Šoupal J, Petruželková L, Grunberger G, et al. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR study. Diabetes Care 2020;43:37–43 [DOI] [PubMed] [Google Scholar]
- 10. Jovanovič L, Savas H, Mehta M, Trujillo A, Pettitt DJ. Frequent monitoring of A1C during pregnancy as a treatment tool to guide therapy. Diabetes Care 2011;34:53–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020;98(4S):S1–S115 [DOI] [PubMed] [Google Scholar]
- 12. Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1C alone to assess glycemic control can be misleading. Diabetes Care 2017;40:994–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D; A1c-Derived Average Glucose Study Group . Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wei N, Zheng H, Nathan DM. Empirically establishing blood glucose targets to achieve HbA1c goals. Diabetes Care 2014;37:1048–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Selvin E. Are there clinical implications of racial differences in HbA1c? A difference, to be a difference, must make a difference. Diabetes Care 2016;39:1462–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bergenstal RM, Gal RL, Connor CG, et al.; T1D Exchange Racial Differences Study Group . Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med 2017;167:95–102 [DOI] [PubMed] [Google Scholar]
- 17. Khosla L, Bhat S, Fullington LA, Horlyck-Romanovsky MF. HbA1c performance in african descent populations in the United States with normal glucose tolerance, prediabetes, or diabetes: a scoping review. Prev Chronic Dis 2021;18:E22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lacy ME, Wellenius GA, Sumner AE, Correa A, Carnethon MR, Liem RI, et al. Association of sickle cell trait with hemoglobin A1c in African Americans. JAMA 2017;317:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rohlfing C, Hanson S, Little RR. Measurement of hemoglobin A1c in patients with sickle cell trait. JAMA 2017;317:2237. [DOI] [PubMed] [Google Scholar]
- 20. Wheeler E, Leong A, Liu CT, et al.; EPIC-CVD Consortium; EPIC-InterAct Consortium; Lifelines Cohort Study . Impact of common genetic determinants of hemoglobin A1c on type 2 diabetes risk and diagnosis in ancestrally diverse populations: a transethnic genome-wide meta-analysis. PLoS Med 2017;14:e1002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diabetes Research in Children Network (DirecNet) Study Group . Relationship of A1C to glucose concentrations in children with type 1 diabetes: assessments by high-frequency glucose determinations by sensors. Diabetes Care 2008;31:381–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buse JB, Kaufman FR, Linder B, Hirst K, El Ghormli L; HEALTHY Study Group . Diabetes screening with hemoglobin A(1c) versus fasting plasma glucose in a multiethnic middle-school cohort. Diabetes Care 2013;36:429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kamps JL, Hempe JM, Chalew SA. Racial disparity in A1C independent of mean blood glucose in children with type 1 diabetes. Diabetes Care 2010;33:1025–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Advani A. Positioning time in range in diabetes management. Diabetologia 2020;63:242–252 [DOI] [PubMed] [Google Scholar]
- 25. Avari P, Uduku C, George D, Herrero P, Reddy M, Oliver N. Differences for percentage times in glycemic range between continuous glucose monitoring and capillary blood glucose monitoring in adults with type 1 diabetes: analysis of the REPLACE-BG dataset. Diabetes Technol Ther 2020;22:222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21:81–85 [DOI] [PubMed] [Google Scholar]
- 27. Kröger J, Reichel A, Siegmund T, Ziegler R. Clinical recommendations for the use of the ambulatory glucose profile in diabetes care. J Diabetes Sci Technol 2020;14:586–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Livingstone R, Boyle JG, Petrie JR. How tightly controlled do fluctuations in blood glucose levels need to be to reduce the risk of developing complications in people with type 1 diabetes? Diabet Med 2020;37:513–521 [DOI] [PubMed] [Google Scholar]
- 29. Messer LH, Berget C, Vigers T, et al. Real world hybrid closed-loop discontinuation: Predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes 2020;21:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mayeda L, Katz R, Ahmad I, et al. Glucose time in range and peripheral neuropathy in type 2 diabetes mellitus and chronic kidney disease. BMJ Open Diabetes Res Care 2020;8:e000991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoo JH, Choi MS, Ahn J, et al. Association between continuous glucose monitoring-derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther 2020;22:768–776 [DOI] [PubMed] [Google Scholar]
- 32. Lu J, Ma X, Shen Y, et al. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther 2020;22:72–78 [DOI] [PubMed] [Google Scholar]
- 33. Diabetes Control and Complications Trial Research Group; Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 34. Holt RIG, DeVries JH, Hess-Fischl A, et al. The management of type 1 diabetes in adults. A Consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2021;44:2589–2625 [DOI] [PubMed] [Google Scholar]
- 35. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tchero H, Kangambega P, Briatte C, Brunet-Houdard S, Retali GR, Rusch E. Clinical effectiveness of telemedicine in diabetes mellitus: a meta-analysis of 42 randomized controlled trials. Telemed J E Health 2019;25:569–583 [DOI] [PubMed] [Google Scholar]
- 37. Salabelle C, Ly Sall K, Eroukhmanoff J, et al. COVID-19 pandemic lockdown in young people with type 1 diabetes: positive results of an unprecedented challenge for patients through telemedicine and change in use of continuous glucose monitoring. Prim Care Diabetes 2021;15:884–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Prabhu Navis J, Leelarathna L, Mubita W, et al. Impact of COVID-19 lockdown on flash and real-time glucose sensor users with type 1 diabetes in England. Acta Diabetol 2021;58:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seidu S, Kunutsor SK, Topsever P, Hambling CE, Cos FX, Khunti K. Deintensification in older patients with type 2 diabetes: a systematic review of approaches, rates and outcomes. Diabetes Obes Metab 2019;21:1668–1679 [DOI] [PubMed] [Google Scholar]
- 40. Khunti K, Davies MJ. Clinical inertia–time to reappraise the terminology? Prim Care Diabetes 2017;11:105–106 [DOI] [PubMed] [Google Scholar]
- 41. Whitehead L, Glass C, Coppell K. The effectiveness of goal setting on glycaemic control for people with type 2 diabetes and prediabetes: a systematic review and meta-analysis. J Adv Nurs 2022;78:1212–1227 [DOI] [PubMed] [Google Scholar]
- 42. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group; Lachin JM, White NH, Hainsworth DP, et al. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015;64:631–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group; Lachin JM, Genuth S, Cleary P, Davis MD, Nathan DM. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med 2000;342:381–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ohkubo Y, Kishikawa H, Araki E, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995;28:103–117 [DOI] [PubMed] [Google Scholar]
- 45. UK Prospective Diabetes Study (UKPDS) Group . Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352:854–865 [PubMed] [Google Scholar]
- 46. UK Prospective Diabetes Study (UKPDS) Group . Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 47. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 48. Lind M, Pivodic A, Svensson AM, Ólafsdóttir AF, Wedel H, Ludvigsson J. HbA1c level as a risk factor for retinopathy and nephropathy in children and adults with type 1 diabetes: Swedish population based cohort study. BMJ 2019;366:l4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adler AI, Stratton IM, Neil HAW, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 51. Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 52. Ismail-Beigi F, Craven T, Banerji MA, et al.; ACCORD trial group . Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010;376:419–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Buse JB, Bain SC, Mann JFE, et al.; LEADER Trial Investigators . Cardiovascular risk reduction with liraglutide: an exploratory mediation analysis of the LEADER trial. Diabetes Care 2020;43:1546–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nathan DM, Cleary PA, Backlund JYC, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nathan DM, Zinman B, Cleary PA, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group . Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Emerging Risk Factors Collaboration; Di Angelantonio E, Kaptoge S, Wormser D, et al. Association of cardiometabolic multimorbidity with mortality. JAMA 2015;314:52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yeung RO, Zhang Y, Luk A, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol 2014;2:935–943 [DOI] [PubMed] [Google Scholar]
- 58. Sattar N, Rawshani A, Franzén S, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation 2019;139:2228–2237 [DOI] [PubMed] [Google Scholar]
- 59. Zabala A, Darsalia V, Holzmann MJ, et al. Risk of first stroke in people with type 2 diabetes and its relation to glycaemic control: a nationwide observational study. Diabetes Obes Metab 2020;22:182–190 [DOI] [PubMed] [Google Scholar]
- 60. Zoungas S, Woodward M, Li Q, et al.; ADVANCE Collaborative group . Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 2014;57:2465–2474 [DOI] [PubMed] [Google Scholar]
- 61. Skyler JS, Bergenstal R, Bonow RO, et al.; American Diabetes Association; American College of Cardiology Foundation; American Heart Association . Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zoungas S, Chalmers J, Neal B, et al.; ADVANCE-ON Collaborative Group . Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med 2014;371:1392–1406 [DOI] [PubMed] [Google Scholar]
- 64. Hayward RA, Reaven PD, Wiitala WL, et al.; VADT Investigators . Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;372:2197–2206 [DOI] [PubMed] [Google Scholar]
- 65. Turnbull FM, Abraira C, Anderson RJ, et al.; Control Group . Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia 2009;52:2288–2298 [DOI] [PubMed] [Google Scholar]
- 66. Duckworth WC, Abraira C, Moritz TE, et al.; Investigators of the VADT . The duration of diabetes affects the response to intensive glucose control in type 2 subjects: the VA Diabetes Trial. J Diabetes Complications 2011;25:355–361 [DOI] [PubMed] [Google Scholar]
- 67. Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA Intern Med 2015;175:356–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vijan S, Sussman JB, Yudkin JS, Hayward RA. Effect of patients’ risks and preferences on health gains with plasma glucose level lowering in type 2 diabetes mellitus. JAMA Intern Med 2014;174:1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lee AK, Warren B, Lee CJ, et al. The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care 2018;41:104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140–149 [DOI] [PubMed] [Google Scholar]
- 72. American Diabetes Association . Postprandial blood glucose. Diabetes Care 2001;24:775–778 [DOI] [PubMed] [Google Scholar]
- 73. Raz I, Wilson PWF, Strojek K, et al. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009;32:381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Agiostratidou G, Anhalt H, Ball D, et al. Standardizing clinically meaningful outcome measures beyond HbA1c for type 1 diabetes: a consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care 2017;40:1622–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Polonsky WH, Fortmann AL, Price D, Fisher L. “Hyperglycemia aversiveness”: investigating an overlooked problem among adults with type 1 diabetes. J Diabetes Complications 2021;35:107925. [DOI] [PubMed] [Google Scholar]
- 76. Ghandi K, Pieri B, Dornhorst A, Hussain S. A comparison of validated methods used to assess impaired awareness of hypoglycaemia in type 1 diabetes: an observational study. Diabetes Ther 2021;12:441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Li P, Geng Z, Ladage VP, Wu J, Lorincz I, Doshi JA. Early hypoglycaemia and adherence after basal insulin initiation in a nationally representative sample of Medicare beneficiaries with type 2 diabetes. Diabetes Obes Metab 2019;21:2486–2495 [DOI] [PubMed] [Google Scholar]
- 78. Hendrieckx C, Ivory N, Singh H, Frier BM, Speight J. Impact of severe hypoglycaemia on psychological outcomes in adults with type 2 diabetes: a systematic review. Diabet Med 2019;36:1082–1091 [DOI] [PubMed] [Google Scholar]
- 79. Amiel SA, Potts L, Goldsmith K, et al. A parallel randomised controlled trial of the Hypoglycaemia Awareness Restoration Programme for adults with type 1 diabetes and problematic hypoglycaemia despite optimised self-care (HARPdoc). Nat Commun 2022;13:2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Khunti K, Alsifri S, Aronson R, et al. Impact of hypoglycaemia on patient-reported outcomes from a global, 24-country study of 27,585 people with type 1 and insulin-treated type 2 diabetes. Diabetes Res Clin Pract 2017;130:121–129 [DOI] [PubMed] [Google Scholar]
- 81. Choudhary P, Amiel SA. Hypoglycaemia in type 1 diabetes: technological treatments, their limitations and the place of psychology. Diabetologia 2018;61:761–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hopkins D, Lawrence I, Mansell P, Thompson G, Amiel S, Campbell M, et al. Improved biomedical and psychological outcomes 1 year after structured education in flexible insulin therapy for people with type 1 diabetes: the U.K. DAFNE experience. Diabetes Care 2012;35:1638–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yang W, Ma J, Yuan G, et al. Determining the optimal fasting glucose target for patients with type 2 diabetes: results of the multicentre, open-label, randomized-controlled FPG GOAL trial. Diabetes Obes Metab 2019;21:1973–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA 2009;301:1565–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Punthakee Z, Miller ME, Launer LJ, et al.; ACCORD Group of Investigators; ACCORD-MIND Investigators . Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care 2012;35:787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Jacobson AM, Musen G, Ryan CM, et al.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group . Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med 2007;356:1842–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Karter AJ, Moffet HH, Liu JY, Lipska KJ. Surveillance of hypoglycemia—limitations of emergency department and hospital utilization data. JAMA Intern Med 2018;178:987–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lee AK, Lee CJ, Huang ES, Sharrett AR, Coresh J, Selvin E. risk factors for severe hypoglycemia in black and white adults with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care 2017;40:1661–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Karter AJ, Lipska KJ, O’Connor PJ, et al.; SUPREME-DM Study Group . High rates of severe hypoglycemia among African American patients with diabetes: the surveillance, prevention, and Management of Diabetes Mellitus (SUPREME-DM) network. J Diabetes Complications 2017;31:869–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zoungas S, Patel A, Chalmers J, et al.; ADVANCE Collaborative Group . Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 91. McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care 2012;35:1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cahn A, Zuker I, Eilenberg R, et al. Machine learning based study of longitudinal HbA1c trends and their association with all-cause mortality: analyses from a national diabetes registry. Diabetes Metab Res Rev 2022;38:e3485. [DOI] [PubMed] [Google Scholar]
- 93. DuBose SN, Weinstock RS, Beck RW, et al. Hypoglycemia in older adults with type 1 diabetes. Diabetes Technol Ther 2016;18:765–771 [DOI] [PubMed] [Google Scholar]
- 94. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Bergenstal RM, Klonoff DC, Garg SK, et al.; ASPIRE In-Home Study Group . Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224–232 [DOI] [PubMed] [Google Scholar]
- 96. Hering BJ, Clarke WR, Bridges ND, et al.; Clinical Islet Transplantation Consortium . Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 2016;39:1230–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Harlan DM. Islet transplantation for hypoglycemia unawareness/severe hypoglycemia: caveat emptor. Diabetes Care 2016;39:1072–1074 [DOI] [PubMed] [Google Scholar]
- 98. McTavish L, Wiltshire E. Effective treatment of hypoglycemia in children with type 1 diabetes: a randomized controlled clinical trial. Pediatr Diabetes 2011;12(4pt2):381–387 [DOI] [PubMed] [Google Scholar]
- 99. McTavish L, Corley B, Weatherall M, Wiltshire E, Krebs JD. Weight-based carbohydrate treatment of hypoglycaemia in people with type 1 diabetes using insulin pump therapy: a randomized crossover clinical trial. Diabet Med 2018;35:339–346 [DOI] [PubMed] [Google Scholar]
- 100. Georgakopoulos K, Katsilambros N, Fragaki M, et al. Recovery from insulin-induced hypoglycemia after saccharose or glucose administration. Clin Physiol Biochem 1990;8:267–272 [PubMed] [Google Scholar]
- 101. Layman DK, Clifton P, Gannon MC, Krauss RM, Nuttall FQ. Protein in optimal health: heart disease and type 2 diabetes. Am J Clin Nutr 2008;87:1571S–1575S [DOI] [PubMed] [Google Scholar]
- 102. Pontiroli AE, Ceriani V. Intranasal glucagon for hypoglycaemia in diabetic patients. An old dream is becoming reality? Diabetes Obes Metab 2018;20:1812–1816 [DOI] [PubMed] [Google Scholar]
- 103. Stanton-Fay SH, Hamilton K, Chadwick PM, et al.; DAFNEplus study group . The DAFNEplus programme for sustained type 1 diabetes self management: intervention development using the Behaviour Change Wheel. Diabet Med 2021;38:e14548. [DOI] [PubMed] [Google Scholar]
- 104. Farrell CM, McCrimmon RJ. Clinical approaches to treat impaired awareness of hypoglycaemia. Ther Adv Endocrinol Metab 2021;12:20420188211000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Cox DJ, Gonder-Frederick L, Julian DM, Clarke W. Long-term follow-up evaluation of blood glucose awareness training. Diabetes Care 1994;17:1–5 [DOI] [PubMed] [Google Scholar]
- 106. Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med 2004;350:2272–2279 [DOI] [PubMed] [Google Scholar]
- 107. Mitchell BD, He X, Sturdy IM, Cagle AP, Settles JA. Glucagon prescription patterns in patients with either type 1 or 2 diabetes with newly prescribed insulin. Endocr Pract 2016;22:123–135 [DOI] [PubMed] [Google Scholar]
- 108. Hermanns N, Heinemann L, Freckmann G, Waldenmaier D, Ehrmann D. Impact of CGM on the management of hypoglycemia problems: overview and secondary analysis of the HypoDE study. J Diabetes Sci Technol 2019;13:636–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Heinemann L, Freckmann G, Ehrmann D, Faber-Heinemann G, Guerra S, Waldenmaier D, et al. Heinemann L, Freckmann G, Ehrmann D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet 2018;391:1367–1377 [DOI] [PubMed] [Google Scholar]
- 110. Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317:371–378 [DOI] [PubMed] [Google Scholar]
- 111. Sequeira PA, Montoya L, Ruelas V, et al. Continuous glucose monitoring pilot in low-income type 1 diabetes patients. Diabetes Technol Ther 2013;15:855–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Tumminia A, Crimi S, Sciacca L, et al. Efficacy of real-time continuous glucose monitoring on glycaemic control and glucose variability in type 1 diabetic patients treated with either insulin pumps or multiple insulin injection therapy: a randomized controlled crossover trial. Diabetes Metab Res Rev 2015;31:61–68 [DOI] [PubMed] [Google Scholar]
- 113. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 2016;388:2254–2263 [DOI] [PubMed] [Google Scholar]
- 114. Hermanns N, Schumann B, Kulzer B, Haak T. The impact of continuous glucose monitoring on low interstitial glucose values and low blood glucose values assessed by point-of-care blood glucose meters: results of a crossover trial. J Diabetes Sci Technol 2014;8:516–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Reddy M, Jugnee N, El Laboudi A, Spanudakis E, Anantharaja S, Oliver N. A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with Type 1 diabetes and impaired awareness of hypoglycaemia. Diabet Med 2018;35:483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Riddlesworth T, Price D, Cohen N, Beck RW. Hypoglycemic event frequency and the effect of continuous glucose monitoring in adults with type 1 diabetes using multiple daily insulin injections. Diabetes Ther 2017;8:947–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. van Beers CAJ, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol 2016;4:893–902 [DOI] [PubMed] [Google Scholar]
- 118. Battelino T, Conget I, Olsen B, et al.; SWITCH Study Group . The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia 2012;55:3155–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Deiss D, Bolinder J, Riveline JP, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care 2006;29:2730–2732 [DOI] [PubMed] [Google Scholar]
- 120. Tamborlane WV, Beck RW, Bode BW, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 121. O’Connell MA, Donath S, O’Neal DN, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia 2009;52:1250–1257 [DOI] [PubMed] [Google Scholar]
- 122. Beck RW, Hirsch IB, Laffel L, et al.; Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group . The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care 2011;34:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ludvigsson J, Hanas R. Continuous subcutaneous glucose monitoring improved metabolic control in pediatric patients with type 1 diabetes: a controlled crossover study. Pediatrics 2003;111:933–938 [DOI] [PubMed] [Google Scholar]
- 125. Pratley RE, Kanapka LG, Rickels MR, Ahmann A, Aleppo G, Beck R, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA 2020;323:2397–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Dicembrini I, Mannucci E, Monami M, Pala L. Impact of technology on glycemic control in type 2 diabetes: a meta-analysis of randomized trials on continuous glucose monitoring and continuous subcutaneous insulin infusion. Diabetes Obes Metab 2019;21:2619–2625 [DOI] [PubMed] [Google Scholar]
- 127. Beck RW, Riddlesworth TD, Ruedy K, et al.; DIAMOND Study Group . Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med 2017;167:365–374 [DOI] [PubMed] [Google Scholar]
- 128. Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ, Vigersky RA. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol 2011;5:668–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 2017;8:55–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yoo HJ, An HG, Park SY, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract 2008;82:73–79 [DOI] [PubMed] [Google Scholar]
- 131. Garg S, Zisser H, Schwartz S, et al. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care 2006;29:44–50 [DOI] [PubMed] [Google Scholar]
- 132. New JP, Ajjan R, Pfeiffer AFH, Freckmann G. Continuous glucose monitoring in people with diabetes: the randomized controlled Glucose Level Awareness in Diabetes Study (GLADIS). Diabet Med 2015;32:609–617 [DOI] [PubMed] [Google Scholar]
- 133. Bergenstal RM, Johnson M, Passi R, et al. Automated insulin dosing guidance to optimise insulin management in patients with type 2 diabetes: a multicentre, randomised controlled trial. Lancet 2019;393:1138–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Kitabchi AE, Umpierrez GE, Miles JM, Fisher JN. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32:1335–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]