Abstract
The American Diabetes Association (ADA) “Standards of Care in Diabetes” includes the ADA’s current clinical practice recommendations and is intended to provide the components of diabetes care, general treatment goals and guidelines, and tools to evaluate quality of care. Members of the ADA Professional Practice Committee, a multidisciplinary expert committee, are responsible for updating the Standards of Care annually, or more frequently as warranted. For a detailed description of ADA standards, statements, and reports, as well as the evidence-grading system for ADA’s clinical practice recommendations and a full list of Professional Practice Committee members, please refer to Introduction and Methodology. Readers who wish to comment on the Standards of Care are invited to do so at professional.diabetes.org/SOC.
The management of diabetes in children and adolescents (individuals <18 years of age) cannot simply be derived from care routinely provided to adults with diabetes. The epidemiology, pathophysiology, developmental considerations, and response to therapy in pediatric diabetes are often different from those of adult diabetes. There are also differences in recommended care for children and adolescents with type 1 diabetes, type 2 diabetes, and other forms of pediatric diabetes. This section is divided into two major parts: the first part addresses care for children and adolescents with type 1 diabetes, and the second part addresses care for children and adolescents with type 2 diabetes. Monogenic diabetes (neonatal diabetes and maturity-onset diabetes in the young [MODY]) and cystic fibrosis–related diabetes, which are often present in youth, are discussed in Section 2, “Classification and Diagnosis of Diabetes.” Table 14.1A and Table 14.1B provide an overview of the recommendations for screening and treatment of complications and related conditions in pediatric type 1 diabetes and type 2 diabetes, respectively. In addition to comprehensive diabetes care, youth with diabetes should receive age-appropriate and developmentally appropriate pediatric care, including vaccines and immunizations as recommended by the Centers for Disease Control and Prevention (CDC) (1). To ensure continuity of care as an adolescent with diabetes becomes an adult, guidance is provided at the end of this section on the transition from pediatric to adult diabetes care.
Table 14.1A.
Recommendations for screening and treatment of complications and related conditions in pediatric type 1 diabetes
| Thyroid disease | Celiac disease | Hypertension | Dyslipidemia | Nephropathy | Retinopathy | Neuropathy | |
|---|---|---|---|---|---|---|---|
| Corresponding recommendations | 14.29 and 14.30 | 14.31–14.33 | 14.34–14.37 | 14.38–14.42 | 14.45 and 14.46 | 14.47–14.49 | 14.50 |
| Method | Thyroid-stimulating hormone; consider antithyroglobulin and antithyroid peroxidase antibodies | IgA tTG if total IgA normal; IgG tTG and deamidated gliadin antibodies if IgA deficient | Blood pressure monitoring | Lipid profile, nonfasting acceptable initially | Albumin-to-creatinine ratio; random sample acceptable initially | Dilated fundoscopy or retinal photography | Foot exam with foot pulses, pinprick, 10-g monofilament sensation tests, vibration, and ankle reflexes |
| When to start | Soon after diagnosis | Soon after diagnosis | At diagnosis | Soon after diagnosis; preferably after glycemia has improved and ≥2 years old | Puberty or >10 years old, whichever is earlier, and diabetes duration of 5 years | Puberty or ≥11 years old, whichever is earlier, and diabetes duration of 3–5 years | Puberty or ≥10 years old, whichever is earlier, and diabetes duration of 5 years |
| Follow-up frequency | Every 1–2 years if thyroid antibodies negative; more often if symptoms develop or presence of thyroid antibodies | Within 2 years and then at 5 years after diagnosis; sooner if symptoms develop | Every visit | If LDL ≤100 mg/dL, repeat at 9–11 years old; then, if <100 mg/dL, every 3 years | If normal, annually; if abnormal, repeat with confirmation in two of three samples over 6 months | If normal, every 2 years; consider less frequently (every 4 years) if A1C <8% and eye professional agrees | If normal, annually |
| Target | NA | NA | <90th percentile for age, sex, and height; if ≥13 years old, <120/80 mmHg | LDL <100 mg/dL | Albumin-to-creatinine ratio <30 mg/g | No retinopathy | No neuropathy |
| Treatment | Appropriate treatment of underlying thyroid disorder | After confirmation, start gluten-free diet | Lifestyle modification for elevated blood pressure (90th to <95th percentile for age, sex, and height or, if ≥13 years old, 120–129/<80 mmHg); lifestyle modification and ACE inhibitor or ARB* for hypertension (≥95th percentile for age, sex, and height or, if ≥13 years old, ≥130/80 mmHg) | If abnormal, optimize glycemia and medical nutrition therapy; if after 6 months LDL >160 mg/dL or >130 mg/dL with cardiovascular risk factor(s), initiate statin therapy (for those aged >10 years)* | Optimize glycemia and blood pressure; ACE inhibitor* if albumin-to-creatinine ratio is elevated in two of three samples over 6 months | Optimize glycemia; treatment per ophthalmology | Optimize glycemia; referral to neurology |
ARB, angiotensin receptor blocker; NA, not applicable; tTG, tissue transglutaminase.
Due to the potential teratogenic effects, individuals of childbearing age should receive reproductive counseling, and medication should be avoided in individuals of childbearing age who are not using reliable contraception.
Table 14.1B.
Recommendations for screening and treatment of complications and related conditions in pediatric type 2 diabetes
| Hypertension | Nephropathy | Neuropathy | Retinopathy | Nonalcoholic fatty liver disease | Obstructive sleep apnea | Polycystic ovarian syndrome (for adolescent female individuals) | Dyslipidemia | |
|---|---|---|---|---|---|---|---|---|
| Corresponding recommendations | 14.77–14.80 | 14.81–14.86 | 14.87 and 14.88 | 14.89–14.92 | 14.93 and 14.94 | 14.95 | 14.96–14.98 | 14.100–14.104 |
| Method | Blood pressure monitoring | Albumin-to-creatinine ratio; random sample acceptable initially | Foot exam with foot pulses, pinprick, 10-g monofilament sensation tests, vibration, and ankle reflexes | Dilated fundoscopy | AST and ALT measurement | Screening for symptoms | Screening for symptoms; laboratory evaluation if positive symptoms | Lipid profile |
| When to start | At diagnosis | At diagnosis | At diagnosis | At/soon after diagnosis | At diagnosis | At diagnosis | At diagnosis | Soon after diagnosis, preferably after glycemia has improved |
| Follow-up frequency | Every visit | If normal, annually; if abnormal, repeat with confirmation in two of three samples over 6 months | If normal, annually | If normal, annually | Annually | Every visit | Every visit | Annually |
| Target | <90th percentile for age, sex, and height; if ≥13 years old, <130/80 mmHg | <30 mg/g | No neuropathy | No retinopathy | NA | NA | NA | LDL <100 mg/dL, HDL >35 mg/dL, triglycerides <150 mg/dL |
| Treatment | Lifestyle modification for elevated blood pressure (90th to <95th percentile for age, sex, and height or, if ≥13 years old, 120–129/<80 mmHg); lifestyle modification and ACE inhibitor or ARB* for hypertension (≥95th percentile for age, sex, and height or, if ≥13 years, ≥130/80 mmHg) | Optimize glycemia and blood pressure; ACE inhibitor* if albumin-to-creatinine ratio is elevated in two of three samples over 6 months | Optimize glycemia; referral to neurology | Optimize glycemia; treatment per ophthalmology | Refer to gastroenterology for persistently elevated or worsening transaminases | If positive symptoms, refer to sleep specialist and polysomnogram | If no contraindications, oral contraceptive pills; medical nutrition therapy; metformin | If abnormal, optimize glycemia and medical nutrition therapy; if after 6 months, LDL >130 mg/dL, initiate statin therapy (for those aged >10 years)*; if triglycerides >400 mg/dL fasting or >1,000 mg/dL nonfasting, begin fibrate |
ARB, angiotensin receptor blocker; NA, not applicable.
Due to the potential teratogenic effects, individuals of childbearing age should receive reproductive counseling, and medication should be avoided in individuals of childbearing age who are not using reliable contraception.
Due to the nature of pediatric clinical research, the recommendations for children and adolescents with diabetes are less likely to be based on clinical trial evidence. However, expert opinion and a review of available and relevant experimental data are summarized in the American Diabetes Association (ADA) position statements “Type 1 Diabetes in Children and Adolescents” (2) and “Evaluation and Management of Youth-Onset Type 2 Diabetes” (3). Finally, other sections in the Standards of Care may have recommendations that apply to youth with diabetes and are referenced in the narrative of this section.
Type 1 Diabetes
Type 1 diabetes is the most common form of diabetes in youth (4), although data suggest that it may account for a large proportion of cases diagnosed in adult life (5). The health care professional must consider the unique aspects of care and management of children and adolescents with type 1 diabetes, such as changes in insulin sensitivity related to physical growth and sexual maturation, ability to provide self-care, supervision in the childcare and school environment, neurological vulnerability to hypoglycemia and hyperglycemia in young children, and possible adverse neurocognitive effects of diabetic ketoacidosis (DKA) (6,7). Attention to family dynamics, developmental stages, and physiologic differences related to sexual maturity is essential in developing and implementing an optimal diabetes treatment plan (8).
A multidisciplinary team trained in pediatric diabetes management and sensitive to the challenges of children and adolescents with type 1 diabetes and their families should provide diabetes-specific care for this population. It is essential that diabetes self-management education and support, medical nutrition therapy, and psychosocial support be provided at diagnosis and regularly thereafter in a developmentally appropriate format that builds on prior knowledge by a team of health care professionals experienced with the biological, educational, nutritional, behavioral, and emotional needs of the growing child and family. The diabetes team, taking into consideration the youth’s developmental and psychosocial needs, should ask about and advise the youth and parents/caregivers about diabetes management responsibilities on an ongoing basis.
Diabetes Self-Management Education and Support
Recommendation
14.1 Youth with type 1 diabetes and their parents/caregivers (for patients aged <18 years) should receive culturally sensitive and developmentally appropriate individualized diabetes self-management education and support according to national standards at diagnosis and routinely thereafter. B
Self-management in pediatric diabetes involves both the youth and their parents/adult caregivers. No matter how sound the medical plan is, it can only be effective if the family and/or affected individuals are able to implement it. Family involvement is a vital component of optimal diabetes management throughout childhood and adolescence. As parents/caregivers are critical to diabetes self-management in youth, diabetes care requires an approach that places the youth and their parents/caregivers at the center of the care model. The pediatric diabetes care team must be capable of evaluating the educational, behavioral, emotional, and psychosocial factors that impact the implementation of a treatment plan and must work with the youth and family to overcome barriers or redefine goals as appropriate. Diabetes self-management education and support requires periodic reassessment, especially as the youth grows, develops, and acquires the need and desire for greater independent self-care skills. The pediatric diabetes team should work with the youth and their parents/caregivers to ensure there is not a premature transfer of self-management tasks to the youth during this time. In addition, it is necessary to assess the educational needs and skills of, and provide training to, day care workers, school nurses, and school personnel who are responsible for the care and supervision of the child with diabetes (9–11).
Nutrition Therapy
Recommendations
14.2 Individualized medical nutrition therapy is recommended for youth with type 1 diabetes as an essential component of the overall treatment plan. A
14.3 Monitoring carbohydrate intake, whether by carbohydrate counting or experience-based estimation, is a key component to optimizing glycemic management. B
14.4 Comprehensive nutrition education at diagnosis, with annual updates, by an experienced registered dietitian nutritionist, is recommended to assess caloric and nutrition intake in relation to weight status and cardiovascular disease risk factors and to inform macronutrient choices. E
Nutrition management should be individualized: family habits, food preferences, religious or cultural needs, finances, schedules, physical activity, and the youth’s and family’s abilities in numeracy, literacy, and self-management should be considered. Visits with a registered dietitian nutritionist should include assessment for changes in food preferences over time, access to food, growth, and development, weight status, cardiovascular risk, and potential for disordered eating. Following recommended nutrition plans is associated with better glycemic outcomes in youth with type 1 diabetes (12).
Physical Activity and Exercise
Recommendations
14.5 Physical activity is recommended for all youth with type 1 diabetes with the goal of 60 min of moderate- to vigorous-intensity aerobic activity daily, with vigorous muscle-strengthening and bone-strengthening activities at least 3 days per week. C
14.6 Frequent glucose monitoring before, during, and after exercise, via blood glucose meter or continuous glucose monitoring, is important to prevent, detect, and treat hypoglycemia and hyperglycemia associated with exercise. C
14.7 Youth and their parents/caregivers should receive education on targets and management of glycemia before, during, and after physical activity, individualized according to the type and intensity of the planned physical activity. E
14.8 Youth and their parents/caregivers should be educated on strategies to prevent hypoglycemia during, after, and overnight following physical activity and exercise, which may include reducing prandial insulin dosing for the meal/snack preceding (and, if needed, following) exercise, reducing basal insulin doses, increasing carbohydrate intake, eating bedtime snacks, and/or using continuous glucose monitoring. Treatment for hypoglycemia should be accessible before, during, and after engaging in activity. C
Physical activity and exercise positively impact metabolic and psychological health in children with type 1 diabetes (13). While it affects insulin sensitivity, physical fitness, strength building, weight management, social interaction, mood, self-esteem building, and the creation of healthful habits for adulthood, it also has the potential to cause both hypoglycemia and hyperglycemia.
See below for strategies to mitigate hypoglycemia risk and minimize hyperglycemia associated with exercise. For an in-depth discussion, see reviews and guidelines (14–16).
Overall, it is recommended that youth participate in 60 min of moderate-intensity (e.g., brisk walking, dancing) to vigorous-intensity (e.g., running, jumping rope) aerobic activity daily, including resistance and flexibility training (17). Although uncommon in the pediatric population, patients should be medically evaluated for comorbid conditions or diabetes complications that may restrict participation in an exercise program. As hyperglycemia can occur before, during, and after physical activity, it is important to ensure that the elevated glucose level is not related to insulin deficiency that would lead to worsening hyperglycemia with exercise and ketosis risk. Intense activity should be postponed with marked hyperglycemia (glucose ≥350 mg/dL [19.4 mmol/L]), moderate to large urine ketones, and/or β-hydroxybutyrate (B-OHB) >1.5 mmol/L. Caution may be needed when B-OHB levels are ≥0.6 mmol/L (12,14).
The prevention and treatment of hypoglycemia associated with physical activity include decreasing the prandial insulin for the meal/snack before exercise and/or increasing food intake. Youth on insulin pumps can lower basal rates by ∼10–50% or more or suspend for 1–2 h during exercise (18). Decreasing basal rates or long-acting insulin doses by ∼20% after exercise may reduce delayed exercise-induced hypoglycemia (19). Accessible rapid-acting carbohydrates and frequent blood glucose monitoring before, during, and after exercise, with or without continuous glucose monitoring (CGM), maximize safety with exercise. The use of hybrid closed-loop systems may improve time in range (70–180 mg/dL) during exercise, and youth can use “exercise mode” to prevent hypoglycemia (20).
Blood glucose targets prior to physical activity and exercise should be 126–180 mg/dL (7.0–10.0 mmol/L) but should be individualized based on the type, intensity, and duration of activity (14,16). Consider additional carbohydrate intake during and/or after exercise, depending on the duration and intensity of physical activity, to prevent hypoglycemia. For low- to moderate-intensity aerobic activities (30–60 min), and if the youth is fasting, 10–15 g of carbohydrate may prevent hypoglycemia (21). After insulin boluses (relative hyperinsulinemia), consider 0.5–1.0 g of carbohydrates/kg per hour of exercise (∼30–60 g), which is similar to carbohydrate requirements to optimize performance in athletes without type 1 diabetes (22–24).
In addition, obesity is as common in youth with type 1 diabetes as in those without diabetes. It is associated with a higher frequency of cardiovascular risk factors, and it disproportionately affects racial/ethnic minorities in the U.S. (25–29). Therefore, diabetes health care professionals should monitor weight status and encourage a healthy eating pattern, physical activity, and healthy weight as key components of pediatric type 1 diabetes care.
School and Child Care
As a large portion of a youth’s day is spent in school and/or day care, training of school or day care personnel to provide care in accordance with the child’s individualized diabetes medical management plan is essential for optimal diabetes management and safe access to all school or day care-sponsored opportunities (10,11,30). In addition, federal and state laws require schools, day care facilities, and other entities to provide needed diabetes care to enable the child to safely access the school or day care environment. Refer to the ADA position statements “Diabetes Care in the School Setting” (10) and “Care of Young Children With Diabetes in the Child Care Setting” (11) and ADA’s Safe at School website (diabetes.org/resources/know-your-rights/safe-at-school-state-laws) for additional details.
Psychosocial Care
Recommendations
14.9 At diagnosis and during routine follow-up care, screen for psychosocial issues and family stresses that could impact diabetes management and provide appropriate referrals to trained mental health professionals, preferably experienced in childhood diabetes. C
14.10 Mental health professionals should be considered integral members of the pediatric diabetes multidisciplinary team. E
14.11 Encourage developmentally appropriate family involvement in diabetes management tasks for children and adolescents, recognizing that premature transfer of diabetes care responsibility to the youth can result in diabetes burnout, suboptimal diabetes management, and deterioration in glycemia. A
14.12 Health care professionals should screen for food security, housing stability/homelessness, health literacy, financial barriers, and social/community support and apply that information to treatment decisions. E
14.13 Health care professionals should consider asking youth and their parents/caregivers about social adjustment (peer relationships) and school performance to determine whether further intervention is needed. B
14.14 Screen youth with diabetes for psychosocial and diabetes-related distress, generally starting at 7–8 years of age. Refer to a qualified mental health professional for further assessment and treatment if indicated. B
14.15 Offer adolescents time by themselves with their health care professional(s) starting at age 12 years or when developmentally appropriate. E
14.16 Starting at puberty, preconception counseling should be incorporated into routine diabetes care for all individuals of childbearing potential. A
14.17 Begin screening youth with type 1 diabetes for disordered eating between 10 and 12 years of age. Refer to a qualified mental health professional for further assessment and treatment if indicated. B
Rapid and dynamic cognitive, developmental, and emotional changes occur during childhood, adolescence, and emerging adulthood. Diabetes management during childhood and adolescence places substantial burdens on the youth and family, necessitating ongoing assessment of psychosocial status, social determinants of health, and diabetes distress in the youth and the parents/caregivers during routine diabetes visits (31–41). It is important to consider the impact of diabetes on quality of life as well as the development of mental health problems related to diabetes distress, fear of hypoglycemia (and hyperglycemia), symptoms of anxiety, disordered eating behaviors and eating disorders, and symptoms of depression (42). Consider screening youth for diabetes distress, generally starting at 7 or 8 years of age (43). Consider screening for depression and disordered eating behaviors using available screening tools (44,45). Early detection of depression, anxiety, disordered eating, and learning disabilities can facilitate effective treatment options and help minimize adverse effects on diabetes management and disease outcomes (35,43). There are validated tools that can be used in assessing diabetes-specific distress in youth starting at age 8 years and in their parents/caregivers (36,46). Furthermore, the complexities of diabetes management require ongoing parental involvement in care throughout childhood with developmentally appropriate family teamwork between the growing child/teen and parent in order to maintain engagement in self-management behaviors and to prevent deterioration in glycemia (47,48). It is appropriate to inquire about diabetes-specific family conflict during visits and to either help to negotiate a plan for resolution or refer to an appropriate mental health professional (49). Such professionals can conduct further assessment and deliver evidence-based behavioral interventions to support developmentally appropriate, collaborative family involvement in diabetes self-management (50,51). Monitoring of social adjustment (peer relationships) and school performance can facilitate both well-being and academic achievement (52). Elevated A1C is a risk factor for underperformance at school and increased absenteeism (53).
Shared decision-making with youth regarding the adoption of management plan components and self-management behaviors can improve diabetes self-efficacy, participation in diabetes care, and metabolic outcomes (26,54). Although cognitive abilities vary, the ethical position often adopted is the “mature minor rule,” whereby children after age 12 or 13 years who appear to be “mature” have the right to consent or withhold consent to general medical treatment, except in cases in which refusal would significantly endanger health (55).
Beginning at the onset of puberty or at diagnosis of diabetes, all individuals with childbearing potential should receive education about the risks of fetal malformations associated with elevated A1C and the use of effective contraception to prevent unplanned pregnancy. Preconception counseling using developmentally appropriate educational and behavioral strategies enables individuals of childbearing potential to make well-informed decisions (56). Preconception counseling resources tailored for adolescents are available at no cost through the ADA (57). Refer to the ADA position statement “Psychosocial Care for People With Diabetes” for further details (43).
Youth with type 1 diabetes have an increased risk of disordered eating behavior as well as clinical eating disorders with serious short-term and long-term negative effects on diabetes outcomes and health in general. It is important to recognize the unique and dangerous disordered eating behavior of insulin omission for weight management in type 1 diabetes (58) using tools such as the Diabetes Eating Problems Survey-Revised (DEPS-R) to allow for early diagnosis and intervention (45,59–61). Given the complexity of treating disordered eating behaviors, collaboration between the diabetes health care team and a mental health professional, ideally with expertise in disordered eating behaviors and diabetes, is recommended.
The presence of a mental health professional on pediatric multidisciplinary teams highlights the importance of attending to the psychosocial issues of diabetes. These psychosocial factors are significantly related to self-management difficulties, elevated A1C, reduced quality of life, and higher rates of acute and chronic diabetes complications.
Glycemic Monitoring, Insulin Delivery, and Targets
Recommendations
14.18 All youth with type 1 diabetes should monitor glucose levels multiple times daily (up to 6–10 times/day by blood glucose meter or continuous glucose monitoring), including prior to meals and snacks, at bedtime, and as needed for safety in specific situations such as physical activity, driving, or the presence of symptoms of hypoglycemia. B
14.19 Real-time continuous glucose monitoring B or intermittently scanned continuous glucose monitoring E should be offered for diabetes management in youth with diabetes on multiple daily injections or insulin pump therapy who are capable of using the device safely (either by themselves or with caregivers). The choice of device should be made based on the individual’s and family’s circumstances, desires, and needs.
14.20 Automated insulin delivery systems should be offered for diabetes management to youth with type 1 diabetes who are capable of using the device safely (either by themselves or with caregivers). The choice of device should be made based on the individual’s and family’s circumstances, desires, and needs. A
14.21 Insulin pump therapy alone should be offered for diabetes management to youth on multiple daily injections with type 1 diabetes who are capable of using the device safely (either by themselves or with caregivers). The choice of device should be made based on the individual’s and family’s circumstances, desires, and needs. A
14.22 Students must be supported at school in the use of diabetes technology, including continuous glucose monitors, insulin pumps, connected insulin pens, and automated insulin delivery systems as prescribed by their diabetes care team. E
14.23 A1C goals must be individualized and reassessed over time. An A1C of <7% (53 mmol/mol) is appropriate for many children and adolescents. B
14.24 Less stringent A1C goals (such as <7.5% [58 mmol/mol]) may be appropriate for youth who cannot articulate symptoms of hypoglycemia; have hypoglycemia unawareness; lack access to analog insulins, advanced insulin delivery technology, and/or continuous glucose monitoring; cannot check blood glucose regularly; or have nonglycemic factors that increase A1C (e.g., high glycators). B
14.25 Even less stringent A1C goals (such as <8% [64 mmol/mol]) may be appropriate for individuals with a history of severe hypoglycemia, limited life expectancy, or where the harms of treatment are greater than the benefits. B
14.26 Health care professionals may reasonably suggest more stringent A1C goals (such as <6.5% [48 mmol/mol]) for selected individuals if they can be achieved without significant hypoglycemia, negative impacts on well-being, or undue burden of care or in those who have nonglycemic factors that decrease A1C (e.g., lower erythrocyte life span). Lower targets may also be appropriate during the honeymoon phase. B
14.27 Continuous glucose monitoring metrics derived from continuous glucose monitor use over the most recent 14 days (or longer for youth with more glycemic variability), including time in range (70–180 mg/dL), time below target (<70 and <54 mg/dL), and time above target (>180 and >250 mg/dL), are recommended to be used in conjunction with A1C whenever possible. E
Current standards for diabetes management reflect the need to minimize hyperglycemia as safely as possible. The Diabetes Control and Complications Trial (DCCT), which did not enroll children <13 years of age, demonstrated that near normalization of blood glucose levels was more difficult to achieve in adolescents than in adults. Nevertheless, the increased use of basal-bolus regimens, insulin pumps, frequent blood glucose monitoring, CGM, automated insulin delivery systems, goal setting, and improved patient education has been associated with more children and adolescents reaching the blood glucose targets recommended by the ADA (62–64), particularly in families in which both the parents/caregivers and the child with diabetes participate jointly to perform the required diabetes-related tasks.
Lower A1C in adolescence and young adulthood is associated with a lower risk and rate of microvascular and macrovascular complications (65–68) and demonstrates the effects of metabolic memory (69–72).
In addition, type 1 diabetes can be associated with adverse effects on cognition during childhood and adolescence (6,73–75), and neurocognitive imaging differences related to hyperglycemia in children provide another motivation for achieving glycemic targets (6). DKA has been shown to cause adverse effects on brain development and function. Additional factors (76–79) that contribute to adverse effects on brain development and function include young age, severe hypoglycemia at <6 years of age, and chronic hyperglycemia (80–82). However, meticulous use of therapeutic modalities such as rapid- and long-acting insulin analogs, technological advances (e.g., CGM, sensor-augmented pump therapy, and automated insulin delivery systems), and intensive self-management education now make it more feasible to achieve glycemic goals while reducing the incidence of severe hypoglycemia (83–106). Please refer to Section 7, “Diabetes Technology,” for more information on technology to support people with diabetes.
In selecting individualized glycemic targets, the long-term health benefits of achieving a lower A1C should be balanced against the risks of hypoglycemia and the developmental burdens of intensive treatment plans in youth (107). Recent data with newer devices and insulins indicate that the risk of hypoglycemia with lower A1C is less than it was before (108–117). Some data suggest that there could be a threshold where lower A1C is associated with more hypoglycemia (118,119); however, the confidence intervals were large, suggesting great variability. In addition, achieving lower A1C levels is likely facilitated by setting lower A1C targets (120,121). Lower goals may be possible during the “honeymoon” phase of type 1 diabetes. Special consideration should be given to the risk of hypoglycemia in young children (aged <6 years) who are often unable to recognize, articulate, and/or manage hypoglycemia.However, registry data indicate that A1C targets can be achieved in children, including those aged <6 years, without increased risk of severe hypoglycemia (109,120). Recent data have demonstrated that the use of real-time CGM lowered A1C and increased time in range in adolescents and young adults and, in children aged <8 years old, was associated with a lower risk of hypoglycemia (122,123). Please refer to Section 6, “Glycemic Targets,” for more information on glycemic assessment.
A strong relationship exists between the frequency of blood glucose monitoring and glycemic management (84–86,124–130). Glucose levels for all children and adolescents with type 1 diabetes should be monitored multiple times daily by blood glucose monitoring and/or CGM. In the U.S., real-time CGM is approved for nonadjunctive use in children aged 2 years and older and intermittently scanned CGM is approved for nonadjunctive use in children aged 4 years and older. Parents/caregivers and youth should be offered initial and ongoing education and support for CGM use. Behavioral support may further improve ongoing CGM use (123). Metrics derived from CGM include percent time in target range, below target range, and above target range (131). While studies indicate a relationship between time in range and A1C (132,133), it is still uncertain what the ideal target time in range should be for children, and further studies are needed. Please refer to Section 7, “Diabetes Technology,” for more information on the use of blood glucose meters, CGM, and insulin pumps. More information on insulin injection technique can be found in Section 9, “Pharmacologic Approaches to Glycemic Treatment.”
Key Concepts in Setting Glycemic Targets
Targets should be individualized, and lower targets may be reasonable based on a benefit–risk assessment.
Blood glucose targets should be modified in children with frequent hypoglycemia or hypoglycemia unawareness.
Postprandial blood glucose values should be measured when there is a discrepancy between preprandial blood glucose values and A1C levels and to assess preprandial insulin doses in those on basal-bolus or pump regimens.
Autoimmune Conditions
Recommendation
14.28 Assess for additional autoimmune conditions soon after the diagnosis of type 1 diabetes and if symptoms develop. B
Because of the increased frequency of other autoimmune diseases in type 1 diabetes, screening for thyroid dysfunction and celiac disease should be considered (134–138). Periodic screening in asymptomatic individuals has been recommended, but the optimal frequency of screening is unclear.
Although much less common than thyroid dysfunction and celiac disease, other autoimmune conditions, such as Addison disease (primary adrenal insufficiency), autoimmune hepatitis, autoimmune gastritis, dermatomyositis, and myasthenia gravis, occur more commonly in the population with type 1 diabetes than in the general pediatric population and should be assessed and monitored as clinically indicated. In addition, relatives of youth with type 1 diabetes should be offered testing for islet autoantibodies through research studies (e.g., TrialNet) and national programs for early diagnosis of preclinical type 1 diabetes (stages 1 and 2).
Thyroid Disease
Recommendations
14.29 Consider testing children with type 1 diabetes for antithyroid peroxidase and antithyroglobulin antibodies soon after diagnosis. B
14.30 Measure thyroid-stimulating hormone concentrations at diagnosis when clinically stable or soon after optimizing glycemia. If normal, suggest rechecking every 1–2 years or sooner if the youth has positive thyroid antibodies or develops symptoms or signs suggestive of thyroid dysfunction, thyromegaly, an abnormal growth rate, or unexplained glycemic variability. B
Autoimmune thyroid disease is the most common autoimmune disorder associated with diabetes, occurring in 17–30% of individuals with type 1 diabetes (135,139,140). At the time of diagnosis, ∼25% of children with type 1 diabetes have thyroid autoantibodies (141), the presence of which is predictive of thyroid dysfunction—most commonly hypothyroidism, although hyperthyroidism occurs in ∼0.5% of people with type 1 diabetes (142,143). For thyroid autoantibodies, a study from Sweden indicated that antithyroid peroxidase antibodies were more predictive than antithyroglobulin antibodies in multivariate analysis (144). Thyroid function tests may be misleading (euthyroid sick syndrome) if performed at the time of diagnosis owing to the effect of previous hyperglycemia, ketosis or ketoacidosis, weight loss, etc. Therefore, if performed at diagnosis and slightly abnormal, thyroid function tests should be repeated soon after a period of metabolic stability and achievement of glycemic targets. Subclinical hypothyroidism may be associated with an increased risk of symptomatic hypoglycemia (145) and a reduced linear growth rate. Hyperthyroidism alters glucose metabolism and usually causes deterioration of glycemia.
Celiac Disease
Recommendations
14.31 Screen youth with type 1 diabetes for celiac disease by measuring IgA tissue transglutaminase (tTG) antibodies, with documentation of normal total serum IgA levels, soon after the diagnosis of diabetes, or IgG tTG and deamidated gliadin antibodies if IgA is deficient. B
14.32 Repeat screening within 2 years of diabetes diagnosis and then again after 5 years and consider more frequent screening in youth who have symptoms or a first-degree relative with celiac disease. B
14.33 Individuals with confirmed celiac disease should be placed on a gluten-free diet for treatment and to avoid complications; they should also have a consultation with a registered dietitian nutritionist experienced in managing both diabetes and celiac disease. B
Celiac disease is an immune-mediated disorder that occurs with increased frequency in people with type 1 diabetes (1.6–16.4% of individuals compared with 0.3–1% in the general population) (134,137,138,146–150). Screening people with type 1 diabetes for celiac disease is further justified by its association with osteoporosis, iron deficiency, growth failure, and potential increased risk of retinopathy and albuminuria (151–154).
Screening for celiac disease includes measuring serum levels of IgA and tissue transglutaminase (tTG) IgA antibodies, or, with IgA deficiency, screening can include measuring tTG IgG antibodies or deamidated gliadin peptide IgG antibodies. Because most cases of celiac disease are diagnosed within the first 5 years after the diagnosis of type 1 diabetes, screening should be considered at the time of diagnosis and repeated at 2 and then 5 years (148) or if clinical symptoms indicate, such as poor growth or increased hypoglycemia (149,151).
Although celiac disease can be diagnosed more than 10 years after diabetes diagnosis, there are insufficient data after 5 years to determine the optimal screening frequency. Measurement of tTG antibody should be considered at other times in individuals with symptoms suggestive of celiac disease (148). Monitoring for symptoms should include an assessment of linear growth and weight gain (149,151). A small bowel biopsy in antibody-positive children is recommended to confirm the diagnosis (155). European guidelines on screening for celiac disease in children (not specific to children with type 1 diabetes) suggest that biopsy may not be necessary in symptomatic children with high antibody titers (i.e., greater than 10 times the upper limit of normal) provided that further testing is performed (verification of endomysial antibody positivity on a separate blood sample) (156). Whether this approach may be appropriate for asymptomatic children in high-risk groups remains an open question, though evidence is emerging (157). It is also advisable to check for celiac disease-associated HLA types in patients who are diagnosed without a small intestinal biopsy. In symptomatic children with type 1 diabetes and confirmed celiac disease, gluten-free diets reduce symptoms and rates of hypoglycemia (158). The challenging dietary restrictions associated with having both type 1 diabetes and celiac disease place a significant burden on individuals. Therefore, a biopsy to confirm the diagnosis of celiac disease is recommended, especially in asymptomatic children, before establishing a diagnosis of celiac disease (159) and endorsing significant dietary changes. A gluten-free diet was beneficial in asymptomatic adults with positive antibodies confirmed by biopsy (160).
Management of Cardiovascular Risk Factors
Hypertension Screening
Recommendation
14.34 Blood pressure should be measured at every routine visit. In youth with high blood pressure (blood pressure ≥90th percentile for age, sex, and height or, in adolescents aged ≥13 years, blood pressure ≥120/80 mmHg) on three separate measurements, ambulatory blood pressure monitoring should be strongly considered. B
Hypertension Treatment
Recommendations
14.35 Treatment of elevated blood pressure (defined as 90th to <95th percentile for age, sex, and height or, in adolescents aged ≥13 years, 120–129/<80 mmHg) is lifestyle modification focused on healthy nutrition, physical activity, sleep, and, if appropriate, weight management. C
14.36 In addition to lifestyle modification, ACE inhibitors or angiotensin receptor blockers should be started for treatment of confirmed hypertension (defined as blood pressure consistently ≥95th percentile for age, sex, and height or, in adolescents aged ≥13 years, ≥130/80 mmHg). Due to the potential teratogenic effects, individuals of childbearing age should receive reproductive counseling, and ACE inhibitors and angiotensin receptor blockers should be avoided in individuals of childbearing age who are not using reliable contraception. B
14.37 The goal of treatment is blood pressure <90th percentile for age, sex, and height or, in adolescents aged ≥13 years, <130/80 mmHg. C
Blood pressure measurements should be performed using the appropriate size cuff with the youth seated and relaxed. Elevated blood pressure should be confirmed on at least three separate days, and ambulatory blood pressure monitoring should be considered. Evaluation should proceed as clinically indicated (161,162). Treatment is generally initiated with an ACE inhibitor, but an angiotensin receptor blocker can be used if the ACE inhibitor is not tolerated (e.g., due to cough) (163).
Dyslipidemia Screening
Recommendations
14.38 Initial lipid profile should be performed soon after diagnosis, preferably after glycemia has improved and age is ≥2 years. If initial LDL cholesterol is ≤100 mg/dL (2.6 mmol/L), subsequent testing should be performed at 9–11 years of age. B Initial testing may be done with a nonfasting lipid level with confirmatory testing with a fasting lipid panel.
14.39 If LDL cholesterol values are within the accepted risk level (<100 mg/dL [2.6 mmol/L]), a lipid profile repeated every 3 years is reasonable. E
Dyslipidemia Treatment
Recommendations
14.40 If lipids are abnormal, initial therapy should consist of optimizing glycemia and medical nutrition therapy to limit the amount of calories from fat to 25–30% and saturated fat to <7%, limit cholesterol to <200 mg/day, avoid trans fats, and aim for ∼10% calories from monounsaturated fats. A
14.41 After the age of 10 years, addition of a statin may be considered in youth with type 1 diabetes who, despite medical nutrition therapy and lifestyle changes, continue to have LDL cholesterol >160 mg/dL (4.1 mmol/L) or LDL cholesterol >130 mg/dL (3.4 mmol/L) and one or more cardiovascular disease risk factors. E Due to the potential teratogenic effects, individuals of childbearing age should receive reproductive counseling, and statins should be avoided in individuals of childbearing age who are not using reliable contraception. B
14.42 The goal of therapy is an LDL cholesterol value <100 mg/dL (2.6 mmol/L). E
Population-based studies estimate that 14–45% of children with type 1 diabetes have two or more atherosclerotic cardiovascular disease (ASCVD) risk factors (164–166), and the prevalence of cardiovascular disease (CVD) risk factors increase with age (166) and among racial/ethnic minorities (25), with girls having a higher risk burden than boys (165).
Pathophysiology.
The atherosclerotic process begins in childhood, and although ASCVD events are not expected to occur during childhood, observations using a variety of methodologies show that youth with type 1 diabetes may have subclinical CVD within the first decade of diagnosis (167–169). Studies of carotid intima-media thickness have yielded inconsistent results (162,163).
Screening.
Diabetes predisposes to the development of accelerated arteriosclerosis. Lipid evaluation for these patients contributes to risk assessment and identifies an important proportion of those with dyslipidemia. Therefore, initial screening should be done soon after diagnosis. If the initial screen is normal, subsequent screening may be done at 9–11 years of age, which is a stable time for lipid assessment in children (170). Children with a primary lipid disorder (e.g., familial hyperlipidemia) should be referred to a lipid specialist. Non-HDL cholesterol level has been identified as a significant predictor of the presence of atherosclerosis—as powerful as any other lipoprotein cholesterol measure in children and adolescents. For both children and adults, non-HDL cholesterol level seems to be more predictive of persistent dyslipidemia and, therefore, atherosclerosis and future events than total cholesterol, LDL cholesterol, or HDL cholesterol levels alone. A major advantage of non-HDL cholesterol is that it can be accurately calculated in a nonfasting state and therefore is practical to obtain in clinical practice as a screening test (171). Youth with type 1 diabetes have a high prevalence of lipid abnormalities (164,172).
Even if normal, screening should be repeated within 3 years, as A1C and other cardiovascular risk factors can change dramatically during adolescence (173).
Treatment.
Pediatric lipid guidelines provide some guidance relevant to children with type 1 diabetes and secondary dyslipidemia (162,170,174,175); however, there are few studies on modifying lipid levels in children with type 1 diabetes. A 6-month trial of dietary counseling produced a significant improvement in lipid levels (176); likewise, a lifestyle intervention trial with 6 months of exercise in adolescents demonstrated improvement in lipid levels (177). Data from the SEARCH for Diabetes in Youth (SEARCH) study show that improved glucose over a 2-year period is associated with a more favorable lipid profile; however, improved glycemia alone will not normalize lipids in youth with type 1 diabetes and dyslipidemia (173).
Although intervention data are sparse, the American Heart Association categorizes children with type 1 diabetes in the highest tier for cardiovascular risk and recommends both lifestyle and pharmacologic treatment for those with elevated LDL cholesterol levels (175,178). Initial therapy should include a nutrition plan that restricts saturated fat to 7% of total calories and dietary cholesterol to 200 mg/day (170). Data from randomized clinical trials in children as young as 7 months of age indicate that this diet is safe and does not interfere with normal growth and development (179).
Neither long-term safety nor cardiovascular outcome efficacy of statin therapy has been established for children; however, studies have shown short-term safety equivalent to that seen in adults and efficacy in lowering LDL cholesterol levels in familial hypercholesterolemia or severe hyperlipidemia, improving endothelial function and causing regression of carotid intimal thickening (180,181). Statins are not approved for children aged <10 years, and statin treatment should generally not be used in children with type 1 diabetes before this age. Statins are contraindicated in pregnancy; therefore, the prevention of unplanned pregnancies is of paramount importance. Statins should be avoided in individuals of childbearing age who are not using reliable contraception (see Section 15, “Management of Diabetes in Pregnancy,” for more information). The multicenter, randomized, placebo-controlled Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial (AdDIT) provides safety data on pharmacologic treatment with an ACE inhibitor and statin in adolescents with type 1 diabetes (162).
Smoking
Recommendations
14.43 Elicit a smoking history at initial and follow-up diabetes visits; discourage smoking in youth who do not smoke and encourage smoking cessation in those who do smoke. A
14.44 Electronic cigarette use should be discouraged. A
The adverse health effects of smoking are well recognized with respect to future cancer and CVD risk. Despite this, smoking rates are significantly higher among youth with diabetes than among youth without diabetes (182,183). In youth with diabetes, it is important to avoid additional CVD risk factors. Smoking increases the risk of the onset of albuminuria; therefore, smoking avoidance is important to prevent both microvascular and macrovascular complications (170,184). Discouraging cigarette smoking, including electronic cigarettes (185,186), is an important part of routine diabetes care. In light of CDC evidence of deaths related to electronic cigarette use (187,188), no individuals should be advised to use electronic cigarettes, either as a way to stop smoking tobacco or as a recreational drug. In younger children, it is important to assess exposure to cigarette smoke in the home because of the adverse effects of secondhand smoke and to discourage youth from ever smoking.
Microvascular Complications
Nephropathy Screening
Recommendation
14.45 Annual screening for albuminuria with a random (morning sample preferred to avoid effects of exercise) spot urine sample for albumin-to-creatinine ratio should be considered at puberty or at age >10 years, whichever is earlier, once the child has had diabetes for 5 years. B
Nephropathy Treatment
Recommendation
14.46 An ACE inhibitor or an angiotensin receptor blocker, titrated to normalization of albumin excretion, may be considered when elevated urinary albumin-to-creatinine ratio (>30 mg/g) is documented (two of three urine samples obtained over a 6-month interval following efforts to improve glycemia and normalize blood pressure). E Due to the potential teratogenic effects, individuals of childbearing age should receive reproductive counseling, and ACE inhibitors and angiotensin receptor blockers should be avoided in individuals of childbearing age who are not using reliable contraception. B
Data from 7,549 participants <20 years of age in the T1D Exchange clinic registry emphasize the importance of meeting glycemic and blood pressure goals, particularly as diabetes duration increases, in order to reduce the risk of diabetic kidney disease. The data also underscore the importance of routine screening to ensure early diagnosis and timely treatment of albuminuria (189). An estimation of glomerular filtration rate (GFR), calculated using GFR estimating equations from the serum creatinine, height, age, and sex (190), should be considered at baseline and repeated as indicated based on clinical status, age, diabetes duration, and therapies. Improved methods are needed to screen for early GFR loss since estimated GFR is inaccurate at GFR >60 mL/min/1.73 m2 (190,191). The AdDIT study in adolescents with type 1 diabetes demonstrated the safety of ACE inhibitor treatment, but the treatment did not change the albumin-to-creatinine ratio over the course of the study (162).
Retinopathy
Recommendations
14.47 An initial dilated and comprehensive eye examination is recommended once youth have had type 1 diabetes for 3–5 years, provided they are aged ≥11 years or puberty has started, whichever is earlier. B
14.48 After the initial examination, repeat dilated and comprehensive eye examination every 2 years. Less frequent examinations, every 4 years, may be acceptable on the advice of an eye care professional and based on risk factor assessment, including a history of A1C <8%. B
14.49 Programs that use retinal photography (with remote reading or use of a validated assessment tool) to improve access to diabetic retinopathy screening can be appropriate screening strategies for diabetic retinopathy. Such programs need to provide pathways for timely referral for a comprehensive eye examination when indicated. E
Retinopathy (like albuminuria) most commonly occurs after the onset of puberty and after 5–10 years of diabetes duration (192). It is currently recognized that there is a low risk of development of vision-threatening retinal lesions prior to 12 years of age (193,194). A 2019 publication based on the follow-up of the DCCT adolescent cohort supports a lower frequency of eye examinations than previously recommended, particularly in adolescents with A1C closer to the target range (195,196). Referrals should be made to eye care professionals with expertise in diabetic retinopathy and experience in counseling pediatric patients and families on the importance of prevention, early detection, and intervention.
Neuropathy
Recommendation
14.50 Consider an annual comprehensive foot exam at the start of puberty or at age ≥10 years, whichever is earlier, once the youth has had type 1 diabetes for 5 years. The examination should include inspection, assessment of foot pulses, pinprick, and 10-g monofilament sensation tests, testing of vibration sensation using a 128-Hz tuning fork, and ankle reflex tests. B
Diabetic neuropathy rarely occurs in prepubertal children or after only 1–2 years of diabetes (192), although data suggest a prevalence of distal peripheral neuropathy of 7% in 1,734 youth with type 1 diabetes and association with the presence of CVD risk factors (197,198). A comprehensive foot exam, including inspection, palpation of dorsalis pedis and posterior tibial pulses, and determination of proprioception, vibration, and monofilament sensation, should be performed annually along with an assessment of symptoms of neuropathic pain (198). Foot inspection can be performed at each visit to educate youth regarding the importance of foot care (see Section 12, “Retinopathy, Neuropathy, and Foot Care”).
Type 2 Diabetes
For information on risk-based screening for type 2 diabetes and prediabetes in children and adolescents, please refer to Section 2, “Classification and Diagnosis of Diabetes.” For additional support for these recommendations, see the ADA position statement “Evaluation and Management of Youth-Onset Type 2 Diabetes” (3).
The prevalence of type 2 diabetes in youth has continued to increase over the past 20 years (4). The CDC published projections for type 2 diabetes prevalence using the SEARCH database; assuming a 2.3% annual increase, the prevalence in those under 20 years of age will quadruple in 40 years (199,200).
Evidence suggests that type 2 diabetes in youth is different not only from type 1 diabetes but also from type 2 diabetes in adults and has unique features, such as a more rapidly progressive decline in β-cell function and accelerated development of diabetes complications (3,201). Long-term follow-up data from the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study showed that a majority of individuals with type 2 diabetes diagnosed as youth had microvascular complications by young adulthood (202). Type 2 diabetes disproportionately impacts youth of ethnic and racial minorities and can occur in complex psychosocial and cultural environments, which may make it difficult to sustain healthy lifestyle changes and self-management behaviors (26,203–206). Additional risk factors associated with type 2 diabetes in youth include adiposity, family history of diabetes, female sex, and low socioeconomic status (201).
As with type 1 diabetes, youth with type 2 diabetes spend much of the day in school. Therefore, close communication with and the cooperation of school personnel are essential for optimal diabetes management, safety, and maximal academic opportunities.
Screening and Diagnosis
Recommendations
14.51 Risk-based screening for prediabetes and/or type 2 diabetes should be considered after the onset of puberty or ≥10 years of age, whichever occurs earlier, in youth with overweight (BMI ≥85th percentile) or obesity (BMI ≥95th percentile) and who have one or more additional risk factors for diabetes (see Table 2.4 for evidence grading of other risk factors).
14.52 If screening is normal, repeat screening at a minimum of 3-year intervals E, or more frequently if BMI is increasing. C
14.53 Fasting plasma glucose, 2-h plasma glucose during a 75-g oral glucose tolerance test, and A1C can be used to test for prediabetes or diabetes in children and adolescents. B
14.54 Children and adolescents with overweight or obesity in whom the diagnosis of type 2 diabetes is being considered should have a panel of pancreatic autoantibodies tested to exclude the possibility of autoimmune type 1 diabetes. B
In the last decade, the incidence and prevalence of type 2 diabetes in adolescents has increased dramatically, especially in racial and ethnic minority populations (170,207). A few studies suggest oral glucose tolerance tests or fasting plasma glucose values as more suitable diagnostic tests than A1C in the pediatric population, especially among certain ethnicities (208), although fasting glucose alone may overdiagnose diabetes in children (209,210). In addition, many of these studies do not recognize that diabetes diagnostic criteria are based on long-term health outcomes, and validations are not currently available in the pediatric population (211). An analysis of National Health and Nutrition Examination Survey (NHANES) data suggests using A1C for screening of high-risk youth (212).
The ADA acknowledges the limited data supporting A1C for diagnosing type 2 diabetes in children and adolescents. Although A1C is not recommended for diagnosis of diabetes in children with cystic fibrosis or symptoms suggestive of acute onset of type 1 diabetes, and only A1C assays without interference are appropriate for children with hemoglobinopathies, the ADA continues to recommend A1C for diagnosis of type 2 diabetes in this population (213,214).
Diagnostic Challenges
Given the current obesity epidemic, distinguishing between type 1 and type 2 diabetes in children can be difficult. Overweight and obesity are common in children with type 1 diabetes (27), and diabetes-associated autoantibodies and ketosis may be present in pediatric individuals with clinical features of type 2 diabetes (including obesity and acanthosis nigricans) (209). The presence of islet autoantibodies has been associated with faster progression to insulin deficiency (209). At the onset, DKA occurs in ∼6% of youth aged 10–19 years with type 2 diabetes (215). Although uncommon, type 2 diabetes has been observed in prepubertal children under the age of 10 years, and thus it should be part of the differential in children with suggestive symptoms (216). Finally, obesity contributes to the development of type 1 diabetes in some individuals (217), which further blurs the lines between diabetes types. However, accurate diagnosis is critical, as treatment plans, educational approaches, dietary advice, and outcomes differ markedly between patients with the two diagnoses. The significant diagnostic difficulties posed by MODY are discussed in Section 2, “Classification and Diagnosis of Diabetes.” In addition, there are rare and atypical diabetes cases that represent a challenge for clinicians and researchers.
Management
Lifestyle Management
Recommendations
14.55 All youth with type 2 diabetes and their families should receive comprehensive diabetes self-management education and support that is specific to youth with type 2 diabetes and is culturally appropriate. B
14.56 Youth with overweight/obesity and type 2 diabetes and their families should be provided with developmentally and culturally appropriate comprehensive lifestyle programs that are integrated with diabetes management to achieve a 7–10% decrease in excess weight. C
14.57 Given the necessity of long-term weight management for youth with type 2 diabetes, lifestyle intervention should be based on a chronic care model and offered in the context of diabetes care. E
14.58 Youth with prediabetes and type 2 diabetes, like all children and adolescents, should be encouraged to participate in at least 60 min of moderate to vigorous physical activity daily (with muscle and bone strength training at least 3 days/week) B and to decrease sedentary behavior. C
14.59 Nutrition for youth with prediabetes and type 2 diabetes, like for all children and adolescents, should focus on healthy eating patterns that emphasize consumption of nutrient-dense, high-quality foods and decreased consumption of calorie-dense, nutrient-poor foods, particularly sugar-added beverages. B
Glycemic Targets
Recommendations
14.60 Blood glucose monitoring should be individualized, taking into consideration the pharmacologic treatment of the patient. E
14.61 Real-time continuous glucose monitoring or intermittently scanned continuous glucose monitoring should be offered for diabetes management in youth with type 2 diabetes on multiple daily injections or insulin pumps who are capable of using the device safely (either by themselves or with a caregiver). The choice of device should be made based on an individual’s and family’s circumstances, desires, and needs. E
14.62 Glycemic status should be assessed every 3 months. E
14.63 A reasonable A1C target for most children and adolescents with type 2 diabetes is <7% (53 mmol/mol). More stringent A1C targets (such as <6.5% [48 mmol/mol]) may be appropriate for selected individuals if they can be achieved without significant hypoglycemia or other adverse effects of treatment. Appropriate individuals might include those with a short duration of diabetes and lesser degrees of β-cell dysfunction and individuals treated with lifestyle or metformin only who achieve significant weight improvement. E
14.64 Less stringent A1C goals (such as 7.5% [58 mmol/mol]) may be appropriate if there is an increased risk of hypoglycemia. E
14.65 A1C targets for individuals on insulin should be individualized, taking into account the relatively low rates of hypoglycemia in youth-onset type 2 diabetes. E
Pharmacologic Management
Recommendations
14.66 Initiate pharmacologic therapy, in addition to behavioral counseling for healthful nutrition and physical activity changes, at diagnosis of type 2 diabetes. A
14.67 In individuals with incidentally diagnosed or metabolically stable diabetes (A1C <8.5% [69 mmol/mol] and asymptomatic), metformin is the initial pharmacologic treatment of choice if renal function is normal. A
14.68 Youth with marked hyperglycemia (blood glucose ≥250 mg/dL [13.9 mmol/L], A1C ≥8.5% [69 mmol/mol]) without acidosis at diagnosis who are symptomatic with polyuria, polydipsia, nocturia, and/or weight loss should be treated initially with long-acting insulin while metformin is initiated and titrated. B
14.69 In individuals with ketosis/ketoacidosis, treatment with subcutaneous or intravenous insulin should be initiated to rapidly correct the hyperglycemia and the metabolic derangement. Once acidosis is resolved, metformin should be initiated while subcutaneous insulin therapy is continued. A
14.70 In individuals presenting with severe hyperglycemia (blood glucose ≥600 mg/dL [33.3 mmol/L]), consider assessment for hyperglycemic hyperosmolar nonketotic syndrome. A
14.71 If glycemic targets are no longer met with metformin (with or without long-acting insulin), glucagon-like peptide 1 receptor agonist therapy approved for youth with type 2 diabetes should be considered in children 10 years of age or older if they have no past medical history or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2. A
14.72 Individuals treated with metformin, a glucagon-like peptide 1 receptor agonist, and long-acting insulin who do not meet glycemic targets should be moved to multiple daily injections with long-acting and prandial insulins or insulin pump therapy. E
14.73 In individuals initially treated with insulin and metformin who are meeting glucose targets based on blood glucose monitoring, insulin can be tapered over 2–6 weeks by decreasing the insulin dose 10–30% every few days. B
14.74 Use of medications not approved by the U.S. Food and Drug Administration for youth with type 2 diabetes is not recommended outside of research trials. B
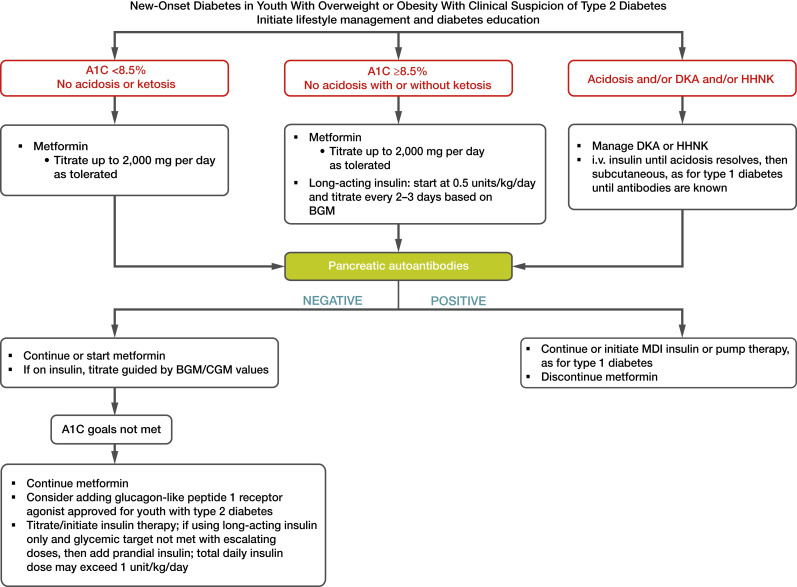
Treatment of youth-onset type 2 diabetes should include lifestyle management, diabetes self-management education and support, and pharmacologic treatment. Initial treatment of youth with obesity and diabetes must take into account that diabetes type is often uncertain in the first few weeks of treatment due to overlap in presentation and that a substantial percentage of youth with type 2 diabetes will present with clinically significant ketoacidosis (218). Therefore, initial therapy should address the hyperglycemia and associated metabolic derangements irrespective of ultimate diabetes type, with adjustment of therapy once metabolic compensation has been established and subsequent information, such as islet autoantibody results, becomes available. Figure 14.1 provides an approach to the initial treatment of new-onset diabetes in youth with overweight or obesity with clinical suspicion of type 2 diabetes.
Figure 14.1.
Management of new-onset diabetes in youth with overweight or obesity with clinical suspicion of type 2 diabetes. A1C 8.5% = 69 mmol/mol. Adapted from the ADA position statement “Evaluation and Management of Youth-Onset Type 2 Diabetes” (3). BGM, blood glucose monitoring; CGM, continuous glucose monitoring; DKA, diabetic ketoacidosis; HHNK, hyperosmolar hyperglycemic nonketotic syndrome; i.v., intravenous; MDI, multiple daily injections.
Glycemic targets should be individualized, taking into consideration the long-term health benefits of more stringent targets and risk for adverse effects, such as hypoglycemia. A lower target A1C in youth with type 2 diabetes when compared with those recommended in type 1 diabetes is justified by a lower risk of hypoglycemia and higher risk of complications (202,219–222).
Self-management in pediatric diabetes involves both the youth and their parents/adult caregivers. Individuals and their families should receive education and support for healthful nutrition and physical activity such as a balanced meal plan, achieving and maintaining a healthy weight, and regular physical activity. Physical activity should include aerobic, muscle-strengthening, and bone-strengthening activities (17). A family-centered approach to nutrition and lifestyle modification is essential in children and adolescents with type 2 diabetes, and nutrition recommendations should be culturally appropriate and sensitive to family resources (see Section 5, “Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes”). Given the complex social and environmental context surrounding youth with type 2 diabetes, individual-level lifestyle interventions may not be sufficient to target the complex interplay of family dynamics, mental health, community readiness, and the broader environmental system (3).
A multidisciplinary diabetes team, including a physician, diabetes care and education specialist, registered dietitian nutritionist, and psychologist or social worker, is essential. In addition to achieving glycemic targets and self-management education (223–225), initial treatment must include management of comorbidities such as obesity, dyslipidemia, hypertension, and microvascular complications.
Current pharmacologic treatment options for youth-onset type 2 diabetes are limited to three approved drugs classes: insulin, metformin, and glucagon-like peptide 1 receptor agonists. Presentation with ketoacidosis or marked ketosis requires a period of insulin therapy until fasting and postprandial glycemia have been restored to normal or near-normal levels. Insulin pump therapy may be considered as an option for those on long-term multiple daily injections who are able to safely manage the device. Initial treatment should also be with insulin when the distinction between type 1 diabetes and type 2 diabetes is unclear and in patients who have random blood glucose concentrations ≥250 mg/dL (13.9 mmol/L) and/or A1C ≥8.5% (69 mmol/mol) (226). Metformin therapy should be added after resolution of ketosis/ketoacidosis.
When initial insulin treatment is not required, initiation of metformin is recommended. The TODAY study found that metformin alone provided durable glycemic control (A1C ≤8% [64 mmol/mol] for 6 months) in approximately half of the subjects (227). The Restoring Insulin Secretion (RISE) Consortium study did not demonstrate differences in measures of glucose or β-cell function preservation between metformin and insulin, but there was more weight gain with insulin (228).
To date, the TODAY study is the only trial combining lifestyle and metformin therapy in youth with type 2 diabetes; the combination did not perform better than metformin alone in achieving durable glycemic control (227).
A randomized clinical trial in youth aged 10–17 years with type 2 diabetes demonstrated the addition of subcutaneous liraglutide (up to 1.8 mg daily) to metformin (with or without long-acting insulin) as safe and effective to decrease A1C (estimated decrease of 1.06 percentage points at 26 weeks and 1.30 percentage points at 52 weeks), although it did increase the frequency of gastrointestinal side effects (229). Liraglutide and once-weekly exenatide extended release are approved for the treatment of type 2 diabetes in youth aged 10 years or older (230–232).
Blood glucose monitoring plans should be individualized, taking into consideration the pharmacologic treatment of the person. Although data on CGM in youth with type 2 diabetes are sparse (233), CGM could be considered in individuals requiring frequent blood glucose monitoring for diabetes management.
Metabolic Surgery
Recommendations
14.75 Metabolic surgery may be considered for the treatment of adolescents with type 2 diabetes who have severe obesity (BMI >35 kg/m2) and who have elevated A1C and/or serious comorbidities despite lifestyle and pharmacologic intervention. A
14.76 Metabolic surgery should be performed only by an experienced surgeon working as part of a well-organized and engaged multidisciplinary team, including a surgeon, endocrinologist, registered dietitian nutritionist, behavioral health specialist, and nurse. A
The results of weight loss and lifestyle interventions for obesity in children and adolescents have been disappointing, and treatment options as adjuncts to lifestyle therapy are limited. Recent U.S. Food and Drug Administration–approved medications for youth ages 12 and older include phentermine and topiramate extended-release capsules and liraglutide (234–236). Over the last decade, weight loss surgery has been increasingly performed in adolescents with obesity. Small retrospective analyses and a prospective multicenter, nonrandomized study suggest that bariatric or metabolic surgery may have benefits in adolescents with obesity and type 2 diabetes similar to those observed in adults. Teenagers experience similar degrees of weight loss, diabetes remission, and improvement of cardiometabolic risk factors for at least 3 years after surgery (237). A secondary data analysis from the Teen-Longitudinal Assessment of Bariatric Surgery (Teen-LABS) and TODAY studies suggests surgical treatment of adolescents with severe obesity and type 2 diabetes is associated with improved glycemia (238); however, no randomized trials have yet compared the effectiveness and safety of surgery to those of conventional treatment options in adolescents (239). The guidelines used as an indication for metabolic surgery in adolescents generally include BMI >35 kg/m2 with comorbidities or BMI >40 kg/m2 with or without comorbidities (240–251). A number of groups, including the Pediatric Bariatric Study Group and Teen-LABS study, have demonstrated the effectiveness of metabolic surgery in adolescents (244–250).
Prevention and Management of Diabetes Complications
Hypertension
Recommendations
14.77 Blood pressure should be measured at every visit. In youth with high blood pressure (blood pressure ≥90th percentile for age, sex, and height or, in adolescents aged ≥13 years, ≥120/80 mmHg) on three separate measurements, ambulatory blood pressure monitoring should be strongly considered. B
14.78 Treatment of elevated blood pressure (defined as 90th to <95th percentile for age, sex, and height or, in adolescents aged ≥13 years, 120–129/<80 mmHg) is lifestyle modification focused on healthy nutrition, physical activity, sleep, and, if appropriate, weight management. C
14.79 In addition to lifestyle modification, ACE inhibitors or angiotensin receptor blockers should be started for treatment of confirmed hypertension (defined as blood pressure consistently ≥95th percentile for age, sex, and height or, in adolescents aged ≥13 years, ≥130/80 mmHg). Due to the potential teratogenic effects, individuals of childbearing age should receive reproductive counseling, and ACE inhibitors and angiotensin receptor blockers should be avoided in individuals of childbearing age who are not using reliable contraception. B
14.80 The goal of treatment is blood pressure <90th percentile for age, sex, and height or, in adolescents aged ≥13 years, <130/80 mmHg. C
Nephropathy
Recommendations
14.81 Protein intake should be at the recommended daily allowance of 0.8 g/kg/day. E
14.82 Urine albumin-to-creatinine ratio should be obtained at the time of diagnosis and annually thereafter. An elevated urine albumin-to-creatinine ratio (>30 mg/g creatinine) should be confirmed on two of three samples. B
14.83 Estimated glomerular filtration rate should be determined at the time of diagnosis and annually thereafter. E
14.84 In youth with diabetes and hypertension, either an ACE inhibitor or an angiotensin receptor blocker is recommended for those with modestly elevated urinary albumin-to-creatinine ratio (30–299 mg/g creatinine) and is strongly recommended for those with urinary albumin-to-creatinine ratio >300 mg/g creatinine and/or estimated glomerular filtration rate <60 mL/min/1.73 m2. E Due to the potential teratogenic effects, individuals of childbearing age should receive reproductive counseling, and ACE inhibitors and angiotensin receptor blockers should be avoided in individuals of childbearing age who are not using reliable contraception. B
14.85 For those with nephropathy, continued monitoring (yearly urinary albumin-to-creatinine ratio, estimated glomerular filtration rate, and serum potassium) may aid in assessing engagement and detecting progression of disease. E
14.86 Referral to nephrology is recommended in case of uncertainty of etiology, worsening urinary albumin-to-creatinine ratio, or decrease in estimated glomerular filtration rate. E
Neuropathy
Recommendations
14.87 Youth with type 2 diabetes should be screened for the presence of neuropathy by foot examination at diagnosis and annually. The examination should include inspection, assessment of foot pulses, pinprick and 10-g monofilament sensation tests, testing of vibration sensation using a 128-Hz tuning fork, and ankle reflex tests. C
14.88 Prevention should focus on achieving glycemic targets. C
Retinopathy
Recommendations
14.89 Screening for retinopathy should be performed by dilated fundoscopy at or soon after diagnosis and annually thereafter. C
14.90 Optimizing glycemia is recommended to decrease the risk or slow the progression of retinopathy. B
14.91 Less frequent examination (every 2 years) may be considered if achieving glycemic targets and a normal eye exam. C
14.92 Programs that use retinal photography (with remote reading or use of a validated assessment tool) to improve access to diabetic retinopathy screening can be appropriate screening strategies for diabetic retinopathy. Such programs need to provide pathways for timely referral for a comprehensive eye examination when indicated. E
Nonalcoholic Fatty Liver Disease
Recommendations
14.93 Evaluation for nonalcoholic fatty liver disease (by measuring AST and ALT) should be done at diagnosis and annually thereafter. B
14.94 Referral to gastroenterology should be considered for persistently elevated or worsening transaminases. B
Obstructive Sleep Apnea
Recommendation
14.95 Screening for symptoms of sleep apnea should be done at each visit, and referral to a pediatric sleep specialist for evaluation and a polysomnogram, if indicated, is recommended. Obstructive sleep apnea should be treated when documented. B
Polycystic Ovary Syndrome
Recommendations
14.96 Evaluate for polycystic ovary syndrome in female adolescents with type 2 diabetes, including laboratory studies, when indicated. B
14.97 Oral contraceptive pills for treatment of polycystic ovary syndrome are not contraindicated for female individuals with type 2 diabetes. C
14.98 Metformin, in addition to lifestyle modification, is likely to improve the menstrual cyclicity and hyperandrogenism in female individuals with type 2 diabetes. E
Cardiovascular Disease
Recommendation
14.99 Intensive lifestyle interventions focusing on weight loss, dyslipidemia, hypertension, and dysglycemia are important to prevent overt macrovascular disease in early adulthood. E
Dyslipidemia
Recommendations
14.100 Lipid screening should be performed initially after optimizing glycemia and annually thereafter. B
14.101 Optimal goals are LDL cholesterol <100 mg/dL (2.6 mmol/L), HDL cholesterol >35 mg/dL (0.91 mmol/L), and triglycerides <150 mg/dL (1.7 mmol/L). E
14.102 If lipids are abnormal, initial therapy should consist of optimizing glycemia and medical nutritional therapy to limit the amount of calories from fat to 25–30% and saturated fat to <7%, limit cholesterol to <200 mg/day, avoid trans fats, and aim for ∼10% calories from monounsaturated fats for elevated LDL. For elevated triglycerides, medical nutrition therapy should also focus on decreasing simple sugar intake and increasing dietary n-3 fatty acids in addition to the above changes. A
14.103 If LDL cholesterol remains >130 mg/dL after 6 months of dietary intervention, initiate therapy with statin, with a goal of LDL <100 mg/dL. Due to the potential teratogenic effects, individuals of childbearing age should receive reproductive counseling, and statins should be avoided in individuals of childbearing age who are not using reliable contraception. B
14.104 If triglycerides are >400 mg/dL (4.7 mmol/L) fasting or >1,000 mg/dL (11.6 mmol/L) nonfasting, optimize glycemia and begin fibrate, with a goal of <400 mg/dL (4.7 mmol/L) fasting to reduce risk for pancreatitis. C
Cardiac Function Testing
Recommendation
14.105 Routine screening for heart disease with electrocardiogram, echocardiogram, or stress testing is not recommended in asymptomatic youth with type 2 diabetes. B
Comorbidities may already be present at the time of diagnosis of type 2 diabetes in youth (201,252). Therefore, blood pressure measurement, a fasting lipid panel, assessment of random urine albumin-to-creatinine ratio, and a dilated eye examination should be performed at diagnosis. Additional medical conditions that may need to be addressed include polycystic ovary disease and other comorbidities associated with pediatric obesity, such as sleep apnea, hepatic steatosis, orthopedic complications, and psychosocial concerns. The ADA position statement “Evaluation and Management of Youth-Onset Type 2 Diabetes” (3) provides guidance on the prevention, screening, and treatment of type 2 diabetes and its comorbidities in children and adolescents.
Youth-onset type 2 diabetes is associated with significant microvascular and macrovascular risk burden and a substantial increase in the risk of cardiovascular morbidity and mortality at an earlier age than in those diagnosed later in life (202,253). The higher complication risk in earlier-onset type 2 diabetes is likely related to prolonged lifetime exposure to hyperglycemia and other atherogenic risk factors, including insulin resistance, dyslipidemia, hypertension, and chronic inflammation. There is a low risk of hypoglycemia in youth with type 2 diabetes, even if they are being treated with insulin (254), and there are high rates of complications (219–222). These diabetes comorbidities also appear to be higher than in youth with type 1 diabetes despite shorter diabetes duration and lower A1C (252). In addition, the progression of vascular abnormalities appears to be more pronounced in youth-onset type 2 diabetes than with type 1 diabetes of similar duration, including ischemic heart disease and stroke (255).
Psychosocial Factors
Recommendations
14.106 Health care professionals should screen for food insecurity, housing instability/homelessness, health literacy, financial barriers, and social/community support and apply that information to treatment decisions. E
14.107 Use age-appropriate standardized and validated tools to screen for diabetes distress, depressive symptoms, and mental/behavioral health in youth with type 2 diabetes, with attention to symptoms of depression and disordered eating, and refer to a qualified mental health professional when indicated. B
14.108 When choosing glucose-lowering or other medications for youth with overweight or obesity and type 2 diabetes, consider medication-taking behavior and the medications’ effect on weight. E
14.109 Starting at puberty, preconception counseling should be incorporated into routine diabetes clinic visits for all individuals of childbearing potential because of the adverse pregnancy outcomes in this population. A
14.110 Adolescents and young adults should be screened for tobacco, electronic cigarettes, and alcohol use at diagnosis and regularly thereafter. C
Most youth with type 2 diabetes come from racial/ethnic minority groups, have low socioeconomic status, and often experience multiple psychosocial stressors (26,43,205,206). Consideration of the sociocultural context and efforts to personalize diabetes management are of critical importance to minimize barriers to care, enhance participation, and maximize response to treatment.
Evidence about psychiatric disorders and symptoms in youth with type 2 diabetes is limited (256–260), but given the sociocultural context for many youth and the medical burden and obesity associated with type 2 diabetes, ongoing surveillance of mental health/behavioral health is indicated. Symptoms of depression and disordered eating are common and associated with poorer glycemic control (39,257,261,262).
Many of the medications prescribed for diabetes and psychiatric disorders are associated with weight gain and can increase concerns about eating, body shape, and weight (263,264).
The TODAY study documented high rates of maternal complications during pregnancy and low rates of preconception counseling and contraception use (265).
Transition from Pediatric to Adult Care
Recommendations
14.111 Pediatric diabetes care teams should begin to prepare youth for transition to adult health care in early adolescence and, at the latest, at least 1 year before the transition. E
14.112 Both pediatric and adult diabetes care professionals should provide support and resources for transitioning young adults. E
14.113 Youth with type 2 diabetes should be transferred to an adult-oriented diabetes specialist when deemed appropriate by the young adult and health care professional. E
Care and close supervision of diabetes management are increasingly shifted from parents and other adults to the youth with type 1 or type 2 diabetes throughout childhood and adolescence. The shift from pediatric to adult health care professionals, however, often occurs abruptly as the older teen enters the next developmental stage, referred to as emerging adulthood (266), which is a critical period for young people who have diabetes. During this period of major life transitions, youth may begin to move out of their parents’ homes and become increasingly responsible for their diabetes care. Their new responsibilities include self-management of their diabetes, making medical appointments, and financing health care once they are no longer covered by their parents’ health insurance plans (ongoing coverage until age 26 years is currently available under provisions of the U.S. Affordable Care Act). In addition to lapses in health care, this is also a period associated with deterioration in glycemic stability; increased occurrence of acute complications; psychosocial, emotional, and behavioral challenges; and the emergence of chronic complications (267–272). The transition period from pediatric to adult care is prone to fragmentation in health care delivery, which may adversely impact health care quality, cost, and outcomes (273). Worsening diabetes health outcomes during the transition to adult care and early adulthood have been documented (274,275).
Although scientific evidence is limited, it is clear that comprehensive and coordinated planning that begins in early adolescence is necessary to facilitate a seamless transition from pediatric to adult health care (267,268,276,277). New technologies and other interventions are being tried to support the transition to adult care in young adulthood (278–282). Given the behavioral, psychosocial, and developmental factors that relate to this transition, diabetes care teams addressing transition should include social workers, psychologists, and other behavioral health professionals, as available (51,283). A comprehensive discussion regarding the challenges faced during this period, including specific recommendations, is found in the ADA position statement “Diabetes Care for Emerging Adults: Recommendations for Transition From Pediatric to Adult Diabetes Care Systems” (268).
The Endocrine Society, in collaboration with the ADA and other organizations, has developed transition tools for clinicians and youth and families (277).
Footnotes
Disclosure information for each author is available at https://doi.org/10.2337/dc23-SDIS.
Suggested citation: ElSayed NA, Aleppo G, Aroda VR, et al., American Diabetes Association. 14. Children and adolescents: Standards of Care in Diabetes—2023. Diabetes Care 2023;46(Suppl. 1):S230–S253
References
- 1. Centers for Disease Control and Prevention . Vaccines site: Healthcare Providers/Professionals. 2021. Accessed 21 October 2022. Available from https://www.cdc.gov/vaccines/hcp/index.html
- 2. Chiang JL, Maahs DM, Garvey KC, et al. Type 1 diabetes in children and adolescents: a position statement by the American Diabetes Association. Diabetes Care 2018;41:2026–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and management of youth-onset type 2 diabetes: a position statement by the American Diabetes Association. Diabetes Care 2018;41:2648–2668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lawrence JM, Divers J, Isom S, et al.; SEARCH for Diabetes in Youth Study Group . Trends in prevalence of type 1 and type 2 diabetes in children and adolescents in the US, 2001-2017. JAMA 2021;326:717–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thomas NJ, Jones SE, Weedon MN, Shields BM, Oram RA, Hattersley AT. Frequency and phenotype of type 1 diabetes in the first six decades of life: a cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol 2018;6:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnea-Goraly N, Raman M, Mazaika P, et al.; Diabetes Research in Children Network (DirecNet) . Alterations in white matter structure in young children with type 1 diabetes. Diabetes Care 2014;37:332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cameron FJ, Scratch SE, Nadebaum C, et al.; DKA Brain Injury Study Group . Neurological consequences of diabetic ketoacidosis at initial presentation of type 1 diabetes in a prospective cohort study of children. Diabetes Care 2014;37:1554–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Markowitz JT, Garvey KC, Laffel LMB. Developmental changes in the roles of patients and families in type 1 diabetes management. Curr Diabetes Rev 2015;11:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Driscoll KA, Volkening LK, Haro H, et al. Are children with type 1 diabetes safe at school? Examining parent perceptions. Pediatr Diabetes 2014 [DOI] [PubMed] [Google Scholar]
- 10. Jackson CC, Albanese-O’Neill A, Butler KL, et al. Diabetes care in the school setting: a position statement of the American Diabetes Association. Diabetes Care 2015;38:1958–1963 [DOI] [PubMed] [Google Scholar]
- 11. Siminerio LM, Albanese-O’Neill A, Chiang JL, et al.; American Diabetes Association . Care of young children with diabetes in the child care setting: a position statement of the American Diabetes Association. Diabetes Care 2014;37:2834–2842 [DOI] [PubMed] [Google Scholar]
- 12. Mehta SN, Volkening LK, Anderson BJ, et al.; Family Management of Childhood Diabetes Study Steering Committee . Dietary behaviors predict glycemic control in youth with type 1 diabetes. Diabetes Care 2008;31:1318–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Absil H, Baudet L, Robert A, Lysy PA. Benefits of physical activity in children and adolescents with type 1 diabetes: a systematic review. Diabetes Res Clin Pract 2019;156:107810. [DOI] [PubMed] [Google Scholar]
- 14. Riddell MC, Gallen IW, Smart CE, et al. Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol 2017;5:377–390 [DOI] [PubMed] [Google Scholar]
- 15. Colberg SR, Sigal RJ, Yardley JE, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2065–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moser O, Riddell MC, Eckstein ML, et al. Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: position statement of the European Association for the Study of Diabetes (EASD) and of the International Society for Pediatric and Adolescent Diabetes (ISPAD) endorsed by JDRF and supported by the American Diabetes Association (ADA). Diabetologia 2020;63:2501–2520 [DOI] [PubMed] [Google Scholar]
- 17. U.S. Department of Health and Human Services . Physical activity guidelines for Americans, 2nd ed., 2018. Accessed 21 October 2022. Available from https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
- 18. Tsalikian E, Kollman C, Tamborlane WB, et al.; Diabetes Research in Children Network (DirecNet) Study Group . Prevention of hypoglycemia during exercise in children with type 1 diabetes by suspending basal insulin. Diabetes Care 2006;29:2200–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taplin CE, Cobry E, Messer L, McFann K, Chase HP, Fiallo-Scharer R. Preventing post-exercise nocturnal hypoglycemia in children with type 1 diabetes. J Pediatr 2010;157:784–8.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eckstein ML, Weilguni B, Tauschmann M, et al. Time in range for closed-loop systems versus standard of care during physical exercise in people with type 1 diabetes: a systematic review and meta-analysis. J Clin Med 2021;10:2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riddell MC, Milliken J. Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: an observational field study. Diabetes Technol Ther 2011;13:819–825 [DOI] [PubMed] [Google Scholar]
- 22. Francescato MP, Stel G, Stenner E, Geat M. Prolonged exercise in type 1 diabetes: performance of a customizable algorithm to estimate the carbohydrate supplements to minimize glycemic imbalances. PLoS One 2015;10:e0125220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baker LB, Rollo I, Stein KW, Jeukendrup AE. Acute effects of carbohydrate supplementation on intermittent sports performance. Nutrients 2015;7:5733–5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adolfsson P, Mattsson S, Jendle J. Evaluation of glucose control when a new strategy of increased carbohydrate supply is implemented during prolonged physical exercise in type 1 diabetes. Eur J Appl Physiol 2015;115:2599–2607 [DOI] [PubMed] [Google Scholar]
- 25. Redondo MJ, Libman I, Cheng P, et al.; Pediatric Diabetes Consortium . Racial/ethnic minority youth with recent-onset type 1 diabetes have poor prognostic factors. Diabetes Care 2018;41:1017–1024 [DOI] [PubMed] [Google Scholar]
- 26. Liu LL, Lawrence JM, Davis C, et al.; SEARCH for Diabetes in Youth Study Group . Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes 2010;11:4–11 [DOI] [PubMed] [Google Scholar]
- 27. DuBose SN, Hermann JM, Tamborlane WV, Beck RW, Dost A, DiMeglio LA, et al. Obesity in youth with type 1 diabetes in Germany, Austria, and the United States. J Pediatr 2015;167:627–632.e1–4 [DOI] [PubMed] [Google Scholar]
- 28. Corbin KD, Driscoll KA, Pratley RE, Smith SR, Maahs DM; Advancing Care for Type 1 Diabetes and Obesity Network (ACT1ON) . Obesity in type 1 diabetes: pathophysiology, clinical impact, and mechanisms. Endocr Rev 2018;39:629–663 [DOI] [PubMed] [Google Scholar]
- 29. Redondo MJ, Foster NC, Libman IM, et al. Prevalence of cardiovascular risk factors in youth with type 1 diabetes and elevated body mass index. Acta Diabetol 2016;53:271–277 [DOI] [PubMed] [Google Scholar]
- 30. American Association of Diabetes Educators . Management of children with diabetes in the school setting. Diabetes Educ 2019;45:54–59 [DOI] [PubMed] [Google Scholar]
- 31. Hood KK, Beavers DP, Yi-Frazier J, et al. Psychosocial burden and glycemic control during the first 6 years of diabetes: results from the SEARCH for Diabetes in Youth study. J Adolesc Health 2014;55:498–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ducat L, Philipson LH, Anderson BJ. The mental health comorbidities of diabetes. JAMA 2014;312:691–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hagger V, Hendrieckx C, Sturt J, Skinner TC, Speight J. Diabetes distress among adolescents with type 1 diabetes: a systematic review. Curr Diab Rep 2016;16:9. [DOI] [PubMed] [Google Scholar]
- 34. Anderson BJ, Laffel LM, Domenger C, et al. Factors associated with diabetes-specific health-related quality of life in youth with type 1 diabetes: the Global TEENs Study. Diabetes Care 2017;40:1002–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hilliard ME, De Wit M, Wasserman RM, et al. Screening and support for emotional burdens of youth with type 1 diabetes: strategies for diabetes care providers. Pediatr Diabetes 2018;19:534–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shapiro JB, Vesco AT, Weil LEG, Evans MA, Hood KK, Weissberg-Benchell J. Psychometric properties of the problem areas in diabetes: teen and parent of teen versions. J Pediatr Psychol 2018;43:561–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iturralde E, Rausch JR, Weissberg-Benchell J, Hood KK. Diabetes-related emotional distress over time. Pediatrics 2019;143:e20183011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hill-Briggs F, Adler NE, Berkowitz SA, et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 2020;44:258–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monaghan M, Mara CA, Kichler JC, et al. Multisite examination of depression screening scores and correlates among adolescents and young adults with type 2 diabetes. Can J Diabetes 2021;45:411–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mulvaney SA, Mara CA, Kichler JC, et al. A retrospective multisite examination of depression screening practices, scores, and correlates in pediatric diabetes care. Transl Behav Med 2021;11:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rechenberg K, Koerner R. Cognitive behavioral therapy in adolescents with type 1 diabetes: an integrative review. J Pediatr Nurs 2021;60:190–197 [DOI] [PubMed] [Google Scholar]
- 42. Lawrence JM, Yi-Frazier JP, Black MH, et al.; SEARCH for Diabetes in Youth Study Group . Demographic and clinical correlates of diabetes-related quality of life among youth with type 1 diabetes. J Pediatr 2012;161:201–207.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Young-Hyman D, de Groot M, Hill-Briggs F, Gonzalez JS, Hood K, Peyrot M. Psychosocial care for people with diabetes: a position statement of the American Diabetes Association. Diabetes Care 2016;39:2126–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Corathers SD, Kichler J, Jones NHY, Houchen A, Jolly M, Morwessel N, et al. Improving depression screening for adolescents with type 1 diabetes. Pediatrics 2013;132:e1395–e1402 [DOI] [PubMed] [Google Scholar]
- 45. Markowitz JT, Butler DA, Volkening LK, Antisdel JE, Anderson BJ, Laffel LMB. Brief screening tool for disordered eating in diabetes: internal consistency and external validity in a contemporary sample of pediatric patients with type 1 diabetes. Diabetes Care 2010;33:495–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Evans MA, Weil LEG, Shapiro JB, et al. Psychometric properties of the parent and child problem areas in diabetes measures. J Pediatr Psychol 2019;44:703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Katz ML, Volkening LK, Butler DA, Anderson BJ, Laffel LM. Family-based psychoeducation and Care Ambassador intervention to improve glycemic control in youth with type 1 diabetes: a randomized trial. Pediatr Diabetes 2014;15:142–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laffel LMB, Vangsness L, Connell A, Goebel-Fabbri A, Butler D, Anderson BJ. Impact of ambulatory, family-focused teamwork intervention on glycemic control in youth with type 1 diabetes. J Pediatr 2003;142:409–416 [DOI] [PubMed] [Google Scholar]
- 49. Anderson BJ, Vangsness L, Connell A, Butler D, Goebel-Fabbri A, Laffel LMB. Family conflict, adherence, and glycaemic control in youth with short duration type 1 diabetes. Diabet Med 2002;19:635–642 [DOI] [PubMed] [Google Scholar]
- 50. Hilliard ME, Powell PW, Anderson BJ. Evidence-based behavioral interventions to promote diabetes management in children, adolescents, and families. Am Psychol 2016;71:590–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kichler JC, Harris MA, Weissberg-Benchell J. Contemporary roles of the pediatric psychologist in diabetes care. Curr Diabetes Rev 2015;11:210–221 [DOI] [PubMed] [Google Scholar]
- 52. Helgeson VS, Palladino DK. Implications of psychosocial factors for diabetes outcomes among children with type 1 diabetes: a review. Soc Personal Psychol Compass 2012;6:228–242 [Google Scholar]
- 53. McCarthy AM, Lindgren S, Mengeling MA, Tsalikian E, Engvall J. Factors associated with academic achievement in children with type 1 diabetes. Diabetes Care 2003;26:112–117 [DOI] [PubMed] [Google Scholar]
- 54. Kuther TL. Medical decision-making and minors: issues of consent and assent. Adolescence 2003;38:343–358 [PubMed] [Google Scholar]
- 55. Coleman DL, Rosoff PM. The legal authority of mature minors to consent to general medical treatment. Pediatrics 2013;131:786–793 [DOI] [PubMed] [Google Scholar]
- 56. Charron-Prochownik D, Sereika SM, Becker D, et al. Long-term effects of the booster-enhanced READY-Girls preconception counseling program on intentions and behaviors for family planning in teens with diabetes. Diabetes Care 2013;36:3870–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Charron-Prochownik D, Downs J. Diabetes and Reproductive Health for Girls. Alexandria, VA, American Diabetes Association, 2016 [Google Scholar]
- 58. Wisting L, Frøisland DH, Skrivarhaug T, Dahl-Jørgensen K, Rø O. Disturbed eating behavior and omission of insulin in adolescents receiving intensified insulin treatment: a nationwide population-based study. Diabetes Care 2013;36:3382–3387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Goebel-Fabbri AE. Disturbed eating behaviors and eating disorders in type 1 diabetes: clinical significance and treatment recommendations. Curr Diab Rep 2009;9:133–139 [DOI] [PubMed] [Google Scholar]
- 60. Atik Altınok Y, Özgür S, Meseri R, Özen S, Darcan Ş, Gökşen D. Reliability and validity of the diabetes eating problem survey in turkish children and adolescents with type 1 diabetes mellitus. J Clin Res Pediatr Endocrinol 2017;9:323–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Saßmann H, Albrecht C, Busse-Widmann P, et al. Psychometric properties of the German version of the Diabetes Eating Problem Survey-Revised: additional benefit of disease-specific screening in adolescents with type 1 diabetes. Diabet Med 2015;32:1641–1647 [DOI] [PubMed] [Google Scholar]
- 62. Gerhardsson P, Schwandt A, Witsch M, et al. The SWEET Project 10-year benchmarking in 19 countries worldwide is associated with improved HbA1c and increased use of diabetes technology in youth with type 1 diabetes. Diabetes Technol Ther 2021;23:491–499 [DOI] [PubMed] [Google Scholar]
- 63. Cameron FJ, de Beaufort C, Aanstoot HJ, et al.; Hvidoere International Study Group . Lessons from the Hvidoere International Study Group on childhood diabetes: be dogmatic about outcome and flexible in approach. Pediatr Diabetes 2013;14:473–480 [DOI] [PubMed] [Google Scholar]
- 64. Miller KM, Beck RW, Foster NC, Maahs DM. HbA1c levels in type 1 diabetes from early childhood to older adults: a deeper dive into the influence of technology and socioeconomic status on HbA1c in the T1D Exchange clinic registry findings. Diabetes Technol Ther 2020;22:645–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Diabetes Control and Complications Trial Research Group . Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. J Pediatr 1994;125:177–188 [DOI] [PubMed] [Google Scholar]
- 66. White NH, Cleary PA, Dahms W, Goldstein D, Malone J; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Beneficial effects of intensive therapy of diabetes during adolescence: outcomes after the conclusion of the Diabetes Control and Complications Trial (DCCT). J Pediatr 2001;139:804–812 [DOI] [PubMed] [Google Scholar]
- 67. Samuelsson U, Steineck I, Gubbjornsdottir S. A high mean-HbA1c value 3-15 months after diagnosis of type 1 diabetes in childhood is related to metabolic control, macroalbuminuria, and retinopathy in early adulthood—a pilot study using two nation-wide population based quality registries. Pediatr Diabetes 2014;15:229–235 [DOI] [PubMed] [Google Scholar]
- 68. Carlsen S, Skrivarhaug T, Thue G, et al. Glycemic control and complications in patients with type 1 diabetes—a registry-based longitudinal study of adolescents and young adults. Pediatr Diabetes 2017;18:188–195 [DOI] [PubMed] [Google Scholar]
- 69. Genuth SM, Backlund JYC, Bayless M, et al.; DCCT/EDIC Research Group . Effects of prior intensive versus conventional therapy and history of glycemia on cardiac function in type 1 diabetes in the DCCT/EDIC. Diabetes 2013;62:3561–3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group . Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA 2003;290:2159–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Writing Team for the DCCT/EDIC Research Group, Gubitosi-Klug RA, Sun W, Cleary PA, et al. Effects of prior intensive insulin therapy and risk factors on patient-reported visual function outcomes in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort. JAMA Ophthalmol 2016;134:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Orchard TJ, Nathan DM, Zinman B, et al.; Writing Group for the DCCT/EDIC Research Group . Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA 2015;313:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Foland-Ross LC, Reiss AL, Mazaika PK, et al.; Diabetes Research in Children Network (DirecNet) . Longitudinal assessment of hippocampus structure in children with type 1 diabetes. Pediatr Diabetes 2018;19:1116–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mauras N, Mazaika P, Buckingham B, et al.; Diabetes Research in Children Network (DirecNet) . Longitudinal assessment of neuroanatomical and cognitive differences in young children with type 1 diabetes: association with hyperglycemia. Diabetes 2015;64:1770–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Foland-Ross LC, Tong G, Mauras N, et al.; Diabetes Research in Children Network (DirecNet) . Brain function differences in children with type 1 diabetes: a functional MRI study of working memory. Diabetes 2020;69:1770–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pourabbasi A, Tehrani-Doost M, Qavam SE, Arzaghi SM, Larijani B. Association of diabetes mellitus and structural changes in the central nervous system in children and adolescents: a systematic review. J Diabetes Metab Disord 2017;16:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Perantie DC, Wu J, Koller JM, et al. Regional brain volume differences associated with hyperglycemia and severe hypoglycemia in youth with type 1 diabetes. Diabetes Care 2007;30:2331–2337 [DOI] [PubMed] [Google Scholar]
- 78. Arbelaez AM, Semenkovich K, Hershey T. Glycemic extremes in youth with T1DM: Effects on the developing brain’s structural and functional integrity. Pediatr Diabetes 2013;14:541–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Broadley MM, White MJ, Andrew B. A systematic review and meta-analysis of executive function performance in type 1 diabetes mellitus. Psychosom Med 2017;79:684–696 [DOI] [PubMed] [Google Scholar]
- 80. Ryan CM. Why is cognitive dysfunction associated with the development of diabetes early in life? The diathesis hypothesis. Pediatr Diabetes 2006;7:289–297 [DOI] [PubMed] [Google Scholar]
- 81. Cameron FJ. The impact of diabetes on brain function in childhood and adolescence. Pediatr Clin North Am 2015;62:911–927 [DOI] [PubMed] [Google Scholar]
- 82. Mauras N, Buckingham B, White NH, et al.; Diabetes Research in Children Network (DirecNet) . Impact of type 1 diabetes in the developing brain in children: a longitudinal study. Diabetes Care 2021;44:983–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Campbell MS, Schatz DA, Chen V, et al.; T1D Exchange Clinic Network . A contrast between children and adolescents with excellent and poor control: the T1D Exchange clinic registry experience. Pediatr Diabetes 2014;15:110–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bergenstal RM, Garg S, Weinzimer SA, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 86. Kovatchev B, Cheng P, Anderson SM, et al. Feasibility of long-term closed-loop control: a multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther 2017;19:18–24 [DOI] [PubMed] [Google Scholar]
- 87. Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bergenstal RM, Nimri R, Beck RW, et al.; FLAIR Study Group . A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet 2021;397:208–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Breton MD, Kanapka LG, Beck RW, et al.; iDCL Trial Research Group . A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020;383:836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Dorando E, Haak T, Pieper D. Correction: continuous glucose monitoring for glycemic control in children and adolescents diagnosed with diabetes type 1: a systematic review and meta-analysis. Exp Clin Endocrinol Diabetes 2022;130:e1–e3 [DOI] [PubMed] [Google Scholar]
- 91. Brown SA, Forlenza GP, Bode BW, et al.; Omnipod 5 Research Group . Multicenter trial of a tubeless, on-body automated insulin delivery system with customizable glycemic targets in pediatric and adult participants with type 1 diabetes. Diabetes Care 2021;44:1630–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Carlson AL, Sherr JL, Shulman DI, et al. Safety and glycemic outcomes during the minimed advanced hybrid closed-loop system pivotal trial in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2022;24:178–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Prahalad P, Ding VY, Zaharieva DP, et al. Teamwork, targets, technology, and tight control in newly diagnosed type 1 diabetes: the Pilot 4T Study. J Clin Endocrinol Metab 2022;107:998–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Champakanath A, Akturk HK, Alonso GT, Snell-Bergeon JK, Shah VN. Continuous glucose monitoring initiation within first year of type 1 diabetes diagnosis is associated with improved glycemic outcomes: 7-year follow-up study. Diabetes Care 2022;45:750–753 [DOI] [PubMed] [Google Scholar]
- 95. Johnson SR, Holmes-Walker DJ, Chee M, et al.; ADDN Study Group . Universal subsidized continuous glucose monitoring funding for young people with type 1 diabetes: uptake and outcomes over 2 years, a population-based study. Diabetes Care 2022;45:391–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rose S, Styles SE, Wiltshire EJ, et al. Use of intermittently scanned continuous glucose monitoring in young people with high-risk type 1 diabetes-Extension phase outcomes following a 6-month randomized control trial. Diabet Med 2022;39:e14756. [DOI] [PubMed] [Google Scholar]
- 97. Beato-Víbora PI, Gallego-Gamero F, Ambrojo-López A, Gil-Poch E, Martín-Romo I, Arroyo-Díez FJ. Rapid improvement in time in range after the implementation of an advanced hybrid closed-loop system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2021;23:609–615 [DOI] [PubMed] [Google Scholar]
- 98. Breton MD, Kovatchev BP. One year real-world use of the Control-IQ advanced hybrid closed-loop technology. Diabetes Technol Ther 2021;23:601–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Forlenza GP, Ekhlaspour L, DiMeglio LA, et al. Glycemic outcomes of children 2-6 years of age with type 1 diabetes during the pediatric MiniMed 670G system trial. Pediatr Diabetes 2022;23:324–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Messer LH, Berget C, Pyle L, et al. Real-world use of a new hybrid closed loop improves glycemic control in youth with type 1 diabetes. Diabetes Technol Ther 2021;23:837–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Varimo T, Pulkkinen MA, Hakonen E, Hero M, Miettinen PJ, Tuomaala AK. First year on commercial hybrid closed-loop system-experience on 111 children and adolescents with type 1 diabetes. Pediatr Diabetes 2021;22:909–915 [DOI] [PubMed] [Google Scholar]
- 102. Ware J, Allen JM, Boughton CK, et al.; KidsAP Consortium . Randomized trial of closed-loop control in very young children with type 1 diabetes. N Engl J Med 2022;386:209–219 [DOI] [PubMed] [Google Scholar]
- 103. Isganaitis E, Raghinaru D, Ambler-Osborn L, et al.; iDCL Trial Research Group . Closed-loop insulin therapy improves glycemic control in adolescents and young adults: outcomes from the international diabetes closed-loop trial. Diabetes Technol Ther 2021;23:342–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Schoelwer MJ, Kanapka LG, Wadwa RP, et al.; iDCL Trial Research Group . Predictors of time-in-range (70-180 mg/dL) achieved using a closed-loop control system. Diabetes Technol Ther 2021;23:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sherr JL, Bode BW, Forlenza GP, et al.; Omnipod 5 in Preschoolers Study Group . Safety and glycemic outcomes with a tubeless automated insulin delivery system in very young children with type 1 diabetes: a single-arm multicenter clinical trial. Diabetes Care 2022;45:1907–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Marigliano M, Eckert AJ, Guness PK, et al.; SWEET Study Group . Association of the use of diabetes technology with HbA1c and BMI-SDS in an international cohort of children and adolescents with type 1 diabetes: the SWEET project experience. Pediatr Diabetes 2021;22:1120–1128 [DOI] [PubMed] [Google Scholar]
- 107. Redondo MJ, Libman I, Maahs DM, et al. The evolution of hemoglobin A1c targets for youth with type 1 diabetes: rationale and supporting evidence. Diabetes Care 2021;44:301–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cooper MN, O’Connell SM, Davis EA, Jones TW. A population-based study of risk factors for severe hypoglycaemia in a contemporary cohort of childhood-onset type 1 diabetes. Diabetologia 2013;56:2164–2170 [DOI] [PubMed] [Google Scholar]
- 109. Haynes A, Hermann JM, Miller KM, et al.; T1D Exchange, WACDD and DPV Registries . Severe hypoglycemia rates are not associated with HbA1c: a cross-sectional analysis of 3 contemporary pediatric diabetes registry databases. Pediatr Diabetes 2017;18:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Haynes A, Hermann JM, Clapin H, et al.; WACDD and DPV Registries . Decreasing trends in mean HbA1c are not associated with increasing rates of severe hypoglycemia in children: a longitudinal analysis of two contemporary population-based pediatric type 1 diabetes registries from Australia and Germany/Austria between 1995 and 2016. Diabetes Care 2019;42:1630–1636 [DOI] [PubMed] [Google Scholar]
- 111. Fredheim S, Johansen A, Thorsen SU, et al.; Danish Society for Diabetes in Childhood and Adolescence . Nationwide reduction in the frequency of severe hypoglycemia by half. Acta Diabetol 2015;52:591–599 [DOI] [PubMed] [Google Scholar]
- 112. Birkebaek NH, Drivvoll AK, Aakeson K, et al. Incidence of severe hypoglycemia in children with type 1 diabetes in the Nordic countries in the period 2008-2012: association with hemoglobin A1c and treatment modality. BMJ Open Diabetes Res Care 2017;5:e000377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. JAMA 2013;310:1240–1247 [DOI] [PubMed] [Google Scholar]
- 114. Downie E, Craig ME, Hing S, Cusumano J, Chan AKF, Donaghue KC. Continued reduction in the prevalence of retinopathy in adolescents with type 1 diabetes: role of insulin therapy and glycemic control. Diabetes Care 2011;34:2368–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Karges B, Rosenbauer J, Kapellen T, et al. Hemoglobin A1c levels and risk of severe hypoglycemia in children and young adults with type 1 diabetes from Germany and Austria: a trend analysis in a cohort of 37,539 patients between 1995 and 2012. PLoS Med 2014;11:e1001742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case-control study. Diabetologia 2013;56:2392–2400 [DOI] [PubMed] [Google Scholar]
- 117. Karges B, Kapellen T, Wagner VM, et al.; DPV Initiative . Glycated hemoglobin A1c as a risk factor for severe hypoglycemia in pediatric type 1 diabetes. Pediatr Diabetes 2017;18:51–58 [DOI] [PubMed] [Google Scholar]
- 118. Saydah S, Imperatore G, Divers J, et al. Occurrence of severe hypoglycaemic events among US youth and young adults with type 1 or type 2 diabetes. Endocrinol Diabetes Metab 2019;2:e00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ishtiak-Ahmed K, Carstensen B, Pedersen-Bjergaard U, Jørgensen ME. Incidence trends and predictors of hospitalization for hypoglycemia in 17,230 adult patients with type 1 diabetes: a Danish Register Linkage cohort study. Diabetes Care 2017;40:226–232 [DOI] [PubMed] [Google Scholar]
- 120. Maahs DM, Hermann JM, DuBose SN, et al.; DPV Initiative; T1D Exchange Clinic Network . Contrasting the clinical care and outcomes of 2,622 children with type 1 diabetes less than 6 years of age in the United States T1D Exchange and German/Austrian DPV registries. Diabetologia 2014;57:1578–1585 [DOI] [PubMed] [Google Scholar]
- 121. Swift PGF, Skinner TC, de Beaufort CE, et al.; Hvidoere Study Group on Childhood Diabetes . Target setting in intensive insulin management is associated with metabolic control: the Hvidoere childhood diabetes study group centre differences study 2005. Pediatr Diabetes 2010;11:271–278 [DOI] [PubMed] [Google Scholar]
- 122. Laffel LM, Kanapka LG, Beck RW, Bergamo K, Clements MA, Criego A, et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA 2020;323:2388–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Strategies to Enhance New CGM Use in Early Childhood (SENCE) Study Group . A randomized clinical trial assessing continuous glucose monitoring (CGM) use with standardized education with or without a family behavioral intervention compared with fingerstick blood glucose monitoring in very young children with type 1 diabetes. Diabetes Care 2021;44:464–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bergenstal RM, Klonoff DC, Garg SK, et al.; ASPIRE In-Home Study Group . Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med 2013;369:224–232 [DOI] [PubMed] [Google Scholar]
- 125. Abraham MB, Davey R, O’Grady MJ, et al. Effectiveness of a predictive algorithm in the prevention of exercise-induced hypoglycemia in type 1 diabetes. Diabetes Technol Ther 2016;18:543–550 [DOI] [PubMed] [Google Scholar]
- 126. Buckingham BA, Bailey TS, Christiansen M, et al. Evaluation of a predictive low-glucose management system in-clinic. Diabetes Technol Ther 2017;19:288–292 [DOI] [PubMed] [Google Scholar]
- 127. Nimri R, Muller I, Atlas E, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care 2014;37:3025–3032 [DOI] [PubMed] [Google Scholar]
- 128. El-Khatib FH, Balliro C, Hillard MA, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. Lancet 2017;389:369–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Levine BS, Anderson BJ, Butler DA, Antisdel JE, Brackett J, Laffel LM. Predictors of glycemic control and short-term adverse outcomes in youth with type 1 diabetes. J Pediatr 2001;139:197–203 [DOI] [PubMed] [Google Scholar]
- 130. Miller KM, Beck RW, Bergenstal RM, et al.; T1D Exchange Clinic Network . Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care 2013;36:2009–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther 2019;21:81–85 [DOI] [PubMed] [Google Scholar]
- 133. Petersson J, Åkesson K, Sundberg F, Särnblad S. Translating glycated hemoglobin A1c into time spent in glucose target range: a multicenter study. Pediatr Diabetes 2019;20:339–344 [DOI] [PubMed] [Google Scholar]
- 134. Warncke K, Fröhlich-Reiterer EE, Thon A, Hofer SE, Wiemann D; DPV Initiative of the German Working Group for Pediatric Diabetology; German BMBF Competence Network for Diabetes Mellitus . Polyendocrinopathy in children, adolescents, and young adults with type 1 diabetes: a multicenter analysis of 28,671 patients from the German/Austrian DPV-Wiss database. Diabetes Care 2010;33:2010–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Nederstigt C, Uitbeijerse BS, Janssen LGM, Corssmit EPM, de Koning EJP, Dekkers OM. Associated auto-immune disease in type 1 diabetes patients: a systematic review and meta-analysis. Eur J Endocrinol 2019;180:135–144 [DOI] [PubMed] [Google Scholar]
- 136. Kozhakhmetova A, Wyatt RC, Caygill C, et al. A quarter of patients with type 1 diabetes have co-existing non-islet autoimmunity: the findings of a UK population-based family study. Clin Exp Immunol 2018;192:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hughes JW, Riddlesworth TD, DiMeglio LA, Miller KM, Rickels MR, McGill JB. Autoimmune diseases in children and adults with type 1 diabetes from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2016;101:4931–4937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Kahaly GJ, Hansen MP. Type 1 diabetes associated autoimmunity. Autoimmun Rev 2016;15:644–648 [DOI] [PubMed] [Google Scholar]
- 139. Roldán MB, Alonso M, Barrio R. Thyroid autoimmunity in children and adolescents with type 1 diabetes mellitus. Diabetes Nutr Metab 1999;12:27–31 [PubMed] [Google Scholar]
- 140. Shun CB, Donaghue KC, Phelan H, Twigg SM, Craig ME. Thyroid autoimmunity in Type 1 diabetes: systematic review and meta-analysis. Diabet Med 2014;31:126–135 [DOI] [PubMed] [Google Scholar]
- 141. Triolo TM, Armstrong TK, McFann K, et al. Additional autoimmune disease found in 33% of patients at type 1 diabetes onset. Diabetes Care 2011;34:1211–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kordonouri O, Deiss D, Danne T, Dorow A, Bassir C, Grüters-Kieslich A. Predictivity of thyroid autoantibodies for the development of thyroid disorders in children and adolescents with type 1 diabetes. Diabet Med 2002;19:518–521 [DOI] [PubMed] [Google Scholar]
- 143. Dost A, Rohrer TR, Fröhlich-Reiterer E, et al.; DPV Initiative and the German Competence Network Diabetes Mellitus . Hyperthyroidism in 276 children and adolescents with type 1 diabetes from Germany and Austria. Horm Res Paediatr 2015;84:190–198 [DOI] [PubMed] [Google Scholar]
- 144. Jonsdottir B, Larsson C, Carlsson A, et al.; Better Diabetes Diagnosis Study Group . Thyroid and islet autoantibodies predict autoimmune thyroid disease at type 1 diabetes diagnosis. J Clin Endocrinol Metab 2017;102:1277–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Mohn A, Di Michele S, Di Luzio R, Tumini S, Chiarelli F. The effect of subclinical hypothyroidism on metabolic control in children and adolescents with type 1 diabetes mellitus. Diabet Med 2002;19:70–73 [DOI] [PubMed] [Google Scholar]
- 146. Holmes GKT. Screening for coeliac disease in type 1 diabetes. Arch Dis Child 2002;87:495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Rewers M, Liu E, Simmons J, Redondo MJ, Hoffenberg EJ. Celiac disease associated with type 1 diabetes mellitus. Endocrinol Metab Clin North Am 2004;33:197–214 [DOI] [PubMed] [Google Scholar]
- 148. Pham-Short A, Donaghue KC, Ambler G, Phelan H, Twigg S, Craig ME. Screening for celiac disease in type 1 diabetes: a systematic review. Pediatrics 2015;136:e170–e176 [DOI] [PubMed] [Google Scholar]
- 149. Craig ME, Prinz N, Boyle CT, et al.; Australasian Diabetes Data Network (ADDN); T1D Exchange Clinic Network (T1DX); National Paediatric Diabetes Audit (NPDA) and the Royal College of Paediatrics and Child Health; Prospective Diabetes Follow-up Registry (DPV) initiative . Prevalence of celiac disease in 52,721 youth with type 1 diabetes: international comparison across three continents. Diabetes Care 2017;40:1034–104028546222 [Google Scholar]
- 150. Cerutti F, Bruno G, Chiarelli F, Lorini R, Meschi F; Diabetes Study Group of the Italian Society of Pediatric Endocrinology and Diabetology . Younger age at onset and sex predict celiac disease in children and adolescents with type 1 diabetes: an Italian multicenter study. Diabetes Care 2004;27:1294–1298 [DOI] [PubMed] [Google Scholar]
- 151. Simmons JH, Foster NC, Riddlesworth TD, et al.; T1D Exchange Clinic Network . Sex- and age-dependent effects of celiac disease on growth and weight gain in children with type 1 diabetes: analysis of the Type 1 Diabetes Exchange clinic registry. Pediatr Diabetes 2018;19:741–748 [DOI] [PubMed] [Google Scholar]
- 152. Margoni D, Chouliaras G, Duscas G, et al. Bone health in children with celiac disease assessed by dual x-ray absorptiometry: effect of gluten-free diet and predictive value of serum biochemical indices. J Pediatr Gastroenterol Nutr 2012;54:680–684 [DOI] [PubMed] [Google Scholar]
- 153. Rohrer TR, Wolf J, Liptay S, et al.; DPV Initiative and the German BMBF Competence Network Diabetes Mellitus . Microvascular complications in childhood-onset type 1 diabetes and celiac disease: a multicenter longitudinal analysis of 56,514 patients from the German-Austrian DPV database. Diabetes Care 2015;38:801–807 [DOI] [PubMed] [Google Scholar]
- 154. Mollazadegan K, Kugelberg M, Montgomery SM, Sanders DS, Ludvigsson J, Ludvigsson JF. A population-based study of the risk of diabetic retinopathy in patients with type 1 diabetes and celiac disease. Diabetes Care 2013;36:316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Rubio-Tapia A, Hill ID, Kelly CP, Calderwood AH; American College of Gastroenterology . ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol 2013;108:656–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Husby S, Koletzko S, Korponay-Szabó IR, et al.; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition . European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–160 [DOI] [PubMed] [Google Scholar]
- 157. Paul SP, Sandhu BK, Spray CH, Basude D, Ramani P. Evidence supporting serology-based pathway for diagnosing celiac disease in asymptomatic children from high-risk groups. J Pediatr Gastroenterol Nutr 2018;66:641–644 [DOI] [PubMed] [Google Scholar]
- 158. Abid N, McGlone O, Cardwell C, McCallion W, Carson D. Clinical and metabolic effects of gluten free diet in children with type 1 diabetes and coeliac disease. Pediatr Diabetes 2011;12:322–325 [DOI] [PubMed] [Google Scholar]
- 159. Husby S, Koletzko S, Korponay-Szabó IR, et al.; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition . European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr 2012;54:136–160 [DOI] [PubMed] [Google Scholar]
- 160. Kurppa K, Paavola A, Collin P, et al. Benefits of a gluten-free diet for asymptomatic patients with serologic markers of celiac disease. Gastroenterology 2014;147:610–617.e1 [DOI] [PubMed] [Google Scholar]
- 161. Flynn JT, Kaelber DC, Baker-Smith CM, et al.; Subcommittee on Screening and Management of High Blood Pressure in Children . Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017;140:e20171904. [DOI] [PubMed] [Google Scholar]
- 162. Marcovecchio ML, Chiesa ST, Bond S, et al.; AdDIT Study Group . ACE inhibitors and statins in adolescents with type 1 diabetes. N Engl J Med 2017;377:1733–1745 [DOI] [PubMed] [Google Scholar]
- 163. de Ferranti SD, de Boer IH, Fonseca V, et al. Type 1 diabetes mellitus and cardiovascular disease: a scientific statement from the American Heart Association and American Diabetes Association. Diabetes Care 2014;37:2843–2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Rodriguez BL, Fujimoto WY, Mayer-Davis EJ, et al. Prevalence of cardiovascular disease risk factors in U.S. children and adolescents with diabetes: the SEARCH for diabetes in youth study. Diabetes Care 2006;29:1891–1896 [DOI] [PubMed] [Google Scholar]
- 165. Margeirsdottir HD, Larsen JR, Brunborg C, Overby NC; Norwegian Study Group for Childhood Diabetes . High prevalence of cardiovascular risk factors in children and adolescents with type 1 diabetes: a population-based study. Diabetologia 2008;51:554–561 [DOI] [PubMed] [Google Scholar]
- 166. Schwab KO, Doerfer J, Hecker W, et al.; DPV Initiative of the German Working Group for Pediatric Diabetology . Spectrum and prevalence of atherogenic risk factors in 27,358 children, adolescents, and young adults with type 1 diabetes: cross-sectional data from the German diabetes documentation and quality management system (DPV). Diabetes Care 2006;29:218–225 [DOI] [PubMed] [Google Scholar]
- 167. Singh TP, Groehn H, Kazmers A. Vascular function and carotid intimal-medial thickness in children with insulin-dependent diabetes mellitus. J Am Coll Cardiol 2003;41:661–665 [DOI] [PubMed] [Google Scholar]
- 168. Haller MJ, Stein J, Shuster J, et al. Peripheral artery tonometry demonstrates altered endothelial function in children with type 1 diabetes. Pediatr Diabetes 2007;8:193–198 [DOI] [PubMed] [Google Scholar]
- 169. Urbina EM, Wadwa RP, Davis C, et al. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr 2010;156:731–737 [DOI] [PubMed] [Google Scholar]
- 170. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics 2011;128(Suppl. 5):S213–S256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Blaha MJ, Blumenthal RS, Brinton EA; National Lipid Association Taskforce on Non-HDL Cholesterol . The importance of non-HDL cholesterol reporting in lipid management. J Clin Lipidol 2008;2:267–273 [DOI] [PubMed] [Google Scholar]
- 172. Kershnar AK, Daniels SR, Imperatore G, et al. Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr 2006;149:314–319 [DOI] [PubMed] [Google Scholar]
- 173. Maahs DM, Dabelea D, D’Agostino RB Jr, et al.; SEARCH for Diabetes in Youth Study . Glucose control predicts 2-year change in lipid profile in youth with type 1 diabetes. J Pediatr 2013;162:101–7.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Daniels SR; Committee on Nutrition . Lipid screening and cardiovascular health in childhood. Pediatrics 2008;122:198–208 [DOI] [PubMed] [Google Scholar]
- 152. Kavey R-EW, Allada V, Daniels SR, et al.; American Heart Association Expert Panel on Population and Prevention Science; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity and Metabolism; American Heart Association Council on High Blood Pressure Research; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on the Kidney in Heart Disease; Interdisciplinary Working Group on Quality of Care and Outcomes Research . Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation 2006;114:2710–2738 [DOI] [PubMed] [Google Scholar]
- 176. Cadario F, Prodam F, Pasqualicchio S, et al. Lipid profile and nutritional intake in children and adolescents with type 1 diabetes improve after a structured dietician training to a Mediterranean-style diet. J Endocrinol Invest 2012;35:160–168 [DOI] [PubMed] [Google Scholar]
- 177. Salem MA, AboElAsrar MA, Elbarbary NS, ElHilaly RA, Refaat YM. Is exercise a therapeutic tool for improvement of cardiovascular risk factors in adolescents with type 1 diabetes mellitus? A randomised controlled trial. Diabetol Metab Syndr 2010;2:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. McCrindle BW, Urbina EM, Dennison BA, et al.; American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee; American Heart Association Council of Cardiovascular Disease in the Young; American Heart Association Council on Cardiovascular Nursing . Drug therapy of high-risk lipid abnormalities in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in Youth Committee, Council of Cardiovascular Disease in the Young, with the Council on Cardiovascular Nursing. Circulation 2007;115:1948–1967 [DOI] [PubMed] [Google Scholar]
- 179. Salo P, Viikari J, Hämäläinen M, et al. Serum cholesterol ester fatty acids in 7- and 13-month-old children in a prospective randomized trial of a low-saturated fat, low-cholesterol diet: the STRIP baby project. Special Turku Coronary Risk Factor Intervention Project for Children. Acta Paediatr 1999;88:505–512 [DOI] [PubMed] [Google Scholar]
- 180. McCrindle BW, Ose L, Marais AD. Efficacy and safety of atorvastatin in children and adolescents with familial hypercholesterolemia or severe hyperlipidemia: a multicenter, randomized, placebo-controlled trial. J Pediatr 2003;143:74–80 [DOI] [PubMed] [Google Scholar]
- 181. Wiegman A, Hutten BA, de Groot E, et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA 2004;292:331–337 [DOI] [PubMed] [Google Scholar]
- 182. Karter AJ, Stevens MR, Gregg EW, et al. Educational disparities in rates of smoking among diabetic adults: the translating research into action for diabetes study. Am J Public Health 2008;98:365–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Reynolds K, Liese AD, Anderson AM, et al. Prevalence of tobacco use and association between cardiometabolic risk factors and cigarette smoking in youth with type 1 or type 2 diabetes mellitus. J Pediatr 2011;158:594–601.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Scott LJ, Warram JH, Hanna LS, Laffel LM, Ryan L, Krolewski AS. A nonlinear effect of hyperglycemia and current cigarette smoking are major determinants of the onset of microalbuminuria in type 1 diabetes. Diabetes 2001;50:2842–2849 [DOI] [PubMed] [Google Scholar]
- 185. Chaffee BW, Watkins SL, Glantz SA. Electronic cigarette use and progression from experimentation to established smoking. Pediatrics 2018;141:e20173594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Audrain-McGovern J, Stone MD, Barrington-Trimis J, Unger JB, Leventhal AM. Adolescent E-cigarette, hookah, and conventional cigarette use and subsequent marijuana use. Pediatrics 2018;142:e20173616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Centers for Disease Control and Prevention . Smoking and tobacco use: outbreak of lung injury associated with e-cigarette use, or vaping. Accessed 21 October 2022. Available from https://www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html
- 188. Miech R, Johnston L, O’Malley PM, Bachman JG, Patrick ME. Trends in adolescent vaping, 2017-2019. N Engl J Med 2019;381:1490–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Daniels M, DuBose SN, Maahs DM, et al.; T1D Exchange Clinic Network . Factors associated with microalbuminuria in 7,549 children and adolescents with type 1 diabetes in the T1D Exchange clinic registry. Diabetes Care 2013;36:2639–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 2009;4:1832–1843 [DOI] [PubMed] [Google Scholar]
- 191. Inker LA, Schmid CH, Tighiouart H, et al.; CKD-EPI Investigators . Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 2012;367:20–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Cho YH, Craig ME, Hing S, et al. Microvascular complications assessment in adolescents with 2- to 5-yr duration of type 1 diabetes from 1990 to 2006. Pediatr Diabetes 2011;12:682–689 [DOI] [PubMed] [Google Scholar]
- 193. Scanlon PH, Stratton IM, Bachmann MO, Jones C; Four Nations Diabetic Retinopathy Screening Study Group . Risk of diabetic retinopathy at first screen in children at 12 and 13 years of age. Diabet Med 2016;33:1655–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Beauchamp G, Boyle CT, Tamborlane WV, et al.; T1D Exchange Clinic Network . Treatable diabetic retinopathy is extremely rare among pediatric T1D Exchange clinic registry participants. Diabetes Care 2016;39:e218–e219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Nathan DM, Bebu I, Hainsworth D, et al.; DCCT/EDIC Research Group . Frequency of evidence-based screening for retinopathy in type 1 diabetes. N Engl J Med 2017;376:1507–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Gubitosi-Klug RA, Bebu I, White NH, et al.; Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group . Screening eye exams in youth with type 1 diabetes under 18 years of age: once may be enough? Pediatr Diabetes 2019;20:743–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Jaiswal M, Divers J, Dabelea D, Isom S, Bell RA, Martin CL, et al. Prevalence of and risk factors for diabetic peripheral neuropathy in youth with type 1 and type 2 diabetes: SEARCH for Diabetes in Youth Study. Diabetes Care 2017;40:1226–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Pop-Busui R, Boulton AJM, Feldman EL, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care 2017;40:136–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Imperatore G, Boyle JP, Thompson TJ, et al.; SEARCH for Diabetes in Youth Study Group . Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care 2012;35:2515–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Pettitt DJ, Talton J, Dabelea D, et al.; SEARCH for Diabetes in Youth Study Group . Prevalence of diabetes in U.S. youth in 2009: the SEARCH for diabetes in youth study. Diabetes Care 2014;37:402–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Copeland KC, Zeitler P, Geffner M, et al.; TODAY Study Group . Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011;96:159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Bjornstad P, Drews KL, Caprio S, et al.; TODAY Study Group . Long-term complications in youth-onset type 2 diabetes. N Engl J Med 2021;385:416–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Arslanian SA. Metabolic differences between Caucasian and African-American children and the relationship to type 2 diabetes mellitus. J Pediatr Endocrinol Metab 2002;15(Suppl. 1):509–517 [PubMed] [Google Scholar]
- 204. Naughton MJ, Ruggiero AM, Lawrence JM, et al.; SEARCH for Diabetes in Youth Study Group . Health-related quality of life of children and adolescents with type 1 or type 2 diabetes mellitus: SEARCH for Diabetes in Youth Study. Arch Pediatr Adolesc Med 2008;162:649–657 [DOI] [PubMed] [Google Scholar]
- 205. Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation 2012;125:1157–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Whalen DJ, Belden AC, Tillman R, Barch DM, Luby JL. Early adversity, psychopathology, and latent class profiles of global physical health from preschool through early adolescence. Psychosom Med 2016;78:1008–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Dabelea D, Mayer-Davis EJ, Saydah S, et al.; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA 2014;311:1778–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Buse JB, Kaufman FR, Linder B, Hirst K, El Ghormli L; HEALTHY Study Group . Diabetes screening with hemoglobin A(1c) versus fasting plasma glucose in a multiethnic middle-school cohort. Diabetes Care 2013;36:429–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Klingensmith GJ, Pyle L, Arslanian S, et al.; TODAY Study Group . The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: results from the TODAY study. Diabetes Care 2010;33:1970–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Hannon TS, Arslanian SA. The changing face of diabetes in youth: lessons learned from studies of type 2 diabetes. Ann N Y Acad Sci 2015;1353:113–137 [DOI] [PubMed] [Google Scholar]
- 211. Kapadia C; Drugs and Therapeutics Committee of the Pediatric Endocrine Society . Hemoglobin A1c measurement for the diagnosis of type 2 diabetes in children. Int J Pediatr Endocrinol 2012;2012:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Wallace AS, Wang D, Shin JI, Selvin E. Screening and diagnosis of prediabetes and diabetes in US children and adolescents. Pediatrics 2020;146:e20200265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Kester LM, Hey H, Hannon TS. Using hemoglobin A1c for prediabetes and diabetes diagnosis in adolescents: can adult recommendations be upheld for pediatric use? J Adolesc Health 2012;50:321–323 [DOI] [PubMed] [Google Scholar]
- 214. Wu EL, Kazzi NG, Lee JM. Cost-effectiveness of screening strategies for identifying pediatric diabetes mellitus and dysglycemia. JAMA Pediatr 2013;167:32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Dabelea D, Rewers A, Stafford JM, et al.; SEARCH for Diabetes in Youth Study Group . Trends in the prevalence of ketoacidosis at diabetes diagnosis: the SEARCH for diabetes in youth study. Pediatrics 2014;133:e938–e945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Hutchins J, Barajas RA, Hale D, Escaname E, Lynch J. Type 2 diabetes in a 5-year-old and single center experience of type 2 diabetes in youth under 10. Pediatr Diabetes 2017;18:674–677 [DOI] [PubMed] [Google Scholar]
- 217. Ferrara CT, Geyer SM, Liu YF, et al.; Type 1 Diabetes TrialNet Study Group . Excess BMI in childhood: a modifiable risk factor for type 1 diabetes development? Diabetes Care 2017;40:698–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Pinhas-Hamiel O, Dolan LM, Zeitler PS. Diabetic ketoacidosis among obese African-American adolescents with NIDDM. Diabetes Care 1997;20:484–486 [DOI] [PubMed] [Google Scholar]
- 219. TODAY Study Group . Safety and tolerability of the treatment of youth-onset type 2 diabetes: the TODAY experience. Diabetes Care 2013;36:1765–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. TODAY Study Group . Retinopathy in youth with type 2 diabetes participating in the TODAY clinical trial. Diabetes Care 2013;36:1772–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. TODAY Study Group . Lipid and inflammatory cardiovascular risk worsens over 3 years in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 2013;36:1758–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. TODAY Study Group . Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care 2013;36:1735–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Grey M, Schreiner B, Pyle L. Development of a diabetes education program for youth with type 2 diabetes. Diabetes Educ 2009;35:108–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. American Diabetes Association . Be Healthy Today; Be Healthy For Life. Accessed 21 October 2022. Available from http://main.diabetes.org/dorg/PDFs/Type-2-Diabetes-in-Youth/Type-2-Diabetes-in-Youth.pdf
- 225. Atkinson A, Radjenovic D. Meeting quality standards for self-management education in pediatric type 2 diabetes. Diabetes Spectr 2007;20:40–46 [Google Scholar]
- 226. Copeland KC, Silverstein J, Moore KR, et al.; American Academy of Pediatrics . Management of newly diagnosed type 2 Diabetes Mellitus (T2DM) in children and adolescents. Pediatrics 2013;131:364–382 [DOI] [PubMed] [Google Scholar]
- 227. Zeitler P, Hirst K, Pyle L, et al.; TODAY Study Group . A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012;366:2247–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. RISE Consortium . Impact of insulin and metformin versus metformin alone on β-cell function in youth with impaired glucose tolerance or recently diagnosed type 2 diabetes. Diabetes Care 2018;41:1717–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Tamborlane WV, Barrientos-Pérez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, et al. Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med 2019;381:637–646 [DOI] [PubMed] [Google Scholar]
- 230. U.S. Food and Drug Administration . FDA approves treatment for pediatric patients with type 2 diabetes - drug information update. 2021. Accessed 21 October 2022. Available from https://content.govdelivery.com/accounts/USFDA/bulletins/2e98d66
- 231. U.S. Food and Drug Administration . FDA approves new treatment for pediatric patients with type 2 diabetes. 2019. Accessed 21 October 2022. Available from https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-pediatric-patients-type-2-diabetes
- 232. Tamborlane WV, Bishai R, Geller D, et al. Once-weekly exenatide in youth with type 2 diabetes. Diabetes Care 2022;45:1833–1840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Chan CL. Use of continuous glucose monitoring in youth-onset type 2 diabetes. Curr Diab Rep 2017;17:66. [DOI] [PubMed] [Google Scholar]
- 234. Kelly AS, Auerbach P, Barrientos-Perez M, et al.; NN8022-4180 Trial Investigators . A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med 2020;382:2117–2128 [DOI] [PubMed] [Google Scholar]
- 235. U.S. Food and Drug Administration . FDA approves weight management drug for patients aged 12 and older. 2021. Accessed 21 October 2022. Available from https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-weight-management-drug-patients-aged-12-and-older
- 236. U.S. Food and Drug Administration . FDA approves treatment for chronic weight management in pediatric patients aged 12 years and older. Accessed 27 August 2022. Available from https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-treatment-chronic-weight-management-pediatric-patients-aged-12-years-and-older
- 237. Inge TH, Courcoulas AP, Jenkins TM, et al.; Teen-LABS Consortium . Weight loss and health status 3 years after bariatric surgery in adolescents. N Engl J Med 2016;374:113–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Inge TH, Laffel LM, Jenkins TM, et al.; Teen–Longitudinal Assessment of Bariatric Surgery (Teen-LABS) and Treatment Options of Type 2 Diabetes in Adolescents and Youth (TODAY) Consortia . Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr 2018;172:452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Rubino F, Nathan DM, Eckel RH, et al.; Delegates of the 2nd Diabetes Surgery Summit . Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Diabetes Care 2016;39:861–877 [DOI] [PubMed] [Google Scholar]
- 240. Pratt JSA, Lenders CM, Dionne EA, et al. Best practice updates for pediatric/adolescent weight loss surgery. Obesity (Silver Spring) 2009;17:901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Dolan K, Creighton L, Hopkins G, Fielding G. Laparoscopic gastric banding in morbidly obese adolescents. Obes Surg 2003;13:101–104 [DOI] [PubMed] [Google Scholar]
- 242. Sugerman HJ, Sugerman EL, DeMaria EJ, et al. Bariatric surgery for severely obese adolescents. J Gastrointest Surg 2003;7:102–108 [DOI] [PubMed] [Google Scholar]
- 243. Inge TH, Garcia V, Daniels S, et al. A multidisciplinary approach to the adolescent bariatric surgical patient. J Pediatr Surg 2004;39:442–447 [DOI] [PubMed] [Google Scholar]
- 244. Lawson ML, Kirk S, Mitchell T, et al.; Pediatric Bariatric Study Group . One-year outcomes of Roux-en-Y gastric bypass for morbidly obese adolescents: a multicenter study from the Pediatric Bariatric Study Group. J Pediatr Surg 2006;41:137–143 [DOI] [PubMed] [Google Scholar]
- 245. Inge TH, Zeller M, Harmon C, et al. Teen-longitudinal assessment of bariatric surgery: methodological features of the first prospective multicenter study of adolescent bariatric surgery. J Pediatr Surg 2007;42:1969–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Ells LJ, Mead E, Atkinson G, et al. Surgery for the treatment of obesity in children and adolescents. Cochrane Database Syst Rev 2015;6:CD011740. [DOI] [PubMed] [Google Scholar]
- 247. Michalsky MP, Inge TH, Simmons M, et al.; Teen-LABS Consortium . Cardiovascular risk factors in severely obese adolescents: the Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study. JAMA Pediatr 2015;169:438–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Zeinoddini A, Heidari R, Talebpour M. Laparoscopic gastric plication in morbidly obese adolescents: a prospective study. Surg Obes Relat Dis 2014;10:1135–1139 [DOI] [PubMed] [Google Scholar]
- 249. Göthberg G, Gronowitz E, Flodmark CE, et al. Laparoscopic Roux-en-Y gastric bypass in adolescents with morbid obesity--surgical aspects and clinical outcome. Semin Pediatr Surg 2014;23:11–16 [DOI] [PubMed] [Google Scholar]
- 250. Inge TH, Prigeon RL, Elder DA, et al. Insulin sensitivity and β-cell function improve after gastric bypass in severely obese adolescents. J Pediatr 2015;167:1042–1048.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Styne DM, Arslanian SA, Connor EL, et al. Pediatric obesity-assessment, treatment, and prevention: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2017;102:709–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care 2006;29:1300–1306 [DOI] [PubMed] [Google Scholar]
- 253. Song SH, Hardisty CA. Early onset type 2 diabetes mellitus: a harbinger for complications in later years—clinical observation from a secondary care cohort. QJM 2009;102:799–806 [DOI] [PubMed] [Google Scholar]
- 254. Zeitler P, Fu J, Tandon N, et al.; International Society for Pediatric and Adolescent Diabetes . ISPAD clinical practice consensus guidelines 2014. Type 2 diabetes in the child and adolescent. Pediatr Diabetes 2014;15(Suppl. 20):26–46 [DOI] [PubMed] [Google Scholar]
- 255. Song SH. Complication characteristics between young-onset type 2 versus type 1 diabetes in a UK population. BMJ Open Diabetes Res Care 2015;3:e000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Cefalu WT. “TODAY” reflects on the changing “faces” of type 2 diabetes. Diabetes Care 2013;36:1732–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Lawrence JM, Standiford DA, Loots B, et al.; SEARCH for Diabetes in Youth Study . Prevalence and correlates of depressed mood among youth with diabetes: the SEARCH for Diabetes in Youth study. Pediatrics 2006;117:1348–1358 [DOI] [PubMed] [Google Scholar]
- 258. Levitt Katz LE, Swami S, Abraham M, et al. Neuropsychiatric disorders at the presentation of type 2 diabetes mellitus in children. Pediatr Diabetes 2005;6:84–89 [DOI] [PubMed] [Google Scholar]
- 259. Lewis-Fernández R, Rotheram-Borus MJ, Betts VT, et al. Rethinking funding priorities in mental health research. Br J Psychiatry 2016;208:507–509 [DOI] [PubMed] [Google Scholar]
- 260. Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes 2013;4:270–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Pinhas-Hamiel O, Hamiel U, Levy-Shraga Y. Eating disorders in adolescents with type 1 diabetes: challenges in diagnosis and treatment. World J Diabetes 2015;6:517–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Wilfley D, Berkowitz R, Goebel-Fabbri A, et al.; TODAY Study Group . Binge eating, mood, and quality of life in youth with type 2 diabetes: baseline data from the today study. Diabetes Care 2011;34:858–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Shelton RC. Depression, antidepressants, and weight gain in children. Obesity (Silver Spring) 2016;24:2450–2450 [DOI] [PubMed] [Google Scholar]
- 264. Baeza I, Vigo L, de la Serna E, et al. The effects of antipsychotics on weight gain, weight-related hormones and homocysteine in children and adolescents: a 1-year follow-up study. Eur Child Adolesc Psychiatry 2017;26:35–46 [DOI] [PubMed] [Google Scholar]
- 265. TODAY Study Group . Pregnancy outcomes in young women with youth-onset type 2 diabetes followed in the TODAY Study. Diabetes Care 2021;45:1038–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Arnett JJ. Emerging adulthood. A theory of development from the late teens through the twenties. Am Psychol 2000;55:469–480 [PubMed] [Google Scholar]
- 267. Weissberg-Benchell J, Wolpert H, Anderson BJ. Transitioning from pediatric to adult care: a new approach to the post-adolescent young person with type 1 diabetes. Diabetes Care 2007;30:2441–2446 [DOI] [PubMed] [Google Scholar]
- 268. Peters A; American Diabetes Association Transitions Working Group . Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems: a position statement of the American Diabetes Association, with representation by the American College of Osteopathic Family Physicians, the American Academy of Pediatrics, the American Association of Clinical Endocrinologists, the American Osteopathic Association, the Centers for Disease Control and Prevention, Children with Diabetes, The Endocrine Society, the International Society for Pediatric and Adolescent Diabetes, Juvenile Diabetes Research Foundation International, the National Diabetes Education Program, and the Pediatric Endocrine Society (formerly Lawson Wilkins Pediatric Endocrine Society). Diabetes Care 2011;34:2477–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Bryden KS, Peveler RC, Stein A, Neil A, Mayou RA, Dunger DB. Clinical and psychological course of diabetes from adolescence to young adulthood: a longitudinal cohort study. Diabetes Care 2001;24:1536–1540 [DOI] [PubMed] [Google Scholar]
- 270. Laing SP, Jones ME, Swerdlow AJ, Burden AC, Gatling W. Psychosocial and socioeconomic risk factors for premature death in young people with type 1 diabetes. Diabetes Care 2005;28:1618–1623 [DOI] [PubMed] [Google Scholar]
- 271. Kapellen TM, Müther S, Schwandt A, et al.; DPV initiative and the Competence Network Diabetes Mellitus funded by the German Federal Ministry of Education and Research . Transition to adult diabetes care in Germany—high risk for acute complications and declining metabolic control during the transition phase. Pediatr Diabetes 2018;19:1094–1099 [DOI] [PubMed] [Google Scholar]
- 272. Agarwal S, Raymond JK, Isom S, et al. Transfer from paediatric to adult care for young adults with type 2 diabetes: the SEARCH for Diabetes in Youth Study. Diabet Med 2018;35:504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Mays JA, Jackson KL, Derby TA, et al. An evaluation of recurrent diabetic ketoacidosis, fragmentation of care, and mortality across Chicago, Illinois. Diabetes Care 2016;39:1671–1676 [DOI] [PubMed] [Google Scholar]
- 274. Lotstein DS, Seid M, Klingensmith G, et al.; SEARCH for Diabetes in Youth Study Group . Transition from pediatric to adult care for youth diagnosed with type 1 diabetes in adolescence. Pediatrics 2013;131:e1062–e1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Lyons SK, Becker DJ, Helgeson VS. Transfer from pediatric to adult health care: effects on diabetes outcomes. Pediatr Diabetes 2014;15:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Garvey KC, Foster NC, Agarwal S, et al. Health care transition preparation and experiences in a U.S. national sample of young adults with type 1 diabetes. Diabetes Care 2017;40:317–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. The Endocrine Society . Transitions of Care. Accessed 21 October 2022. Available from https://www.endocrine.org/improving-practice/transitions#t1d
- 278. Reid MW, Krishnan S, Berget C, et al. CoYoT1 clinic: home telemedicine increases young adult engagement in diabetes care. Diabetes Technol Ther 2018;20:370–379 [DOI] [PubMed] [Google Scholar]
- 279. Spaic T, Robinson T, Goldbloom E, et al.; JDRF Canadian Clinical Trial CCTN1102 Study Group . Closing the gap: results of the multicenter Canadian randomized controlled trial of structured transition in young adults with type 1 diabetes. Diabetes Care 2019;42:1018–1026 [DOI] [PubMed] [Google Scholar]
- 280. White M, O’Connell MA, Cameron FJ. Clinic attendance and disengagement of young adults with type 1 diabetes after transition of care from paediatric to adult services (TrACeD): a randomised, open-label, controlled trial. Lancet Child Adolesc Health 2017;1:274–283 [DOI] [PubMed] [Google Scholar]
- 281. Schultz AT, Smaldone A. Components of interventions that improve transitions to adult care for adolescents with type 1 diabetes. J Adolesc Health 2017;60:133–146 [DOI] [PubMed] [Google Scholar]
- 282. Sequeira PA, Pyatak EA, Weigensberg MJ, et al. Let’s Empower and Prepare (LEAP): evaluation of a structured transition program for young adults with type 1 diabetes. Diabetes Care 2015;38:1412–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Monaghan M, Baumann K. Type 1 diabetes: addressing the transition from pediatric to adult-oriented health care. Res Rep Endocr Disord 2016;6:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]