Abstract
Background
Early treatment with caffeine in the delivery room (DR) has been proposed to decrease the need for mechanical ventilation (MV) by limiting episodes of apnoea and improving respiratory mechanics in preterm infants. Our aim was to verify the hypothesis that intravenous or enteral administration of caffeine can be performed in the preterm infant in the DR.
Methods
Infants with 25±0–29±6 weeks of gestational age were enrolled and randomised to receive 20 mg/kg of caffeine citrate intravenously, via the umbilical vein, or enterally, through an orogastric tube, within 10 min of birth. Caffeine blood level was measured at 60 ± 15 min after administration and 60 ± 15 min before the next dose (5 mg/kg). The primary endpoint was evaluation of the success rate of intravenous and enteral administration of caffeine in the DR.
Results
Nineteen patients were treated with intravenous caffeine and 19 with enteral caffeine. In all patients the procedure was successfully performed. Peak blood level of caffeine 60 ± 15 min after administration in the DR was found to be below the therapeutic range (5 µg/mL) in 25 % of samples and above the therapeutic range in 3%. Blood level of caffeine 60 ± 15 min before administration of the second dose was found to be below the therapeutic range in 18% of samples.
Conclusions
Intravenous and enteral administration of caffeine can be performed in the DR without interfering with infants’ postnatal assistance. Some patients did not reach the therapeutic range, raising the question of which dose is the most effective to prevent MV.
Clinical Trial Registration
ClinicalTrials.gov identifier NCT04044976; EudraCT number 2018-003626-91.
Key Points
| This is the first study that demonstrates the actual feasibility of caffeine administration in the delivery room without interfering with infants’ postnatal assistance. |
| For the first time we described the feasibility of enteral administration of caffeine in the delivery room. The effectiveness of the procedure was confirmed by the measurement of caffeine blood level. |
| In some patients, the caffeine therapeutic range was not reached, and this raises the question of which dose is the most effective to prevent MV. |
Introduction
Mechanical ventilation (MV) is one of the most important risk factors for the development of bronchopulmonary dysplasia (BPD) in the preterm infant, due to early pulmonary inflammation from volume- and baro-trauma, and the high risk of ventilator-associated pneumonia (VAP) [1]. Therefore, in recent years particular attention has been paid to reducing the need for MV, and some beneficial interventions such as early application of nasal continuous positive airway pressure (CPAP) and surfactant treatment, have become widespread [1, 2]. These interventions have the common objective of promoting development and maintenance of alveolar functional residual capacity (FRC), improving pulmonary compliance, reducing the work of breathing, and favouring gas exchanges [1, 2]. Unfortunately, it has been noted that nasal CPAP in combination with or without surfactant administration fails to prevent MV in about 45–50% of treated infants [3]. In fact, the need for MV can depend not only on the severity of respiratory distress syndrome (RDS), which remains an important factor, but, especially in mild-moderate forms of RDS, it can be due to the onset of relapsing episodes of apnoea. Therefore, it has been proposed to treat very preterm infants with caffeine in the delivery room (DR) within the first minutes of life. In a recent pilot study, Katheria et al. observed that early treatment with caffeine decreased the need for MV in comparison to late treatment in extremely preterm infants [4]. Moreover, in a similar population Dekker et al. demonstrated that treatment with caffeine in the DR or immediately after arrival in the neonatal intensive care unit (NICU) significantly increased the tidal volume and decreased the need for oxygen-therapy in comparison with later treatment [5]. Although these studies had no sufficient statistical power to assess whether treatment with caffeine in the DR is effective in decreasing the need for MV in preterm infants with RDS, the reported results are promising and suggest the usefulness of continuing research on the possible beneficial effects of very-early treatment with caffeine in the delivery room versus later treatment [2].
Thus, this feasibility study aims to demonstrate the hypothesis that it is operatively possible to administer intravenous (IV) or enteral caffeine in the DR during infants’ postnatal assistance and stabilisation. This study is conceived as preliminary to the planning of a subsequent large randomised controlled trial which will assess whether caffeine administered so early can reduce the risk of MV in very preterm infants.
Patient and Methods
Patient Population
This prospective study was carried out from September 2019 to June 2021 at the NICU of the Careggi University Hospital of Florence, after approval by paediatric ethics committees of Tuscany (ID 58/2019). After informed parental consent was obtained before the delivery, inborn infants aged 25±0–29±6 weeks of gestational age at high risk of developing RDS, who do not require MV in the DR, were enrolled in the study. Exclusion criteria included maternal consumption of caffeine before giving birth (> 2 cups of coffee in the 6-h prior birth) major congenital malformations and chromosomal syndromes.
Study Design
The protocol of the study was previously published [6].
Infants were randomised to receive IV 20 mg/kg (1 mL = 20 mg) of caffeine citrate (Peyona®, Chiesi Farmaceutici Spa, Parma, Italy) via the umbilical vein, or enterally, through an orogastric tube, within 10 min of birth. The allocation sequences consisted of computer-generated random numbers, which allowed allocation concealment from investigators.
The treatment with caffeine was carried out by the neonatologist who attended the delivery. Intravenous administration was performed via a "butterfly" needle (21 G) prefilled with saline and attached to a saline-filled 5-mL syringe, which was inserted in the umbilical vein just above the umbilicus after disinfection with chlorhexidine. Endovascular location of the needle tip was confirmed by flushing in saline and withdrawing blood. Gastric tube insertion length was estimated by measuring the distance from the corner of the mouth to the inferior attachment of the ear lobe to a point mid-way between the xiphoid process and umbilicus [7]. Gastric auscultation verified tube position in the stomach and the tube was attached to a 5-mL syringe. A bolus of caffeine was followed by the administration of a 2-mL "flush" of saline both after IV and enteral administration. Careful instructions were given to nurses to avoid aspiration of gastric residuals in the first two hours after enteral administration of caffeine. Completion or failure of administration was recorded.
Caffeine blood level was measured 60 ± 15 min after the administration, to evaluate its peak value, and 60 ± 15 min before IV administration of the second dose (5 mg/kg/day) in the NICU, to evaluate the achievement of therapeutic blood level (5–20 µg/mL) [8]. Blood samples were collected via vein or heel punctures and measured using the "dried blood spots" method with spectrometry and "tandem-mass" liquid chromatography [9].
Resuscitation in the DR was performed following the guidelines of the AAP/AAH [10]. Consistently, infants who needed respiratory support were assisted with nasal continuous airway pressure (nCPAP) when they autonomously breathed or with positive pressure ventilation (PPV) when their breathing was ineffective, or they were apnoeic or persistently bradycardic (< 100 bpm) [9]. Respiratory support was provided using a T-piece resuscitator. Since all infants studied required resuscitation at birth, delayed umbilical cord clamping was not performed [9]. After admission in the NICU, infants with RDS were assisted with non-invasive supports or MV. Surfactant (Curosurf®, Chiesi, Parma, Italy) was given (200 mg/kg) according to the InSURE (Intubation-SURfactant-Extubation) or LISA (Less-Invasive-Surfactant-Administration) technique in infants requiring FiO2 > 0.30 [11] to maintain a SpO2 90–95% and in all infants who needed MV.
Mechanical ventilation was started if pCO2 was > 65 mmHg and pH < 7.20, or pO2 < 50 mmHg with FiO2 > 0.50 after surfactant administration or in case of apnoea (> 4 episodes in 1 h or > 2 episodes in 1 h requiring manual ventilation [12]) and was continued with the aim of maintaining a pCO2 of 55–65 mmHg and a SpO2 of 90–95%. Patients treated with MV could receive additional doses of surfactant (100 mg/kg) at the discretion of the attending neonatologist. Patients were extubated when a good respiratory autonomy was associated with a FiO2 < 0.30 and a mean airway pressure (MAP) < 8 cmH2O. This policy was the same for infants enrolled in the present study and included in the historical control group.
Data Collection
The following data were recorded for each infant: gestational age; birth weight; birth weight < 10th percentile; sex; type of delivery; Apgar score at 5 min; RDS, diagnosis of which was based on the occurrence of oxygen-dependence, tachypnoea, dyspnoea, exclusion of other causes of respiratory failure, and the presence of a typical radiological pattern; peak of FiO2 and MAP, treatment with surfactant; need, type and duration of respiratory supports; prenatal and postnatal steroid treatment; patent ductus arteriosus (PDA) requiring pharmacological therapy, BPD (defined as oxygen requirement at 36 weeks of post-menstrual age [13]), intraventricular haemorrhage (IVH) ≥ 3 grade [14], necrotising enterocolitis (NEC) [15], retinopathy of prematurity (ROP) > 3 grade [16], and sepsis (defined as positive blood culture). In addition, mortality and duration of hospitalisation were reported. The examined maternal variables included clinical chorioamnionitis, placental abruption, hypertensive disorders of pregnancy, and prolonged premature rupture of membranes (pPROM) > 18 h.
Time from birth to 1st and 2nd caffeine doses and from birth to 1st and 2nd blood caffeine level measurements were also recorded.
Main Outcomes
The main outcome of the study was evaluation of the feasibility of IV or enteral administration of caffeine in preterm infants in the DR during stabilisation. We measured caffeine blood level to have objective evidence that caffeine administration had occurred successfully. We also compared the success rate of IV versus enteral caffeine administration and evaluated if it was followed by the achievement of a caffeine therapeutic level (5–20 µg/mL [8]). Moreover, the need for MV in treated infants in comparison with a historical matched control group was assessed. The historical control group includes infants selected consecutively starting September 2017 using the same inclusion and exclusion criteria as infants in the study group. These infants received, as per local procedure, an IV loading dose of 20 mg/kg caffeine citrate (Peyona®, Chiesi Farmaceutici Spa, Parma, Italy) within 6 h of birth followed by an IV maintenance dose of 5–10 mg/kg per day.
Statistical Methods
In the absence of previous studies to use as a reference, it was arbitrarily decided to study 40 patients, 20 of whom were randomly treated with IV caffeine and 20 with enteral caffeine.
The clinical characteristics of infants were described by calculating their mean values and standard deviations, median and ranges or rates and percentages.
The primary endpoint was assessed as treated population calculating the percentage of cases in which caffeine was successfully administered and caffeine blood level could be measured. Comparisons between infants treated with IV or enteral caffeine and between infants treated with caffeine and historical control group were performed using the Student "t" test for continuous parametric variables, the Wilcoxon rank sum test for non-parametric continuous variables and the Fisher test for categorical variables. A p < 0.05 was considered statistically significant.
Results
Forty-two patients were randomised; however we reported data of 38 patients because two patients were erroneously randomised twice, one patient was randomised but pregnancy extended beyond the study inclusion criteria, and one patient was not given caffeine by the neonatologist due to his immediate post-natal transfer to another hospital. Clinical characteristics of infants are detailed in Table 1. Five infants developed 3°–4° IVH (1 in the IV group and 4 in the enteral group) and three infants in the enteral group died (2 from respiratory failure and 1 from sepsis).
Table 1.
Clinical characteristics of infants treated with intravenous or enteral caffeine
| Intravenous caffeine (n = 19) | Enteral caffeine (n = 19) | |
|---|---|---|
| Gestational age (week) | 27.9 ± 1.2 | 27.4 ± 1.2 |
| 25–26 | 4 (21) | 5 (26) |
| 27–29 | 15 (79) | 14 (74) |
| Birth weight (g) | 983 ± 230 | 1003 ± 206 |
| < 10° percentile | 1 (5) | 1 (5) |
| Female | 11 (58) | 11 (58) |
| Antenatal steroids | 19 (100) | 19 (100) |
| Vaginal delivery | 4 (21) | 6 (32) |
| Apgar score at 5 min | 8 (8–9) | 8 (6–8) |
| Respiratory distress syndrome | 14 (74) | 16 (84) |
| Peak FiO2 | 0.52 ± 0.26 | 0.58 ± 0.25 |
| Peak MAP (cm H2O) | 6.8 ± 2.2 | 8.2 ± 4.9 |
| Surfactant | 14 (74) | 15 (79) |
| Non-invasive ventilation | 19 (100) | 19 (100) |
| nCPAP duration (day) | 21 ± 10 | 20 ± 15 |
| nIPPV duration (day) | 13 ± 8 | 7 ± 8 |
| Mechanical ventilation < 72 h of age | 4 (21) | 3 (16) |
| Age at the start (h) | 25 ± 33 | 27 ± 39 |
| PTV | 3 (16) | 2 (11) |
| PTV + HFOV | 1 (5) | 1 (5) |
| Mechanical ventilation during hospitalisation | 6 (33) | 7 (37) |
| PTV | 6 (33) | 7 (33) |
| HFOV | 1 (5) | 4 (21) |
| PTV duration (day) | 16 ± 9 | 15 ± 18 |
| HFOV duration (day) | 1 | 1 ± 1.5 |
| Postnatal steroids | 5 (26) | 4 (21) |
| Patent ductus arteriosus | 15 (79) | 17 (89) |
| Severe bronchopulmonary dysplasia | 2 (10) | 0 |
| Necrotising enterocolitis | 1 (5) | 1 (5) |
| Sepsis | 9 (47) | 6 (32) |
| 3°–4° grade intraventricular haemorrhage | 1 (5) | 4 (20) |
| > 3° grade retinopathy of prematurity | 0 | 0 |
| Death | 0 | 3 (16) |
| Duration of NICU stay (day) | 80 ± 24 | 77 ± 30 |
| Duration of hospital stay (day) | 49 ± 21 | 41 ± 21 |
Mean (± SD), rate (%), or median (interquartile range)
HFOV high frequency oscillatory ventilation, nCPAP nasal continuous airway pressure, NICU neonatal intensive care unit, nIPPV nasal intermittent positive pressure ventilation, pPROM prolonged premature rupture of membranes, PTV patient-triggered ventilation
The occurrence of clinical chorioamnionitis (0% vs 2%), placental abruption (16% vs 5%), hypertensive disorders of pregnancy (21% vs 11%), and pPROM > 18 h (42% vs 53%) were similar in the IV and enteral caffeine groups.
Nineteen patients were treated with IV caffeine and 19 with enteral caffeine. The procedure was performed within the expected time of 10 min (7.6 ± 1.3 vs 7.1 ± 2.0 min) in all patients. Peak blood level of caffeine 60 ± 15 min after administration in the DR was measured in 36 (95%) patients (two patients in the enteral group had low quality samples, which did not allow measurements) and ranged from 1.3 to 22.4 µg/mL in IV-treated infants and from 0.4 to 18.1 µg/mL in infants enterally treated (Fig. 1). Nine samples out of 36 (25%) showed a caffeine blood level below the therapeutic range (5 µg/mL) and one (3%) over the therapeutic range (Table 2). Blood level of caffeine 60 ± 15 min before administration of the second dose in NICU was measured in 33 patients (three patients in the IV group and two patients in the enteral group had low quality samples which did not allow measurements) and ranged from 2.3 to 19.8 µg/mL in IV-treated infants and from 2.8 to 15.0 µg/mL in infants enterally treated (Fig. 1). Six samples out of 33 (18 %) showed a caffeine blood level below the therapeutic range (Table 2).
Fig. 1.
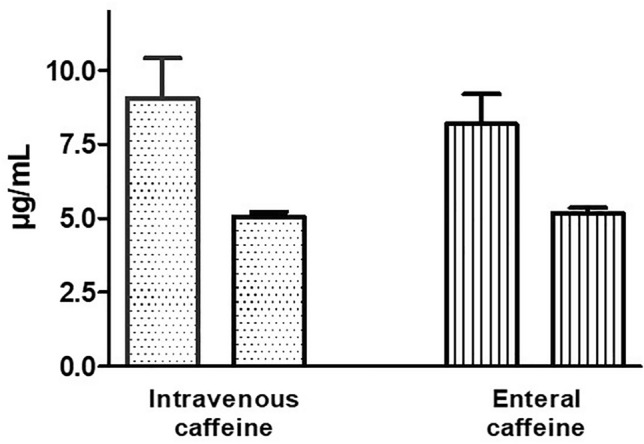
Caffeine blood level (µg/mL) measured 60 ± 15 min after intravenous or enteral administration in the delivery room (first bar) and 60 ± 15 min before the administration of the 2nd dose in neonatal intensive care unit (NICU) (second bar). Mean and standard deviation (SD)
Table 2.
Time from birth and the 1st and 2nd dose of caffeine and caffeine blood level measured 60 ± 15 min after the 1st dose and 60 ± 15 min before the second dose
| Intravenous caffeine (n = 19) | Enteral caffeine (n = 19) | p value | |
|---|---|---|---|
| Time from birth to 1st caffeine dose (min) | 7.6 ± 1.3 | 7.1 ± 2.0 | 0.367 |
| Time from birth to 1st caffeine level measurement (min) | 61.1 ± 3.0 | 61.4 ± 2.7 | 0.748 |
| Caffeine blood level 60 ± 15 min after administration in the DR (µg/mL) | 9.1 ± 5.2 | 8.3 ± 4.1 | 0.602 |
| Caffeine blood level < 5 µg/mL | 6/19 (32) | 3/17 (18) | 0.451 |
| Caffeine blood level > 20 µg/mL | 1/19 (5) | 0 | 1.000 |
| Time from birth to 2nd caffeine dose (h) | 23.5 ± 7.1 | 25.5 ± 2.3 | 0.250 |
| Time from birth to 2nd caffeine level measurement (h) | 22.0 ± 5.4 | 23.1 ± 0.5 | 0.383 |
| Caffeine blood level 60 ± 15 min before the second dose (µg/mL) | 5.0 ± 1.3 | 5.1 ± 1.0 | 0.792 |
| Caffeine blood level < 5 µg/mL | 2/16 (13) | 4/17 (24) | 0.656 |
| Caffeine blood level > 20 µg/mL | 1 (5) | 0 | 1.000 |
Mean ± standard deviation (SD)
DR delivery room, SD standard deviation
The frequency of MV ≤ 72 h of age (7/38, 18 %) was similar in infants who had caffeine blood level < or ≥ 5 µg/mL 60 ± 15 min after the administration in the DR (2/9, 22% vs 5/27, 19%; p = 1.000) and in infants who had caffeine blood level < or ≥ 5 µg/mL 60 ± 15 min before the second dose (1/6, 17% vs 6/27, 22%; p = 1.000).
Fifteen infants in total had both measurements of caffeine blood levels in both the IV and enteral groups and changes in each patient are presented in Fig. 2.
Fig. 2.
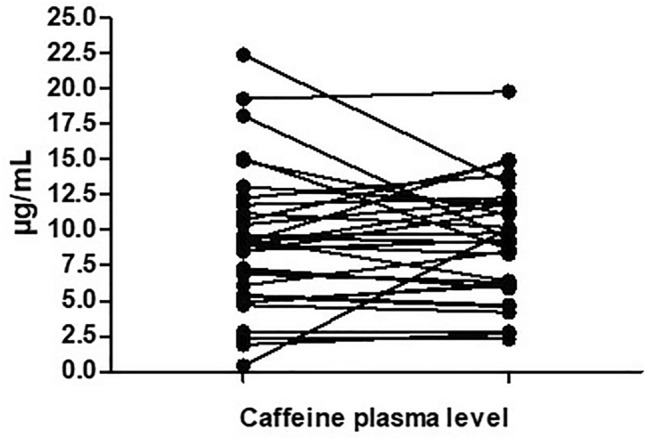
Changes in each patient of caffeine blood level (µg/mL) measured 60 ± 15 min after the intravenous or enteral administration in the delivery room and 60 ± 15 min before the administration of the 2nd dose in the neonatal intensive care unit (NICU)
Eighteen percent (7/38) of our patients needed MV within the first 72 h of life, at the mean age of 26 ± 32 h of life, and 34% (13/38) needed MV during hospitalisation. These values are similar to those found in a historical matched control group since 18% (7/38) of these infants needed MV within the first 72 h of life, at the mean age of 24 ± 21 h of life, and 29 % (11/38) were treated with MV during hospitalisation (Table 3).
Table 3.
Clinical characteristics of infants treated with caffeine in the delivery room versus a historical control group
| Caffeine group (n = 38) | Control group (n = 38) | p value | |
|---|---|---|---|
| Gestational age (week) | 27.7 ± 1.2 | 27.4 ± 1.3 | 0.299 |
| Birth weight (g) | 993 ± 215 | 982 ± 220 | 0.826 |
| Female | 22 (58) | 26 (68) | 0.476 |
| Antenatal steroids | 38 (100) | 38 (100) | 1.000 |
| Vaginal delivery | 10 (26) | 8 (21) | 0.788 |
| Apgar score at 5 min | 8 (5–9) | 8 (6–9) | |
| Peak FiO2 | 0.55 ± 0.25 | 0.48 ± 0.22 | 0.199 |
| Peak MAP (cm H2O) | 7.5 ± 3.8 | 8.5 ± 2.4 | 0.174 |
| Respiratory distress syndrome | 30 (79) | 32 (84) | 0.768 |
| Non-invasive ventilation | 38 (100) | 38 (100) | 1.000 |
| Mechanical ventilation < 72 h of age | 7 (18) | 7 (18) | 1.000 |
| Age at the start (h) | 26 ± 32 | 24 ± 21 | 0.748 |
| Mechanical ventilation during hospitalisation | 13 (34) | 11 (29) | 0.805 |
| Surfactant | 29 (76) | 30 (79) | 1.000 |
| Postnatal steroids | 9 (47) | 2 (5) | 0.047 |
| Patent ductus arteriosus | 22 (58) | 24 (63) | 0.815 |
| Severe bronchopulmonary dysplasia | 2 (5) | 3 (8) | 1.000 |
| Necrotising enterocolitis | 2 (5) | 0 | 0.493 |
| Sepsis | 15 (39) | 10 (26) | 0.329 |
| 3°–4° grade intraventricular haemorrhage | 5 (13) | 2 (5) | 0.430 |
| > 3° grade retinopathy of prematurity | 0 | 0 | N/A |
| Death | 3 (8) | 1 (3) | 0.615 |
| Duration of NICU stay (day) | 45 ± 21 | 40 ± 20 | 0.291 |
| Duration of hospital stay (day) | 78 ± 27 | 77 ± 22 | 0.860 |
| Placental abruption | 4 (11) | 4 (11) | 1.000 |
| Clinical chorioamnionitis | 2 (5) | 2 (5) | 1.000 |
| Hypertensive disorders of pregnancy | 6 (16) | 9 (24) | 0.567 |
| pPROM > 18 h | 18 (47) | 16 (42) | 0.818 |
Mean (± SD), rate (%), or median (interquartile range)
MAP mean airway pressure, NICU neonatal intensive care unit, pPROM prolonged premature rupture of membranes
Discussion
In this study we evaluated the possibility of giving IV caffeine to very preterm infants in the DR, through the umbilical vein, or, for the first time, enterally, through an orogastric tube. We found that both routes of administration allowed a 100% effective caffeine administration as evidenced by the findings in all cases of measurable caffeine blood levels. Moreover, caffeine administration was always performed within the expected 10 min from delivery without interfering with postnatal assistance and stabilisation; thus, supporting the feasibility of the procedure.
The peak of caffeine blood level measured 60 ± 15 min after administration in the DR was similar in infants IV or enterally treated suggesting that both routes are effective options, the latter being less invasive and, in our opinion, slightly faster and more manageable. Infants who received IV caffeine had a higher occurrence of blood level below the therapeutic range (5–20 µg/mL) in comparison with infants who received enteral caffeine (32% vs 16%), but this difference was not statistically significant. These results were expected since it is well known that oral caffeine is almost completely bioavailable and is rapidly and completely absorbed from the gastrointestinal tract [17, 18]. Our data, if confirmed, might raise the relevant question of the most effective dose of caffeine, because failure of CPAP due to the onset of relapsing episodes of apnoea often occurs in the first hours of life when an effective caffeine blood level might not yet been reached, despite the early treatment. Therefore, to prevent MV, it might be useful to increase the loading dose of caffeine since many studies have shown that a high dose (loading doses 20–80 mg/kg/day, maintenance dose 3–20 mg/kg/day) of caffeine is more effective than the standard dose with negligible adverse effects (i.e., tachycardia not leading to discontinuation of caffeine treatment) [19]. On the other hand, we found that early administration of a standard dose of caffeine was safe since only one patient in the IV treatment group showed a blood level over the therapeutic range (22.4 µg/mL). However, this value is not dangerous because it has been reported that slight toxicity manifesting as temporary jitteriness was not detected until 50–84 µg/mL [17, 20], and adverse effects did not occur when caffeine plasma level ranged from 35 to 69 µg/mL [21].
The caffeine blood level measured 60 ± 15 min before administration of caffeine in the NICU was similar in IV or enterally treated infants. We observed that six patients (16%, two patients in the IV group and four in the enteral group) did not reach the therapeutic range, confirming that in some patients a higher dose of caffeine would have been necessary to obtain a therapeutic level. However, these results are consistent with the previously described high interindividual variability of caffeine clearance and volume of distribution, which has been reported to be 18.8% and 22.3%, respectively, in preterm infants and this, ultimately, can explain the observed differences of caffeine blood level [21].
Although it is not the objective of our study, we compared some respiratory outcomes in our population to those of a historical control group. We found that the need for MV in the first 72-h of life (18% vs 18%) and during the hospitalisation (29% vs 34%), and the age at the start of MV (26 ± 32 vs 24 ± 21 h) were similar between the groups. These results contradict the finding of Katheria et al. who found in a small randomised controlled study in 21 infants with gestational age < 29 weeks that a loading dose of 20 mg/kg of caffeine citrate within the first 2-h of life decreased the need for MV at 12-h of life from 70 to 27% in comparison with treatment at 12-h of life [4]. Although our study is not a randomised controlled study, this different effect of caffeine on the need for MV can be explained by the higher occurrence of MV in the Katheria study [4] compared to ours. Conversely, our results agree with Dekker et al. who randomised 30 infants with gestational age of 24–30 weeks to receive 10 mg/kg of IV caffeine based in the DR or later in the NICU and did not find a decrease in MV rate during hospitalisation in the early treatment group (54% vs 40%) [5].
Limitations
The limitations of our study include the size and design, which allowed evaluation of respiratory outcomes in our population only in comparison with a historical control group. Moreover, we did not study pulmonary mechanics and could not evaluate possible correlation between these measurements and caffeine blood level. However, these were not the objectives of our study, which was planned to assess the feasibility of IV or enteral caffeine administration in the DR preliminary to the planning of a large randomised controlled trial to investigate whether early caffeine can reduce the risk of MV in very preterm infants. The strengths of the study include its originality: for the first time we described the feasibility of enteral administration of caffeine in the DR and the measurement of caffeine blood levels as confirmation of an effective administration.
Conclusions
We have found that in our population, IV and enteral administration of caffeine can be performed in the DR without interfering with infants’ postnatal assistance and stabilisation. The effectiveness of the procedure was confirmed by the finding in 95% of cases of measurable caffeine blood level. We observed that in some patients the therapeutic range was not reached, and this raises the question of which dose is the most effective to prevent MV. Thus, our study can contribute to the planning of a randomised controlled trial to assess the effect of very-early caffeine in the DR, the optimal route of administration, and dosage.
Declarations
Funding
Open access funding provided by Università degli Studi di Firenze within the CRUI-CARE Agreement. The study was partially funded by a liberal contribute from Chiesi Farmaceutici Spa.
Conflict of Interest
CD received honoraria from Chiesi Farmaceutici Spa and Vyaire Medical Inc. for scientific consultancy. Remaining authors declare that they have no competing interests.
Ethical Approval
Local ethics committees approved the study.
Consent to Participate
Parental consent was obtained prior to delivery.
Consent for Publication
Not applicable.
Availability of Data and Materials
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
Author Contributions
All authors made substantive intellectual contributions to the trial design and manuscript. All revised the manuscript critically. CD and AC conceived of the study. AC, MC, FM, ML, and GR was responsible for patients’ enrolment, neonatal care to new-borns enrolled, and data collection. GLM and MDB were responsible for the laboratory measurements. LB provided statistical expertise and developed the web-based electronic case report form. CD wrote the manuscript. All authors revised and approved the final manuscript.
Footnotes
The original online version of this article was revised to correct the affiliations for authors Maria Della Bona and Giancarlo la Marca .
Change history
2/16/2024
A Correction to this paper has been published: 10.1007/s40272-024-00619-9
References
- 1.Foglia EE, Jensen EA, Kirpalani H. Delivery room interventions to prevent bronchopulmonary dysplasia in extremely preterm infants. J Perinatol. 2017;37:1171–1179. doi: 10.1038/jp.2017.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kribs A, HummLer H. Ancillary therapies to enhance success of non-invasive modes of respiratory support—approaches to delivery room use of surfactant and caffeine? Semin Fetal Neonatal Med. 2016;21:212–218. doi: 10.1016/j.siny.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Wright CJ, Polin RA, Kirpalani H. Continuous positive airway pressure to prevent neonatal lung injury: how did we get here, and how do we improve? J Pediatr. 2016;173:17–24.e2. doi: 10.1016/j.jpeds.2016.02.059. [DOI] [PubMed] [Google Scholar]
- 4.Katheria AC, Sauberan JB, Akotia D, et al. A pilot randomised controlled trial of early versus routine caffeine in extremely premature infants. Am J Perinatol. 2015;32:879–886. doi: 10.1055/s-0034-1543981. [DOI] [PubMed] [Google Scholar]
- 5.Dekker J, Hooper SB, van Vonderen JJ, et al. Caffeine to improve breathing effort of preterm infants at birth: a randomized controlled trial. Pediatr Res. 2017;82:290–296. doi: 10.1038/pr.2017.45. [DOI] [PubMed] [Google Scholar]
- 6.Dani C, Cecchi A, Remaschi G, Mercadante D, et al. Study protocol: treatment with caffeine of the very preterm infant in the delivery room: a feasibility study. BMJ Open. 2020;10:e040105. doi: 10.1136/bmjopen-2020-040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman D, Saxton V, Holberton J. A weight-based formula for the estimation of gastric tube insertion length in newborns. Adv Neonatal Care. 2012;12:179–182. doi: 10.1097/ANC.0b013e318256bb13. [DOI] [PubMed] [Google Scholar]
- 8.Long JY, Guo HL, He X, et al. caffeine for the pharmacological treatment of apnea of prematurity in the NICU: dose selection conundrum, therapeutic drug monitoring and genetic factors. Front Pharmacol. 2021;12:681842. doi: 10.3389/fphar.2021.681842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel P, Mulla H, Kairamkonda V, et al. Dried blood spots and sparse sampling: a practical approach to estimating pharmacokinetic parameters of caffeine in preterm infants. Br J Clin Pharmacol. 2013;75:805–813. doi: 10.1111/j.1365-2125.2012.04392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wyckoff MH, Aziz K, Escobedo MB, et al. Part 13: neonatal resuscitation: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care (reprint) Pediatrics. 2015;136:S196–218. doi: 10.1542/peds.2015-3373G. [DOI] [PubMed] [Google Scholar]
- 11.Sweet DG, Carnielli V, Greisen G, et al. European consensus guidelines on the management of respiratory distress syndrome—2019 update. Neonatology. 2019;115:432–450. doi: 10.1159/000499361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandri F, Plavka R, Ancora G, et al. Prophylactic or early selective surfactant combined with nCPAP in very preterm infants. Pediatrics. 2010;125:e1402–e1409. doi: 10.1542/peds.2009-2131. [DOI] [PubMed] [Google Scholar]
- 13.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116:1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 14.Papile LS, Burstein J, Burstein R, et al. Incidence and evolution of the sub-ependymal intraventricular hemorrhage: a study of infants weighing less than 1500 grams. J Pediatr. 1978;92:529–534. doi: 10.1016/S0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 15.Bell MJ, Ternberg JL, Feigin RD, et al. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978;187:1–12. doi: 10.1097/00000658-197801000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Committee for the Classification of the Retinopathy of Prematurity An international classification of infection or inflammation of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 17.Aranda JV, Cook CE, Gorman W, et al. Pharmacokinetic profile of caffeine in the premature newborn infant with apnea. J Pediatr. 1979;94:663–668. doi: 10.1016/S0022-3476(79)80047-5. [DOI] [PubMed] [Google Scholar]
- 18.Blanchard J, Sawers SJA. The absolute bioavailability of caffeine in man. Eur J Clin Pharmacol. 1983;24:93–98. doi: 10.1007/BF00613933. [DOI] [PubMed] [Google Scholar]
- 19.Brattström P, Russo C, Ley D, et al. High-versus low-dose caffeine in preterm infants: a systematic review and meta-analysis. Acta Paediatr. 2019;108:401–410. doi: 10.1111/apa.14586. [DOI] [PubMed] [Google Scholar]
- 20.Aranda JV, Turmen T. Methylxanthines in apnea of prematurity. Clin Perinatol. 1979;6:87–108. doi: 10.1016/S0095-5108(18)31165-5. [DOI] [PubMed] [Google Scholar]
- 21.Lee TC, Charles B, Steer P, et al. Population pharmacokinetics of intravenous caffeine in neonates with apnea of prematurity. Clin Pharmacol Ther. 1997;61:628–640. doi: 10.1016/S0009-9236(97)90097-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


