Abstract
Background
Due to undesired environmental impact of insecticides as well as resistant of vectors to them, the development of organic and natural insecticides has been more considered. In the current study, we developed nanoemulsion of eucalyptus and investigated lavicidal activity of it against malaria vector, Anopheles stephensi and Culex pipiens under laboratory as well as semi-field conditions.
Methods
An optimized nanoemulsion was prepared by mixing Eucalyptus oil, Tween 80 and ethanol at ratio of 1:2:1.5 in distilled water, then, stirred for 20 minutes at room temperature. The product was then used for bioassay tests against 3–4th instar larvae of Anopheles stephensi as well as Culex pipiens. Furthermore, a semi-field trial was carried out to evaluate larvicidal activity of nanoemulsion of eucalyptus.
Results
Nanoemulsion of eucalyptus showed significantly high lavicidal activity comparing with bulk eucalyptus essential oil. The LC50 and LC90 value of nanoemulsion against An. stephensi were 111.0 and 180.8 ppm respectively and 29.5 and 73.7 ppm for Cx. pipiens, respectively. In the semi field condition, the Nanoemulsion of eucalyptus decreased 1–2nd instar larval density of Culicines and Anophelines to 90.1% and 85.2%, respectively.
Conclusion
The nano formulation of eucalyptus oil showed high larvicidal activity. Therefore, nanoemulsion of eucalyptus oil can be used as an eco-friendly larvicide against mosquitoes.
Keywords: Eucalyptus, Essential oil, Nanoemulsion, Larvicide, Anopheles stephensi, Culex pipiens
Introduction
Mosquitoes are a serious threat to public health as they act as vectors that help in transmission of diseases that can be lethal (1,2). Owing to the lack of proper medication and vaccines for treating mosquito-borne diseases, an alternative and effective approach used to control the vector population at the larvicidal stage is necessary because, during this stage, the mosquitoes are in a stationary phase (3–5). The resistance to synthetic pesticides and harmful effects of their accumulation in the environment has created the need for natural and non-persistent insecticides. The essential oils extracted from plants are suitable as they are economically reasonable and have high activity in certain cases and are also bio-degradable (4,6,7). Eucalyptus is a diverse genus of flowering trees and shrubs in the Myrtle family, Myrtaceae. The oil extracted from eucalyptus leaves possesses allelopathic property and prevents insects from attacking it, thereby, acting as a natural pesticide (8,9). The fumigation activity (10) and repellency (11) and insecticidal activity of eucalyptus oil has been demonstrated (12). Nanoemulsion of natural oils could enhance pesticide activity of the component (13). Nanoemulsions are emulsions whose droplet size is uniform and extremely small with the size ranging from 20 to 100 nm. Nanoemulsions are metastable and their stability is determined by the method of preparation (14). Nanoemulsions can be formulated by two kinds of methods such as high-energy and low-energy emulsification methods. The high-energy emulsification method comprises high-pressure homogenization and ultrasonication (15). Ultrasonication is the most widely used method owing to its ease of use and it is an economical method. The low-energy emulsification technique comprises methods that exploit the chemical properties of a system to convert a microemulsion into a nanoemulsion (16,17). The use of nanopesticides would be a contemporary measure for the control of pests and reducing the toxic effect of synthetic bulk pesticides on the environment (18). Recently, controlling for vector borne diseases using neem oil nanoemulsion will be of good alternative against Culex quinquefasciatus compared to the synthetic pesticides (13). The present study was carried out to develop a nano formulation of eucalyptus oil as an ecofriendly larvicide and evaluate its larvicidal activity against the larvae of malaria vector, Anopheles stephensi and vectors of West Nile virus (WNV), Culex pipiens in laboratory and semi-field conditions.
Material and Methods
Preparation of nanoemulsion
Eucalyptus oil (Eucalyptus globulus) was purchased from Barich Co., Iran and stored at room temperature under laboratory conditions; Tween 80 (Polyoxyethylene 20 monooleate) was supplied from Sigma. All other chemicals used were of analytical reagent grade. The oil-in-water nanoemulsion was formulated using eucalyptus oil, non- ionic surfactant (tween 80) and water. The concentration of eucalyptus oil (6%, v/v) was fixed for all the formulations. Initially, coarse emulsion was prepared by adding water to organic phase containing oil, surfactant and cosurfactant in ratios 1:1: 1.5 (v/v) using a magnetic stirrer, which was then subjected to ultrasonic emulsification using a 20 kHz Sonicator (Ultrasonics, USA) with a power output of 750 W. Energy input was given through sonotrode containing a piezoelectric crystal with a probe diameter of 13 mm. Sonicator probe generates disruptive forces that reduce the droplet diameter converting coarse emulsion to nanoemulsion. Then the characterization of nanoemulsions was carried out and the emulsion stability was investigated.
Droplet size distribution and polydispersity index
The droplet size distribution (analysis by volume) and poly dispersity index (PDI) of eucalyptus oil nanoemulsion formulation (1:2) was determined using a 90-plus particle size analyzer. The PDI is a measure of the homogeneity and stability of the droplet size in the nanoemulsion system. PDI values below 0.2 indicate a narrow size distribution and thus provide long-term stability to the formulated nanoemulsion. Prior to experiment, formulated emulsion was diluted with milli-Q (Millipore corporation) double-distilled water to trim down multiple scattering effects.
Morphology of emulsion droplets
To visualize the shape and morphology of the formulated nanoemulsions, Atomic force microscopy (AFM) was carried out. One drop of emulsion was negatively stained with phosphotungstic acid and positioned on a copper grid.
Larvicidal bioassay
Mosquito rearing: Mosquitoes consisting of An. stephensi and Cx. pipiens were reared under a uniform condition including larvae and adult nutrition, temperature (28±2 °C), humidity (70±10 %) and on a 12-h light-dark cycle. Larvae were grown in bowls at a density of 200 larvae / 500 ml of distilled water with 0.01% table salt, and fed on fish food. The pupae were transferred to cages made of muslin cloth before eclosion to the adult stage. The female mosquitoes were fed on 10% fructose and the females were fed on guinea pig blood.
Larvicidal activity: The larvicidal activity of eucalyptus nanoemulsion against third instar larvae of An. stephensi and Culex pipiens were treated with different concentrations of nano and bulk eucalyptus oil emulsions. The bioassays were carried according to the guide line of World Health Organization for laboratory and field testing of mosquito larvicides (19). Initially, serial dilutions of nanoemulsion (5, 50, 100, 160, and 240 ppm) were prepared in sterile glass beakers (250 mL) containing 200 ml of water. Then 20 larvae (stage 3–4) of the mosquito species were placed into each beaker. The same procedure was conducted for the bulk of eucalyptus oil. The mortality rate of larvae was recorded after 24 and 48 hours. Each test was performed in four replicates. The percentages of larval mortality and standard deviation were calculated for each concentration of nanoemulsion and bulk emulsion.
Semi-field larval bioassays
Twelve artificial breeding places each 1×1 meters were provided in a semi-field condition in the Kazeroun area, southern Iran and allow the wild mosquitoes to lay their eggs on the surface of breeding places. The density of larvae 10 per dipper were measured in each breeding place before any intervention. The spray of three times of obtained lethal concentration (LC90) of bulk-eucalyptus oil (≈114 ppm) and nano-eucalyptus oil (≈90 ppm) were sprayed randomly on the surface of each breeding place. Each treatment was replicated in four breeding places and four breeding places of free oils as the control.
Statistical analysis
The mortality quantities of 50% and 90 % of imagicides (LC50 and LC90) and the level of confidence of 95%, the equation of the regression line were estimated using a regression probit analysis as described by Finney (20).
Mosquito identifications
All the adults females were identified using morphological identification key identification (21).
Results
Characterization of the selected nanoemulsion
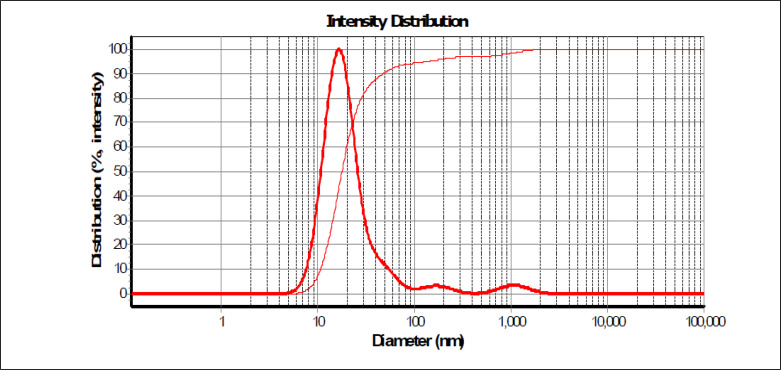
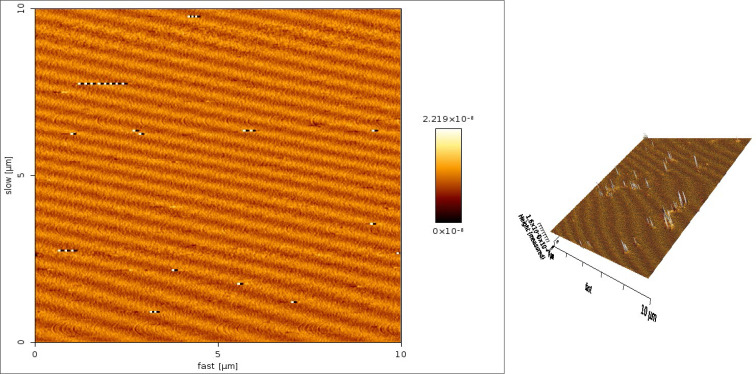
Based on the thermodynamic stability study, 1:2 ratio nanoemulsions was selected, and their average diameter is 18 nm and their size distribution as presented in Fig. 1. The polydispersity index (PI) of the nanoemulsions is 0.060 which shows that it is uniform. The particles are presented in Atomic force microscopy (AFM) from 22 to 40 nm, and the droplets are spherical in nature (Fig. 2).
Fig. 1.
Size distribution of eucalyptus nanoemulsion oil measured by Dynamic Light Scattering
Fig. 2.
Atomic force microscopy image and size of eucalyptus nanoemulsion oil
Larvicidal activity of eucalyptus oil nanoemulsion
The larvicidal activity of both nanoemulsion eucalyptus oil and bulk eucalyptus oil against larvae of An. stephensi and Cx. pipiens was varied. High mortality rate was observed among larvae of An. stephensi exposed to 160 ppm of nanoemulsion of eucalyptus oil within 24 hours, while the mortality rate for eucalyptus oil was 74% at the concentration of 160 ppm after 24 hours (Table 1). Moreover, 100% mortality occurred for the larvae exposed to both nanoemulsion eucalyptus oil and eucalyptus oil at a concentration of 240 ppm. Overall, the larvicidal activity of nanoemulsion oil against larvae of An. stephensi within 24 hours was significantly higher than eucalyptus at 160 ppm, but absolute mortality was observed among the larvae of An. stephensi after 48 hours in both nanoemulsion formulation and bulk oil of eucalyptus (Table 1). Similarity, the larvicidal activity of nanoemulsion oil against larvae of Cx. pipiens was higher than eucalyptus oil at 160 ppm within 24 hours while 100% mortality occurred by 240 ppm for both formulations of eucalyptus oils (Table 2). Lethal concentration (LC50 and LC90) for nanoemulsion of eucalyptus oil and eucalyptus oil were significantly different within 24 and 48 hours after treatment (Table 3). Moreover, LC50 and LC90 of nanoemulsion of eucalyptus oil against larvae of An. stephensi significantly lower than eucalyptus oil within both 24 and 48 hours treatment (Table 3). Similarly, lower LC50 and LC90 was observed for larvae of Cx. pipense exposed to nanoemulsion of eucalyptus oil which was significantly different with LC50 and LC90 for the larvae exposed to eucalyptus oil (Table 4).
Table 1.
Probit analysis of larvicidal activity of essential and nanoemlusion of Eucalyptus globulus oil on 3rd and 4th instar larvae of Anopheles stephensi after 24 hours
| Concentration (ppm) | Essential oil | Nanoemlusion oil | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mortality (%) | Observed probit mortality | Expected probit mortality | Mortality (%) | Observed probit mortality | Expected probit mortality | |
| 60 | 9 | 3.659 | 3.246 | 8 | 3.595 | 3.384 |
| 160 | 74 | 5.643 | 5.658 | 91 | 5.025 | 5.204 |
| 240 | 100 | 7.526 | 6.654 | 100 | 7.576 | 7.024 |
| 360 | 100 | 7.526 | 7.651 | 100 7.576 | 8.089 | |
| Control | 0 | - | - | 0 | - | - |
Table 2.
Probit analysis of larvicidal activity of essential and nanoemlusion of Eucalyptus globulus oil on 3rd and 4th instar larvae of Culex pipiens after 24 hours
| Concentration (ppm) | Essential oil | Nanoemlusion oil | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Mortality (%) | Observed probit mortality | Expected probit mortality | Mortality (%) | Observed probit mortality | Expected probit mortality | |
| 60 | 8 | 3.595 | 3.384 | 11 | 3.773 | 3.520 |
| 160 | 51 | 5.025 | 5.204 | 86 | 4.900 | 5.161 |
| 240 | 100 | 7.576 | 7.024 | 100 | 7.576 | 6.802 |
| 360 | 100 | 7.576 | 8.089 | 100 | 7.576 | 8.443 |
| Control | 0 | - | - | 0 | - | - |
Table 3.
Lethal concentration (LC50 and LC90) and in the 24 and 48 hours bioassay tests of Eucalyptus oil and Nano-Eucalyptus against 3rd and 4th instar larvae of Anopheles stephensi
| Exposure time (hours) | p-Value | χ2 table (df) | χ2 (Heterogeneity) | LC50 (ppm) ± 95%C.L | LC90 (ppm) ± 95% C.L | b ± SE | |
|---|---|---|---|---|---|---|---|
| Eucalyptus oil | 24 | 0.01 | 15.086 (5) | 15.611* | 103.7845 | 169.1937 | 5.6608 ± 0.764 |
| 122.8343 | 206.2336 | ||||||
| 145.0833 | 295.0632 | ||||||
| 48 | 0.00 | 15.035 (5) | 14.623* | 93.761 | 142.402 | 2.399 ± 1.023 | |
| 101.663 | 155.973 | ||||||
| 145.0833 | 175.732 | ||||||
|
| |||||||
| Nano-Eucalyptus oil | 24 | 0.01 | 9.210 (2) | 5.1802* | 63.7924 | 102.8420 | 6.0457 ± 0.539 |
| 80.8730 | 111.0171 | ||||||
| 105.7719 | 119.7206 | ||||||
| 48 | 0.00 | 9.145 (2) | 4.258* | 68.235 | 46.235 | 7.0457 ± 0.652 | |
| 76.869 | 58.595 | ||||||
| 95.582 | 65.548 | ||||||
Table 4.
Lethal concentration (LC50 and LC90) and in the 24 and 48 hours bioassay tests of Eucalyptus oil and Nano-Eucalyptus against 3rd and 4th instar larvae of Culex pipiens
| Exposure time (hours) | p-Value | χ2 table (df) | χ2 (Heterogeneity) | LC50 (ppm) ± 95%C.L | LC90 (ppm) ± 95%C.L. | b ± SE | |
|---|---|---|---|---|---|---|---|
| Eucalyptus oil | 24 | 0.01 | 8.235 (2) | 10.546* | 47.3526 | 20.3120 | 6.5324 ± 1.034 |
| 68.6321 | 39.4112 | ||||||
| 76.1356 | 78.6321 | ||||||
| 48 | 0.01 | 9.210 (2) | 10.678* | 42.4037 | 14.7673 | .4508 ± 1.127 | |
| 64.2180 | 37.3711 | ||||||
| 74.0175 | 86.4146 | ||||||
|
| |||||||
| Nano-Eucalyptus oil | 24 | 0.01 | 9.210 (2) | 15.611* | 43.3775 | 25.7675 | 3.2200 ± 0.313 |
| 53.6733 | 29.4644 | ||||||
| 70.0869 | 33.0781 | ||||||
| 48 | 0.01 | 9.451 (2) | 15.646* | 31.6885 | 19.9645 | 5.1234 ± 0.4123 | |
| 34.5681 | 23.2523 | ||||||
| 39.0263 | 26.2879 | ||||||
Semi field larval bioassays
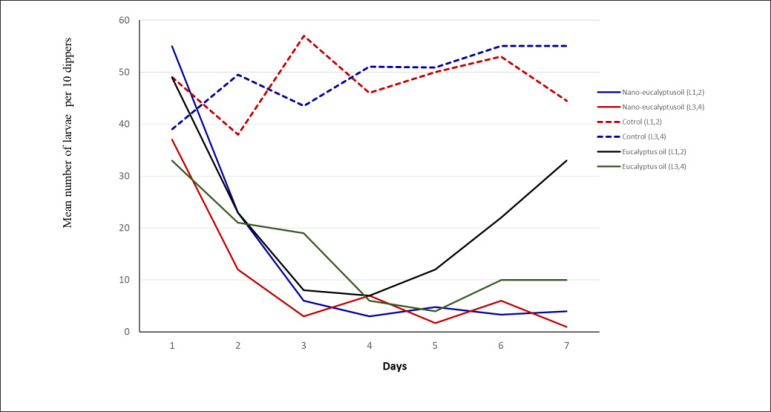
Both nano-eucalyptus and bulk eucalyptus oils decreased the density of mosquito larvae in the treated breeding places one day after spraying. The high larvae population at stage 1 and 2 in the control breeding sites indicated that the wild mosquitoes laid eggs frequently during the bioassay period (Fig. 3). Therefore, the density of mosquito larvae was relatively high in the control breeding site while rapidly declining in the treated sites. However, nano-eucalyptus emulsion kept the larvae density low for 6 days post spraying while the number of larvae at stage 1 and 2 increased two days after eucalyptus oil spraying (Fig. 3). The results indicating that nano-eucalyptus oil has slightly longer residual larvicidal activity rather than bulk eucalyptus.
Fig. 3.
Residual larvicidal activity of Nanoemulsion of eucalyptus oil and bulk eucalyptus oil against different stages of mosquito larvae at semi-field condition. Twelve artificial breeding places (1×1 meters) were prepared and then allowed the wild mosquitoes laid eggs. L1, 2 = Larvae stage 1 & 2, L 3,4= Larvae stage 3 & 4
Discussion
Due to numerous limitations to control of adult mosquitoes, the ideal method to control them is targeting the larval stage. Control of mosquito larvae is based largely on the use of synthetic chemicals. Generally, synthetic pesticides have some disadvantages such as health problems, harmful to the environment, pests may develop tolerance to certain chemicals over time and contamination of soil and water resources (22–24). However, the indiscriminate and injudicious use of pesticides has led to the widespread development of resistance among pests as well as insect vectors (25). Thus, alternative components to be needed for controlling mosquito vectors. Botanical pesticides are considered as safe, easily biodegradable, environmentally friendly and with low toxicity (26,27). However, usage of them is often limited due to instability and rapid degradation, application frequency as well as requiring higher application rates. Hence, the use of botanical insecticides associated with nanotechnology offers considerable potential for increasing efficacy of plant based insecticide (27–29). Eucalyptus oil is the oil distilled from the leaves of eucalyptus, a genus of the plant family Myrtaceae. The repellent activity of eucalyptus oil was demonstrated against Cx. quinquefasciatus (30,31). The seed and leaf extract of eucalyptus oil contain compounds that are toxic to mosquito larvae (32,33). Since they do not cause any adverse health effects; they are used as insect repellents and a safe and eco-friendly alternative to synthetic pesticides (34). Therefore, in this study, we used eucalyptus oil as a safe and non-toxic larvicide. Generally, botanic pesticides have low effective durability; therefore, we utilized nanotechnology to overcome this problem. We synthesized nano-eucalyptus oil to increase larvicidal durability and effectiveness. The method of preparation determines the stability of the formulated nanoemulsion. Based on atomic force microscopy analysis, the size of our product was from 22 to 40 nm (Fig. 2), and the larvae mortality we achieved in laboratory and semi field bioassays (Tables 1, 2 and Fig. 3) was as the results of these nano-size. Nevertheless, the larvicidal effects of nano-eucalyptus oil are likely to change as the nano-particle size changes.
The stabilization of the nanoemulsions is also dependent on the steric effect of the non-ionic surfactant (35). Therefore, the effectiveness of nano-eucalyptus oil may be influenced by chemical component of water in the breeding sites. In semi-field assay, we did not observe 100% mortality of mosquito larvae a day after treatment of nano-eucalyptus oil and eucalyptus. This result may be due to components of water in the breeding sites. In addition, only a single concentration of nano-eucalyptus oil (≈90 ppm) was tested against wild mosquito larvae. According to WHO guide line, the larvicide dosage for the field trial should be three times of LD90 concentration in a laboratory scale. However, we did not observe convenient results for larvicidal effect of nano-eucalyptus oil in the field condition, Thus, more serial concentrations of the nano emulsion should be assays to overcome the highest larvae mortality in the field condition. In this study, eucalyptus oil (6%, v/v) is mixed with a non-ionic surfactant, tween 80 along with water, which is used as an aqueous phase. This emulsion is then subjected to ultrasonication that breaks down the bulk emulsion into an emulsion comprising droplets having the size in the nanometer range. This increases the surface area of the droplets, thereby, increasing the reactivity; thus, making nanoemulsions more effective than its bulk counterpart. However, the insecticidal and antimicrobial activity of eucalyptus oil is linked to its chemical composition such as 8-cineole (eucalyptol), which its concentration ratio varies in eucalyptus trees from different locations (36).
Conclusion
The nano formulation of eucalyptus oil showed high larvicidal activity against mosquito larvae when compared to its bulk counterpart. Therefore, nanoemulsion of eucalyptus oil can be used as an eco-friendly larvicide against mosquitoes. From this study, it can be concluded that eucalyptus oil nanoemulsion is a safe and effective alternative larvicide to control mosquito-borne diseases.
Acknowledgements
The authors would like to thank the School of Public Health, Tehran University of Medical Sciences, for their support in carrying out this research. This study was supported by the Deputy of Research, Tehran University of Medical Sciences, Grant No. 29565. The ethical approval was obtained from the ethics committee of Tehran University of Medical Sciences.
Footnotes
Competing interests
The authors declare that they have no competing interests.
References
- 1. Lajeunesse MJ, Avello DA, Behrmann MS, Buschbacher TJ, Carey K, Carroll J, Chafin TJ, Elkott F, Faust AM, Fauver H. ( 2020) Infected mosquitoes have altered behavior to repellents: A systematic review and meta-analysis. J Med Entomol. 57: 542– 550. [DOI] [PubMed] [Google Scholar]
- 2. Nadim SS, Ghosh I, Martcheva M, Chattopadhyay J. ( 2020) Impact of venereal transmission on the dynamics of vertically transmitted viral diseases among mosquitoes. Math Biosci. 325: 108366. [DOI] [PubMed] [Google Scholar]
- 3. Stoops CA, Qualls WA, Nguyen TT, Richards SL. ( 2019) A review of studies evaluating insecticide barrier treatments for mosquito control from 1944 to 2018. Environ Health Insights. 13, 1178630219859004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sukumar K, Perich MJ, Boobar LR. ( 1991) Botanical derivatives in mosquito control: a review. J Am Mosq Control Assoc. 7: 210– 237. [PubMed] [Google Scholar]
- 5. Tamarina NA. ( 1975) Natural control of blood-sucking mosquito population densities (review of literature). Med Parazitol (Mosk). 44: 603– 607. [PubMed] [Google Scholar]
- 6. Ochoa SA, Sanchez-Torres LE, Nevarez-Moorillon GV, Camacho AD, Nogueda-Torres B. ( 2017) Essential oils and their components as an alternative in the control of mosquito vectors of disease. Biomedica. 37: 224– 243. [DOI] [PubMed] [Google Scholar]
- 7. Fallatah SA, Khater EI. ( 2010) Potential of medicinal plants in mosquito control. J Egypt Soc Parasitol. 40: 1– 26. [PubMed] [Google Scholar]
- 8. Park JH, Lee HS. ( 2018) Toxicities of Eucalyptus dives oil, 3-Carvomenthenone, and its analogues against stored-product insects. J Food Prot. 81: 653– 658. [DOI] [PubMed] [Google Scholar]
- 9. Batish DR, PalSing H, Kumar Kohli R, Kaura S. ( 2008) Eucalyptus essential oil as a natural pesticide. For Ecol Manag. 256: 2166– 2174. [Google Scholar]
- 10. Juan LW, Lucia A, Zerba EN, Harrand L, Marco M, Masuh HM. ( 2011) Chemical composition and fumigant toxicity of the essential oils from 16 species of Eucalyptus against Haematobia irritans (Diptera: Muscidae) adults. J Econ Entomol. 104: 1087– 1092. [DOI] [PubMed] [Google Scholar]
- 11. Mossi AJ, Astolfi V, Kubiak G, Lerin L, Zanella C, Toniazzo G, Oliveira D, Treichel H, Devilla IA, Cansian R, Restello R. ( 2011) Insecticidal and repellency activity of essential oil of Eucalyptus sp. against Sitophilus zeamais Motschulsky (Coleoptera, Curculionidae). J Sci Food Agric. 91: 273– 277. [DOI] [PubMed] [Google Scholar]
- 12. Alzogaray RA, Lucia A, Zerba EN, Masuh HM. ( 2011) Insecticidal activity of essential oils from eleven Eucalyptus spp. and two hybrids: lethal and sublethal effects of their major components on Blattella germanica. J Econ Entomol. 104: 595– 600. [DOI] [PubMed] [Google Scholar]
- 13. Anjali CH, Sharma Y, Mukherjee A, Chandrasekaran N. ( 2012) Neem oil (Azadirachta indica) nanoemulsion--a potent larvicidal agent against Culex quinquefasciatus. Pest Manag Sci. 68: 158– 163. [DOI] [PubMed] [Google Scholar]
- 14. Salvia-Trujillo L, Soliva-Fortuny R, Rojas-Grau MA, McClements DJ, Martin-Belloso O. ( 2017) Edible Nanoemulsions as Carriers of Active Ingredients: A Review. Annu Rev Food Sci Technol. 8: 439– 466. [DOI] [PubMed] [Google Scholar]
- 15. Kumar M, Bishnoi RS, Shukla AK, Jain CP. ( 2019) Techniques for formulation of nanoemulsion drug delivery system: A review. Prev Nutr Food Sci. 24: 225– 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sarheed O, Shouqair D, Ramesh K, Khaleel T, Amin M, Boateng J, Drechsler M. ( 2020) Formation of stable nanoemulsions by ultrasound-assisted two-step emulsification process for topical drug delivery: Effect of oil phase composition and surfactant concentration and loratadine as ripening inhibitor. Int J Pharm. 576: 118952. [DOI] [PubMed] [Google Scholar]
- 17. Wang L, Mutch KJ, Eastoe J, Heenan RK, Dong J. ( 2008) Nanoemulsions prepared by a two-step low-energy process. Langmuir. 24: 6092– 6099. [DOI] [PubMed] [Google Scholar]
- 18. Kumar S, Nehra M, Dilbaghi N, Marrazza G, Hassan AA, Kim KH. ( 2019) Nano-based smart pesticide formulations: Emerging opportunities for agriculture. J Control Release, 294, 131– 153. [DOI] [PubMed] [Google Scholar]
- 19. World Health Organization ( 2005) Guidelines for laboratory and field testing of mosquito larvicides. WHO/CDS/WHOPES/GCDPP/2005.13. pp39
- 20. Finney DJ. ( 1971) Probit Analysis. 3rd ed. Cambridge University press, London. pp 333 [Google Scholar]
- 21. Azarian-Hamidi S, Harbach RE. (2009). Keys to the adult females and fourth-instar larvae of the mosquitoes of Iran (Diptera: Culicidae). Zootaxa, 2078( 1), 1– 33. [Google Scholar]
- 22. Yassin M, Ton J, Rolfe SA, Valentine TA, Cromey M, Holden N, Newton AC. ( 2021) The rise, fall and resurrection of chemical-induced resistance agents. Pest Manag Sci. 10.1002/ps.6370 [DOI] [PubMed] [Google Scholar]
- 23. Gurusubramanian G, Rahman A, Sarmah M, Ray S, Bora S. ( 2008) Pesticide usage pattern in tea ecosystem, their retrospects and alternative measures. J Environ Biol. 29: 813– 826. [PubMed] [Google Scholar]
- 24. Casida JE. ( 2016) Unexpected metabolic reactions and secondary targets of pesticide action. J Agric Food Chem. 64: 4471– 4477. [DOI] [PubMed] [Google Scholar]
- 25. Smith RF, Calvert DJ. ( 1976) Health-related aspects of integrated pest management. Environ Health Perspect. 14: 185– 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jaoko V, Nji Tizi Taning C, Backx S, Mulatya J, Van den Abeele J, Magomere T, Olubayo F, Mangelinckx S, Werbrouck SPO, Smagghe G. ( 2020) The phytochemical composition of Melia volkensii and its potential for insect pest management. Plants (Basel). 9( 2): 143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Casida JE. ( 2012) The greening of pesticide-environment interactions: some personal observations. Environ Health Perspect. 120: 487– 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chaudhari AK, Singh VK, Kedia A, Das S, Dubey NK. ( 2021) Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: prospects and retrospects. Environ Sci Pollut Res Int. 28: 18918– 18940. [DOI] [PubMed] [Google Scholar]
- 29. Luiz de Oliveira J, Ramos Campos EV, Fraceto LF. ( 2018) Recent developments and challenges for nanoscale formulation of botanical pesticides for use in sustainable agriculture. J Agric Food Chem. 66: 8898– 8913. [DOI] [PubMed] [Google Scholar]
- 30. Tian Y, Dong F, Zhou X, Yang X. ( 2020) Repellent, insecticidal and antimicrobial activities of leaf essential oils from three eucalyptus species. Chem Biodivers. 17: e1900580. [DOI] [PubMed] [Google Scholar]
- 31. Magesa SM, Kamugisha ML. ( 2006) Evaluation of the bio-efficacy of three brands of repellents against wild populations of anthropophilic mosquitoes. Tanzan Health Res Bull. 8: 145– 148. [PubMed] [Google Scholar]
- 32. Cheng SS, Huang CG, Chen YJ, Yu JJ, Chen WJ, Chang ST. ( 2009) Chemical compositions and larvicidal activities of leaf essential oils from two eucalyptus species. Bioresour Technol. 100: 452– 456. [DOI] [PubMed] [Google Scholar]
- 33. Senthil Nathan S. ( 2007) The use of Eucalyptus tereticornis Sm. (Myrtaceae) oil (leaf extract) as a natural larvicidal agent against the malaria vector Anopheles stephensi Liston (Diptera: Culicidae). Bioresour Technol. 98: 1856– 1860. [DOI] [PubMed] [Google Scholar]
- 34. Kweka EJ, Mosha F, Lowassa A, Mahande AM, Kitau J, Matowo J, Mahande MJ, Massenga CP, Tenu F, Feston E., Lyatuu E, Mboya M, Mndeme R, Chuwa G, Temu E. ( 2008) Ethnobotanical study of some of mosquito repellent plants in north-eastern Tanzania. Malar J. 7: 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perugini L, Cinelli G, Cofelice M, Ceglie A, Lopez F, Cuomo F. ( 2018) Effect of the coexistence of sodium caseinate and Tween 20 as stabilizers of food emulsions at acidic pH. Colloids Surf B Biointerfaces. 168: 163– 168. [DOI] [PubMed] [Google Scholar]
- 36. Gonzalez A, Gutierrez-Cutino M, Moenne A. ( 2014) Oligo-carrageenan kappa-induced reducing redox status and increase in TRR/TRX activities promote activation and reprogramming of terpenoid metabolism in Eucalyptus trees. Molecules. 19: 7356– 7367. [DOI] [PMC free article] [PubMed] [Google Scholar]