Abstract
In vitro infection of macrophages with Legionella pneumophila induced interleukin-1α (IL-1α), IL-10, monocyte chemotactic protein 1 (MCP-1), and MCP-3 but not IL-12. The lipopolysaccharide (LPS)-induced production of IL-12 was down-regulated by infection with virulent L. pneumophila, but other cytokines were not affected. In contrast, avirulent L. pneumophila or UV-killed, virulent L. pneumophila did not induce any suppression of IL-12. The IL-12 suppression occurred at the level of mRNA accumulation for IL-12 genes in response to LPS stimulation, but the infection induced a marked accumulation of mRNA for both MCP-1 and MCP-3, which are known to suppress IL-12 production in LPS-stimulated macrophages. However, pretreatment of macrophages with MCP-1 did not suppress LPS-induced IL-12 production at the concentrations induced by L. pneumophila infection. These results suggest that L. pneumophila selectively suppresses IL-12 production induced by LPS from macrophages in vitro by an MCP-independent mechanism.
Legionella pneumophila is a gram-negative, facultative intracellular pathogen and is the causative agent of Legionnaires' disease, a severe form of pneumonia. Once L. pneumophila enters the respiratory tract and causes pneumonia, most of the invading bacteria locate within inflammatory phagocytic cells. However, the mechanism by which L. pneumophila infection of the lung is controlled is not yet clear, but the activation of macrophages to suppress intracellular bacterial growth is thought to be an essential effector mechanism of cell-mediated immunity (CMI) in the resolution of legionellosis (16, 23). Th1 cells are essential for the development of CMI and may play a pivotal role in the defense against L. pneumophila infection. Interleukin-12 (IL-12), which is one of the key cytokines in the regulation of development of Th1 responses (26, 28), is a heterodimeric cytokine composed of two disulfide-linked subunits, p35 and p40. Both subunits have to be expressed within the same cell to produce the biologically active p70 heterodimer (13). It has been shown that p40 mRNA accumulation is up-regulated in the cells producing IL-12, whereas p35 mRNA is constitutively expressed in various cells (10). Previous studies have demonstrated that IL-12 is critical for resolution of infection by some replicative intracellular pathogens, including Leishmania major (15, 24), Mycobacterium tuberculosis (8), Listeria monocytogenes (17), Toxoplasma gondii (12), and L. pneumophila (4). Furthermore, it has been shown that the Th1 cytokine gamma interferon γ (IFN-γ) could activate macrophages and monocytes to inhibit L. pneumophila growth (2, 22). Therefore, regulation of IL-12 production may eventually regulate, at least somewhat, the outcome of L. pneumophila infection.
The production of IL-12 by monocytes and macrophages is induced by exposing responsive cells to a variety of microbial products. Lipopolysaccharide (LPS) of gram-negative bacteria has been the most clearly defined inducer of IL-12 (10). On the other hand, the production of IL-12 is regulated by multiple mechanisms; these include cytokines, chemoattractants, endogenous pharmacoactive substances, and activation-induced deactivation pathways (3, 18, 26). Furthermore, some intracellular pathogens have been shown to suppress macrophage IL-12 production. For example, the interaction of Leishmania spp. (1, 5, 14, 27), measles virus (19), Histoplasma capsulatum (21), and human immunodeficiency virus (HIV) (6, 7) with macrophages and monocytes results in a marked decrease in IL-12 production. Therefore, it seems likely that the suppression of IL-12 production may be exploited by these intracellular pathogens as a way to escape from CMI. However, it is not clear how L. pneumophila infection affects IL-12 production. In the study reported here, therefore, the ability of L. pneumophila to regulate macrophage IL-12 production was examined in vitro.
Peritoneal macrophages were obtained from female A/J mice (Jackson Laboratory, Bar Harbor, Maine), 8 to 12 weeks old, 3 days after intraperitoneal injection of 3% thioglycolate broth, as described previously (32). The macrophages were adhered to 6-well tissue culture plates for 2 h in 5% CO2 at 37°C, and the resulting cell monolayers in RPMI 1640 medium with 10% heat-inactivated fetal calf serum (FCS; Hyclone Laboratories, Logan, Utah) were utilized for the experiments. Virulent L. pneumophila M124 serogroup 1 was obtained from a case of fatal legionellosis (11). Avirulent L. pneumophila was prepared by multiple passages of strain M124 as described previously (31). Avirulent L. pneumophila showed no lethal activity for susceptible A/J mice by intraperitoneal infection (31). Both virulent and avirulent L. pneumophila strains were cultured on buffered charcoal yeast extract (BCYE) medium (Gibco Laboratories, Madison, Wis.) for 3 days at 37°C. UV-killed virulent L. pneumophila was prepared by UV irradiation for 20 min. The killed bacteria were tested for viability by plating on BCYE agar medium. The macrophage monolayers were infected with L. pneumophila (infectivity ratio, 10 bacteria per cell) for 30 min, washed to remove nonphagocytized bacteria, and incubated in RPMI 1640 medium containing 10% FCS with or without 1 μg of Escherichia coli LPS (Sigma Chemical Co., St. Louis, Mo.) per ml. To determine the monocyte chemotactic protein 1 (MCP-1) concentration necessary for suppression of IL-12, the macrophages were preincubated with various concentrations of mouse recombinant MCP-1 (PharMingen Int., San Diego, Calif.) for 1 h at 37°C before stimulation with LPS (1 μg/ml). Twenty-four hours later, culture supernatants were collected, and IL-12 levels were determined by enzyme-linked immunosorbent assay (ELISA). The amounts of IL-1α, IL-6, IL-10, IL-12 p40/p70, and MCP-1 in culture supernatants were determined by sandwich ELISA (PharMingen; Genzyme Diagnostics, Cambridge, Mass.). The ELISA for the IL-12 p40/p70 utilized in this study measured both the IL-12 p40 and IL-12 p70 heterodimers. RNA isolation from macrophages and reverse transcription (RT)-PCR with primers for β2-microglobulin (BMG), IL-10, IL-12 p35, IL-12 p40, MCP-1, and MCP-3 were performed as described previously (30). The primer sequences for BMG were also described previously (29). The sequences of the primers for IL-10 were 5′-ACC TGG TAG AAG TGA TGC CCC AGG CA-3′ (sense) and 5′-CTA TGC AGT TGA TGA AGA TGT CAA A-3′ (antisense). The sequences of primers for IL-12 p35 were 5′-AAG ACA TCA CAC GGG ACC AAA CCA-3′ (sense) and 5′-CGC AGA GTC TCG CCA TTA TGA TTC-3′ (antisense). The sequences of primers for IL-12 p40 were 5′-CCA CTC ACA TCT GCT GCT CCA CAA G-3′ (sense) and 5′-ACT TCT CAT AGT CCT TTG GTC CAG-3′ (antisense). The sequences of primers for MCP-1 were 5′-GTG AGC TCC AGA TGC AGT TA-3′ (sense) and 5′-AGC ACA GAC CTC TCT CTT GA-3′ (antisense). The sequences of primers for MCP-3 were 5′-ACC ATG AGG ATC TCT GCC AC-3′ (sense) and 5′-CAT TCC TTA GGC GTG ACC AT-3′ (antisense). The PCR was performed in a Minicycler (MJ Research, Watertown, Mass.) for either 30 cycles and 60°C annealing temperature (BMG) or 40 cycles and 62°C annealing temperature (IL-10, IL-12 p35, IL-12 p40, MCP-1, and MCP-3). PCR products were analyzed on ethidium bromide-stained 2% agarose gels, semiquantitated, and normalized to BMG using densitometry readings (Bio-Rad Laboratories, Hercules, Calif.). Statistical analysis was performed with the paired Student's t test.
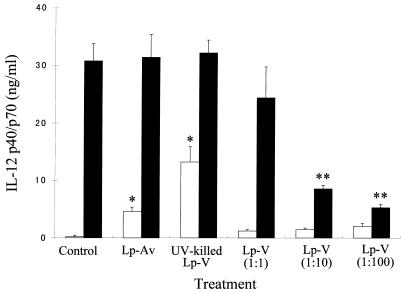
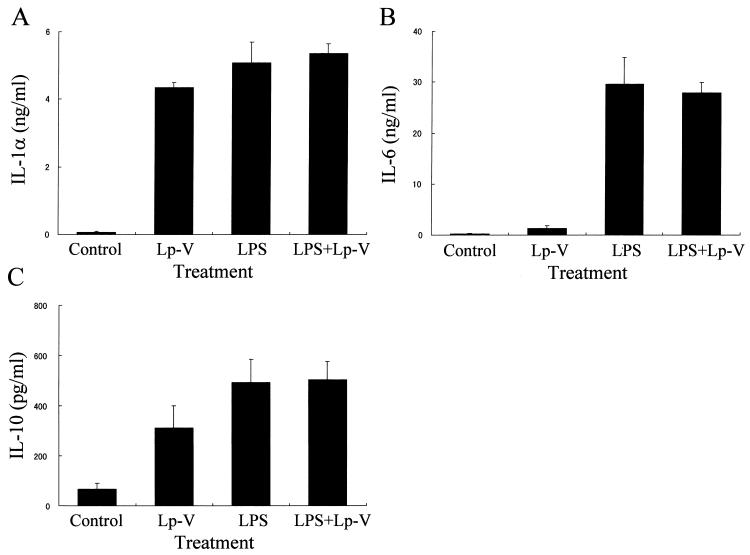
We initially determined how in vitro L. pneumophila infection of macrophages could affect the IL-12 p40/p70 protein production in response to LPS, since it is known that p40 is inducible but p35 is constitutive. The cells were infected with L. pneumophila or stimulated with either LPS alone or LPS in combination with L. pneumophila. As evident in Fig. 1, both avirulent and UV-killed L. pneumophila induced a significant level of IL-12 protein in culture supernatants of the macrophages. However, virulent bacteria did not induce any significant amounts of IL-12, even with a higher infectivity ratio, such as 100 bacteria per macrophage. Furthermore, infection of macrophages with virulent L. pneumophila caused a marked down-regulation of the LPS-induced IL-12 production in a dose-dependent manner. On the other hand, neither avirulent nor UV-killed bacteria showed any down-regulation of LPS-induced IL-12 production. In order to determine whether IL-12 was suppressed in a selective fashion by L. pneumophila infection, levels of IL-1α, IL-6, and IL-10 proteins in the culture supernatants were measured by ELISA. The virulent L. pneumophila infection induced production of IL-1α (Fig. 2A) and IL-10 (Fig. 2C), but induction of IL-6 was minimal (Fig. 2B). Furthermore, the infection did not alter the LPS-induced production of IL-1α, IL-6, and IL-10.
FIG. 1.
Effect of L. pneumophila infection on the protein levels of IL-12 p40/p70 production induced by LPS. The amount of IL-12 p40/p70 protein in the culture supernatants obtained at 24 h after infection was measured by ELISA. The infectivity ratios were 1:10 (macrophage-bacteria) in the case of LP-Av and UV-killed LP-V and 1:1 to 1:100 for LP-V. Results are expressed as means ± SD for three independent experiments. Open column, non-LPS-stimulated group; closed column, LPS-stimulated group; ∗, P < 0.05 compared to noninfected control group; ∗∗, P < 0.05 compared to LPS-stimulated control group.
FIG. 2.
Effect of virulent L. pneumophila infection on the protein levels of IL-1α (A), IL-6 (B), and IL-10 (C) production induced by LPS. The amount of indicated cytokine proteins in the culture supernatants obtained at 24 h after infection was measured by ELISA. Results are expressed as means ± SD for three independent experiments.
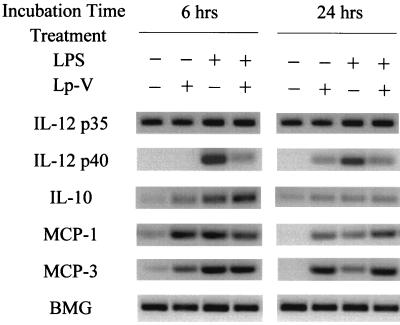
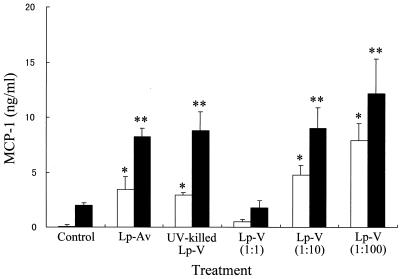
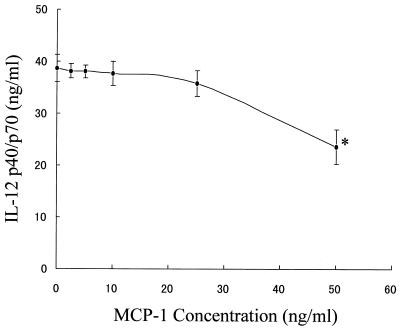
To determine how virulent L. pneumophila infection affects IL-12 production at the level of gene transcription, we also examined steady-state levels of IL-12 p35 and IL-12 p40 mRNA isolated from macrophages infected with L. pneumophila or stimulated with either LPS alone or LPS in combination with bacteria by RT-PCR. Previous studies have demonstrated that IL-10 and MCPs are potent inhibitors of IL-12 production (3, 9, 20, 25); therefore, we also examined the expression levels of IL-10, MCP-1, and MCP-3 mRNA. As shown in Fig. 3, virulent L. pneumophila infection resulted in suppression of mRNA accumulation for the IL-12 p40 gene in response to LPS stimulation but not the IL-12 p35 gene, which was constitutive in all macrophages treated. The virulent L. pneumophila infection induced mRNA accumulation for both MCP-1 and MCP-3 genes in response to LPS stimulation, but IL-10 induction was minimal at 24 h after infection. To determine how the exposure of macrophages to L. pneumophila infection could affect the MCP-1 protein production in response to LPS, we measured the level of MCP-1 in the culture supernatants by ELISA. As shown in Fig. 4, L. pneumophila infection induced production of MCP-1 and up-regulated the LPS-induced production of MCP-1 regardless of the virulence of L. pneumophila and its viability in the macrophages, because both avirulent and killed bacteria up-regulated MCP-1 similarly to virulent bacteria. Furthermore, in order to determine a possible involvement of MCP-1 in the suppression of IL-12 by infection, we also determined the MCP-1 concentration necessary for IL-12 suppression. Macrophages were preincubated with various concentrations of MCP-1 before stimulation with LPS. The significant suppressive effect of MCP-1 occurred at a dose of 50 ng/ml (Fig. 5). However, pretreatment with MCP-1 at the concentrations induced by virulent L. pneumophila, 5 to 15 ng/ml, did not suppress LPS-induced IL-12 production.
FIG. 3.
Effect of virulent L. pneumophila infection on cytokine mRNA expression induced by LPS. Macrophage monolayers were infected with virulent L. pneumophila and incubated with or without LPS. The mRNA expression for cytokines was determined by RT-PCR, as described in Materials and Methods.
FIG. 4.
Effect of L. pneumophila infection on the MCP-1 production induced by LPS. The amount of MCP-1 in the culture supernatants obtained at 24 h after infection was measured by ELISA. See the legend to Fig. 1. Results are expressed as means ± SD for three independent experiments. Open column, non-LPS-stimulated group; closed column, LPS-stimulated group; ∗, P <0.05 compared to noninfected control group; ∗∗, P <0.05 compared to LPS-stimulated control group.
FIG. 5.
Effect of MCP-1 on the IL-12 p40/p70 production induced by LPS. The amount of IL-12 p40/p70 protein in the culture supernatants obtained at 24 h after stimulation in the presence of various concentrations of MCP-1 was measured by ELISA. Results are expressed as means ± SD for three independent experiments. ∗, P <0.05 compared to nonstimulated control group.
Thus, it was demonstrated that virulent L. pneumophila but not avirulent or killed bacteria suppressed macrophage IL-12 protein production induced by LPS. This result indicates that IL-12 suppression may be dependent on L. pneumophila virulency and its viability in the macrophages. The suppression of cytokines by L. pneumophila infection was selective for IL-12, since IL-1α, IL-6, and IL-10, as well as MCP-1 and MCP-3, induced by LPS were not suppressed by the infection. Therefore, it is conceivable that this suppression of IL-12 production by L. pneumophila infection has specificity and is not the result of a generalized failure of macrophage function. It is interesting that the data also indicate that the suppression may not be dependent on L. pneumophila-induced IL-10, which is known to suppress IL-12 production in the presence of LPS (9, 25), because the infection did not enhance the IL-10 production induced by LPS. This is consistent with prior reports of IL-12 suppression by HIV (6, 7) and Leishmania spp. (14). On the other hand, it is possible that L. pneumophila-induced MCPs, which are also known to suppress IL-12 production in the presence of LPS (3), may be related to IL-12 suppression. As shown in Fig. 4, virulent L. pneumophila infection induced production of MCP-1 and, furthermore, up-regulated the LPS-induced production of MCP-1 in a dose-dependent manner. Therefore, it seems likely that MCP-1 induced by infection may play a significant role in down-regulation of IL-12 by infection. However, both avirulent and UV-killed virulent L. pneumophila, which did not induce any suppression of IL-12, also up-regulated the LPS-induced production of MCP-1 similarly to virulent bacteria, which induced IL-12 suppression. Moreover, in the dose-response study concerning the suppression of IL-12 protein by MCP-1, pretreatment with MCP-1 at concentrations induced by virulent L. pneumophila did not suppress LPS-induced IL-12 production. Thus, even though there were no neutralization experiments of MCPs by antibody in this study due to lack of commercially available antibodies, these results indicate that MCP-1 may not be involved in the suppression of LPS-induced IL-12 by L. pneumophila infection.
L. pneumophila suppressed IL-12 production induced by LPS from macrophages at the level of mRNA accumulation, because the suppression was associated with decreased steady-state levels of mRNA for IL-12 p40. These results are consistent with prior reports of IL-12 suppression by Leishmania spp. (14) regarding inhibition of IL-12 at the mRNA level of p40. However, IL-12 suppression by HIV is associated with decreased mRNA accumulation for both p40 and p35 (7). Therefore, mechanisms of IL-12 suppression by infection may depend on the pathogens used.
The finding of IL-12 suppression by L. pneumophila infection in vitro does not agree with the recent study showing that the experimental mouse infection with L. pneumophila induced certain production levels of IL-12 (4). Since in vivo versus in vitro systems are quite different, we cannot directly compare the results of the two systems. However, it can be speculated that the suppression of IL-12 by infection may be overcome by the host defense system, because the infected animals tested were eventually cured. This speculation is supported by the finding that the systemic administration of exogenous IL-12 increases host resistance to several intracellular pathogens (15, 24, 27). Nevertheless, it is obvious that viable virulent L. pneumophila selectively suppresses IL-12 production induced by LPS from macrophages in vitro at the level of both mRNA and protein secretion by an MCP-1-independent mechanism. Although the mechanism of suppression is not yet clear, the suppression of IL-12 production may be exploited by L. pneumophila as a way to escape from cell-mediated immunity.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (AI45169) and the American Lung Association of Florida.
REFERENCES
- 1.Belkaid Y, Butcher B, Sacks D L. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania-infected cells. J Immunol. 1998;28:1389–1400. doi: 10.1002/(SICI)1521-4141(199804)28:04<1389::AID-IMMU1389>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 2.Bhardwaj N, Nash T W, Horwitz D M. IFN-γ activated human monocytes inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1986;137:2662–2669. [PubMed] [Google Scholar]
- 3.Braun M C, Lahey E, Kelsall B L. Selective suppression of IL-12 production by chemoattractants. J Immunol. 2000;164:3009–3017. doi: 10.4049/jimmunol.164.6.3009. [DOI] [PubMed] [Google Scholar]
- 4.Brieland J K, Remick D G, Legendre M L, Engleberg N C, Fantone J C. In vivo regulation of replicative Legionella pneumophila lung infection by endogenous interleukin-12. Infect Immun. 1998;66:65–69. doi: 10.1128/iai.66.1.65-69.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrera L, Gazzinelli R T, Badolato R, Hieny S, Muller W, Kuhn R, Sacks D L. Leishmania promastigotes selectively inhibit interleukin 12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med. 1996;183:515–526. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chehimi J, Starr S E, Frank I, D'Andrea A, Ma X, MacGregor R R, Sennelier J, Trinchieri G. Impaired interleukin 12 production in human immunodeficiency virus-infected patients. J Exp Med. 1994;179:1361–1366. doi: 10.1084/jem.179.4.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chougnet C, Wynn T A, Clerici M, Landay A L, Kessler H A, Rusnak J, Melcher G P, Sher A, Shearer G M. Molecular analysis of decreased interleukin-12 production in persons infected with human immunodeficiency virus. J Infect Dis. 1996;174:46–53. doi: 10.1093/infdis/174.1.46. [DOI] [PubMed] [Google Scholar]
- 8.Cooper A M, Roberts A D, Rhoades E R, Callahan J E, Getzv D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 9.D'Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon-γ production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Andrea A, Rengaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, Chizzonite R, Wolf S F, Trinchieri G. Production of natural killer cell stimulatory factor (NKSF/IL-12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman H, Widen R, Klein T W, Searls L, Cabrian K. Legionella pneumophila-induced blastogenesis of murine lymphoid cells in vitro. Infect Immun. 1984;43:314–319. doi: 10.1128/iai.43.1.314-319.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazzinelli R T, Wysocka M, Hayashi S, Denkers E Y, Hieny S, Casper P, Trinchieri G, Sher A. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J Immunol. 1994;153:2533–2543. [PubMed] [Google Scholar]
- 13.Gubler U A, Chua O, Schoenhaut D S, Dwyer C M, McComas W, Motyka R, Nabavi N, Wolitzky A G, Quinn P M, Familletti P C, Gately M C. Coexpression of two distinct genes is required to generate secreted bioactive cytotoxic lymphocyte maturation factor. Proc Natl Acad Sci USA. 1991;88:4143–4147. doi: 10.1073/pnas.88.10.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gui-Jie F, Goodridge H S, Harnett M M, Wei X, Nikolaev A V, Higson A P, Liew F. Extracellular signal-related kinase (ERK) and p38 mitogen-activated protein (MAP) kinases differentially regulate the lipopolysaccharide-mediated induction of inducible nitric oxide synthase and IL-12 in macrophages; Leishmania phosphoglycans subvert macrophage IL-12 production by targetting ERK MAP kinase. J Immunol. 1999;163:6403–6412. [PubMed] [Google Scholar]
- 15.Heinzel F P, Schoenhaut D S, Rerco R M, Rosser L E, Gately M K. Recombinant interleukin-12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–1512. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horwitz M A. Cell mediated immunity in Legionnaries' disease. J Clin Investig. 1980;66:441–450. [Google Scholar]
- 17.Hsieh C S, Macatonia S E, Tripp C S, Wolf S F, O'Garra A, Murphy K M. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 18.Karp C L, Wysocka M, Ma X, Marovitch M, Factor R E, Nutman T, Armant M, Wahl L M, Cuomo P J, Trinchieri G. Potent suppression of IL-12 production from monocytes and dendritic cells during endotoxin tolerance. Eur J Immunol. 1998;28:3128–3136. doi: 10.1002/(SICI)1521-4141(199810)28:10<3128::AID-IMMU3128>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 19.Karp C L, Wysoska M, Wahl L M, Ahearn J M, Cuomo P J, Sherry B, Trinchieri G, Griffin D E. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–231. doi: 10.1126/science.273.5272.228. [DOI] [PubMed] [Google Scholar]
- 20.Karpus W J, Kennedy K J, Kunkel S L, Lukacs N W. Monocyte chemotactic protein 1 regulates oral tolerance induction by inhibition of T helper cell 1-related cytokines. J Exp Med. 1998;187:773–778. doi: 10.1084/jem.187.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marth T, Kelsall B L. Regulation of interleukin-12 by complement receptor 3 signaling. J Exp Med. 1997;185:1987–1995. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nash T W, Libby D M, Horwitz D M. IFN-γ activated human alveolar macrophages inhibit the intracellular multiplication of Legionella pneumophila. J Immunol. 1988;140:3978–3981. [PubMed] [Google Scholar]
- 23.Skerrett S J, Martin T R. Alveolar macrophage activation in experimental legionellosis. J Immunol. 1991;147:337–345. [PubMed] [Google Scholar]
- 24.Sypek J P, Chung C L, Mayor S E H, Subramanyam J M, Goldman S J, Sieburth D S, Wolf S F, Schaub R G. Resolution of cutaneous leishmaniasis: interleukin-12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takenaka H, Maruo S, Yamamoto N, Wysocka M, Ono S, Kobayashi M, Yagita H, Okumura K, Hamaoka T, Trinchieri G, Fujiwara H. Regulation of T-cell-dependent and independent IL-12 production by the three Th2-type cytokines IL-10, IL-6 and IL-4. J Leukoc Biol. 1997;61:80–87. doi: 10.1002/jlb.61.1.80. [DOI] [PubMed] [Google Scholar]
- 26.Trinchieri G. Interleukin-12 and its role in the generation of Th1 cells. Immunol Today. 1993;14:335–337. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 27.Wagner R D, Steinberg H, Brown J F, Czuprynski C J. Recombinant interleukin-12 enhances resistance of mice to Listeria monocytogenes infection. Microb Pathog. 1994;17:175–186. doi: 10.1006/mpat.1994.1064. [DOI] [PubMed] [Google Scholar]
- 28.Weinstein S L, Sanghera J S, Lemke K, Defranco A L, Pelech S L. Bacterial lipopolysaccharide induces tyrosine phosphorylation and activation of mitogen-activated protein-kinases in macrophages. J Biol Chem. 1992;267:14955–14962. [PubMed] [Google Scholar]
- 29.Wu C Y, Demeure C, Kiniwa M, Gately M, Delepesse G. IL-12 induces the production of IFN-gamma by neonatal human CD4 T cells. J Immunol. 1993;151:1938–1949. [PubMed] [Google Scholar]
- 30.Yamamoto Y, Retzlaff C, He P, Klein T W, Friedman H. Quantitative reverse transcription-PCR analysis of Legionella pneumophila-induced cytokine mRNA in different macrophage populations by high-performance liquid chromatography. Clin Diagn Lab Immunol. 1995;2:18–24. doi: 10.1128/cdli.2.1.18-24.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto Y, Klein T W, Friedman H. Legionella pneumophila virulence conserved after multiple single-colony passage on agar. Curr Microbiol. 1993;27:241–245. [Google Scholar]
- 32.Yamamoto Y, Klein T W, Newton C A, Widen R, Friedman H. Growth of Legionella pneumophila in thioglycolate-elicited peritoneal macrophages from A/J mice. Infect Immun. 1988;56:370–375. doi: 10.1128/iai.56.2.370-375.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]