Abstract
Species from the flowering plant genus Cyclamen are popular amongst consumers. In particular Cyclamen persicum Mill. has been significantly used commercially, and certain small flowering species such as Cyclamen hederifolium and Cyclamen coum are gradually growing in popularity in the potted flower market. Here, the chloroplast genomes of nine Cyclamen samples including four Cyclamen species and five varieties of C. hederifolium were sequenced for genome structure comparison, White green septal striped leaves related gene screening and DNA molecular markers were developed for phylogenetic analysis. In comparing Cyclamen species’ chloroplast genomes, gene content and gene order were found to be highly similar with the length of genomes ranging from 151,626 to 153,058 bp. The chloroplast genome of Cyclamen has 128 genes, including 84 protein-coding genes, 36 transfer RNA genes, and 8 ribosomal RNA genes. Based on intraspecific variation, seven hotspots, including three genes and four intergenic regions, were identified as variable markers for downstream species delimitation and interspecific relationship analyses. Moreover, a phylogenetic tree constructed with complete chloroplast genomes, revealed that Cyclamen are monophyletic with Lysimachia as the closest neighbor. Phylogenetic analyses of the 14 Cyclamen species with the seven variable regions showed five distinct clades within this genus. The highly supported topologies showed these seven regions may be used as candidate DNA barcode sequences to distinguish Cyclamen species. White green septal striped leaves is common in C. hederifolium, however the molecular mechanism of this has not yet been described. Here, we find that the intergenic region rps4-trnT-UGU seems related to white green septal striped leaves.
Subject terms: Evolution, Phylogenetics
Introduction
The plant chloroplast genome is highlight conserved in regards to structure, gene number, and gene composition compared with mitochondrial or nuclear genomes1. Due to uniparental inheritance and small genome size, complete chloroplast genomes have been used extensively in phylogenetic analyses and species identification2,3.
Distributed in and around the Mediterranean region, the genus Cyclamen, within the order Ericales, family Primulaceae, and subfamily Myrsinoideae consists of 24 species4. Some species have large flowers such as C. persicum Mill. which has been bred for more than 150 years, leading to the wide ranges of color seen today along with significant commercial use. Some species of Cyclamen are frost resilient, and some can bloom from late summer until late spring. C. hederifolium is native to Italy, southern France, Greece, and Western Turkey. It has large intraspecific variation and strong cold and heat tolerance. C. hederifolium as the parent to cross with C. persicum is one of the main examples of distant cross breeding of Cyclamen4.
Cyclamen hederifolium has small flowers and great variation in leaf color such as white and green stripes, light green, and green with white in center. White and green striped leaf color is special and has high ornamental value, however, the formation mechanism of white and green striped variation remains unknown. The mechanisms for the formation of plant leaf spots can be roughly divided into two categories: (1) environmental factors, such as pathogens and nutritional defects, and (2) genetic mutation5. Plastid mutation is considered to be one of the main mechanisms for the formation of striped leaves. Under natural conditions, there is a 0.006–0.3% probability of spontaneous mutation of plastid gene which might result in variegated or striped leaf color6.
Some plastid mutants cause chlorotic effects, that is, developmental damage or environmental sensitivity, and showing reversible mottled or albino phenotypes. To date, many leaf color mutants produced by plastid gene mutations have been identified, involving eight plastid genes, including psbE, rbcL, infa, ycf3, matK, and others. Similarly, a mutation in the rps4 chloroplast gene coding region was described in the yellowing mutant of Chinese cabbage (Brassica campestris ssp. pekinensis) and named cdm, consisting of a Val to Gly conversion in the rps4 protein’s 193th amino acid. qRT-PCR showed that the expression level of the rps4 gene was down-regulated in cdm7. The candidate chloroplast genes related to the formation of white green septal striped leaves may be identified through comparative analyses of chloroplast genomes.
In contrast, the taxonomy of Cyclamen remains unclear. Phylogenetic analyses of Primulaceae, Myrsinaceae, and Theophyllastaceae based on three chloroplast gene fragments revealed that the clade composed of Aegiceras, Grammadenia, Myrsine, and Hymenandra has the closest genetic relationship with Cyclamen, but the nodes of phylogenetic tree showed a comb structure and lacked support rates8. In contrast to the phylogenetic reconstructions based on plastid gene sequences, when using ITS sequences, Cyclamen does not appear as a member of the Myrsinaceae-Lysimachieae clade. Therefore, Cyclamen’s exact position on the phylogenetic tree remains unclear9.
A phylogenetic tree constructed with only one gene or several genes has low reliability, and the phylogenetic tree of Myrsinaceae based on chloroplast genomes did not include the genus Cyclamen10. Therefore, little is known about the evolutionary relationships between species within the Cyclamen genus. Cyclamen a resource rich genus, and its value and application covers many aspects, such as ornamental, fragrance, and medicine, and thus the study of intraspecific relationships is of great significance for cross breeding. In recent years, the improvement of sequencing technology and decrease in costs has led to omics data from many systems, however, there genetic data from this genus remains scarce, which thus hinders basic research at a molecular level.
In this work, we sequenced and assembled nine Cyclamen whole chloroplast (cp) genomes and performed a comprehensive analysis, including genome features comparisons, repeats, selective pressures, divergence hotspots, and phylogenetic relationships. Our goals in this study were to: (1) Present the complete cp genome sequences of nine newly assembled nine Cyclamen samples in five species and construct phylogenetic tree of Myrsinaceae; (2) Identify candidate chloroplast genes related to the formation of white green septal striped leaves through a comparative analysis of the chloroplast genome; (3) identify divergence hotspots as potential genetic markers for DNA barcoding and test the effectiveness of these markers; and (4) construct the phylogeny of 14 Cyclamen species using selected DNA markers and explore their interspecific relationships.
Results
Chloroplast genome sequencing and features of cyclamen species
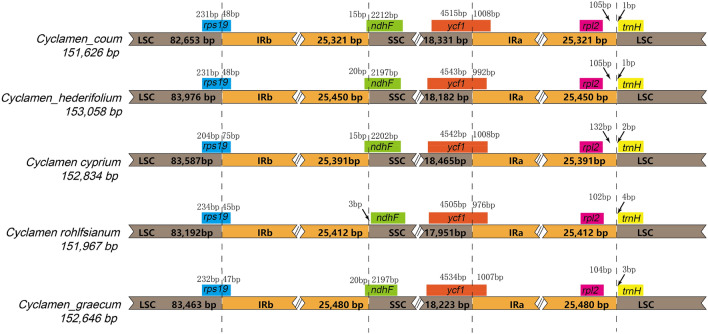
Nine complete chloroplast genome sequences represent five species were deposited in GenBank with the accession numbers: ON480518, ON480519, ON480520, ON480521, ON480522, ON480523, OP957067, OP957068 and OP957069. The assembly results were uniform and the validating of the assembly results by mapping reads to the assembled sequence were showed in supplementary materials (Supplementary Fig. S1). The total chloroplast genome size ranged from 151,626 bp (C. coum) to 153,058 bp (C. hederifolium with white green septal striped leaves) (Fig. 1). The Cyclamen chloroplast genome has a typical quadripartite structure and includes a pair of IR regions (25,321–25,480 bp), LSC regions (82,653–83,976 bp), and SSC regions (18,182–18,465 bp) (Fig. 2).
Figure 1.
Nine sequenced samples in this study. (1) C. coum, (2) C. hederifolium-1, (3) C. hederifolium-2, (4) C. hederifolium-3, (5) C. hederifolium-4, (6) C. hederifolium-5, (7) C. graecum, (8) C. rohlfsianum, (9) C. cyprium (all bars are 2 cm).
Figure 2.
Comparison of the borders of LSC, SSC, and IR regions among five Cyclamen species.
Long repeat and SSR analysis
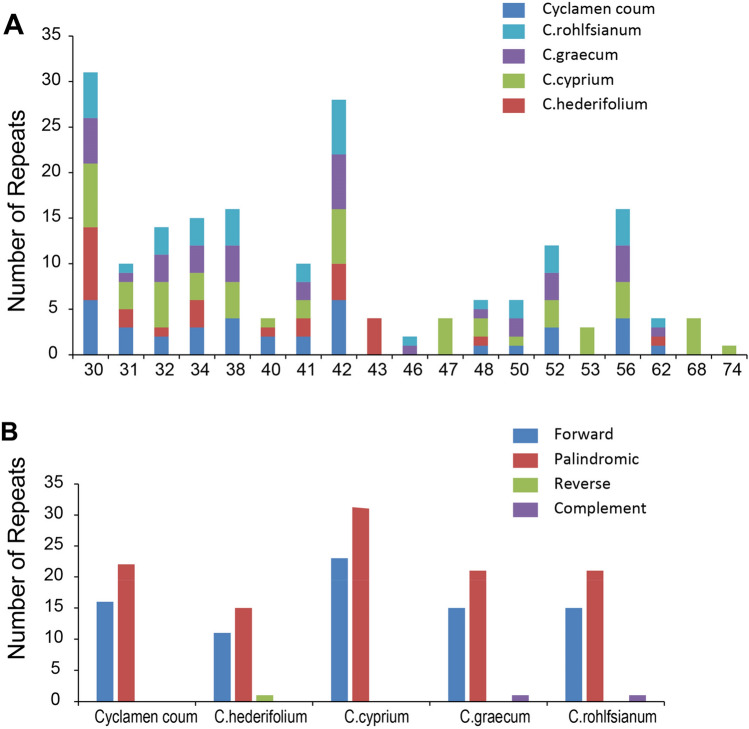
Repeat sequences in Cyclamen cp genomes were detected with REPuter (https://bibiserv.cebitec.uni-bielefeld.de/reputer), with a copy size of 30 bp or longer. 38 long repeats consisting of 16 forward repeats and 22 palindromic repeats were detected in C. coum while 27 long repeats consisting of 11 forward repeats, 15 palindromic repeats, and 1 reverse repeat were detected in C. hederifolium (Fig. 3). Five C. hederifolium samples shared the same SSR features, but there is some variation between the five Cyclamen species in SSR number and type (Table 1).
Figure 3.
The number of long repeats in the whole cp genome sequence of C. coum and C. hederifolium. (A) Frequency of repeats more than 30 bp long. (B) Frequency of repeat type.
Table 1.
SSR type of the five Cyclamen species.
| SSR type | C. coum | C. hederifolium | C. cyprium | C. graecum | C. rohlfsianum |
|---|---|---|---|---|---|
| A/T | 54 | 51 | 54 | 46 | 36 |
| AT/AT | 7 | 8 | 8 | 6 | 5 |
| AAC/GTT | 7 | 7 | 6 | 7 | 7 |
| AAG/CTT | 24 | 25 | 24 | 24 | 24 |
| AAT/ATT | 18 | 22 | 20 | 24 | 26 |
| ACT/AGT | 1 | 1 | 1 | 1 | |
| AGC/CTG | 7 | 7 | 7 | 7 | 6 |
| AGG/CCT | 2 | 2 | 2 | 2 | 2 |
| ATC/ATG | 4 | 5 | 4 | 5 | 5 |
| AAAG/CTTT | 1 | 1 | 1 | 1 | 1 |
| AAAT/ATTT | 4 | 3 | 4 | 4 | 2 |
| AAGG/CCTT | 1 | 1 | 1 | 1 | |
| AATC/ATTG | 1 | 1 | 1 | 1 | 1 |
| AATT/AATT | 1 | 1 | 1 | 1 | 1 |
| AAATT/AATTT | 1 | ||||
| AATAG/ATTCT | 2 | 1 |
The chloroplast genome of Cyclamen has 128 genes, including 84 protein-coding genes, 36 transfer RNA genes, and eight ribosomal RNA genes. Six protein-coding genes (rps7, rps12, rpl2, rpl23, ndhB, and ycf2), seven tRNA genes (trnA-UGC, trnI-GAU, trnI-CAU, trnL-CAA, trnN-GUU, trnR-ACG, and trnV-GAC) and all four rRNA genes are duplicated in the IR regions. Fourteen genes (trnA-UGC, trnI-GAU, trnK-UUU, trnL-UAA, trnV-UAC, rps12, rps16, rpl2, rpl16, rpoC1, petB, petD, atpF, ndhA, and ndhB) contain a single intron and two genes (clpP and ycf3) have two introns (Table 2).
Table 2.
List of annotated genes in Cyclamen chloroplast genomes.
| Gene group | Gene name |
|---|---|
| Ribosomal RNAs | rrn16(2), rrn23(2), rrn4.5(2), rrn5(2) |
| Transfer RNAs | trnA-UGC*(2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnfM-CAU, trnG-GCC, trnH-GUG, trnI-GAU*(2), trnI-CAU(2), trnK-UUU*, trnL-CAA(2), trnL-UAA*, trnL-UAG, trnM-CAU, trnN-GUU(2), trnP-UGG, trnQ-UUG, trnR-ACG(2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC(2), trnV-UAC*, trnW-CCA, trnY-GUA |
| Small ribosomal subunit | rps2, rps3, rps4, rps7(2), rps8, rps11, rps12*(2), rps14, rps15, rps16*, rps18, rps19 |
| Large ribosomal subunit | rpl2*(2), rpl14, rpl16*, rpl20, rpl22, rpl23(2), rpl32, rpl33, rpl36 |
| DNA dependent RNA polymerase | rpoA, rpoB, rpoC1*, rpoC2 |
| Photosystem I | psaA, psaB, psaC, psaI, psaJ |
| Photosystem II |
psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ |
| Cytochrome b/f complex | petA, petB*, petD*, petG, petL, petN |
| Subunits of ATP synthase | atpA, atpB, atpE, atpF*, atpH, atpI |
| Protease | clpP** |
| Large subunit of rubisco | rbcL |
| NADH dehydrogenase |
ndhA*, ndhB*(2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK |
| Maturase | matK |
| Envelope membrane protein | cemA |
| Acetyl-CoA carboxylase | accD |
| c-type cytochrome Synthesis gene | ccsA |
| Open reading frames(ORF, ycf) | ycf1, ycf2(2), ycf3**, ycf4 |
*Indicates gene with one intron and **indicates gene with two introns. (2) indicates that the number of the repeat unit is 2. The rps12 gene is a trans-spliced gene.
Phylogenetic analysis reveals inter- and intraspecific variation
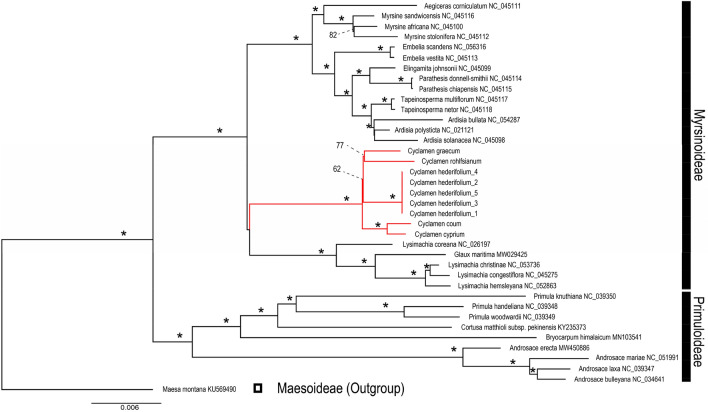
A total of 28 Myrsinoideae and 9 related cp genomes were included in the phylogenetic analysis. The molecular phylogenetic tree showed the Cyclamen is monophyletic, located in Myrsinoideae and is closely related to the genera Lysimachia and Glaux (Fig. 4). Ten genera in Myrsinoideae formed four clades, one on them includes Aegiceras and Myrsine. The second consists of Embellia, Ardisia, Tapeinosperma, and Elingamita. Cyclamen represents one clade and the fourth clade includes Lysimachia and Glaux. In particular, Lysimachia and Glaux are not reciprocal monophyly: Glaux is embedded in Lysimachia which indicates that accurate classification of these two genera requires further study.
Figure 4.
Phylogenetic tree constructed using maximum likelihood method based on the whole chloroplast genomes of 33 different species (The symbol * in the phylogenetic tree indicated that the support value is 100%).
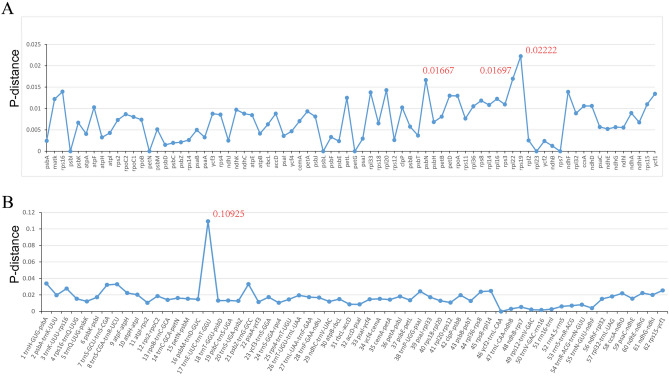
We analyzed the nucleotide diversity (Pi) values to measure the divergence levels of protein-coding genes and intergenic regions of the five Cyclamen species. The level of sequence divergence among protein-coding genes was more conserved than in intergenic regions. The Pi value was from 0 to 0.02222 in protein-coding genes while it ranged from 0 to 0.10925 in intergenic regions (Fig. 5). Three genes and four intergenic regions were selected for interspecific relationship analysis due to their relatively high Pi value and potential success as PCR primers (Table 3).
Figure 5.
Nucleotide variability (Pi) values were calculated in the five Cyclamen species chloroplast genomes. (A) Protein-coding genes. (B) Intergenic regions.
Table 3.
The variability of the hypervariable markers.
| Sequence name | Length range | Variable sites | Variable sites (%) | Nucleotide diversity |
|---|---|---|---|---|
| rpl22 | 448 | 38 | 8.48 | 0.0184 |
| ndhF | 960 | 73 | 7.60 | 0.0177 |
| Ycf1-1 | 1341 | 89 | 6.64 | 0.0148 |
| Ycf1-2 | 1309 | 104 | 7.94 | 0.0179 |
| Ycf1-3 | 1376 | 110 | 7.99 | 0.0161 |
| rpoB-trnC | 696 | 43 | 6.18 | 0.0131 |
| trnS-rps4 | 275 | 16 | 5.82 | 0.0113 |
| rps4-trnT | 278 | 14 | 5.04 | 0.0118 |
| ycf4-petA | 1042 | 53 | 5.09 | 0.0106 |
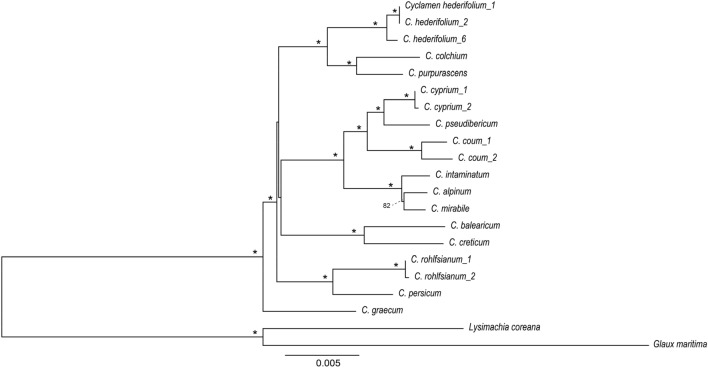
The phylogenetic analysis of the 19 samples represent 14 species was constructed based on three genes (Ycf1 was divided into three fragments as it is too long to amplify and sequence) and four intergenic regions. Different samples of same species like C. hederifolium, C. cyprium, C. coum and C. rohlfsianum formed separated branches indicating the effectiveness of these genes in phylogenetic construction. 14 species in Cyclamen genus formed five clades, one of which includes C. hederifolium, C. colchium and C. purpurascens. The second clade is comprised of C. cyprium, C. pseudibericum, C. coum, C. intaminatum, C. alpinum and C. mirabile. The third clade consisted of C. creticum and C. balearicum. The fourth clade includes C. rohlfsianum and C. persicum. The species C. graecum formed the fifth clade (Fig. 6).
Figure 6.
Phylogenetic relationship among the 14 Cyclamen species based on nine seven segments (The symbol * in the phylogenetic tree indicated that the support value is 100%).
The gene rpl22 has high interspecific variation and low intraspecific variation in Cyclamen. The amplified length of this gene is 448 bp, with 38 basepair variations within the genus. This gene can identify species with a single DNA sequence, which is simple, efficient, and low cost (Supplementary Fig. S2).
Discussion
In this study, we sequenced and annotated the cp genomes of nine Cyclamen samples, compared genomic features among the samples of Cyclamen, constructed cp genome phylogenetic tree to analyze the location of the genus, and identified SSR, long repeats and suitable polymorphic loci of suitable molecular markers for phylogenetic analysis. Using the complete chloroplast genome phylogenetic inference of the nine Cyclamen samples we annotated and the 24 species from Myrsinoideae and nine Primuloideae downloaded from NCBI, helped to elucidate the taxonomic position of the genus Cyclamen within Myrsinoideae and in close relationship with the clade formed by Lysimachia and Glaux.
The cp genomes of Cyclamen species have 128 genes, including 84 protein-coding genes, 36 transfer RNA genes, and eight ribosomal RNA genes. Only one insertion/deletion mutation in the intergenic region rps4-trnT-UGU among the five Cyclamen hederifolium chloroplast genome samples was described. This insertion/ deletion seems to be associated with white green septal striped leaves in samples C. hederifolium1, C. hederifolium3, and C. hederifolium5 which have the -ATCAATAATT insertion have special leaf variegation. In contrast, samples C. hederifolium2, C. hederifolium4, and other four species lack the -ATCAATAATT insertion and do not have white green septal striped leaves. Variegated-leaf plants are ideal materials for studying the mechanism of chloroplast development and interaction between plastid and nucleus, because of the coexistence of green sectors with normal chloroplast development and white or yellow sectors with abnormal chloroplast development in the same leaf11. In this research, insertion/deletion mutations in the intergenic region rps4-trnT-UGU were screened to be related to white green septal striped leaves, however, the mechanism of interaction between chloroplast development and nucleus remains unknown.
The entire cp genome may be used directly as a long barcode for species identification and phylogenetic analysis. Furthermore, the hypervariable regions described here may be screened out as potential molecular markers for species identification12,13.
The phylogenetic tree based on the whole cp genome showed the Cyclamen is monophyletic, in the family Myrsinoideae and closely related to the genera Lysimachia and Glaux. Ten genera of Myrsinoideae formed four clades, one on them including Aegiceras and Myrsine. The second consists of Embellia, Ardisia, Tapeinosperma, and Elingamita, which compose the Myrsinaceae s.str. clade which is a tropical woody representative in Myrsinoideae of Primulaceae10. Cyclamen represents one clade and the fourth clade includes Lysimachia and Glaux. In particular, Lysimachia and Glaux do not exhibit reciprocal monophyly; Glaux is embedded into Lysimachia which indicates that the classification of these two genera needs further investigation.
The repeat variations in plastid genomes are often involved in diverse cellular functions including gene evolution, RNA editing, and gene mobility14. cpSSR usually showed high polymorphism in close related species and within the same species, and which are potentially useful markers for interspecific relationship and population genetics15. Here, SSRs are varying in number and type between five Cyclamen species, and the most abundant repeat type was found to be A/T. The enriched SSRs in five genomes to be tri-nucleotides, followed by mononucleotides, tetra-nucleotides, dinucleotides and and penta-nucleotides. cpSSRs derived from five Cyclamen species are expected to be useful for the genetic diversity and molecular breeding studies.
Three genes and four intergenic regions including rpl22, ndhF, Ycf1, rpoB-trnC, trnS-rps4, rps4-trnT, and ycf4-petA can be successfully amplified and are also effective in phylogenetic analysis. The phylogenetic tree shows that the genetic relationship between individuals within a species is closer than that between species, and each branch has a high support rate, which indicates that the phylogenetic tree has high reliability. C. creticum is closely related to C. balearicum, a species endemic to the Balearic Islands and southern France16,17. Research based on ITS, trnL-F, and rps16 also showed that C. creticum and C. balearicum are closely related; that C. hederifolium, C. colchicum, and C. purpurascens have close genetic affinity; that C. cyprium and C. pseudibericum are positioned within the same clade; and that C. intaminatum is closely related to C. mirable18. Our phylogenetic tree based on seven fragments are consistent with these previous findings, supporting that the tree generated in our study is reliable. It also bears mention that the a majority of branches in the phylogenetic tree of Cyclamen of our study have 100% support, again indicating that the tree is reliable.
DNA barcoding is simple, accurate, and fast, and is therefore the most effective method for species identification19,20. Sequences with high success rates of amplification and sequencing, high variability among species but low nucleotide variation within species (less than 2%) are considered to be excellent DNA markers21. The rpl22 gene is efficient for species identification in the genus as it can identify species with a single gene, as it has high rates of variable sites (8.48%) within genus and only 0.45% nucleotide variation within species.
Our results enrich the data on the cp genomes of the genus Cyclamen and shed light on the phylogenetic relationship between genera and species. Here, candidate chloroplast genes related to the formation of white green septal striped leaves have been identified through comparative analysis of chloroplast genomes. This work lays an important foundation for molecular breeding and cross breeding research.
Methods
Chloroplast plant material and DNA extraction
The leaves of Cyclamen species were obtained from a personal gardener in Chiba-ken, in Japan (Fig. 6). Total genomic DNA was extracted from leaf material with the plant genomic DNA kit (Tiangen Biotech, Beijing, China) according to the manufacturer’s protocol. The final DNA concentration (> 50 ng/μL) was measured using a NanoDrop spectrophotometer, and fragmentation was achieved using sonication.
Sequencing, assembly, and annotation
First, the fragmented DNA was purified and end-repaired, and sizes were determined by gel electrophoresis. Paired-end libraries with insert sizes of 350 bp were prepared following Illumina’s standard genomic DNA library preparation procedure, and subsequently, a control library quality for sequencing was prepared. Whole genome samples were sequenced on a Illumina Novaseq 6000 platform (Illumina, USA).
Paired-end Illumina raw reads where cleaned and quality filtered using Trimmmatic with the following settings: trim from both ends, remove individual bases with Phred quality score < 20, remove read if three consecutive uncalled bases22. Entire reads with a median quality score lower than 21 or less than 40 bp in length after trimming were discarded. After quality filtering, reads were mapped using Bowtie2 v.2.2.6 to the chloroplast genome of the closest related species in order to exclude reads of nuclear and mitochondrial origins23. Subsequently, all putative chloroplast reads which mapped to the reference sequence were de novo assembled to reconstruct the chloroplast genomes using GetOrganelle 1.7.524. Finally, clean reads were mapped to the complete plastome again to examine and correct mis-assemblies manually. Automatic annotation of chloroplast genomes was generated with CpGAVAS225 and manually corrected by referring to previously published plastomes on Geneious26. Finally, a circular representation of both sequences was drawn using the online tool OGDRAW27 (https://chlorobox.mpimp-golm.mpg.de/OGDraw.html).
Comparison of the chloroplast genomes and divergent hotspot identification
The chloroplast genome composition of LSC, SSC, and IRs were compared according to their annotations. Genome rearrangements were performed with the Mauve Genome Alignment v2.3.128 Plugin and the progressive Mauve algorithm29.
Phylogenetic analysis
Thirty-seven complete chloroplast sequences were used in the phylogenetic analysis, including nine Cyclamen samples and 28 samples of other species of Myrsinoideae and Primuloideae from GenBank.
All of the cp genome sequences were aligned using MAFFTv730 and adjusted manually as needed. Maximum likelihood (ML) analyses were implemented with RAxML version 8.2.1231. RAxML is based on the general time reversible (GTR) model of nucleotide substitution with the gamma model of rate heterogeneity. Non-parametric bootstrapping was implemented with the fast bootstrap algorithm of RAxML used 1000 replicates.
Identification of variable DNA markers and phylogenetic relationships within the genus
Variable DNA marker genetic sequences were first aligned using MAFFT v730 and then DnaSP version 5.132 was used to evaluate the nucleotide variability (Pi) of gene. Genes and intergenic regions with high nucleotide variability were used to design primers. Primers were amplified in 14 species of the Cyclamen genus to test validity and construct phylogenetic relationships.
Sample collection and experiment statement
All the methods including plant leaves collection and experiment were carried out in accordance with relevant national/international/legislative and institutional guidelines and regulations.
Supplementary Information
Author contributions
Conceptualization, L.H.M. and Z.S.S.; methodology, L.H.M., Q.C.Z.; software, L.H.M. and Z.S.S.; validation, L.H.M., Q.D. and X.R.C. and Z.Z.—original draft preparation, L.H.M.; writing—review and editing, L.H.M., Q.D., X.R.C.; visualization, Z.S.S.; supervision, Q.C.Z.; funding acquisition, Q.C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Key Research and Development Plan of Zhejiang Province, Grant number 2019c02025.
Data availability
The complete chloroplast sequence generated and analyzed during the current study is available in GenBank (https://www.ncbi.nlm.nih.gov, with the accession numbers: ON480518, ON480519, ON480520, ON480521, ON480522, ON480523, OP957067, OP957068 and OP957069).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lihui Mao, Email: mlh@mail.zaas.ac.cn.
Qingcheng Zou, Email: qczou@aliyun.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-27163-1.
References
- 1.Dong W, Xu C, Cheng T, Lin K, Zhou S. Sequencing angiosperm plastid genomes made easy: A complete set of universal primers and a case study on the phylogeny of Saxifragales. Genome Biol. Evol. 2013;5:989997. doi: 10.1093/gbe/evt063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong W, et al. Resolving the systematic positions of enigmatic taxa: Manipulating the chloroplast genome data of Saxifragales. Mol. Phylogenet. Evol. 2018;126:321–330. doi: 10.1016/j.ympev.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 3.Li W, et al. Interspecific chloroplast genome sequence diversity and genomic resources in Diospyros. BMC Plant Biol. 2018;18:210. doi: 10.1186/s12870-018-1421-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grey-Wilson, C. Cyclamen (A Guide for Gardeners, Horticulturists and Botanists) 182–197 (Bastsford, 2015).
- 5.Yu F, et al. Variegation mutants and mechanisms of chloroplast biogenesis. Plant Cell Environ. 2007;30:350–365. doi: 10.1111/j.1365-3040.2006.01630.x. [DOI] [PubMed] [Google Scholar]
- 6.Greiner S. Plastome mutants of higher plants. In: Bock R, Knoop V, editors. Genomics of Chloroplasts and Mitochondria. Springer; 2012. pp. 237–266. [Google Scholar]
- 7.Tang, X. Y. Cloing and functional analysis of chloroplast mutant gene rps4 in Chinese Cabbage (Brassica campestris ssp pekinensis). Doctoral Dissertation of Shenyang Agricultural University (2018).
- 8.Kllersj M, Bergqvist G, Anderberg AA. Generic realignment in primuloid families of the Ericales s.l.: A phylogenetic analysis based on DNA sequences from three chloroplast genes and morphology. Am. J. Bot. 2000;87:1325–1341. doi: 10.2307/2656725. [DOI] [PubMed] [Google Scholar]
- 9.Martins L, Oberpreieler C, Hellwig FH. A phylogenetic analysis of Primulaceae s.l. based on internal transcribed spacer (ITS) DNA sequence data. Plant Syst. Evol. 2003;237:75–85. doi: 10.1007/s00606-002-0258-1. [DOI] [Google Scholar]
- 10.Yan X, et al. Chloroplast genomes and comparative analyses among thirteen taxa within Myrsinaceae s.str. Clade (Myrsinoideae, Primulaceae) Int. J. Mol. Sci. 2019;20:4534. doi: 10.3390/ijms20184534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng M, et al. Chloroplast translation initiation factors regulated leaf variegation and development. Plant Physiol. 2016;172:1117–1130. doi: 10.1104/pp.15.02040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, et al. Plant DNA barcoding: From gene to genome. Biol. Rev. Camb. Philos. Soc. 2015;90:157–166. doi: 10.1111/brv.12104. [DOI] [PubMed] [Google Scholar]
- 13.Celiński K, Kijak H, Wiland-Szymańska J. Complete chloroplast genome sequence and phylogenetic inference of the canary islands dragon tree (Dracaena draco L.) Forests. 2020;11:309. doi: 10.3390/f11030309. [DOI] [Google Scholar]
- 14.George B, Bhatt BS, Awasthi M, George B, Singh AK. Comparative analysis of microsatellites in chloroplast genomes of lower and higher plants. Curr. Genet. 2015;61:665–667. doi: 10.1007/s00294-015-0495-9. [DOI] [PubMed] [Google Scholar]
- 15.Eguiluz M, Rodrigues NF, Guzman F, Yuyama P, Margis R. The chloroplast genome sequence from Eugenia uniflora, a Myrtaceae from Neotropics. Plant Syst. Evol. 2017;303:1199–1212. doi: 10.1007/s00606-017-1431-x. [DOI] [Google Scholar]
- 16.Anderberg AA. Phylogeny and subgeneric classification of Cyclamen L. (Primulaceae) Kew Bull. 1993;49:455–467. doi: 10.2307/4114469. [DOI] [Google Scholar]
- 17.Gielly L, Thompson M. Geographic isolation and evolution of Mediterranean endemic Cyclamen: Insights from chloroplast trn L (UAA) intron sequence variation. Plant Syst. Evol. 2001;230:75–88. doi: 10.1007/s006060170005. [DOI] [Google Scholar]
- 18.Yesson C, Toomey NH, Culham A. Cyclamen: time, sea and speciation biogeography using a temporally calibrated phylogeny. J. Biogeogr. 2009;36:1234–1252. doi: 10.1111/j.1365-2699.2008.01971.x. [DOI] [Google Scholar]
- 19.Bringloe TT, Saunders GW. DNA barcoding of the marine macroalgae from Nome, Alaska (Northern Bering Sea) reveals many trans-Arctic. Polar Biol. 2019;42:851–864. doi: 10.1007/s00300-019-02478-4. [DOI] [Google Scholar]
- 20.Chen KC, et al. DNA barcoding of fish species reveals low rate of package mislabeling in Qatar. Genome. 2019;62:69–76. doi: 10.1139/gen-2018-0101. [DOI] [PubMed] [Google Scholar]
- 21.Kowalska Z, Pniewski F, Latała A. DNA barcoding-A new device in phycologist's toolbox. Ecohydrol. Hydrobiol. 2019;19:417–427. doi: 10.1016/j.ecohyd.2019.01.002. [DOI] [Google Scholar]
- 22.Bolger A, Lohse M, Usadel B. Trimmomatic: A fexible trimmer for Illumina Sequence Data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin JJ, et al. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020;21:241. doi: 10.1186/s13059-020-02154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi LC, et al. CPGAVAS2, an integrated plastome sequence annotator and analyzer. Nucleic Acids Res. 2019;47:W65–W73. doi: 10.1093/nar/gkz345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kearse M, et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohse M, Drechsel O, Kahlau S, Bock R. OrganellarGenomeDRAW—A suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013;41:W575–W581. doi: 10.1093/nar/gkt289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Darling AE, Mau B, Blatter FR, Perna NT. Mauve: Multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 2004;14:1394–1403. doi: 10.1101/gr.2289704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darling AE, Mau B, Perna NT. progressiveMauve: Multiple genome alignment with gene gain, loss, and rearrangement. PLoS One. 2010;5:e11147. doi: 10.1371/journal.pone.0011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katoh K, Kuma KI, Toh H, Miyata T. MAFFT version 5: Improvement in accuracy of multiple sequence alignment. Nucleic Acids Res. 2005;33:511–518. doi: 10.1093/nar/gki198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stamatakis A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Librado P, Rozas J. DnaSP v5: Software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete chloroplast sequence generated and analyzed during the current study is available in GenBank (https://www.ncbi.nlm.nih.gov, with the accession numbers: ON480518, ON480519, ON480520, ON480521, ON480522, ON480523, OP957067, OP957068 and OP957069).