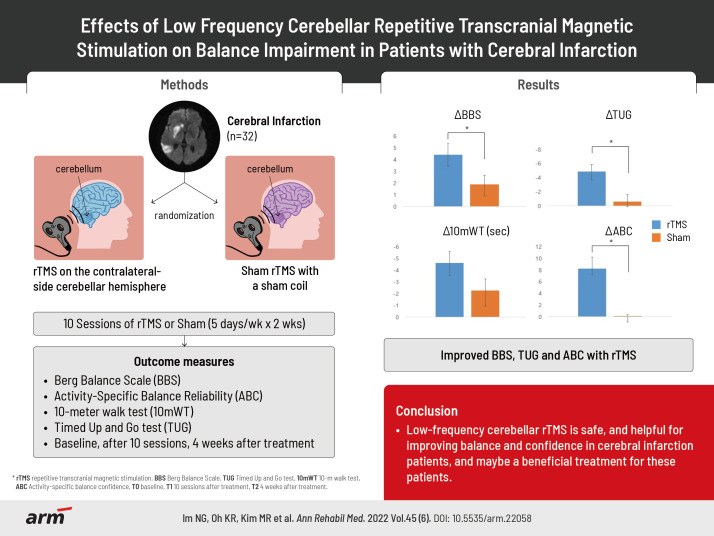
Abstract
Objective
To investigate the effect of low frequency cerebellar repetitive transcranial magnetic stimulation (rTMS) on balance impairment in patients with cerebral infarction.
Methods
Thirty-two patients were randomly divided into two groups: rTMS group (n=16) and control (n=16). In the rTMS group, treatment was performed five times per week for 2 weeks (10 sessions), and in the control group, a sham coil was used with the sound and sensation of scalp similar to the rTMS coil. Patients in both groups underwent a conventional rehabilitation program. Berg Balance Scale (BBS) was used as the primary outcome measurement. Timed Up and Go test (TUG), 10-m walk test (10mWT), and Activity-specific Balance Confidence scale (ABC) were used as the secondary outcome measurement. All scales were measured at baseline (T0), after 10 sessions of rTMS (T1), and at 4 weeks after treatment completion (T2) by therapists with over 5 years of clinical experience.
Results
There were significant improvements between T0 and T1, and between T0 and T2, for all assessed items in the rTMS group. Whereas there were significant improvements between T0 and T1, and between T0 and T2, for the BBS and 10mWT in the control group. TUG (-4.87±5.05 vs. -0.50±2.97 seconds) and ABC score (8.10±8.33 vs. 0.16±0.97) were observed significant differences in comparison of the changes from T0 to T1 between the two group. BBS score (4.40±3.66 vs. 1.88±3.14), TUG (-4.87±4.56 vs. -0.62±2.96 seconds) and ABC score (8.22±7.70 vs. -0.09±0.86) differed significantly from T0 to T2 between the two groups.
Conclusion
Our findings suggest that low-frequency cerebellar rTMS is helpful for improving balance in patients with cerebral infarction, and maybe a beneficial treatment for these patients.
Keywords: Repetitive transcranial magnetic stimulation (rTMS), Stroke, Balance
GRAPHICAL ABSTRACT
INTRODUCTION
Balance impairment after cerebral infarction is a common complication. Approximately 83% of stroke survivors reportedly suffer from balance impairment [1]. Balance impairment causes inconvenience in walking and daily activities for patients with cerebral infarction. In addition, balance impairments are characterized by a short support time, differences on both sides of the body, and a slow gait speed, which may increase the risk of falls [2].
Deficiencies in motor and proprioceptive control have been regarded as the main mechanism for balance impairment in cerebral infarction. As treatment for these balance impairments, whole-body vibration [3], virtual reality [4], mirror therapy [5], and ankle-foot orthoses [6] are being studied. Additionally, some studies have shown that stair climbing [7], trampoline training [8], etc., may also be helpful for improving balancing ability.
With the recent introduction of repetitive transcranial magnetic stimulation (rTMS) as a non-invasive treatment, various studies are attempting to broaden its target area and therapeutic effects. Depending on the site of treatment, rTMS is used as an alternative treatment for improving muscular strength [9], depression [10], aphasia [11], and dysphagia [12].
In the previous study, Fierro et al. [13] reported that low frequency cerebellar rTMS induced a long-lasting modulatory effect on the excitability of the interconnected motor area in healthy people. They hypothesized that suppression mechanisms resulting from inhibition of the dentate thalamo-cortical pathway to the corticospinal tract were induced by activation of the Purkinje cell. A dentate corticospinal tract emerges from the dentate nuclei, which receives the most input from Purkinje cell, and a vestibule-spinal tract from the lateral vestibular nuclei, which receives some input. In another study, low-frequency cerebellar rTMS was shown to be effective for ataxia in patients with posterior cerebral artery (PCA) territory infarction [14]. Additionally, walking ability was improved in patients with spinocerebellar degeneration when low-frequency rTMS was applied to the cerebellum [15,16]. Furthermore, a previous study reported the effect of cerebellar intermittent theta-burst stimulation on balance impairment in patients with unilateral cerebral infarction [17]. However, to our knowledge, no such studies have been conducted on the application of low-frequency cerebellar rTMS when the location of stroke was in a lesion other than the cerebellum or PCA territory region.
We hypothesized that low-frequency cerebellar rTMS would be effective in improving balance function in patients with cerebral infarction except the cerebellum or PCA territory region. We then conducted a study to investigate the effects of low-frequency rTMS on the cerebellum in terms of balance and function in patients with balance impairment after cerebral infarction.
MATERIALS AND METHODS
Study design and patients
The study was designed as a randomized, double-blinded placebo-controlled study. Forty-three subjects were recruited from the inpatients and outpatients of the Department of Rehabilitation at Gwangju Veteran Hospital between August 2021 and May 2022. We included the following subjects: (1) patients aged ≥19 years, (2) patients who had experienced cerebral infarction for the first time, (3) patients with unilateral cerebral infarction, (4) impaired balance (Berg Balance Scale [BBS] ≤45), (5) patients with functional ambulation categories ≥3, who could walk on their own, and (6) patients with a Mini-Mental State Examination (MMSE) score of ≥24 who could understand and respond to questionnaires. We excluded those (1) who could not undergo rTMS (history of seizures or epilepsy, intracranial metallic or magnetic material, pacemakers, and other implantable medical devices, taking tricyclic antidepressant (TCA) and neuroleptics, pregnant women), (2) with balance impairment due to reasons other than cerebral infarction (fractures, peripheral nerve damage, visual impairment, vestibular dysfunction or disorder), (3) those where the infarction was located in the cerebellum or PCA territory area, and (4) those with cognitive disability that impeded responding to the questionnaires. Among the 43 patients, 32 patients were included, excluding those who had cerebral infarction in the cerebellum or PCA territory region (n=3), who could not understand the questions due to cognitive impairment (n=7), and who could not undergo rTMS due to a pacemaker (n=1). Among these 32 patients, 16 patients were randomly assigned to the rTMS group while the other 16 patients were assigned to the control group (Fig. 1). The therapist who conducted rTMS and the personnel who performed outcome measurements were different, thereby making the protocol double blind.
Fig. 1.
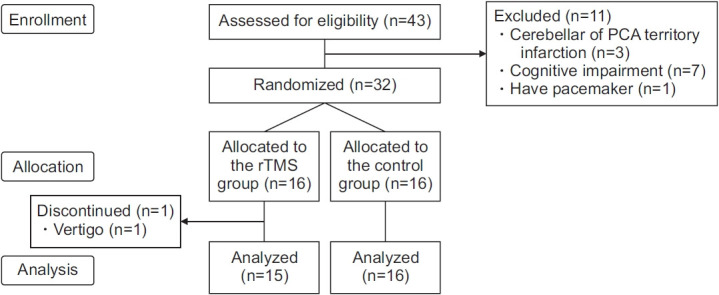
Study flowchart. rTMS, repetitive transcranial magnetic stimulation; PCA, posterior cerebral artery.
This study was approved by the Institutional Review Board of Gwangju Veteran Hospital (No. 2021-17-2). The rights of all study participants were protected in accordance with the ethical principles of the Declaration of Helsinki and informed consent was obtained in advance.
Intervention
Patients in both groups underwent a conventional rehabilitation program five times a week. Each day, patients performed passive and active mobilization exercises, gait training, balance training, and muscle strengthening for both upper and lower extremities for 30 minutes, followed by 30 minutes of training in grasp power, hand dexterity, and activities of daily living.
In the rTMS group, the coil of the machine used for treatment was placed 2 cm below and 2 cm lateral to the inion by targeting the cerebellar hemisphere contralateral to the site of cerebral infarction [13] (Fig. 2). In one session, 900 pulses of 1 Hz at 90% of the resting exercise threshold (RMT) were delivered for 15 minutes [14]. We determined the RMT by stimulating a motor hotspot with the lowest energy capable of generating at least 5 evoked potentials ≥0.05 mV within 10 stimuli [18]. Sessions were conducted five times per week for 2 weeks (total of 10 sessions). For rTMS treatment, Magpro R30 (Magventure, Farum, Denmark) and a figure-of-8 coil were used. It was performed by an experienced therapist. For the control group, a sham coil that did not induce a magnetic field was used, although the sound and scalp sensation were similar to those produced by the real rTMS coil [19].
Fig. 2.
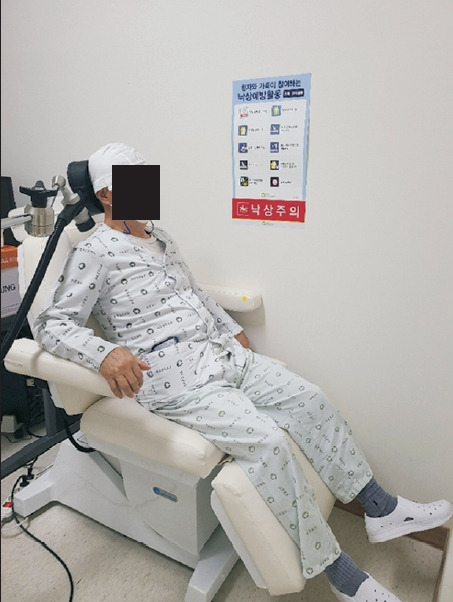
Cerebellar repetitive transcranial magnetic stimulation. The coil of the machine used for treatment was placed 2 cm below and 2 cm lateral to the inion by targeting the cerebellar hemisphere contralateral to the site of cerebral infarction. The photo was posted with the consent of the patient.
Outcome measurements
The BBS score was used as the primary outcome measure. BBS is an objective measure of dynamic balance taken while performing 14 functional tasks. Items in the BBS are scored on a 4-point scale, with a maximum combined score of 56, where higher scores indicate better balance and functional independence with respect to the activities tested [20].
The 10-m walk test (10mWT), Timed Up and Go test (TUG), and Activity-specific Balance Confidence scale (ABC) scores were used as secondary outcome measures. The 10mWT is a gait assessment that determines gait speed. A stopwatch is used to measure the time taken to walk 10 m and gait speed is calculated by dividing the distance walked by the time taken (m/s). The higher the 10mWT value, the slower is the gait speed [21]. In the TUG test, the patient begins in a sitting position in a standard armchair (seat height of 46 cm). The patient is then instructed to rise from the chair, walk 3 m (usually with a walking aid) to colored tape placed on the floor, turn around, return to the chair, and sit back down again. The stopwatch is started when the patient's hips leave the seat and is stopped when the hips touch the seat again. A higher TUG value indicates a lower gait speed [22]. The ABC scale consists of 16 items, each representing a variety of indoor and outdoor activities that require different levels of balance skills. Respondents are asked to indicate their level of confidence in performing each activity, using a scale distribution from 0% to 100%, and the values are averaged. Higher scores indicate a higher confidence in performing the activity [23,24]. Assessments were performed by the patient’s attending therapist, who did not know if the patient was in the rTMS or control group. Depending on the timepoint, all assessment items were recorded at baseline (T0), after 10 sessions of rTMS (T1), and at 4 weeks after treatment completion (T2).
Statistical analysis
All statistical analyzes were performed using R software, version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org). Statistical significance was based on a p-value of <0.05. For continuous variables, normality of data distribution was tested using the Shapiro-Wilk test. If assumptions of normality were satisfied, repeated-measure analysis of variance (ANOVA) was used. If not, the Wilcoxon test was performed. In addition, two-sample t-test and Mann-Whitney U test were used to compare the rTMS group and the control group.
RESULTS
Among the 16 subjects in the rTMS group, one subject complained of vertigo and discontinued treatment (Fig. 1).
The two groups did not differ significantly in terms of demographic variables, post-stroke duration, and baseline balance and gait function (Table 1).
Table 1.
Baseline characteristics of patients
| rTMS group (n=15) | Control group (n=16) | p-value | |
|---|---|---|---|
| Age (yr) | 75.13±2.75 | 75.94±4.57 | 0.952 |
| Height (cm) | 164.07±6.63 | 162.38±6.62 | 0.483 |
| Weight (kg) | 67.87±9.95 | 68.81±8.85 | 0.781 |
| BMI (kg/m2) | 25.16±2.93 | 26.12±3.02 | 0.38 |
| Post-stroke duration (mo) | 35.67±43.27 | 35.75±45.12 | 0.722 |
| Sex | |||
| Male | 15 | 15 | |
| Female | 0 | 1 | |
| Stroke region | |||
| Right | 8 | 5 | |
| Left | 7 | 11 | |
| Supratentorium | 13 | 13 | |
| Infratentorium | 2 | 3 | |
| BBS score | 36.40±11.51 | 38.94±10.64 | 0.529 |
| TUG (s) | 28.07±14.41 | 26.12±17.97 | 0.743 |
| 10mWT (s) | 27.27±17.54 | 52.50±53.01 | 0.088 |
| ABC score | 47.67±22.60 | 51.95±18.96 | 0.572 |
Values are presented as mean±standard deviation.
rTMS, repetitive transcranial magnetic stimulation; BMI, body mass index; BBS, Berg Balance Scale; TUG, Timed Up and Go test; 10mWT, 10-m walk test; ABC, Activity-specific Balance Confidence scale.
Two sample t-test or Mann-Whitney U test were used for continuous variables, Fisher exact tests were used for sex.
Table 2 shows the records for all assessment items for the rTMS and control groups at T0, T1, and T2. In the rTMS group, all assessed items improved significantly from T0 to T1, and from T0 to T2. In the control group, BBS and 10mWT differed significantly from T0 to T1, and from T0 to T2.
Table 2.
Outcome measurement for each group before and after treatment
| rTMS group |
Control group |
|||||
|---|---|---|---|---|---|---|
| T0 | T1 | T2 | T0 | T1 | T2 | |
| BBS score | 36.40±11.51 | 40.73±12.06* | 40.80±11.53** | 38.94±10.64 | 40.81±9.67* | 40.81±9.67** |
| TUG (s) | 28.07±14.41 | 23.20±12.91* | 23.20±12.68** | 26.12±17.97 | 25.62±16.57 | 25.50±16.57 |
| 10mWT (s) | 27.27±17.54 | 22.80±16.38* | 22.67±16.37** | 52.50±53.01 | 49.44±48.69* | 50.25±50.54** |
| ABC score | 47.67±22.60 | 55.77±22.98* | 55.90±21.78** | 51.95±18.96 | 52.11±18.90 | 51.87±18.88 |
Values are presented as mean±standard deviation.
rTMS, repetitive transcranial magnetic stimulation; BBS, Berg Balance Scale; TUG, Timed Up and Go test; 10mWT, 10-m walk test; ABC, Activity-specific Balance Confidence scale; T0, baseline; T1, 10 sessions after treatment; T2, 4 weeks after treatment.
p<0.05 comparison between T0 and T1 by post hoc test with Tukey method after repeated-measures ANOVA.
p<0.05 comparison between T0 and T2 by post hoc test with Tukey method after repeated-measures ANOVA.
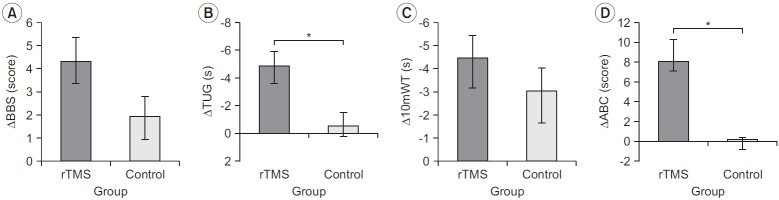
Fig. 3 shows the results of the comparison of the changes from T0 to T1 between the two groups. The difference between the rTMS (4.33±3.90) and control (1.94±3.43) groups was not significant for the BBS score (p>0.05), while the changes in the TUG score differed significantly (p<0.05) between the rTMS (-4.87±5.05) and control (-0.50±2.97) groups. The difference in the change in the 10mWT was not significant (p>0.05) between the rTMS (-4.47±4.93) and control (-3.06±5.60) groups, whereas the difference in the changes in the ABC score between the rTMS (8.10±8.33) and control (0.16±0.97) groups was significant (p<0.05).
Fig. 3.
Results of the comparison of the changes from T0 to T1 between the two groups: (A) BBS, (B) TUG, (C) 10mWT, and (D) ABC. rTMS, repetitive transcranial magnetic stimulation; BBS, Berg Balance Scale; TUG, Timed Up and Go test; 10mWT, 10-m walk test; ABC, Activity-specific Balance Confidence scale; T0, baseline; T1, 10 sessions after treatment. *p<0.05 comparison between rTMS group and control group by repeated-measures ANOVA.
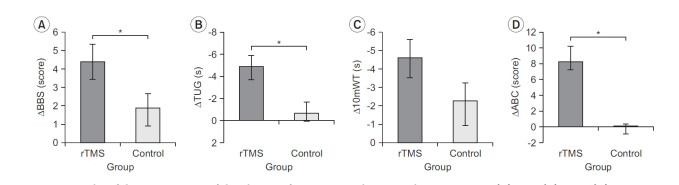
Fig. 4 shows the results of the comparison of the changes from T0 to T2 between the two groups. The BBS changes differed significantly (p<0.05) between the rTMS (4.40±3.66) and control (1.88±3.14) groups, and similar results were observed for TUG value (rTMS -4.87±4.56 vs. control -0.62±2.96; p<0.05). The changes in the 10mWT were not significantly different (p>0.05) between the rTMS (-4.60±4.14) and control (-2.25±5.20) groups. On the other hand, the ABC score changes were significantly different (p<0.05) between the rTMS (8.22±7.70) and control (-0.09±0.86) groups.
Fig. 4.
Results of the comparison of the changes from T0 to T2 between the two groups: (A) BBS, (B) TUG, (C) 10mWT, and (D) ABC. rTMS, repetitive transcranial magnetic stimulation; BBS, Berg Balance Scale; TUG, Timed Up and Go test; 10mWT, 10-m walk test; ABC, Activity-specific Balance Confidence scale; T0, baseline; T1, 10 sessions after treatment. *p<0.05 comparison between rTMS group and control group by repeated-measures ANOVA.
DISCUSSION
Our study showed that from T1 to T2, all outcome measurements differed significantly in the rTMS group. BBS and 10mWT in the control group improved significantly, but not TUG and ABC. However, the scores for the BBS, 10mWT, TUG, and ABC were improved, from T0 to T1 and T2 in both the rTMS and control groups. This seems to be the result of the conventional treatment in both groups.
When we compared changes from T0 to T1, the rTMS group differed significantly in TUG and ABC compared to the control group. When comparing changes from T0 to T2, the rTMS group differed significantly compared to the control group in BBS, TUG and ABC.
In previous studies, BBS and 10mWT differed significantly when low-frequency cerebellar rTMS was applied to patients with PCA territory infarction [14]. However, our study showed that in the assessments conducted at T1 and T2, 10mWT, which simply assessed gait, did not differed significantly between the rTMS and control groups. In another previous study, BBS in patients with stroke who received cerebellar intermittent theta-burst stimulation improved significantly [17].
Our study showed that the change in BBS scores from T0 to T2 was significantly different between the two groups, although the BBS score change from T0 to T1 did not differ significantly between the groups. The TUG and BBS reflect proprioception and a higher-level sense of balance, in addition to reflecting simple gait function, and both showed significant differences. Taken together, these results indicate that low frequency cerebellar rTMS in cerebral infarction affected balance rather than gait function. It is not clear why the treatment effect on BBS in the rTMS group was not as marked as compared to the control group immediately after completing the treatment course. However, the effect was statistically significant by 4 weeks after completing the treatment, suggesting that rTMS may have a lasting effect. ABC is an assessment of subjective confidence, and the rTMS group showed significant improvement in these scores as compared to the control group, demonstrating efficacy.
In previous research, Iwata and Ugawa [25] confirmed changes in corticomotor excitability due to the changes in cerebello-cerebral inhibition after low-frequency cerebellar rTMS was applied to healthy people. Additionally, Fierro et al. [13] also found that low-frequency cerebellar rTMS induces a long-lasting modulatory effect on the excitability of the interconnected motor area in healthy people. Furthermore, Minks et al. [26] demonstrated that low-frequency cerebellar rTMS enhanced task performance in patients with Parkinson's disease. Based on those previous studies, we expected that low-frequency cerebellar rTMS can improve balance and walking abilities even in patients with cerebral infarction, not with cerebellar infarction.
Neurophysiologically, Purkinje cells play a role in inhibiting excitatory neuron transmission by releasing gamma-aminobutyric acid (GABA) [27], which exerts an inhibitory effect on the deep nucleus of the cerebellum. Thus, when low-frequency rTMS of the cerebellum inhibits the inhibitory Purkinje cells, an excitatory effect may occur from the dentate nuclei and interpositus nuclei to the motor cortex through the ventrolateral nucleus of the thalamus [28,29]. Since cerebellar motor deficits in patients with cerebellar dysfunction arise due to an imbalance between excitation and inhibition of the corticospinal pathway [30], we made on assumption that low-frequency rTMS may improve the motor and balance ability in patients with spinocerebellar degeneration.
Symptoms of patients with cerebral infarction include hemiplegic-side motor weakness, sensory deficits, speech impairment, ataxia, dysphagia, and dementia. In these patients, balance impairment may occur due to motor weakness, sensory deficits, and ataxia. The application of cerebellar rTMS may have had an effect on motor weakness and ataxia in our patients, thus improving the balance impairment in patients with cerebral infarction.
Among the 16 subjects in the rTMS group, one subject discontinued treatment due to vertigo. Symptoms improved after discontinuation of the treatment, with no recurrence for at least 1 month. The side effects of cerebellar rTMS shown in previous studies are as follows: Satow et al. [31] reported that nausea was induced in two of eight healthy subjects when 900 rTMS pulses were applied to the right cerebellum at 0.9 Hz for 10 minutes. Brighina et al. [32] reported mild muscle and neck stiffness among two of 17 subjects with migraine. In the present study, side effects other than vertigo, including nausea and migraine, were not observed. Nevertheless, future studies should monitor these side effects. To the best of our knowledge, no previous study has investigated the effects of low-frequency cerebellar rTMS, delivered outside the cerebellum and PCA territory regions, on balance in patients with cerebral infarction.
There were several limitations to this study. First, the number of cases in our study is relatively small. Second, long-term effects could not be verified due to the short follow-up period of 4 weeks. Third, the duration of cerebral infarction varied significantly among subjects. Fourth, we did not evaluate the motor and NIHSS (National Institutes of Health Stroke Scale) in our study, thereby not showing the improvement was achieved. Fifth, we did not classify the causes of balance disorders after stroke. Finally, most patients in this study were man. In subsequent studies, it is recommended to increase the sample size, study the effects of rTMS after 4 weeks, categorize the infarction duration, and study the effects of rTMS according to sex.
In conclusion, low-frequency cerebellar rTMS is safe, and helpful for improving balance and confidence in patients with cerebral infarction. Therefore, it may be a beneficial treatment for these patients.
Footnotes
No potential conflict of interest relevant to this article was reported.
None.
Conceptualization: Yoon SR, Im NG. Methodology: Yoon SR, Im NG. Formal analysis: Lim NN, Oh KR, Kim MG, Lee Y. Funding acquisition: Yoon SR, Im NG. Project administration: Yoon SR, Im NG, Cho TH. Visualization: Yoon SR, Ryu SR, Im NG. Writing - original draft: Yoon SR, Im NG. Writing - review and editing: Yoon SR, Im NG, Lim NN. Approval of final manuscript: all authors.
REFERENCES
- 1.Li J, Zhong D, Ye J, He M, Liu X, Zheng H, et al. Rehabilitation for balance impairment in patients after stroke: a protocol of a systematic review and network meta-analysis. BMJ Open. 2019;9:e026844. doi: 10.1136/bmjopen-2018-026844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forster A, Young J. Incidence and consequences of falls due to stroke: a systematic inquiry. BMJ. 1995;311:83–6. doi: 10.1136/bmj.311.6997.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Wang P, Liu C, He C, Reinhardt JD. The effect of whole body vibration on balance, gait performance and mobility in people with stroke: a systematic review and meta-analysis. Clin Rehabil. 2015;29:627–38. doi: 10.1177/0269215514552829. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Han XG, Sheng J, Ma SJ. Virtual reality for improving balance in patients after stroke: a systematic review and meta-analysis. Clin Rehabil. 2016;30:432–40. doi: 10.1177/0269215515593611. [DOI] [PubMed] [Google Scholar]
- 5.Broderick P, Horgan F, Blake C, Ehrensberger M, Simpson D, Monaghan K. Mirror therapy for improving lower limb motor function and mobility after stroke: a systematic review and meta-analysis. Gait Posture. 2018;63:208–20. doi: 10.1016/j.gaitpost.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 6.Tyson SF, Kent RM. Effects of an ankle-foot orthosis on balance and walking after stroke: a systematic review and pooled meta-analysis. Arch Phys Med Rehabil. 2013;94:1377–85. doi: 10.1016/j.apmr.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Park KH, Kim DY, Kim TH. The effect of step climbing exercise on balance and step length in chronic stroke patients. J Phys Ther Sci. 2015;27:3515–8. doi: 10.1589/jpts.27.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn J, Shin S, Lee W. The effect of modified trampoline training on balance, gait, and falls efficacy of stroke patients. J Phys Ther Sci. 2015;27:3351–4. doi: 10.1589/jpts.27.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu WY, Cheng CH, Liao KK, Lee IH, Lin YY. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. 2012;43:1849–57. doi: 10.1161/STROKEAHA.111.649756. [DOI] [PubMed] [Google Scholar]
- 10.Shen X, Liu M, Cheng Y, Jia C, Pan X, Gou Q, et al. Repetitive transcranial magnetic stimulation for the treatment of post-stroke depression: a systematic review and meta-analysis of randomized controlled clinical trials. J Affect Disord. 2017;211:65–74. doi: 10.1016/j.jad.2016.12.058. [DOI] [PubMed] [Google Scholar]
- 11.Ren CL, Zhang GF, Xia N, Jin CH, Zhang XH, Hao JF, et al. Effect of low-frequency rTMS on aphasia in stroke patients: a meta-analysis of randomized controlled trials. PLoS One. 2014;9:e102557. doi: 10.1371/journal.pone.0102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JW, Oh JC, Lee JW, Yeo JS, Ryu KH. The effect of 5Hz high-frequency rTMS over contralesional pharyngeal motor cortex in post-stroke oropharyngeal dysphagia: a randomized controlled study. Neurogastroenterol Motil. 2013;25:324. doi: 10.1111/nmo.12063. [DOI] [PubMed] [Google Scholar]
- 13.Fierro B, Giglia G, Palermo A, Pecoraro C, Scalia S, Brighina F. Modulatory effects of 1 Hz rTMS over the cerebellum on motor cortex excitability. Exp Brain Res. 2007;176:440–7. doi: 10.1007/s00221-006-0628-y. [DOI] [PubMed] [Google Scholar]
- 14.Kim WS, Jung SH, Oh MK, Min YS, Lim JY, Paik NJ. Effect of repetitive transcranial magnetic stimulation over the cerebellum on patients with ataxia after posterior circulation stroke: a pilot study. J Rehabil Med. 2014;46:418–23. doi: 10.2340/16501977-1802. [DOI] [PubMed] [Google Scholar]
- 15.Shiga Y, Tsuda T, Itoyama Y, Shimizu H, Miyazawa KI, Jin K, et al. Transcranial magnetic stimulation alleviates truncal ataxia in spinocerebellar degeneration. J Neurol Neurosurg Psychiatry. 2002;72:124–6. doi: 10.1136/jnnp.72.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu H, Tsuda T, Shiga Y, Miyazawa K, Onodera Y, Matsuzaki M, et al. Therapeutic efficacy of transcranial magnetic stimulation for hereditary spinocerebellar degeneration. Tohoku J Exp Med. 1999;189:203–11. doi: 10.1620/tjem.189.203. [DOI] [PubMed] [Google Scholar]
- 17.Koch G, Bonni S, Casula EP, Iosa M, Paolucci S, Pellicciari MC, et al. Effect of cerebellar stimulation on gait and balance recovery in patients with hemiparetic stroke: a randomized clinical trial. JAMA Neurol. 2019;76:170–8. doi: 10.1001/jamaneurol.2018.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application: an updated report from an I.F.C.N. Committee. Clin Neurophysiol. 2015;126:1071–107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theoret H, Haque J, Pascual-Leone A. Increased variability of paced finger tapping accuracy following repetitive magnetic stimulation of the cerebellum in humans. Neurosci Lett. 2001;306:29–32. doi: 10.1016/s0304-3940(01)01860-2. [DOI] [PubMed] [Google Scholar]
- 20.Blum L, Korner-Bitensky N. Usefulness of the Berg Balance Scale in stroke rehabilitation: a systematic review. Phys Ther. 2008;88:559–66. doi: 10.2522/ptj.20070205. [DOI] [PubMed] [Google Scholar]
- 21.Dean CM, Richards CL, Malouin F. Task-related circuit training improves performance of locomotor tasks in chronic stroke: a randomized, controlled pilot trial. Arch Phys Med Rehabil. 2000;81:409–17. doi: 10.1053/mr.2000.3839. [DOI] [PubMed] [Google Scholar]
- 22.Ng SS, Hui-Chan CW. The timed up & go test: its reliability and association with lower-limb impairments and locomotor capacities in people with chronic stroke. Arch Phys Med Rehabil. 2005;86:1641–7. doi: 10.1016/j.apmr.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Botner EM, Miller WC, Eng JJ. Measurement properties of the Activities-specific Balance Confidence Scale among individuals with stroke. Disabil Rehabil. 2005;27:156–63. doi: 10.1080/09638280400008982. [DOI] [PubMed] [Google Scholar]
- 24.Jang SN, Cho SI, Ou SW, Lee ES, Baik HW. The validity and reliability of Korean Fall Efficacy Scale (FES) and Activities-specific Balance Confidence Scale (ABC) J Korean Geriatr Soc. 2003;7:255–68. [Google Scholar]
- 25.Iwata NK, Ugawa Y. The effects of cerebellar stimulation on the motor cortical excitability in neurological disorders: a review. Cerebellum. 2005;4:218–23. doi: 10.1080/14734220500277007. [DOI] [PubMed] [Google Scholar]
- 26.Minks E, Marecek R, Pavlik T, Ovesna P, Bares M. Is the cerebellum a potential target for stimulation in Parkinson’s disease? Results of 1-Hz rTMS on upper limb motor tasks. Cerebellum. 2011;10:804–11. doi: 10.1007/s12311-011-0290-1. [DOI] [PubMed] [Google Scholar]
- 27.Duguid IC, Smart TG. Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron-Purkinje cell synapses. Nat Neurosci. 2004;7:525–33. doi: 10.1038/nn1227. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa T, Tomatsu S, Tsunoda Y, Lee J, Hoffman DS, Kakei S. Releasing dentate nucleus cells from Purkinje cell inhibition generates output from the cerebrocerebellum. PLoS One. 2014;9:e108774. doi: 10.1371/journal.pone.0108774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindeman S, Hong S, Kros L, Mejias JF, Romano V, Oostenveld R, et al. Cerebellar Purkinje cells can differentially modulate coherence between sensory and motor cortex depending on region and behavior. Proc Natl Acad Sci U S A. 2021;118:e2015292118. doi: 10.1073/pnas.2015292118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamburin S, Fiaschi A, Andreoli A, Marani S, Manganotti P, Zanette G. Stimulus-response properties of motor system in patients with cerebellar ataxia. Clin Neurophysiol. 2004;115:348–55. doi: 10.1016/s1388-2457(03)00357-2. [DOI] [PubMed] [Google Scholar]
- 31.Satow T, Mima T, Hara H, Oga T, Ikeda A, Hashimoto N, et al. Nausea as a complication of low-frequency repetitive transcranial magnetic stimulation of the posterior fossa. Clin Neurophysiol. 2002;113:1441–3. doi: 10.1016/s1388-2457(02)00187-6. [DOI] [PubMed] [Google Scholar]
- 32.Brighina F, Palermo A, Panetta ML, Daniele O, Aloisio A, Cosentino G, et al. Reduced cerebellar inhibition in migraine with aura: a TMS study. Cerebellum. 2009;8:260–6. doi: 10.1007/s12311-008-0090-4. [DOI] [PubMed] [Google Scholar]