Abstract
The Shiga toxins (Stx) are critical virulence factors for Escherichia coli O157:H7 and other serotypes of enterohemorrhagic E. coli (EHEC). These potent toxins are encoded in the genomes of temperate lambdoid bacteriophages. We recently demonstrated that induction of the resident Stx2-encoding prophage in an O157:H7 clinical isolate is required for toxin production by this strain. Since several factors produced by human cells, including hydrogen peroxide (H2O2), are capable of inducing lambdoid prophages, we hypothesized that such molecules might also induce toxin production by EHEC. Here, we studied whether H2O2 and also human neutrophils, an important endogenous source of H2O2, induced Stx2 expression by an EHEC clinical isolate. Both H2O2 and neutrophils were found to augment Stx2 production, raising the possibility that these agents may lead to prophage induction in vivo and thereby contribute to EHEC pathogenesis.
Enterohemorrhagic Escherichia coli (EHEC) strains, including E. coli O157:H7, are emerging pathogens responsible for outbreaks and sporadic cases of diarrhea (11). EHEC isolates often share numerous virulence factors with other pathogenic E. coli strains, but are distinguished by their production of Shiga toxins (Stx). The activity of these A-B-type toxins in the human microvasculature can result in the most severe consequences of EHEC infection, including hemorrhagic colitis and hemolytic-uremic syndrome (11). Two main types of Stx, Stx1 and Stx2, have been described, each consisting of human glycolipid-binding B subunits and enzymatic A subunits that inhibit protein synthesis by cleaving eukaryotic 28S rRNA. More than 60 serotypes of E. coli associated with human disease have been found to encode the Shiga toxins (1).
The stx genes in most, if not all, EHEC strains are carried by lysogenic bacteriophages of the lambdoid family (17, 22, 26, 28). The toxin genes from lysogens of several of these phages were cloned and sequenced (2, 9, 20), and primer extension analyses were used to identify functional promoters immediately 5′ of each toxin-coding sequence (5, 27). Although the Stx1 promoter was found to be inducible in low-iron growth media by virtue of an operator for the iron-dependent Fur transcriptional repressor (3, 21), no environmental parameters for transcriptional regulation at the Stx2 promoter have been found (18).
Superimposed on the transcriptional regulation of these toxin gene-associated promoters is the contribution of the phage lytic cycle to Stx production. Whereas most toxin-encoding bacteriophages are thought to serve primarily as vectors for dissemination of toxin genes among bacterial strains, it has become clear that the phage life cycle has a central role in the regulation of Stx production by EHEC. Recent work has shown that phage induction may contribute to EHEC pathogenesis in a number of ways, including (i) increasing toxin gene copy number as a result of phage genome replication, (ii) increasing toxin transcription from phage promoters that are repressed during lysogeny, and (iii) leading to toxin release in the process of phage-mediated bacterial lysis (19, 23, 29). In fact, very little toxin is produced by an EHEC mutant strain which contains a deletion of the late phage promoter, PR′, suggesting that phage induction is critical for toxin expression (P. Wagner, M. Neely, X. Zhang, D. Acheson, M. Waldor, and D. Friedman, submitted for publication).
The molecular event that initiates phage λ induction is cleavage of the repressor cI, which holds phage transcription silent during lysogeny (24). cI cleavage is RecA dependent and occurs as a consequence of activating the bacterial SOS response to DNA damage (13). Mitomycin C (18) and fluoroquinolone (16, 31) antibiotics are DNA-damaging agents known to induce the SOS response and are capable of inducing lambdoid phages, as well as Stx production by EHEC strains. However, most patients who develop the severe sequelae of EHEC infection are not exposed to these agents. Therefore, some other, perhaps endogenous, agent in the human intestine may activate the lytic cycle of Stx-encoding bacteriophages and thereby trigger in vivo Stx production by EHEC.
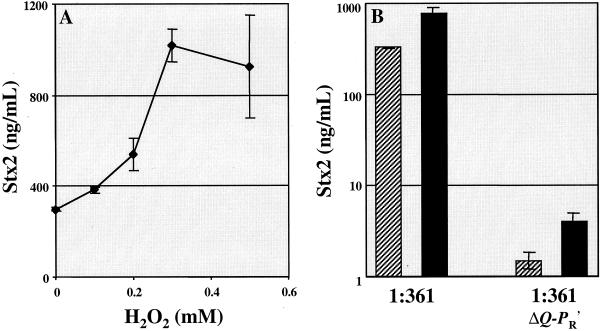
Human neutrophils and other cell types release a variety of antibacterial molecules, some of which—including H2O2 and NO—are known to damage bacterial DNA in a way that induces the SOS response (10, 15). We thus hypothesized that human neutrophils and their products might induce Stx-encoding phages and Stx production by EHEC. To test this hypothesis, we first exposed the O157:H7 EHEC clinical isolate 1:361 to a range of concentrations of H2O2 in vitro. A dose-dependent relationship between H2O2 concentration and Stx2 production by strain 1:361 organisms was observed, as shown in Fig. 1A. Concomitant with the observed increase in toxin production, a >10-fold increase in phage titer was also observed upon treatment of 1:361 with 0.25 mM H2O2 (not shown). The isogenic 1:361 derivative, 1:361ΔQ-PR′, which lacks sequences necessary for late phage transcription and is known to be impaired in toxin production when treated with mitomycin C (Wagner et al., submitted), was similarly impaired in the presence of H2O2 (Fig. 1B). These results suggest that the H2O2-mediated increase in Stx2 production depends upon late phage transcription.
FIG. 1.
H2O2 induces Stx2 expression by the E. coli O157:H7 clinical isolate 1:361. (A) An overnight culture of 1:361 was diluted 1/5,000 in L broth containing H2O2 (Sigma, St. Louis, Mo.) at the indicated concentrations. 1:361 cultures did not grow in L broth containing >0.5 mM H2O2. (B) 1:361 and 1:361ΔQ-PR′ cultures were diluted 1/5,000 in L broth ( ) or L broth supplemented with 0.25 mM H2O2 (■). Stx2 levels (nanograms/milliliter) were measured from sonicated overnight cultures by using a previously described enzyme-linked immunosorbent assay (6). Mean values from three independent cultures are shown along with standard deviations. The addition of 0.25 mM H2O2 resulted in a statistically significant (P < 0.004) increase in Stx2 production by 1:361.
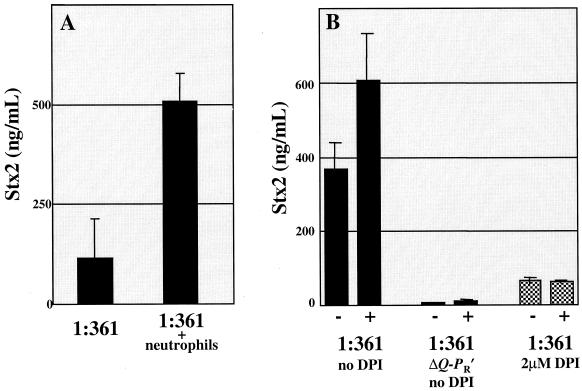
We next cocultured strain 1:361 with human neutrophils isolated from healthy donors. A significant inducing effect of neutrophils on bacterial Stx2 production was observed, relative to growth in medium alone (Fig. 2A). A reasonable explanation for this observation is that neutrophil-derived factors damage bacterial DNA and thereby activate the SOS response and subsequent phage induction. If this hypothesis is correct, then 1:361ΔQ-PR′, which is defective in late phage transcription, should be impaired in Stx2 production when cocultured with neutrophils. Furthermore, inhibitors of neutrophil enzymes required for the generation of H2O2 and other factors that activate the SOS response should reduce the effect of neutrophil induction of Stx2 production. Each of these predictions proved correct. 1:361ΔQ-PR′ made very small amounts of Stx2 in the presence of neutrophils (Fig. 2B). Oxygen-derived free radical production by neutrophils can be antagonized by diphenyleneiodonium (DPI), an agent known to inhibit NADPH oxidase (4) but not the production of other microbicidal factors by human neutrophils (7). DPI prevented the neutrophil-associated increase in Stx2 production by 1:361 organisms (Fig. 2B), but not mitomycin C-associated Stx2 induction (not shown). Thus, despite the fact that 1:361 organisms made less Stx2 overall when grown in DPI, these results suggest that DPI inhibited the production of phage-inducing factors by neutrophils rather than the response of strain 1:361 organisms to these factors. Our data are consistent with the hypothesis that the production of NADPH oxidase-dependent reactive oxygen intermediates by neutrophils induces the bacterial SOS response and thereby augments production of Stx2.
FIG. 2.
Coculture of 1:361 with human neutrophils results in increased Stx2 production. (A) An overnight culture of 1:361 was diluted 1/5,000 in a solution consisting of one part L broth and one part Hank's buffered saline solution or one part L broth and one part Hank's buffered saline solution containing 106 neutrophils. The addition of neutrophils resulted in a statistically significant (P = 0.01) increase in Stx2 production by 1:361 organisms. (B) 1:361 or 1:361ΔQ-PR′ was diluted 1/5,000 from an overnight culture as described above, without (−) or with (+) 106 neutrophils and with or without DPI (Sigma) at a final concentration of 2 μM. Neutrophils were isolated as previously described (8), and Stx2 levels were measured as described for Fig. 1.
We recently found that Shiga toxin production is regulated as part of the lytic cycle of the toxin-encoding bacteriophages (Wagner et al., submitted). Therefore, an important next step in understanding EHEC pathogenesis is the identification of host factors that induce Stx-encoding prophages and thereby toxin production. Our current data suggest that neutrophils may be a source of such factors. Besides inducing Stx production by EHEC, several recent observations implicate neutrophils in other aspects of EHEC pathogenesis. Epidemiologic studies have revealed that EHEC disease severity correlates with peripheral blood neutrophil counts (25). In vitro data suggest that neutrophil infiltration into the intestinal lumen enhances Stx uptake (B. P. Hurley, C. M. Thorpe, A. J. King, G. T. Keusch, and D. W. K. Acheson, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., abstr. B-101, 2000). Furthermore, Stx2 can induce the respiratory burst of neutrophils (12) and inhibit neutrophil apoptosis (14). Taken together, these observations suggest a model in which neutrophils act to facilitate both Stx production and absorption. Such a model, if correct, has important clinical implications. If host factors such as neutrophils and their products contribute to EHEC pathogenesis, then it is reasonable to consider inhibition of these factors as a novel therapeutic strategy for EHEC treatment. The need for new approaches is underscored by the fact that many antibiotics commonly used to treat diarrhea are known to induce bacteriophages and are associated with increased morbidity in patients with EHEC infections (30).
Acknowledgments
We are grateful to B. P. Hurley for invaluable technical advice and critical reading of the manuscript, to B. Davis and A. Kane for critical reading of the manuscript, and to A. Kane and the NEMC GRASP Digestive Disease Center for preparing the microbiologic media for our studies.
This work was supported by grants AI-42347 to M.K.W., AI-39067 to D.W.K.A., and P30DK-34928 for the NEMC GRASP Digestive Center. M.K.W. is an Assistant Investigator of the Howard Hughes Medical Institute and a Pew Scholar in the Biomedical Sciences. P.L.W. was supported by a Howard Hughes Medical Institute Research Training Fellowship for Medical Students.
REFERENCES
- 1.Acheson D W K, Keusch G T. Which Shiga-toxin producing types of E. coli are important? ASM News. 1996;62:302–306. [Google Scholar]
- 2.Calderwood S B, Auclair F, Donohue-Rolfe A, Keusch G T, Mekalanos J J. Nucleotide sequence of the Shiga-like toxin genes of Escherichia coli. Proc Natl Acad Sci USA. 1987;84:4364–4368. doi: 10.1073/pnas.84.13.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calderwood S B, Mekalanos J J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the furlocus. J Bacteriol. 1987;169:4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cross A R, Jones O T. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Specific labelling of a component polypeptide of the oxidase. Biochem J. 1986;237:111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Grandis S, Ginsberg J, Toone M, Climie S, Friesen J, Brunton J. Nucleotide sequence and promoter mapping of the Escherichia coliShiga-like toxin operon of bacteriophage H-19B. J Bacteriol. 1987;169:4313–4319. doi: 10.1128/jb.169.9.4313-4319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donohue-Rolfe A, Acheson D W K, Kane A V, Keusch G T. Purification of Shiga toxin and Shiga-like toxins I and II by receptor analog affinity chromatography with immobilized P1 glycoprotein and production of cross-reactive monoclonal antibodies. Infect Immun. 1989;57:3888–3893. doi: 10.1128/iai.57.12.3888-3893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis J A, Mayer S J, Jones O T G. The effect of the NADPH oxidase inhibitor diphenyleneiodonium on aerobic and anaerobic microbicidal activities of human neutrophils. Biochem J. 1988;251:887–891. doi: 10.1042/bj2510887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.English D, Anderson B R. Single step separation of red blood cells, granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974;5:249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- 9.Huang A, Friesen J, Brunton J L. Characterization of a bacteriophage that carries the genes for production of Shiga-like toxin 1 in Escherichia coli. J Bacteriol. 1987;169:4308–4312. doi: 10.1128/jb.169.9.4308-4312.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imlay J A, Linn S S. Mutagenesis and stress responses induced in Escherichia coliby hydrogen peroxide. J Bacteriol. 1987;167:2967–2976. doi: 10.1128/jb.169.7.2967-2976.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaper J B, O'Brien A D, editors. Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. Washington, D.C.: ASM Press; 1998. [Google Scholar]
- 12.King A J, Sundaram S, Cendoroglo M, Acheson D W K, Keusch G T. Shiga toxin induces superoxide production in polymorphonuclear cells with subsequent impairment of phagocytosis and responsiveness to phorbol esters. J Infect Dis. 1999;179:503–507. doi: 10.1086/314579. [DOI] [PubMed] [Google Scholar]
- 13.Little J W. The SOS regulatory system. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. R. G. Georgetown, Tex: Landes; 1996. pp. 453–479. [Google Scholar]
- 14.Liu J, Akahoshi T, Sasahana T, Kitasato H, Namai R, Sasaki T, Inoue M, Kondo H. Inhibition of neutrophil apoptosis by verotoxin 2 derived from Escherichia coliO157:H7. Infect Immun. 1999;67:6203–6205. doi: 10.1128/iai.67.11.6203-6205.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lobysheva I I, Stupakova M V, Mikoyan V D, Vasilieva S V, Vanin A F. Induction of the SOS DNA repair response in Escherichia coliby nitric oxide donating agents: dinitrosyl iron complexes with thiol-containing ligands and S-nitrosothiols. FEBS Lett. 1999;454:177–180. doi: 10.1016/s0014-5793(99)00777-2. [DOI] [PubMed] [Google Scholar]
- 16.Matsushiro A, Sato K, Miyamoto H, Yamamura T, Honda T. Induction of prophages of enterohemorrhagic Escherichia coliO157:H7 with norfloxacin. J Bacteriol. 1999;181:2257–2260. doi: 10.1128/jb.181.7.2257-2260.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizutani S, Nakazono N, Sugino Y. The so-called chromosomal verotoxin genes are actually carried by defective prophages. DNA Res. 1999;6:141–143. doi: 10.1093/dnares/6.2.141. [DOI] [PubMed] [Google Scholar]
- 18.Muhldorfer I, Hacker J, Keusch G T, Acheson D W K, Tschape H, Kane A V, Ritter A, Olschlager T, Donohue-Rolfe A. Regulation of the Shiga-like toxin II operon in Escherichia coli. Infect Immun. 1996;64:495–502. doi: 10.1128/iai.64.2.495-502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neely M N, Friedman D I. Functional and genetic analysis of regulatory regions of coliphage H-19B: location of Shiga-like toxin and lysis genes suggest a role for phage functions in toxin release. Mol Microbiol. 1998;28:1255–1267. doi: 10.1046/j.1365-2958.1998.00890.x. [DOI] [PubMed] [Google Scholar]
- 20.Newland J W, Strockbine N A, Neill R J. Cloning of genes for production of Escherichia coliShiga-like toxin type II. Infect Immun. 1987;55:2675–2680. doi: 10.1128/iai.55.11.2675-2680.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Brien A D, LaVech G D, Thompson M R, Formal S B. Production of Shigella dysenteriae type 1-like cytotoxin by Escherichia coli. J Infect Dis. 1982;146:763–769. doi: 10.1093/infdis/146.6.763. [DOI] [PubMed] [Google Scholar]
- 22.O'Brien A D, Newland J W, Miller S F, Holmes R K, Smith H W, Formal S B. Shiga-like toxin-converting phages from Escherichia colistrains that cause hemorrhagic colitis or infantile diarrhea. Science. 1984;226:694–696. doi: 10.1126/science.6387911. [DOI] [PubMed] [Google Scholar]
- 23.Plunkett G, III, Rose D J, Durfee T J, Blattner F R. Sequence of Shiga toxin 2 phage 933W from Escherichia coliO157:H7: Shiga toxin as a phage late-gene product. J Bacteriol. 1999;181:1767–1778. doi: 10.1128/jb.181.6.1767-1778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ptashne M. A genetic switch: phage λ and higher organisms. Cambridge, Mass: Cell Press; 1992. [Google Scholar]
- 25.Slutsker L, Ries A A, Greene K D, Wells J G, Hutwagner L, Griffin P M. Escherichia coliO157:H7 diarrhea in the United States: clinical and epidemiologic features. Ann Intern Med. 1997;126:505–513. doi: 10.7326/0003-4819-126-7-199704010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Smith H W, Green P, Parsell Z. Vero cell toxins in Escherichia coliand related bacteria: transfer by phage and conjugation and toxin action in laboratory animals, chickens and pigs. J Gen Microbiol. 1983;129:3121–3137. doi: 10.1099/00221287-129-10-3121. [DOI] [PubMed] [Google Scholar]
- 27.Sung L M, Jackson M P, O'Brien A D, Holmes R K. Transcription of the Shiga-like toxin type II and Shiga-like toxin type II variant operons of Escherichia coli. J Bacteriol. 1990;172:6386–6395. doi: 10.1128/jb.172.11.6386-6395.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unkmeir A, Schmidt H. Structural analysis of phage-borne stx genes and their flanking sequences in Shiga toxin-producing Escherichia coli and Shigella dysenteriaetype 1 strains. Infect Immun. 2000;68:4856–4864. doi: 10.1128/iai.68.9.4856-4864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waldor M K. Bacteriophage biology and bacterial virulence. Trends Microbiol. 1998;6:295–297. doi: 10.1016/s0966-842x(98)01320-1. [DOI] [PubMed] [Google Scholar]
- 30.Wong C S, Jelacic S, Habeeb R L, Watkins S L, Tarr P I. The risk of the hemolytic-uremic syndrome after antibiotic treatment of Escherichia coliO157:H7 infections. N Engl J Med. 2000;342:1930–1936. doi: 10.1056/NEJM200006293422601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, McDaniel A D, Wolf L E, Keusch G T, Waldor M K, Acheson D W K. Quinolone antibiotic induces Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J Infect Dis. 2000;181:664–670. doi: 10.1086/315239. [DOI] [PubMed] [Google Scholar]