Non-alcoholic fatty liver disease (NAFLD) is highly prevalent globally and requires multidisciplinary care. Here, we report key findings of a NAFLD care workshop, address knowledge gaps and highlight a path to optimise healthcare resource use, to improve outcomes in patients with steatotic liver disease.
Subject terms: Non-alcoholic fatty liver disease, Non-alcoholic steatohepatitis, Health services
Schattenberg et al. outline discussions from a recent workshop on NAFLD care and advocate for a multidisciplinary approach to managing this complex and multifactorial disease. The authors highlight gaps in current models of care and make recommendations on optimising a multistakeholder approach in steatotic liver diseases.
NAFLD, the most common chronic liver disease, affects around 33% of adults worldwide1. Given the growing global prevalence of obesity and type 2 diabetes mellitus (T2DM), and their association with NAFLD, the burden of this liver condition and its socio-economic costs are only expected to grow2.
NAFLD encompasses a disease spectrum that is defined on the basis of liver histology with fatty infiltration of liver tissue and gradual progression towards chronic inflammation (non-alcoholic steatohepatitis, NASH), fibrosis, cirrhosis and hepatocellular carcinoma (HCC)3. There are no approved therapies for NASH, but a growing evidence base supports the effectiveness of non-pharmacological interventions in halting progression or causing remission, highlighting the importance of early diagnosis and clinical management. Additionally, drugs licenced for cardiovascular disease (CVD), like statins, or T2DM, such as glucagon-like peptide 1 (GLP-1) receptor agonists and sodium-glucose cotransporter-2 inhibitors, hold early promise for NAFLD patients4.
However, NAFLD and NASH, along with other steatotic (fatty) liver diseases, are largely underdiagnosed worldwide and epidemiological data are scarce5, limiting the provision of good practice care and implementation of national preparedness plans. Furthermore, at present, liver biopsy is the most reliable method for diagnosing fibrosis and steatohepatitis, which is limited by cost, sampling error and procedure-related morbidity and mortality3, hindering clinical trials and drug development programmes. Several non-invasive tests (NITs), including serum and genetic biomarkers and imaging modalities, have been proposed as options for diagnosing NAFLD and NASH6.
NAFLD typically develops in the context of metabolic syndrome (MetS), which includes obesity, T2DM, dyslipidaemia and hypertension3. Comprehensive care therefore requires a multidisciplinary and multistakeholder approach, to address the range of comorbidities affecting patients with NAFLD. Recent analyses have shown that a wide range of NAFLD models of care (MoCs) exist, varying in most aspects such as in who provides care, what care is provided considering the disease stage, which healthcare settings care is provided in and to what extent care is coordinated across the healthcare system7. Importantly, the availability of auxiliary healthcare services, such as peer-to-peer patient programmes, physician assistants or nurse practitioners, differs widely across healthcare systems.
National preparedness plans are deficient globally, indicating systemic gaps in delivering NAFLD care8. While adequate policies and civil society engagement, guidelines9, epidemiological data and care management are pivotal to the provision of good care, these are suboptimal to varying degrees in different countries, underscoring a need for dialogue among all relevant stakeholders, to prompt the integration of NAFLD into national public health agendas.
Building on a global public health and NAFLD consensus statement10, in May 2022, a multidisciplinary group of some 100 stakeholders met in Barcelona, Spain, and online, to exchange their experience on public health approaches to NAFLD and NASH. The discussions focused on diverse MoCs that currently exist or should be developed, in order to set out priorities for healthcare providers and policymakers, to optimise the diagnosis and management of NAFLD. This unique programme provided an interactive platform for primary care physicians, hepatologists, endocrinologists, nutritionists, public health experts, nurses and patients. Here, we summarise the discussions from this meeting.
The fatal triple
Obesity, T2DM and CVD are closely linked with and impact each other and NAFLD. However, each of these metabolic diseases also has its own unique features, resulting in several sub-types of patients that require a personalised, multidisciplinary approach (Fig. 1). Obesity is a major risk factor for NAFLD, and exposure to obesity increases the risk of HCC11. NAFLD increases the risk of developing T2DM, whereas improvement of hepatic steatosis, or fat in the liver, decreases the incidence of T2DM12. Furthermore, approximately 55% of patients with T2DM have NAFLD13. The presence of NAFLD in patients with T2DM hampers glycaemic control, requiring the use of multiple anti-diabetic therapies and increasing the risk of both micro- and macro-vascular complications14. The potential impact of NAFLD on CVD remains controversial. Several mechanisms can explain how NASH can impact the cardiovascular system, offering biological plausibility15. However, an independent contribution of NAFLD to the development of CVD is difficult to disentangle from the impact of other factors that are well-established risk factors for clinical CVD events, such as obesity, T2DM, dyslipidaemia and hypertension3. The majority of drugs used to prevent or treat CVD, e.g., statins, aspirin and other anti-aggregant drugs, have benefit on the risk factors for NAFLD15, but therapies to treat NASH directly are required. Public health measures to address NAFLD include raising awareness and education, in addition to close collaboration between hepatologists, diabetologists, nutritionists and cardiologists, in care provision.
Fig. 1. The fatal triple association between obesity, T2DM and CVD, and NAFLD.
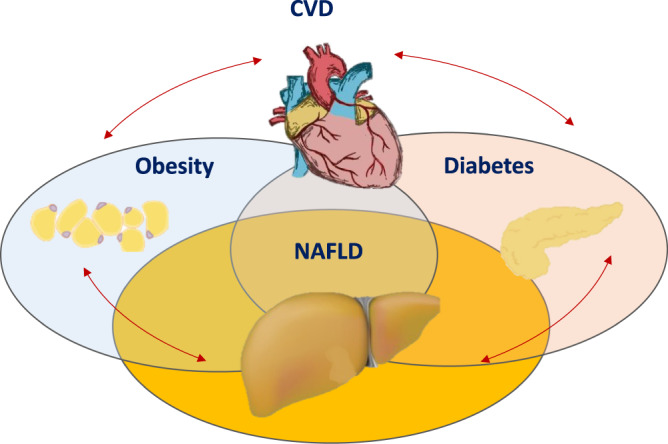
The conditions are closely associated but without a complete overlap: the estimated prevalence of obesity among T2DM is approximately 80% in the US and the estimated prevalence of obesity among patients with NAFLD is 51% in Europe and the US. The prevalence of NAFLD among T2DM is approximately 55%, whereas the prevalence of NAFLD among patients with obesity varies from 50 to 90%, depending on the severity of obesity. The potential impact of NAFLD on CVD remains controversial, with several mechanisms explaining how NASH can impact the cardiovascular system; however, the independent contribution of NAFLD to the development of CVD is difficult to disentangle from the impact of other factors that are well-established risk factors for CVD. Given these interconnections, a personalised multidisciplinary approach is needed for the optimal care of patients with NAFLD. CVD cardiovascular disease, NAFLD non-alcoholic fatty liver disease, NASH non-alcoholic steatohepatitis, T2DM type 2 diabetes mellitus.
Screening and risk stratification
People admitted to hospital with decompensated cirrhosis, characterised by liver failure and/or liver-related issues like ascites or jaundice9, have a 25% chance of 60-day mortality; approximately 70% of new cirrhosis diagnoses are made on such an admission, making a case for the importance of early detection. Detection strategies based on case finding should focus on risk factors such as MetS features, like T2DM and obesity, or routinely performed liver function tests, and aim to improve the efficiency of existing patient interactions (e.g., automated laboratory measurement analysis to provide decision support to clinicians)16. The key to the success of such strategies is risk stratification by focusing on fibrosis markers, as the amount and pattern of fibrosis are indicators of disease progression9.
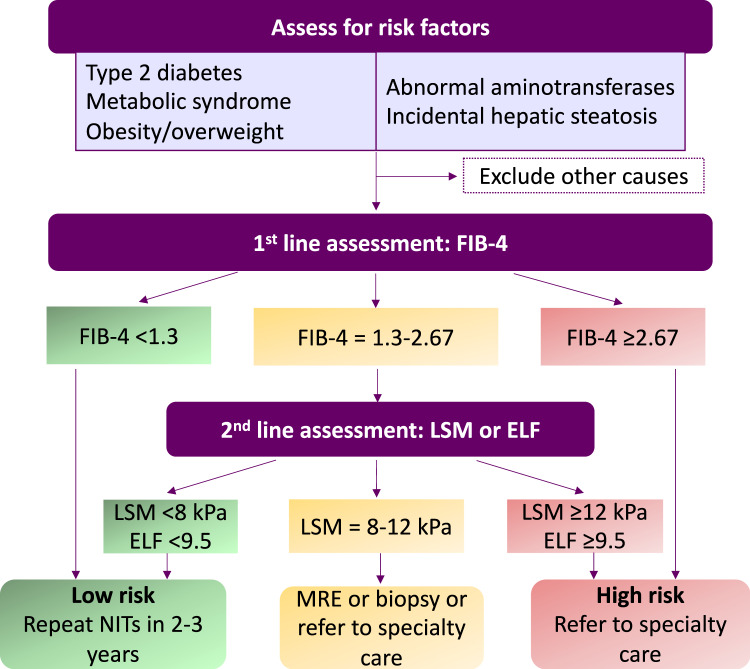
Both the European Association for the Study of the Liver and the American Gastroenterological Association recommend a two-tiered approach to risk stratification in those suspected or incidentally found to have hepatic steatosis on imaging, using sequential NITs (Fig. 2)17,18. Although seemingly straightforward and consistent, the success of clinical pathways depends on the level of awareness and action in primary care and by other non-hepatology physicians, who must recognise risk factors, order liver enzyme tests, calculate and interpret NIT results and refer patients to specialists, when appropriate. Referral according to Fig. 2 will help to identify patients with end-stage liver disease and offer care in the context of clinical trials. Repeated testing over years can help decrease false negative rates in NAFLD which is, in general, slow progressing. Moving into the arena of precision medicine and supported by technological progress, risk prediction in the future will likely abandon indirect surrogate scores and incorporate the increasing knowledge on genetics and epigenetic changes, to benefit patients with advanced liver disease as a result of NAFLD. These caveats underscore the critical importance of developing and disseminating risk stratification approaches between multiple disciplines and providing robust data demonstrating improvement in patient outcomes in real-world settings.
Fig. 2. Sequential risk stratification of patients with hepatic steatosis or metabolic risk factors.
The sequential use of NITs aiming at the identification of patients with advanced liver disease, histologically defined as stages F2 and F3, allows in a first step to rule-out cases with advanced disease using the FIB-4 score. The presence of metabolic risk factors informs about the at-risk population. This will be crucial to enable the assignation of resources to the group with the highest risk of a detrimental outcome. Patients in the indeterminate category will need to be further evaluated by using a more refined second line test, including LSM or ELF. Patients in the high-risk category should be referred to specialists for further management. Based on the resources and availability of NITs, one-step testing strategies could be used in the future. ELF Enhanced Liver Fibrosis, LSM liver stiffness measurement, MRE magnetic resonance elastography, NIT non-invasive test.
NAFLD management in different settings
As NAFLD is a chronic multisystem disease, its prevention, detection and management require a multidisciplinary approach which should include primary care, hepatology, nutritionists, endocrinology, obesity medicine, bariatric surgery/endoscopy, psychology and exercise physiology19 (Box 1). More studies are needed, but a multidisciplinary metabolic hepatology clinic approach has already proven to be effective, as patients with NAFLD experienced reductions in liver enzyme and stiffness levels and cardio-metabolic parameters like cholesterol, glucose and weight20. At present, lifestyle interventions are the mainstay of available treatment. Comprehensive MoCs need to incorporate such interventions and integrate and define the roles of primary, secondary and tertiary care throughout the patient’s prevention, detection and management journey7.
With a focus on holistic, comprehensive care and secondary prevention, primary care should be ideally placed to find and risk stratify people with NAFLD, while recognising competing workload priorities, positioning NAFLD in the context of existing chronic disease management in different primary healthcare settings and reimbursement schemes and emphasising the need for integrated, centrally approved frameworks.
NAFLD care in the community needs to be part of a wider metabolic multimorbidity management approach, recognising the key role of the primary care nursing team and allied professionals with expertise in preventive hepatology and providing long-term nutrition and lifestyle interventions9. To drive change and optimise outcomes, ideal MoCs would include peer support and a role for a NAFLD/metabolic nurse specialist, to closely monitor disease management.
A crucial trigger for successful nutritional treatment is the active support of physicians. There is a vital role for healthcare providers as educators in explaining the importance of treating NAFLD (including in the broader context of T2DM, CVD and cancer prevention) and its potential to regress, teaching healthy eating skills, enhancing confidence in the benefits of diet, discussing potential barriers (e.g., life stressors and the obesogenic environment) and co-creating solutions21. Web-based interventions may be more inclusive and evidence indicates that web-based treatment tools are beneficial but dropout rates may be high and these may be more accessible to younger patients22. Further studies that integrate available technology with health applications are required.
Finally, although weight loss through traditional lifestyle modifications has been associated with the resolution of NASH and fibrosis regression23, only a minority of patients can lose and maintain weight loss. Bariatric surgery leads to significant weight loss and resolution of NASH in the majority of patients and, more importantly, has been shown to reduce both major adverse cardiac events and liver outcomes24. Non-surgical options include anti-obesity medications (AOMs), to support patients with NAFLD achieve and sustain their weight loss goals. Oral AOMs have low adherence rates, insufficient data on the histologic improvement of NASH/fibrosis and inconclusive data on their effects on the liver25, making them less than ideal for achieving the desired weight loss in fibrotic NASH. Emerging evidence indicates that GLP-1 receptor agonists26 can improve steatohepatitis, but it will remain to be determined if any compound meets the regulatory barrier of conditional approval. Whether through lifestyle, pharmaceuticals, surgery or a combination of these, weight management strategies should involve multidisciplinary input and be tailored to each patient.
Box 1 Recommendations to enable and optimise a multidisciplinary approach in NAFLD care.
Evaluate the patient benefit and cost-effectiveness of multidisciplinary models of care based on non-invasive tests, which should be adopted by first-line providers, such as primary care physicians and endocrinologists, and monitor their use.
Implement context-specific (prevention, screening, risk-stratification, treatment) and resource-specific (for low-, middle- and high-income settings) models of care and always report their effectiveness.
Develop, provide and monitor clear guidance, endorsed by the relevant professional societies, regarding preventive hepatology and specific criteria for referral to specialty care such as hepatology, cardiology, endocrinology, obesity specialists, bariatric surgeons and exercise physiologists.
Delivery models must overcome the time-constraints of primary care physicians and other doctors by decreasing the human burden (by using e.g., automated methods of screening and risk-stratification, multidisciplinary ‘metabolic’ clinics or training, virtual care, nurse coordinators across disciplines, web-based applications for longitudinal care, virtual assistants).
Generate evidence to provide policy-makers the basis to recognise NAFLD as significant public health threat and allocate the appropriate resources to address it.
The World Health Organization should integrate all types of steatotic liver disease into non-communicable disease technical guidance, action plans and strategies, setting out how to best implement a multidisciplinary approach.
NAFLD non-alcoholic fatty liver disease.
Conclusions
Steatotic liver disease is a complex, multisystem disease that requires a multidisciplinary approach to prevention, diagnosis, treatment and care. Such an approach must be bottom-up, with key actors in the health system collaborating and developing efficient MoCs, and top-down, guided by national and global strategies. While no NAFLD- or NASH-specific treatment exists, structured lifestyle interventions and medications for related conditions can have positive effects, and many different stakeholders can and should play a role in improving health outcomes in patients with fatty liver disease.
Acknowledgements
The authors acknowledge the role of the steering committee of the INC BCN 2022 workshop as well as speakers at the event, including (in alphabetical order), Rachel Batterham, Louise Campbell, Javier Crespo, Donna Cryer, Fernando García Pérez, Roger Green, Stephen Harrison, Jean Muris, Marcus Ranney, Emmanuel Tsochatzis and José Willemse.
Author contributions
J.M.S., A.M.A., H.J., S.Z.-S., K.C., J.F.D., C.C., S.M.F., Z.Y., N.A. and J.V.L. were all involved in the writing, editing and approval of the final version of the manuscript, including the authorship list.
Competing interests
J.M.S. reports consultancy for Apollo Endosurgery, Albireo Pharma Inc, Bayer, BMS, Boehringer Ingelheim, Echosens, Genfit, Gilead Sciences, GSK, Heel GmbH, Intercept Pharmaceuticals, Ipsen, Inventiva Pharma, Julius Clinical, Madrigal, MSD, Nordic Bioscience, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, Shinogi, Siemens Healthcare GmbH and Summit Clinical Research; research funding from Gilead Sciences, Boehringer Ingelheim, Nordic Bioscience and Siemens Healthcare GmbH; and speaker honorarium from MedPublico GmbH and Boehringer Ingelheim, all outside of the submitted work. A.M.A. has received research support from Novo Nordisk, Pfizer and Target Pharma and is a consultant for Novo Nordisk and Pfizer, all outside of the submitted work. K.C. has received research support towards the University of Florida as principal investigator from Echosens, Inventiva, Novo Nordisk, Poxel, Labcorp and Zydus and is a consultant for Arrowhead, AstraZeneca, 89Bio, BMS, Lilly, Madrigal, Novo Nordisk, Quest, Sagimet, Sonic Incytes and Terns, all outside of the submitted work. J.F.D. reports grants to his institution from AbbVie, Gilead Sciences and MSD and personal fees from AbbVie, CEPHEID, Gilead Sciences, Intercept, Janssen, Intelligent Ultrasound ltd and MSD, all outside of the submitted work. C.C. received consultant fees from Gilead, NovoNordisk, AstraZeneca, Bayer and Lilly and received grant support from Gilead, all outside of the submitted work. S.M.F. holds a senior clinical investigator fellowship from the Research Foundation Flanders (FWO) (1802154N); his institution has received grants from Astellas, Falk Pharma, Genfit, Gilead Sciences, GlympsBio, Janssens Pharmaceutica, Inventiva, Merck Sharp & Dome, Pfizer and Roche, all outside of the submitted work. S.M.F. has acted as consultant for AbbVie, Actelion, Aelin Therapeutics, AgomAb, Aligos Therapeutics, Allergan, Astellas, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristoll-Meyers Squibb, CSL Behring, Coherus, Echosens, Eisai, Enyo, Galapagos, Galmed, Genetech, Genfit, Gilead Sciences, Intercept, Inventiva, Janssens Pharmaceutica, Julius Clinical, Madrigal, Medimmune, Merck Sharp & Dome, NGM Bio, Novartis, Novo Nordisk, Promethera and Roche; he has also been lecturer for AbbVie, Allergan, Bayer, Eisai, Genfit, Gilead Sciences, Janssens Cilag, Intercept, Inventiva, Merck Sharp & Dome, Novo Nordisk and Promethera, all outside of the submitted work. N.A. has been on the advisory board for Gilead and Allergan; he is on the speaker’s bureau for Intercept and Gilead, all outside of the submitted work. J.V.L. reports grants to his institution from AbbVie, Gilead Sciences and MSD and personal fees from AbbVie, CEPHEID, Gilead Sciences, GSK, Genfit, Intercept, Janssen, MSD and ViiV, all outside of the submitted work. All other authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Riazi K, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet. Gastroenterol. Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 2.Schattenberg JM, et al. Disease burden and economic impact of diagnosed non-alcoholic steatohepatitis in five European countries in 2018: a cost-of-illness analysis. Liver. Int. 2021;41:1227–1242. doi: 10.1111/liv.14825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalasani N, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 4.Polyzos SA, Kechagias S, Tsochatzis EA. Review article: non-alcoholic fatty liver disease and cardiovascular diseases: associations and treatment considerations. Aliment. Pharmacol. Ther. 2021;54:1013–1025. doi: 10.1111/apt.16575. [DOI] [PubMed] [Google Scholar]
- 5.Loomba R, et al. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: a real world analysis of Medicare data. Aliment. Pharmacol. Ther. 2020;51:1149–1159. doi: 10.1111/apt.15679. [DOI] [PubMed] [Google Scholar]
- 6.Wong VW-S, Adams LA, de Lédinghen V, Wong GL-H, Sookoian S. Noninvasive biomarkers in NAFLD and NASH — current progress and future promise. Nat. Rev. Gastroenterol. Hepatol. 2018;15:461–478. doi: 10.1038/s41575-018-0014-9. [DOI] [PubMed] [Google Scholar]
- 7.Lazarus JV, et al. Defining comprehensive models of care for NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2021;18:717–729. doi: 10.1038/s41575-021-00477-7. [DOI] [PubMed] [Google Scholar]
- 8.Lazarus JV, et al. The global NAFLD policy review and preparedness index: are countries ready to address this silent public health challenge? J. Hepatol. 2022;76:771–780. doi: 10.1016/j.jhep.2021.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Francque SM, et al. Non-alcoholic fatty liver disease: a patient guideline. JHEP. Rep. 2021;3:100322. doi: 10.1016/j.jhepr.2021.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lazarus JV, et al. Advancing the global public health agenda for NAFLD: a consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022;19:60–78. doi: 10.1038/s41575-021-00523-4. [DOI] [PubMed] [Google Scholar]
- 11.Li L, et al. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes. Rev. 2016;17:510–519. doi: 10.1111/obr.12407. [DOI] [PubMed] [Google Scholar]
- 12.Mantovani A, et al. Non-alcoholic fatty liver disease and risk of incident diabetes mellitus: an updated meta-analysis of 501 022 adult individuals. Gut. 2021;70:962–969. doi: 10.1136/gutjnl-2020-322572. [DOI] [PubMed] [Google Scholar]
- 13.Younossi ZM, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J. Hepatol. 2019;71:793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 14.Cusi K. Treatment of patients with type 2 diabetes and non-alcoholic fatty liver disease: current approaches and future directions. Diabetologia. 2016;59:1112–1120. doi: 10.1007/s00125-016-3952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Francque SM, van der Graaff D, Kwanten WJ. Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J. Hepatol. 2016;65:425–443. doi: 10.1016/j.jhep.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Dillon JF, et al. Intelligent liver function testing (iLFT): a trial of automated diagnosis and staging of liver disease in primary care. J. Hepatol. 2019;71:699–706. doi: 10.1016/j.jhep.2019.05.033. [DOI] [PubMed] [Google Scholar]
- 17.Kanwal F, et al. Clinical care pathway for the risk stratification and management of patients with nonalcoholic fatty liver disease. Gastroenterology. 2021;161:1657–1669. doi: 10.1053/j.gastro.2021.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis − 2021 update. J. Hepatol. 2021;75:659–689. doi: 10.1016/j.jhep.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet. Gastroenterol. Hepatol. 2021;6:578–588. doi: 10.1016/S2468-1253(21)00020-0. [DOI] [PubMed] [Google Scholar]
- 20.Moolla A, et al. A multidisciplinary approach to the management of NAFLD is associated with improvement in markers of liver and cardio-metabolic health. Frontline. Gastroenterol. 2019;10:337–346. doi: 10.1136/flgastro-2018-101155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haigh L, et al. Barriers and facilitators to mediterranean diet adoption by patients with nonalcoholic fatty liver disease in Northern Europe. Clin. Gastroenterol. Hepatol. 2019;17:1364–1371. doi: 10.1016/j.cgh.2018.10.044. [DOI] [PubMed] [Google Scholar]
- 22.Mazzotti A, et al. An internet-based approach for lifestyle changes in patients with NAFLD: two-year effects on weight loss and surrogate markers. J Hepatol. 2018;69:1155–1163. doi: 10.1016/j.jhep.2018.07.013. [DOI] [PubMed] [Google Scholar]
- 23.Vilar-Gomez E, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149:367–378. doi: 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Aminian A, et al. Association of bariatric surgery with major adverse liver and cardiovascular outcomes in patients with biopsy-proven nonalcoholic steatohepatitis. JAMA. 2021;326:2031–2042. doi: 10.1001/jama.2021.19569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan CS, Stanley TL. Effect of weight loss medications on hepatic steatosis and steatohepatitis: a systematic review. Front. Endocrinol. 2020;11:70. doi: 10.3389/fendo.2020.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubino DM, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327:138–150. doi: 10.1001/jama.2021.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]