Summary
Background
No real-world randomised controlled trials (RCTs) have explored the effectiveness of lifestyle interventions based on multiple behaviour change theories and using combined digital and group-based face-to-face delivery to improve risk factors for type 2 diabetes (T2D).
Methods
We conducted a one-year, multi-centre, unblinded, pragmatic RCT in primary healthcare using the habit formation, self-determination, and self-regulation theories among 2907 adults aged 18–74 years at increased T2D risk randomised into a digital lifestyle intervention group (DIGI, n = 967), a combined digital and group-based lifestyle intervention group (DIGI+GROUP, n = 971), and a control group receiving usual care (CONTROL, n = 969). We collected data on primary outcomes (diet quality by Healthy Diet Index [HDI], physical activity, body weight, fasting plasma glucose, 2-hour plasma glucose) and secondary outcomes (sedentary time, waist circumference, fasting plasma insulin) using digital questionnaires, clinical examinations, fasting blood tests, and 2-hour oral glucose tolerance tests. Main statistical analyses were performed using linear mixed-effects models adjusted for age, sex, and province. This RCT was registered with ClinicalTrials.gov, NCT03156478.
Findings
The 2907 participants assigned were recruited between March 1st, 2017, and February 28th, 2018. Diet quality improved more (3·2 vs. 1·4 HDI points, p<0·001 for difference between groups, p’<0·001 for group*time interaction) and waist circumference tended to decrease more (−1·8 vs. −1·3 cm, p = 0·028, p’ = 0·068) in DIGI+GROUP than in CONTROL. Fasting insulin tended to increase in CONTROL but not in DIGI (1·0 vs. 0·0 mU/L, p = 0·033, p’ = 0·054) or in DIGI+GROUP (1·0 vs. 0·5 mU/L, p = 0·042, p’ = 0·054). Good adherence to DIGI and DIGI+GROUP (≥median of 501 habits/year in DIGI, ≥5 of all 6 sessions in GROUP) was associated with improved diet quality and good adherence to DIGI with increased physical activity and decreased sedentary time.
Interpretation
A lifestyle intervention based on multiple behaviour change theories and combined digital and group-based face-to-face delivery improves diet quality and tends to decrease abdominal adiposity and prevent an increase in insulin resistance. Good adherence improves the results of the interventions.
Funding
Strategic Research Council at Academy of Finland, Academy of Finland, Novo Nordisk Foundation, and Finnish Diabetes Research foundation.
Keywords: Type 2 diabetes, Lifestyle intervention, Prevention, Randomised controlled trial, eHealth, Digital, Behaviour change, Diet, Nutrition, Physical activity, Adiposity, Insulin resistance, Primary healthcare, Habit formation, Self-determination theory
Research in context.
Evidence before this study
We searched PubMed for systematic reviews and meta-analyses of lifestyle interventions as well as original articles in English on mobile and Internet-based interventions to prevent type 2 diabetes (T2D) implemented in a health care setting until April 2020. Interventions in clinical and real-world settings have proven that lifestyle modification programmes based on face-to-face counselling either individually or in groups can be effective in the prevention of T2D. Some evidence also suggests that digital interventions can help in lifestyle modification and weight reduction, but not whether they are effective alone or in combination with group counselling in the prevention of T2D.
Added value of this study
The StopDia study is the first large-scale randomised controlled trial (RCT) that is conducted in primary healthcare as part of its routine practices and that is focused on the effectiveness of digital and group-based face-to-face lifestyle interventions based on habit formation, self-determination and self-regulation theories in adults at increased risk of T2D. The results of our study suggest that a lifestyle intervention based on multiple behaviour change theories and combined digital and group-based face-to-face delivery improves diet quality and tends to decrease abdominal adiposity and prevent an increase in insulin resistance. The outcome appears to depend on adherence to these interventions, emphasising the importance of participant engagement.
Implications of all the available evidence
Given the increasing human and economic burden of T2D and other non-communicable diseases, evidence on the added value of low-cost and scalable digital lifestyle interventions combined with group-based lifestyle interventions in real-world conditions is needed. Our findings emphasise that a lifestyle intervention based on multiple behaviour change theories and using combined digital and group-based face-to-face delivery is effective in improving health behaviour, particularly diet quality, to improve risk factors for T2D. The variable participation in the digital and group-based lifestyle interventions warrant further RCTs in which adapted intervention models optimising the intervention type based on individuals’ characteristics and early adherence will be considered.
Alt-text: Unlabelled box
Introduction
Previous randomised controlled trials (RCTs) have shown the efficacy of lifestyle interventions in the prevention of type 2 diabetes (T2D) among individuals at increased risk1, 2, 3 and the sustainability of the beneficial effects for several years after the discontinuation of the interventions.4,5 These interventions aimed at decreasing the risk of T2D have mainly applied face-to-face lifestyle counselling either individually or in groups. The efficacy of such interventions has been shown to be associated with the number of lifestyle goals achieved,4 which emphasizes the importance of beneficial lifestyle changes in the prevention of T2D.
Lifestyle interventions implemented in real-world conditions, using group-based face-to-face counselling, and delivered by health care professionals have been found to decrease the risk of T2D as compared with usual care in individuals at increased risk, although the effects of these interventions on weight reduction have been small.6 Adherence to these lifestyle interventions appears to be crucial in decreasing body weight and the risk of T2D.6 Digital lifestyle interventions conducted in real-world conditions, such as in healthcare or work places, have been shown to improve diet quality, increase physical activity,7 decrease body weight,7, 8, 9 and improve glucose metabolism8,9 in people at increased risk of T2D. However, little is known about the role of variable adherence on the effects of digital interventions on risk factors for T2D at individuals at increased risk.
In addition to the contents and delivery methods of lifestyle interventions, strategies based on behaviour change theories may also influence the effectiveness of these interventions. Empirical evidence supports a self-determination theoretical approach in promoting autonomous motivation and perceived competence10 and a self-regulation theoretical approach in promoting self-monitoring, goal setting, and action planning in lifestyle modification.11 However, these strategies focus on adoption of novel behaviours, while maintenance of these behaviours as daily routines through habit formation has received less attention.12 Habits are central in reaching sustainable lifestyle changes as they are performed relatively automatically with little conscious consideration.12 Habit formation techniques guide selection of specific simple, contextualized, and frequent behaviours that promote repetition. So far, a minority of digital behaviour change interventions have utilized habit formation techniques.12,13
There are no large-scale RCTs carried out in real-world conditions comparing the effects of digital lifestyle interventions alone and combined with group-based lifestyle interventions that would have been founded on behaviour change theories on various risk factors of T2D in people at increased risk. We therefore conducted a large one-year RCT in primary healthcare as part of its routine practices to compare the effects of a digital intervention and a combined digital and group-based intervention - each founded on habit formation, self-determination, and/or self-regulation theories - against usual care on measures of diet quality, physical activity, sedentary time, anthropometrics, insulin resistance, fasting glycaemia, and glucose tolerance in adults at increased risk of T2D. We also investigated whether adherence to the digital and group-based lifestyle interventions modified these outcomes.
Methods
Study design
As part of the Stop Diabetes (StopDia) study,14 we carried out a one-year, parallel-group, unblinded, multicentre RCT on the effects of a digital lifestyle intervention and a combined digital and group-based lifestyle intervention on diet quality, physical activity, sedentary time, overall adiposity, abdominal adiposity, insulin resistance, and glucose metabolism as compared with usual care in adults at increased risk of T2D in primary healthcare as part of its routine practices. The StopDia study was approved by the Research Ethics Committee of the Hospital District of North Savo (Statement 467/2016) and was carried out according to the principles of the Declaration of Helsinki as revised in 2008 and the guidelines for responsible conduct of research by the Finnish Advisory Board on Research Integrity. There were no protocol deviations or modifications in the protocols and methods after the ethical approval and during the study.
Participants
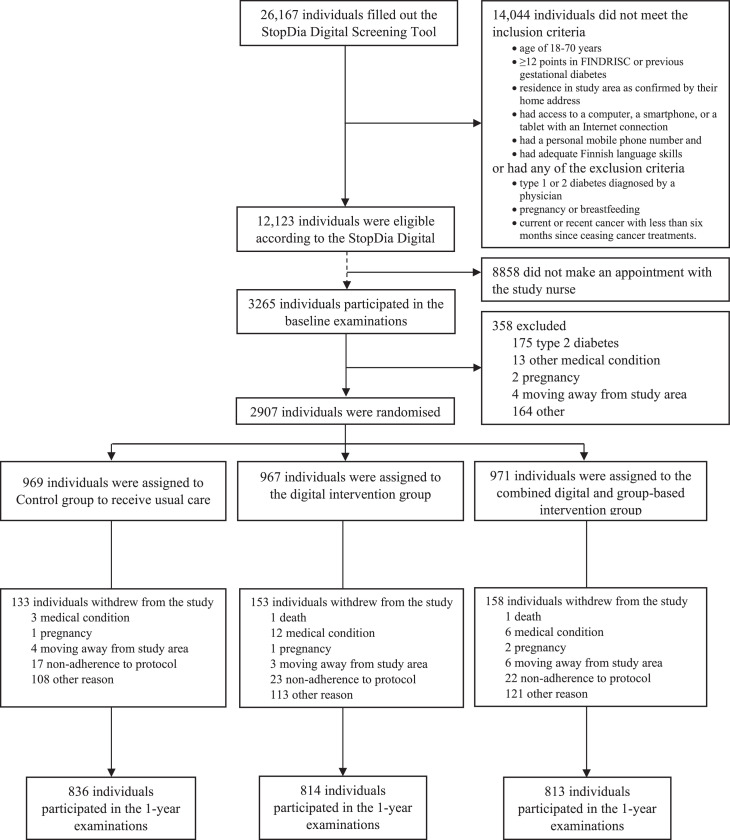
Adults aged 18-74 with a Finnish Diabetes Risk Score (FINDRISC) at least 12 indicating a moderate risk of developing T2D representing a one in six chance of developing T2D within the next 10 years were recruited through multiple channels from three provinces in Finland and screened for eligibility using the anonymous StopDia Digital Screening Tool between March 1st, 2017, and February 28th, 2018, as described in detail earlier.14,15 The inclusion and exclusion criteria are listed in the flow chart (Figure 1). The individuals deemed eligible were invited to participate in the RCT and were provided with instructions on how to book an appointment with the designated nurse in a local healthcare centre for the verification of the inclusion and exclusion criteria and for clinical measurements, including a 2-hour oral glucose tolerance test (OGTT) to rule out prevalent, unknown diabetes. All participants gave their written informed consent at the first study visit.
Figure 1.
Flow chart of the StopDia study.
Randomisation and masking
The participants who met the inclusion criteria and had no exclusion criteria, had filled out the StopDia Digital Questionnaire using a digital Lime Survey platform (Lime Survey GmbH, Hamburg, Germany), had given blood samples in a local laboratory, and had no diabetes according to the results of the 2-hour OGTT were randomly assigned to a digital intervention group (DIGI), a combined digital and group-based face-to-face intervention group (DIGI+GROUP), or a control group (CONTROL) with 1:1:1 allocation. The randomization was performed using a computerized randomisation system by specially trained nurses working in the local healthcare centres. The participants, the nurses who used the computerized randomisation system and performed the clinical assessments, the healthcare professionals who carried out the face-to-face group counselling, or the researchers who performed the statistical analyses were not masked to the group assignment. After randomisation to the study groups, all participants were sent an electronic information letter by email and short message service. The participants in DIGI and DIGI+GROUP were given a description of the contents of the interventions and instructions on how to attend. The participants in CONTROL were given information about lifestyle-related risk factors for T2D and recommendations on a healthy diet and physical activity only once at baseline.
Lifestyle interventions
The lifestyle interventions were carried out between March 1st, 2017, and February 28th, 2019. The lifestyle targets of the interventions were diet quality, physical activity, sedentary time, body weight, sleep, smoking, and alcohol consumption, as described in detail earlier.14 The digital and group-based lifestyle interventions used a self-determination theory approach that supports autonomous motivation and perceived competence10 through emphasis on participants’ freedom of choice in lifestyle change as well as on their existing knowledge, skills and healthy habits as a basis for change. The digital lifestyle intervention that was based on using the BitHabit web app was specifically built on the habit formation theory to promote maintenance of behaviour change by repetition and eventual habit automaticity.12 The group-based lifestyle intervention utilised self-regulation theory to guide in self-monitoring, goal setting, and planning related to lifestyle behaviours.11 Detailed descriptions of both interventions with program contents, behaviour change techniques, and technological functionalities are included in Pihlajamäki et al. 2019.14
The participants in DIGI and DIGI+GROUP got access to the BitHabit web app via a link sent by email and short message service and were instructed to use it throughout the 1-year intervention period. The app provided an extensive evidence-based habit library developed by translating lifestyle guidelines and recommendations into simple habit-forming suggestions of health behaviours that could easily be adopted into daily life.13 The library consisted of 489 behavioural suggestions divided into 13 lifestyle categories, including meal frequency, vegetables, dietary fat, grain products, sugar, alcohol and other drinks, conditioning physical activity, everyday physical activity, sedentary behaviour, sleep, stress management, positive mood, and non-smoking. The app use was possible with all smart devices and did not require installing a separate app. The main functionalities of the app were 1) browsing behavioural suggestions and selecting those that the users wanted to perform, 2) daily self-monitoring of the selected behaviours, and 3) getting summary feedback for habit formation in each of the 13 lifestyle categories. The participants in DIGI+GROUP received a six-session group coaching programme over the first six months of the one-year intervention period. The group sessions were delivered by trained nurses, dietitians, exercise specialists, and other healthcare professionals and were organised in local healthcare centres in groups of 6–15 individuals. Each session lasted for two hours and included 90 minutes of organised activity and 30 minutes of optional activity. All six sessions had their specific topics, including 1) “Orientation to the StopDia group coaching”, 2) “Rhythm of daily life”, 3) “Let's eat well and healthy”, 4) “Enjoying physical activity”, 5) “Automating activity to everyday life”, and 6) “Succeeding in lifestyle management, also after the StopDia study”. The contents of the sessions have been explained in detail earlier.14 Between the face-to-face group counselling sessions, lifestyle changes were supported by homework materials.
Control group
The participants in CONTROL received a digital information package on lifestyle risk factors for T2D and on dietary and physical activity recommendations to decrease the risk of T2D only once at baseline. They were informed that they will have the opportunity to use the BitHabit web app after one year. The participants in CONTROL received standard healthcare as did the participants in DIGI and DIGI+GROUP.
Assessments
At baseline and at one year, the participants completed the StopDia Digital Questionnaire, including validated questions on food consumption16 and questions on physical activity and sedentary time modified from those used in previous studies.14 The study nurse also measured their body weight, body height, and waist circumference, and they underwent blood sampling and the 2-hour OGTT in the local laboratories. Impaired fasting glucose and impaired glucose tolerance were defined according to the criteria of the American Diabetes Association.17 The assessments of primary and secondary outcomes and other variables at baseline and at one-year visits have been described in detail earlier14 and in supplement.
Outcomes
The primary outcomes were one-year changes in diet quality assessed by the Healthy Diet Index (HDI)18 total physical activity, body weight, fasting plasma glucose, and 2-hour plasma glucose from the 2-hour OGTT. The HDI gives an estimate of the adherence to a healthy diet according to the Nordic and Finnish nutrition recommendations, with emphasis on dietary factors associated with the risk of T2D. The HDI is based on a short food frequency questionnaire16 and comprises of following seven domains, which are weighted depending on their importance in a diet to prevent T2D: meal pattern (score range 0–10), grains (0–20), fruit and vegetables (0–20), fats (0–15), fish and meat (0–10), dairy (0–10), and snacks and treats including beverages (0–15). The HDI total score ranges from 0 (lowest quality) to 100 (highest quality). The secondary outcomes were one-year changes in the HDI domains, total sedentary behaviour, waist circumference, fasting plasma insulin, and blood glycated haemoglobin (HbA1c). The nurses of the local healthcare centres followed the condition of the participants for the assessment of safety and adverse events that were unlikely due to the nature of the lifestyle interventions.
Statistical analyses
The sample size calculations have been explained in detail earlier14 and in supplement. We performed all statistical analyses using the IBM SPSS Statistics® software, Version 27·0 (IBM Corp., Armonk, NY, USA). A p-value of <0·05 for a 2-tailed test was used to indicate statistical significance. The normality of the distributions of the outcome variables were evaluated based on visual observation of the histograms. We compared baseline characteristics between DIGI, DIGI+GROUP, and CONTROL by the Analysis of Variance for continuous variables with normal distributions, by the Kruskal-Wallis test for continuous variables with skewed distributions, and by the Chi-square test for categorical variables.
We studied the effects of the interventions on the outcomes using the intention-to-treat principle by including all 2907 participants in the statistical analyses. We analysed the data using linear mixed-effects models according to a 2-level data structure by clustering the repeated outcome variables at baseline and at one year within participants who were considered as subjects in the mixed model structure. We adjusted the data for age, sex, and study province at baseline and included main effects for time and for study group × time interaction in the models. The linear mixed-effects model analyses have been explained in more detail in supplement.
To analyse whether participation in the interventions affected the changes in the outcomes, we divided the participants in DIGI and DIGI+GROUP into those who had higher engagement in the digital intervention (≥median of 501 habits/year, higher 50%) and those with lower engagement (<501 habits/year, lower 50%) and the participants in DIGI+GROUP into those with higher attendance in the group-based intervention (≥5 of all 6 sessions) and those with lower attendance (<5 of all 6 sessions). We performed these linear mixed-effects models similarly to the main analyses, except that the level of engagement in the digital intervention and the level of attendance in the group-based intervention were used instead of the study group variable. This trial was registered with ClinicalTrials.gov, NCT03156478.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit the article for publication.
Results
A total of 26,167 people were screened for eligibility, and 12,123 adults at increased risk of T2D were invited to book an appointment with the study nurse (Figure 1). Altogether 3265 adults (27% of the eligible) attended the baseline examinations. Of them, 358 were excluded at baseline (Figure 1). Of these 358 individuals 178 who were diagnosed with T2D at baseline by elevated fasting plasma glucose (≥7·0 mmol/L) or 2-hour plasma glucose (≥11·1 mmol/L at 2-hour OGTT), were excluded from the RCT, and were directed to diabetes care in local healthcare centres. Finally, 2907 adults at increased T2D risk were randomly allocated to CONTROL (n = 969), DIGI (n = 967), or DIGI+GROUP (n = 971).
Of the participants, 2326 (80%) were women, 581 (20%) were men, and 2879 (99%) were born in Finland. The age ranged between 18 and 74 years with a mean (standard deviation, SD) of 55·1 (10·0) years. The mean (SD) of BMI was 31·1 kg/m2 (5·4), and 349 (12%) of the participants were normal weight, 986 (34%) overweight, and 1571 (54%) obese. Of the participants, 1283 (44%) had normal glucose metabolism, 1040 (36%) isolated impaired fasting glucose (IFG), 161 (6%) isolated impaired glucose tolerance (IGT), and 407 (14%) had both IFG and IGT. The baseline characteristics of participants in the study groups are presented in Table 1. The proportions of dropouts were not different between the study groups (14% in CONTROL, 16% in DIGI, 16% in DIGI+GROUP, p = 0·251).
Table 1.
Baseline characteristics of the participants.
| CONTROL (n = 969) | DIGI (n = 967) | DIGI+GROUP (n = 971) | |
|---|---|---|---|
| Sex (women) | 785 (81%) | 757 (78%) | 784 (81%) |
| Age (y) | 55·0 (9·9) | 55·1 (9·9) | 55·2 (10·1) |
| Study province | |||
| North Savo | 290 (30%) | 290 (30%) | 291 (30%) |
| South Karelia | 279 (29%) | 278 (29%) | 280 (29%) |
| Päijät-Häme | 400 (41%) | 399 (41%) | 400 (41%) |
| Native country | |||
| Finland | 960 (99%) | 958 (99%) | 961 (99%) |
| Other | 9 (1%) | 9 (1%) | 10 (1%) |
| Education level | |||
| Primary, comprehensive, and middle schools | 69 (7%) | 63 (6%) | 85 (9%) |
| Upper secondary and vocational schools | 267 (28%) | 276 (29%) | 253 (26%) |
| Tertiary school including institute, bachelor's, and master's degrees | 633 (65%) | 628 (65%) | 633 (65%) |
| Smoking status | |||
| Never smoker | 510 (53%) | 544 (56%) | 531 (55%) |
| Former smoker | 391 (40%) | 355 (37%) | 368 (38%) |
| Current smoker | 68 (7%) | 68 (7%) | 72 (7%) |
| Body mass index (kg/m2) | 31·3 (5·5) | 31·0 (5·4) | 30·9 (5·4) |
| <25·0 | 105 (11%) | 119 (12%) | 125 (13%) |
| 25·0–29·9 | 326 (34%) | 335 (35%) | 325 (33%) |
| ≥30·0 | 538 (55%) | 512 (53%) | 521 (54%) |
| Medication for hypertension | |||
| No | 647 (67%) | 628 (65%) | 656 (68%) |
| Yes | 322 (33%) | 339 (35%) | 315 (32%) |
| Medication for hypercholesterolaemia | |||
| No | 803 (83%) | 800 (83%) | 816 (84%) |
| Yes | 166 (17%) | 167 (17%) | 155 (16%) |
| Family history of type 2 diabetes | |||
| No | 211 (22%) | 207 (21%) | 205 (21%) |
| Yes | 758 (78%) | 760 (79%) | 766 (79%) |
| History of gestational diabetes | |||
| No | 843 (87·0%) | 841 (87·1%) | 830 (85·6%) |
| Yes | 126 (13·0%) | 125 (12·9%) | 140 (14·4%) |
| Glucose metabolism status | |||
| Normal | 418 (44%) | 443 (46%) | 422 (44%) |
| Isolated IFG | 359 (37%) | 338 (35%) | 343 (35%) |
| Isolated IGT | 50 (5%) | 54 (6%) | 57 (6%) |
| Combined IFG and IGT | 134 (14%) | 128 (13%) | 145 (15%) |
| FINDRISC (range 0–26) | 15·8 (3·6) | 15·7 (3·5) | 15·7 (3·6) |
The data are frequencies (percentages) from the Chi-square test for categorical variables or means (standard deviations) from the Analysis of Variance for continuous variables. The categories of glucose metabolism status are based on the American Diabetes Association classification.17
IFG, impaired fasting glucose; IGT, impaired glucose tolerance; FINDRISC, Finnish Diabetes Risk Score.
Median (interquartile range, IQR) for the total number of reported habits performed over the 1-year intervention period assessed by the app was 382 (48-1205) in DIGI and 396 (60-1110) in DIGI+GROUP. In DIGI+GROUP among participants who answered the question on attendance in the group-based intervention after one year, 28% reported participating in all six meetings, 21% in five meetings, 12% in four meetings, 6% in three meetings, 2% in two meetings, and 2% in one meeting.
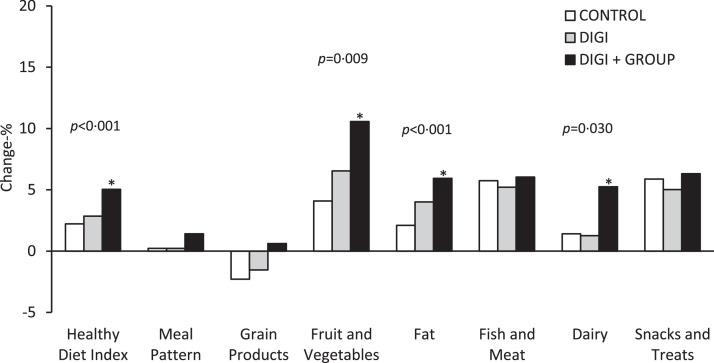
The HDI improved on average more over one year in DIGI+GROUP compared with CONTROL (Table 2: +3·2 vs +1·4 points, p<0·001). There was also an interaction effect between intervention and time (p<0·001), indicating the overall intervention effect on the improved HDI with time. There was no difference in the mean change of total physical activity or total sedentary time across the study groups (Table 2). Of the components of the HDI, the consumption of fruit and vegetables (Figure 2, Table S1: +1·1 vs +0·4 points, p = 0·009) increased on average more and the quality of dietary fat (Figure 2, Table S1: +0·5 vs +0·2 points, p<0·001) and dairy products (Figure 2, Table S1: +0·3 vs +0·1 points, p = 0·030) improved on average more in DIGI+GROUP than in CONTROL.
Table 2.
Effects of the digital intervention (DIGI) and the combined digital and group-based face-to-face intervention (DIGI+GROUP) on the measures of lifestyle factors, anthropometry, and glucose metabolism compared with usual care with no study intervention (CONTROL).
| n | One-year mean change | Regression coefficient β (95% CI) | p-valuea | p- valueb | |
|---|---|---|---|---|---|
| Lifestyle factors | |||||
| Healthy Diet Index (score) | <0·001 | ||||
| CONTROL | 749 | 1·40 | (ref) | ·· | |
| DIGI | 720 | 1·79 | 0·40 (−0·29 to 1·09) | 0·255 | |
| DIGI+GROUP | 736 | 3·17 | 1·63 (0·95 to 2·31) | <0·001 | |
| Total physical activity (h/wk) | 0·194 | ||||
| CONTROL | 725 | 0·34 | (ref) | ·· | |
| DIGI | 693 | 0·77 | 0·48 (−0·25 to 1·20) | 0·196 | |
| DIGI+GROUP | 695 | 1·24 | 0·64 (−0·08 to 1·37) | 0·082 | |
| Total sedentary time (h/d) | 0·555 | ||||
| CONTROL | 770 | −0·25 | (ref) | ·· | |
| DIGI | 745 | −0·16 | 0·04 (−0·11 to 0·18) | 0·609 | |
| DIGI+GROUP | 755 | −0·32 | −0·04 (−0·18 to 0·10) | 0·561 | |
| Anthropometry | |||||
| Body weight (kg) | 0·160 | ||||
| CONTROL | 834 | 0·03 | (ref) | ·· | |
| DIGI | 810 | −0·14 | −0·20 (−0·56 to 0·17) | 0·287 | |
| DIGI+GROUP | 812 | −0·27 | −0·35 (−0·72 to 0·01) | 0·056 | |
| Body mass index (kg/m2) | 0·177 | ||||
| CONTROL | 834 | 0·01 | (ref) | ·· | |
| DIGI | 810 | −0·05 | −0·06 (−0·20 to 0·07) | 0·334 | |
| DIGI+GROUP | 812 | −0·10 | −0·12 (−0·26 to 0·01) | 0·063 | |
| Waist circumference (cm) | 0·068 | ||||
| CONTROL | 829 | −1·32 | (ref) | ·· | |
| DIGI | 808 | −1·37 | −0·10 (−0·56 to 0·36) | 0·665 | |
| DIGI+GROUP | 809 | −1·78 | −0·52 (−0·98 to −0·05) | 0·028 | |
| Glucose metabolism | |||||
| Fasting insulin (mU/L) | 0·054 | ||||
| CONTROL | 774 | 0·99 | (ref) | ·· | |
| DIGI | 759 | −0·01 | −0·89 (−1·70 to −0·07) | 0·033 | |
| DIGI+GROUP | 751 | 0·46 | −0·85 (−1·66 to −0·03) | 0·042 | |
| Fasting glucose (mmol/L) | 0·997 | ||||
| CONTROL | 798 | 0·02 | (ref) | ·· | |
| DIGI | 790 | 0·03 | 0·00 (−0·04 to 0·04) | 0·984 | |
| DIGI+GROUP | 780 | 0·02 | 0·00 (−0·04 to 0·04) | 0·944 | |
| 2-hour glucose (mmol/L) | 0·266 | ||||
| CONTROL | 793 | −0·07 | (ref) | ·· | |
| DIGI | 782 | 0·04 | 0·07 (−0·07 to 0·21) | 0·320 | |
| DIGI+GROUP | 776 | 0·05 | 0·11 (−0·02 to 0·25) | 0·107 | |
| HbA1c (mmol/mol) | 0·131 | ||||
| CONTROL | 798 | 0·91 | (ref) | ·· | |
| DIGI | 779 | 0·81 | −0·07 (−0·32 to 0·17) | 0·562 | |
| DIGI+GROUP | 775 | 0·66 | −0·25 (−0·49 to 0·00) | 0·050 |
The data are unadjusted one-year mean changes in the measures of lifestyle factors, anthropometry, and glucose metabolism in CONTROL, DIGI, and DIGI+GROUP. The regression coefficients β and their 95% confidence intervals for the effects of DIGI and DIGI+GROUP compared with CONTROL are also shown. The p-values are obtained from linear mixed-effects models adjusted for age, sex, study province at baseline, and time between assessments (nurse visits, filling out questionnaires, or laboratory visits) and including the study group*time interaction term in these models.
P-values for the statistical significance of the effects of DIGI and DIGI+GROUP on the measures of lifestyle factors, anthropometry, and glucose metabolism compared with CONTROL.
P-values for the statistical significance of the interaction effects between intervention and time (study group*time interactions) on the measures of lifestyle factors, anthropometry, and glucose metabolism.
HbA1c, glycated haemoglobin. The Healthy Diet Index ranges between 0 (lowest quality) and 100 (highest quality).
Figure 2.
Unadjusted one-year mean percentage changes in the Healthy Diet Index and its components in the control group (CONTROL), the digital intervention group (DIGI), and the combined digital and group-based face-to-face intervention group (DIGI+GROUP). The data are from linear mixed-effects models adjusted for age, sex, study province at baseline, and time between assessments (filling out questionnaires) and including the study group*time interaction term in these models. The asterisks denote the statistical significance (p<0·05) of the effects of DIGI and DIGI+GROUP on the Healthy Diet Index and its components compared with CONTROL. The P-values shown are for the statistical significance of the interaction effects between intervention and time (study group*time interactions) on the Healthy Diet Index and its components.
There were no statistically significant differences in the mean changes of body weight, BMI, fasting glucose, or 2-hour glucose across the study groups (Table 2). Waist circumference tended to decrease on average more in DIGI+GROUP than in CONTROL (Table 2: −1·8 vs −1·3 points, p = 0·068), and fasting insulin tended to increase on average in CONTROL but not in DIGI (Table 2: 1·0 vs. 0·0 mU/L, p = 0·054) or in DIGI+GROUP (1·0 vs. 0·5 mU/L, p = 0·054). Sex, age, BMI, impaired fasting glucose, or impaired glucose tolerance at baseline did not modify the effects of the interventions on the outcomes.
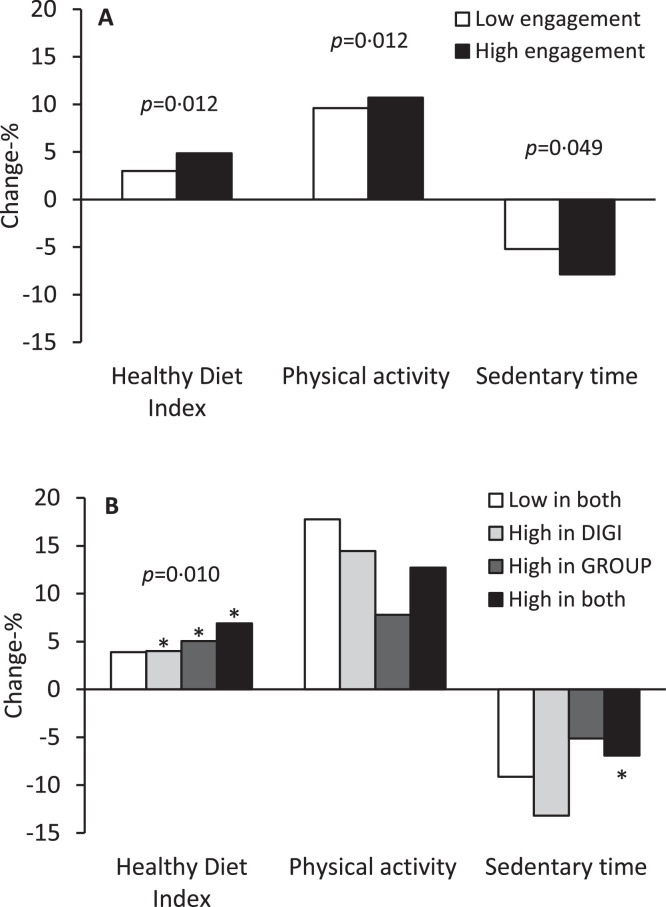
According to the secondary analyses, the HDI increased on average more among those with higher engagement in the digital intervention (Figure 3a, Table S2), among those with higher attendance in the group-based intervention (Figure 3b, Table S3), and particularly among those with more active participation in the combined intervention (Figure 3b, Table S3). Total physical activity increased on average more and total sedentary time decreased on average more among those with higher engagement in the digital intervention (Figure 3a, Table S2).
Figure 3.
(a) Unadjusted one-year mean percentage changes in the Healthy Diet Index, physical activity, and sedentary time among those with high engagement in the digital intervention (≥median of 501 habits/year, higher 50%) and among those with low engagement in the digital intervention (<501 habits/year, lower 50%) in the study groups with digital intervention (DIGI and DIGI+GROUP). The data are from linear mixed-effects models adjusted for age, sex, study province at baseline, and time between assessments (filling out questionnaires). The P-values are for the statistical significance of the differences in the one-year mean percentage changes in the Healthy Diet Index, physical activity, and sedentary time between the low engagement group and the high engagement group. (b): Unadjusted one-year mean percentage changes in the Healthy Diet Index, physical activity, and sedentary time among those with low participation in both study interventions, high engagement in the digital intervention (≥501 habits/year, higher 50%), high attendance in the group-based face-to-face intervention (≥5 of all 6 group sessions), and high participation in both study interventions in the study group with group-based face-to-face intervention (DIGI+GROUP). The data are from linear mixed-effects models adjusted for age, sex, study province at baseline, and time between assessments (filling out questionnaires). The asterisks denote the statistical significance (p<0·05) of the differences in the one-year mean percentage changes in the Healthy Diet Index and sedentary time in the groups of high engagement in the digital intervention, high attendance in the group-based face-to-face intervention, and high participation in both interventions compared with the group of low participation in both interventions. The P-value shown is for the statistical significance of the difference in the one-year mean percentage change in the Healthy Diet Index across the groups of low participation in both interventions, high engagement in the digital intervention, high attendance in the group-based face-to-face intervention, and high participation in both interventions.
Discussion
This one-year RCT carried out in primary healthcare as part of its routine practices demonstrated that a lifestyle intervention based on multiple behaviour change theories and using combined digital and group-based face-to-face delivery improved diet quality in adults at increased risk of T2D. In line with the improved diet quality, the combined digital and group-based lifestyle intervention tended to decrease abdominal adiposity and prevent the increase in insulin resistance. The beneficial effect on diet quality increased with high engagement in the digital intervention and even more so with high attendance in the group-based intervention. Moreover, those who actively engaged in the digital intervention also showed a larger increase in total physical activity and a larger decrease in total sedentary time than those with lower engagement.
To our knowledge, the StopDia study is the first large-scale RCT exploring the effects of digital lifestyle interventions alone and combined with group-based lifestyle interventions on diet quality, physical activity, and sedentary time in primary healthcare as part of its routine practices. The digital intervention combined with the group-based intervention, but not alone, improved the overall diet quality, and more specifically, increased the consumption of fruit and vegetables and improved the quality of dietary fat and dairy products. So far, there are few RCTs reporting the effects of digital lifestyle interventions on health behaviours in real-world conditions, such as in healthcare, among adults at increased risk of T2D.9 While two thirds of the reviewed interventions were found to be effective over the first six months, one third also showed effects extending to 12 months. The effective interventions were more likely to utilise behaviour change techniques, with digital features facilitating health and lifestyle education, behaviour or outcome tracking, and online health coaching being most effective. A more recent non-randomised controlled trial confirmed the importance of features for self-monitoring of diet and physical activity as well as features allowing virtual interaction with a human coach and peer support.19 Our findings suggest that the habit-based digital lifestyle intervention may not be sufficient alone, but support from healthcare professionals and peers received in group-based intervention is necessary for effectiveness. The group-based intervention may have helped the participants apply the dietary contents of the digital intervention in their everyday life. However, the digital intervention alone or combined with the group-based intervention had no effect on total physical activity or total sedentary time. One explanation for this could be that the BitHabit app included more habits for improving diet quality than for increasing physical activity or decreasing sedentary time, resulting in choosing more habits related to diet quality than physical activity or sedentary time.13
Although the improved diet quality could be expected to lead to weight loss, we found only a modest and statistically non-significant decrease in waist circumference, and no reduction in body weight, with the combined digital and group-based lifestyle intervention. There is some evidence from earlier RCTs that lifestyle interventions conducted in real-world conditions, including group counselling organised by community members, can decrease excess body weight in individuals at increased risk of T2D.6 It is important to note that our lifestyle interventions were not explicitly aimed at decreasing energy intake and losing body weight but achieving permanent improvements in daily health behaviours, including diet, physical activity, sedentary time, alcohol consumption, and smoking.14 The effects of our interventions on body weight could thus have been larger if weight loss had been the defined aim in the interventions.
Previous RCTs have demonstrated that group counselling by healthcare professionals can decrease the risk of T2D in real-world conditions in adults at increased risk.6 On the other hand, it is known that the intensity of lifestyle interventions plays a major role in the magnitude of decreased risk of T2D achieved.9,20,21 Therefore, it is not surprising that the few earlier RCTs carried out in healthcare have shown inconsistent results concerning the effects of digital lifestyle interventions on indicators of glucose metabolism in adults at increased risk of T2D.9 In line with this, we observed that the digital intervention alone and combined with group-based intervention modestly decreased fasting insulin but had no effect on fasting glucose, 2-hour glucose, or HbA1c in adults at increased T2D risk. The explanation for this suggestive finding could be that insulin resistance is usually evident long before chronic hyperglycaemia in the pathogenesis of T2D.22 Other reasons for the lack of effect on fasting or 2-hour plasma glucose could be the large proportion of individuals with normal glucose metabolism (44%) in our study, the modest intervention effect on adiposity, and the relatively short intervention period of one year, all of which tend to decrease the likelihood of showing the beneficial effects of the observed lifestyle modifications on glucose metabolism. Moreover, the digital intervention combined with the group-based intervention improved the overall diet quality that appeared to be attributed to the increased consumption of fruit and vegetables and the improved quality of dietary fat and dairy products. However, the consumption of whole grains, which did not change in our study, has been most consistently been associated with T2D risk.23 Finally, it has to be highlighted that the StopDia study was performed as part of routine primary healthcare practices within real-world population setting. Thus, variation in adherence to the interventions and the responses to interventions limit the statistical power to demonstrate effects on glucose metabolism as compared with studies conducted in more controlled conditions.
We observed that high engagement in the digital intervention and high attendance in the group-based intervention resulted in a larger improvement in diet quality. This is expected as active participation in lifestyle interventions has been found to be important for the beneficial effects on health behaviours and their health consequences.6 High engagement in the digital intervention also led to a larger increase in total physical activity and a larger decrease in total sedentary time. These findings provide further evidence for the previous notion that good adherence to lifestyle interventions is important to achieve the expected health behaviour changes and associated health benefits.6 It may be more challenging to maintain good adherence in digital interventions than in more structured group-based interventions in which healthcare professionals motivate and support individuals to improve their health behaviour.19,24
Adopting personalised and adaptive approaches would allow lifestyle interventions to accommodate individual characteristics and preferences.25 Doing so could increase intervention participation and effectiveness as these approaches enable interventions to react to early signs of decreased response.26 In further developments of the BitHabit app, we will utilize the possibility to better adapt the digital intervention based on participants’ personal needs and preferences, utilizing the knowledge of over 1·0 million daily habit selections reported by the participants within the first six months of the intervention.13
The strengths of our study include the large number of adults at increased risk of T2D screened digitally from a general population, the RCT carried out in primary healthcare as part of its routine practices, and the opportunity to investigate the effects of the digital lifestyle intervention alone and combined with the group-based lifestyle intervention on various outcomes relevant for the prevention of T2D. We also had strong theoretical basis of the interventions because we used multiple behaviour change theories, including the self-determination theory,10 the habit-formation theory,12 and the self-regulation theory,11 to improve the adoption and sustainability of health behaviour changes.27,28 About 15% of the participants dropped out from the study, and the proportions of dropouts were similar in all three study groups. This suggests that the interventions, including the app, were well accepted by the participants. Finally, the whole StopDia approach, including the recruitment process, described in detail earlier,14 can be scaled for the prevention of not only T2D but also other non-communicable diseases in real-world healthcare.
A limitation of our study is the relatively low intensity of the lifestyle interventions in a real-world population setting that decreased the likelihood of showing the expected effects on the outcomes. First, we acknowledge that the real-world approach in our study design started with the identification of adults at a moderately increased risk of type 2 diabetes via the inexpensive and feasible FINDRISC. As this was used in place of the more expensive and challenging 2-hour oral glucose tolerance test, there may have been limited power to observe a reduction in risk factors during the trial. Furthermore, the decision about the intensity of interventions has to be balanced on their potential scalability at the population level: implementation of intensive interventions has largely failed in healthcare due to lack of resources. The reason for accepting the lower intensity of the interventions was that we wanted to develop a cost-effective and scalable model for the prevention of T2D in healthcare and society. The assessment of diet quality using a questionnaire instead of food records, the assessment of physical activity and sedentary time using a questionnaire instead of objective measures, and non-fasting measurement of body weight and waist circumference were realistic choices in our large RCT carried out in primary healthcare as part of its routine practices. However, they may have decreased accuracy of the assessment of these outcomes and thereby limited statistical power to find the expected effects of the interventions. At least in case of physical activity there is evidence that pedometers, heart rate monitors, as well as combined heart rate and body movement monitors may motivate participants to increase physical activity.29 The nurses who performed the measurements were not blinded to group assignment. This could have introduced measurement bias. The participants were also recruited to the RCT through workplaces, social media, the internet, newspapers, community pharmacies, and health care15 that could have resulted in larger proportion of women participating the study. Finally, the larger number of women than men in our study sample is a weakness that may limit the generalizability of our findings to men. However, we controlled for sex in all statistical analyses to avoid confounding by sex.
In conclusion, this one-year RCT conducted in primary healthcare as part of its routine practices showed that the digital lifestyle intervention combined with the group-based lifestyle intervention improved diet quality in adults at increased risk of T2D. Although we could not demonstrate a statistically significant effect of the interventions on glucose metabolism, the results of our study suggest that the combined digital and group-based lifestyle intervention tended to decrease waist circumference and prevent the increase in insulin resistance. The variable participation in the digital and group-based lifestyle interventions warrant further RCTs in which adapted intervention models optimising the intervention type based on individuals’ characteristics and early adherence will be considered.
Contributors
J.Pi. is the principal investigator of the StopDia study. J.Pi., T.A.L., L.K., J.M., J.M., K.P., M.E, P.A., and J.L. designed the study. T.T.-T., R.M., M.K., U.S., N.L., R.J. and M.H. assisted in designing the study. T.T.-T., R.M., R.J., and J.L participated in recruiting the participants. R.M., T.T.-T., and P.A. trained the healthcare nurses, and T.T.-T. and R.M. monitored study implementation. K.A., E.J.-R., T.T.-T., R.M., and E.M. were responsible for data management. K.A. and S.M. were responsible for statistical analyses. All authors contributed to writing the manuscript and approved the final version of it.
Data sharing statement
No additional data are available. De-identified data collected for this study and a data dictionary are available from the corresponding author on reasonable request.
Declaration of interests
J.M. is a founding partner of ESiOR Oy and a board member of Siltana Oy. P.A. is a founding partner of Provention Ltd and owner of Collaborative Care Systems Finland. These companies were not involved in carrying out this research. The authors declare that they have no competing interests.
Acknowledgements
STOP DIABETES - from knowledge to solutions project was funded by the Strategic Research Council at the Academy of Finland (http://www.aka.fi/en/about-us/SRC/) in 2016–2019 (303537, 303643, 303644, 303645) and by the Academy of Finland 2020–2023 (T2D-Data project, 332465, 332464, 332466), by the Novo Nordisk Foundation 2018–2020 (33980 and 63753), and by the Finnish Diabetes Research foundation. We acknowledge all health and social care workers in the three participating counties (Hospital district of North Savo, Päijät-Häme and South Karelia) and the stakeholders of the StopDia project (Ministry of Social Affairs and Health, Finnish Social Security Institute Kela, Regional councils of North Savo and Päijät-Häme, Cities of Kuopio, Varkaus, and Siilinjärvi, Ylä-Savon SOTE, Finnish Heart Association, Finnish Diabetes Association, Family Federation of Finland, Association of Finnish Pharmacies, Consumers’ Union of Finland, Etera Mutual Pension Insurance Company, Agency for Rural Affairs Mavi and its partner organisations, Self-care and Digital Value Services project ODA) for participation in planning of the recruitment of the risk individuals and interventions in the StopDia model. We acknowledge Juho Viitasalo and Juha Kekäläinen from the University of Eastern Finland for their extensive work in the development of the StopDia digital tools. We also acknowledge Tiina Laatikainen, Kennet Harald, Markku Peltonen, Pekka Jousilahti, Katri Hemiö, Maliheh Nekouei, Marvi Langari, and Eeva Virtanen from the National Institute for Health and Welfare; Saara Vanhatalo, Johanna Leväsluoto, Adil Umer, Juha Leppänen, Samuli Heinonen, and Eeva Rantala from the Technical Research Centre of Finland VTT; Kari Jalkanen, Suvi Manninen, and Matti Uusitupa from the University of Eastern Finland; and Jaakko Tuomilehto from the University of Helsinki for their role in the development of the StopDia protocols and for participating in the project. We also thank the international advisory board of the StopDia project, including professors Edith Feskens, Theresa Marteau, and Peter Schwarz.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.lanepe.2022.100527.
Appendix. Supplementary materials
References
- 1.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 2.Knowler W, Barrett-Connor E, Fowler S, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49:289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 4.Lindström J, Peltonen M, Eriksson JG, et al. Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS) Diabetologia. 2013;56:284–293. doi: 10.1007/s00125-012-2752-5. [DOI] [PubMed] [Google Scholar]
- 5.Nathan DM, Barrett-Connor E, Crandall JP, et al. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015;3:866–875. doi: 10.1016/S2213-8587(15)00291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galaviz KI, Weber MB, Straus A, Haw JS, Narayan KMV, Ali MK. Global diabetes prevention interventions: a systematic review and network meta-analysis of the real-world impact on incidence, weight, and glucose. Diabetes Care. 2018;41:1526–1534. doi: 10.2337/dc17-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block G, Azar KMJ, Romanelli RJ, et al. Improving diet, activity and wellness in adults at risk of diabetes: randomized controlled trial. Nutr Diabetes. 2016;6:e231. doi: 10.1038/nutd.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block G, Azar KM, Romanelli RJ, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res. 2015;17:e240. doi: 10.2196/jmir.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Rhoon L, Byrne M, Morrissey E, Murphy J, McSharry J. A systematic review of the behaviour change techniques and digital features in technology-driven type 2 diabetes prevention interventions. Digit Heal. 2020;6 doi: 10.1177/2055207620914427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan RM, Deci EL. Self-determination theory and the facilitation of intrinsic motivation, social development, and well-being. Am Psychol. 2000;55:68–78. doi: 10.1037//0003-066x.55.1.68. [DOI] [PubMed] [Google Scholar]
- 11.Mann T, De Ridder D, Fujita K. Self-regulation of health behavior: social psychological approaches to goal setting and goal striving. Heal Psychol. 2013;32:487–498. doi: 10.1037/a0028533. [DOI] [PubMed] [Google Scholar]
- 12.Wood W, Neal DT. Healthy through habit: interventions for initiating & maintaining health behavior change. Behav Sci Policy. 2016;2:71–83. [Google Scholar]
- 13.Harjumaa M, Absetz P, Ermes M, et al. Internet-based lifestyle intervention to prevent type 2 diabetes through healthy habits: design and 6-month usage results of randomized controlled trial. JMIR Diabetes. 2020;5 doi: 10.2196/15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pihlajamäki J, Männikkö R, Tilles-Tirkkonen T, et al. Digitally supported program for type 2 diabetes risk identification and risk reduction in real-world setting: protocol for the StopDia model and randomized controlled trial. BMC Public Health. 2019;19 doi: 10.1186/s12889-019-6574-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jalkanen K, Järvenpää R, Tilles-Tirkkonen T, et al. Comparison of communication channels for large-scale type 2 diabetes risk screening and intervention recruitment: empirical study. JMIR Diabetes. 2021;6 doi: 10.2196/21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemiö K, Pölönen A, Ahonen K, Kosola M, Viitasalo K, Lindström J. A simple tool for diet evaluation in primary health care: validation of a 16-item food intake questionnaire. Int J Environ Res Public Health. 2014;11:2683–2697. doi: 10.3390/ijerph110302683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Association AD Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S14–S31. doi: 10.2337/dc20-S002. [DOI] [PubMed] [Google Scholar]
- 18.Lindström J, Aittola K, Pölönen A, et al. Formation and validation of the healthy diet index (HDI) for evaluation of diet quality in healthcare. Int J Environ Res Public Health. 2021;18:1–22. doi: 10.3390/ijerph18052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golovaty I, Wadhwa S, Fisher L, et al. Reach, engagement and effectiveness of in-person and online lifestyle change programs to prevent diabetes. BMC Public Health. 2021;21 doi: 10.1186/s12889-021-11378-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff. 2012;31:67–75. doi: 10.1377/hlthaff.2011.1009. [DOI] [PubMed] [Google Scholar]
- 21.Dunkley AJ, Bodicoat DH, Greaves CJ, et al. Diabetes prevention in the real world: effectiveness of pragmatic lifestyle interventions for the prevention of type 2 diabetes and of the impact of adherence to guideline recommendations - a systematic review and meta-analysis. Diabetes Care. 2014;37:922–933. doi: 10.2337/dc13-2195. [DOI] [PubMed] [Google Scholar]
- 22.Laakso M. Is insulin resistance a feature of or a primary risk factor for cardiovascular disease? Curr Diab Rep. 2015;15 doi: 10.1007/s11892-015-0684-4. [DOI] [PubMed] [Google Scholar]
- 23.Schwingshackl L, Hoffmann G, Lampousi AM, et al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32:363–375. doi: 10.1007/s10654-017-0246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eysenbach G. The law of attrition. J Med Internet Res. 2005;7 doi: 10.2196/jmir.7.1.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen H, Mackinnon A. The law of attrition revisited [3] J Med Internet Res. 2006;8 doi: 10.2196/jmir.8.3.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almirall D, Nahum-Shani I, Sherwood NE, Murphy SA. Introduction to SMART designs for the development of adaptive interventions: with application to weight loss research. Transl Behav Med. 2014;4:260–274. doi: 10.1007/s13142-014-0265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lally P, Gardner B. Promoting habit formation. Health Psychol Rev. 2013;7:S137–S158. [Google Scholar]
- 28.Wood W, Rünger D. Psychology of habit. Annu Rev Psychol. 2016;67:289–314. doi: 10.1146/annurev-psych-122414-033417. [DOI] [PubMed] [Google Scholar]
- 29.Franssen WMA, Franssen GHLM, Spaas J, Solmi F, Eijnde BO. Can consumer wearable activity tracker-based interventions improve physical activity and cardiometabolic health in patients with chronic diseases? A systematic review and meta-analysis of randomised controlled trials. Int J Behav Nutr Phys Act. 2020;17:57. doi: 10.1186/s12966-020-00955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.