Abstract
Introduction
The severity of lupus nephritis (LN) varies between different ethnicities. However, there are limited data regarding disease severity for LN in patients from the Arabian Gulf region; moreover, there are no treatment guidelines developed specifically for this population. The objective of this review was to characterise the incidence of LN, current treatment practices, the severity of LN, and the pathophysiology and biomarkers associated with LN in the Arabian Gulf region.
Methods
A literature search using EMBASE was conducted in October, 2021 to identify publications reporting on the incidence, treatment practices, severity, pathophysiology or biomarkers associated with LN, from countries in the Arabian Gulf region (including Bahrain, Kuwait, Oman, Qatar, Saudi Arabia and the United Arab Emirates). Additional relevant publications were provided by collaborators. A manual review of the publications was conducted to determine their relevance and data on the outcomes of interest were extracted.
Results
Of 3705 publications, 54 publications were identified as relevant. LN is one of the most commonly diagnosed renal diseases within the Arabian Gulf and approximately 10%–36% of all renal biopsies are for LN. Treatment patterns within the region appear to vary and generally follow treatment guidelines recommended by the Asia Pacific League of Associations for Rheumatology (APLAR), the European Alliance of Associations for Rheumatology (EULAR) and Kidney Disease Improving Global Outcomes (KDIGO). The majority of patients receive cyclophosphamide for induction therapy, whilst others receive mycophenolate mofetil. Most studies showed that the most frequently diagnosed class of LN within the Arabian Gulf region was Class IV (up to 63% of patients with LN). Sustained or increased levels of serum creatinine and proteinuria; and depressed levels of complement C3/C4 were commonly seen among patients with LN from the Arabian Gulf region.
Conclusions
This review identified that LN may manifest more severely among patients from the Arabian Gulf region than in other populations, such as Caucasian populations. A greater understanding of LN and the treatment practices within the region, as well as the development of more specific treatment guidelines for this population may help improve outcomes for patients with LN in the Arabian Gulf region.
Keywords: lupus nephritis, Arabian Gulf, incidence, treatment, severity, biomarkers
Introduction
Lupus nephritis (LN) is a common and severe manifestation of systemic lupus erythematosus (SLE) that can lead to end-stage renal disease (ESRD) and death.1–3 The prevalence of SLE in the Arabian Gulf region has been estimated to be 19–103 per 100,000 persons,4–6 similar to estimates in the USA (40–79 per 100,000 persons);7 despite this, there are limited data regarding the incidence, severity and treatment patterns of patients with LN in the Arabian Gulf region.
Studies have shown that the severity of LN varies between patients of different ethnicities; for example, a significantly greater proportion of African American (51%) and Hispanic patients (57%) with LN had World Health Organization (WHO) Class IV LN compared with Caucasian patients (30%).8 It has yet to be confirmed whether LN also disproportionately affects patients from the Arabian Gulf region. However, approximately 47% of Arabian patients with SLE from Dubai were found to have renal manifestations, whereas data elsewhere suggest 38% of patients with SLE across Europe, North America and Asia have renal manifestations.3,9
Currently, there are no curative therapies for LN and treatment largely focusses on managing the disease, with the aim of remission of symptoms, prevention of organ damage and minimisation of treatment side effects.10 Treatment recommendations for LN by the Asia Pacific League of Associations for Rheumatology (APLAR), the European Alliance of Associations for Rheumatology (EULAR) and Kidney Disease Improving Global Outcomes (KDIGO) indicate that immunosuppressants and glucocorticoids should be used for induction and maintenance phases of therapy. Hydroxychloroquine is also typically recommended. However, the recommendations regarding specific drugs and dosing differs between regions.11–14 Therefore, it may be expected that current treatment practices also vary by region; however, no summary of the LN treatment practice literature from the Arabian Gulf region has been conducted to date.
This review was conducted to analyse the available literature on LN among patients in the Arabian Gulf region to determine the incidence, severity, treatment practices, pathophysiology and biomarkers associated with LN.
Methods
Search strategy
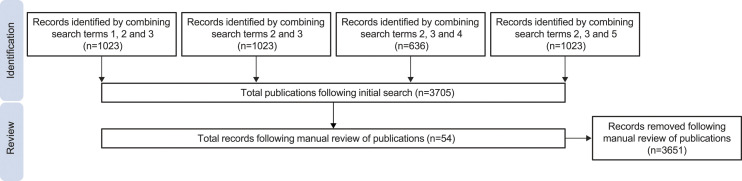
A literature search was performed in October, 2021, using the Excerpta Medica Database (EMBASE) to identify publications reporting on the incidence of LN, current treatment practices, and the severity, and pathophysiology or biomarkers associated with LN from countries in the Arabian Gulf region. Additionally, other relevant references were provided by collaborators. Publications from the following countries/regions were eligible for inclusion: Bahrain, Kuwait, Oman, Qatar, Saudi Arabia and the United Arab Emirates (UAE). Abu Dhabi and Dubai were also used as search terms to capture publications from the UAE. Search terms that were utilised included ‘lupus erythematosus’, ‘lupus erythematosus nephritis’, ‘[respective country/city]’, ‘systemic lupus erythematosus’ and ‘systemic lupus erythematosus’ OR ‘lupus erythematosus’. The search strategy and number of included studies are detailed in Figure 1. The abstracts of the identified references were manually reviewed by one researcher, and then by two other researchers collaboratively to determine whether they were relevant for inclusion in this review. References were excluded if they did not focus on LN or if most of the population were not from the Arabian Gulf region.
Figure 1.
Search strategy and number of publications available from selected regions. The numbers 1–5 correspond to specific search terms; 1, lupus erythematosus; 2, lupus erythematosus nephritis; 3, respective country/city; 4, systemic lupus erythematosus; 5, systemic lupus erythematosus OR lupus erythematosus.
Results
Included studies
Of the 3705 publications identified in the searches, a total of 54 references met the inclusion criteria for this literature review. A comprehensive list of the eligible publications is shown in Supplementary Table 1.
Incidence and prevalence of LN in the Arabian Gulf region
LN identified in renal biopsies
Thirteen studies conducted in Saudi Arabia (n = 5), Bahrain (n = 2), Kuwait (n = 2), Oman (n = 2) and UAE (n = 2) investigated the prevalence of LN among patients who have had renal biopsies. These studies showed that LN was diagnosed in approximately 10%–36% of available renal biopsies.15–27 In some of these studies, LN was the most common cause of secondary glomerulonephritis (GN), constituting between approximately 36%–72% of all secondary GN cases.15–21,27 The two studies conducted in Oman showed that LN was the most frequent glomerular disease, occurring in approximately 30%–36% of all patients who had an available renal biopsy.23,26
Proportion of patients with SLE that develop LN
Five studies determined the proportion of patients with SLE who develop LN. A 26-year observational study (1980–2006) that enrolled 624 patients with SLE from Saudi Arabia found that 299 (approximately 48%) went on to develop LN.28 A separate 15-year Saudi Arabian study (1990–2005) showed that 54% of patients with SLE presented with LN.29 A third Saudi Arabian study similarly found that 55% of patients with SLE presented with LN.30 A 10-year study in Oman (2006–2016) that compared the incidence of LN among patients with SLE between paediatric and adult cohorts found that more children present with LN (64%) compared with adults (33%).31 One review of the literature determined that approximately 80% of paediatric patients with SLE from five separate Arabian patient cohorts had renal involvement, and that 40% of those with nephritis had WHO Class IV LN.32
Summary
Whilst the incidence of LN varies among different cohorts within the Arabian Gulf region, the proportion of patients presenting with LN among those with SLE and those with renal diseases appears to be high. Relative to other renal diseases within the Arabian Gulf region, LN is one of the most commonly diagnosed, with 10%–36% of diagnosed renal biopsies being LN. Additionally, large proportions of patients with adult SLE develop LN; approximately 18%–54% of patients with SLE present with LN in the Arabian Gulf. Similar studies in other regions (Americas, Europe and Asia) estimate that approximately 40% of patients with SLE develop LN,3 whilst two separate Iranian studies estimate that 29% and 67% of patients with SLE have LN.33,34
Treatment practices and procedures in the Arabian Gulf region
Treatment recommendations
Several treatment recommendations for LN have been published, including APLAR, EULAR and KDIGO.11,13,14
Generally, the recommended first line of induction therapy is either mycophenolate mofetil (MMF) or intravenous cyclophosphamide (CYC) in combination with hydroxychloroquine and a moderate dose of glucocorticoids among patients with active/proliferative LN (International Society of Nephrology and the Renal Pathology Society [ISN/RPS] Classes III, IV or V). Alternative doses of CYC (higher or lower) or use of tacrolimus (TAC; alone or in combination with MMF) are recommended alternative induction agents; rituximab can also be considered in patients who do not respond to standard regimens. Maintenance therapies with immunosuppressants should then be continued for 5 years to prevent renal flares, with MMF or azathioprine (AZA) as the recommended agents.11,13,14
Commonly prescribed treatments
Four studies have detailed the treatment practices in Saudi Arabia over the last few decades. One study found that most patients with LN received CYC (66%) as immunosuppressant therapy, followed by MMF (21%).35 Other Saudi Arabian studies have shown that CYC was also the most commonly prescribed induction therapy, and that MMF was most frequently used as maintenance therapy.24,36 A 26-year observational study in Saudi Arabia (1980–2006) also showed that all patients with LN were prescribed prednisolone and more patients received CYC in combination with other drugs (66%) than MMF (12%), consistent with the previous study.28 Among patients receiving CYC, 56% received a high-dose regimen compared to 13% receiving low-dose CYC.28 AZA was prescribed in combination with prednisolone among 12% of patients, whilst CYC and AZA were prescribed in combination with prednisolone among 27% of patients.28
A separate 8-year observational study (2001–2009) showed that induction therapy consisted of pulse steroids in 43% of cases at a mean cumulative dose of 2.9 g, whilst CYC was given to 54% of patients. Maintenance therapy in this cohort consisted of oral steroids for all patients, with a mean cumulative dose of 15.2 mg; other maintenance therapies used were MMF, AZA and cyclosporin among 59%, 15% and 11% of patients, respectively. Angiotensin-converting-enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) were each prescribed to 10% of patients, whilst a combination of the two was prescribed to 23% of patients.37
Another 8-year study (2006–2014) compared the treatment regimens of patients with severe (with crescent formation) LN compared with those without; all patients in the study received steroid induction with either CYC or MMF.38 The authors suggested that those with severe LN should be treated with CYC rather than MMF during induction therapy. A 12-year study (2000–2012) found that that CYC was used in the treatment of 48% and 52% of patients with and without necrosis, respectively.39
Three separate Saudi Arabian studies showed that more patients were prescribed MMF (47%–75%) than CYC (18%–43%) as induction therapy.40–42 The use of hydroxychloroquine was referenced in one of these studies, and was prescribed to all patients in the study population; this is consistent with current APLAR treatment recommendations.13,40 Another study identified that 20% of patients who received induction therapy were prescribed a higher dose of CYC (NIH protocol) versus approximately 12% on a lower dose (Euro Lupus protocol).42 Separately, findings from a randomised clinical trial (RCT) in Saudi Arabia showed that the use of induction therapy of low-dose CYC (5 mg/kg) was as effective as a higher dose (10 mg/kg), resulting in similar rates of remission and fewer side effects in a Saudi Arabian patient population with Class IV LN.43
Additionally, TAC has been used alone, and in combination with MMF and steroids, among 2.3% and 6.8% of patients as induction therapy, respectively.40 Among patients from a Saudi Arabian hospital with Class II LN, 92% of patients were able to achieve complete remission with corticosteroids alone; however, the authors of this study recommend that more aggressive immunosuppressant treatment should be administered to patients with Class II LN with acute kidney injury.44
Three studies have also investigated treatment practices in Kuwait. A 4-year study (2014–2018) investigated the treatment patterns of patients receiving rituximab in Kuwait; when used for the treatment of LN, rituximab was used in conjunction with MMF (with or without calcineurin inhibitors [CNI]) in approximately 81% of cases, alone in 10%, with CNI in 6%, and in conjunction with CYC in just 3% of cases.45 Patients in Kuwait with kidney transplants resulting from ESRD caused by LN were treated with significantly more anti-hypertensives than those whose ESRD was caused by other renal diseases.46 A RCT conducted in Kuwait demonstrated that rituximab use alone during maintenance therapy in patients with Class IV LN was as safe and effective as MMF in combination with low-dose prednisone; all patients received induction therapy of CYC with MMF and prednisone.47
In a 2-year study conducted in the UAE (2016–2018), most patients received a low-dose regimen of CYC (55%) for induction of remission, followed by MMF (29%); rituximab was also used, but in the lowest proportion of patients (11%).48 In Qatar, 74% of patients were prescribed MMF, followed by AZA (14%), with the lowest proportion of patients receiving CYC (6%); all patients were prescribed oral steroids, and 44% were prescribed pulse steroids.49
Paediatric treatment practices
Two studies have investigated treatment patterns amongst paediatric patients with LN in the Arabian Gulf. A 15-year observational study (2000–2015) showed that all patients from a paediatric population in Saudi Arabia received steroids in combination with antimalarials and immunosuppressants; CYC was most commonly prescribed, followed by MMF, and then AZA; rituximab was then used in patients with inadequate response to MMF or CYC; patients in this cohort who progressed to ESRD received regular dialysis.50 In a separate study, also in Saudi Arabia, paediatric patients with Class IV LN were typically treated with steroids and CYC during induction, and AZA and MMF during maintenance.51
Non-standard treatments
Other small studies have investigated the effectiveness of non-standard treatments in patients who were previously unresponsive to therapy. A case study in Kuwait showed that infliximab (in combination with CYC, MMF and steroids) resulted in sustained remission in a patient with Class IV LN.52 Other therapies evaluated in the Arabian Gulf region for the treatment of LN included therapeutic plasma exchange in UAE,53 and intravenous immunoglobulins (IvIg) in Kuwait and Saudi Arabia.28,37,54 In the Kuwaiti study, IvIg treatment resulted in stabilisation of renal function and control of symptoms in patients with Class IV LN.54 Additionally, rituximab use prior to dialysis was shown to restore renal function in one patient in Saudi Arabia.55 Other studies argue that repeat biopsies are critical for the management of LN and other renal diseases in the Arabian Gulf region due to the progressive nature of the disease.20,35,56
Summary
There is variation in treatment practices within and between countries in the Arabian Gulf region; however, most studies investigating the treatment patterns of patients with LN appear consistent with the recommendations of APLAR, EULAR or KDIGO.11,13,14
In most studies, CYC was the most frequently prescribed induction immunosuppressant.35,38,39,48,50,51 However, MMF was prescribed most frequently in Saudi Arabia.40–42 Use of hydroxychloroquine was rarely mentioned as a frequently prescribed medication in the studies referenced here. These practices are similar to those observed in Iran, where several studies have shown that most patients are prescribed CYC alone,57–59 or in combination with MMF.60
These practices differ from those in non-Arabian Gulf region countries; for example, a study showed that over half of patients with LN from a cohort in the United States were prescribed hydroxychloroquine; MMF was the most frequently prescribed immunosuppressant and CYC was prescribed to less than 1% of patients.61 However, these treatment patterns more closely follow the ACR recommendations for the United States, which may partially explain these differences.12
Severity of LN in the Arabian Gulf region
LN is a severe disease in the Arabian Gulf region with a significant healthcare burden; for example, one study in the UAE showed that half of all patients with SLE that were hospitalised had also presented with LN.62,63 Additionally, an epidemiological study in the UAE estimated that LN accounted for approximately 2% of all ESRD cases.64 Publications reporting remission status, incidence of dialysis, ESRD and death are summarised in Table 1.
Table 1.
Publications categorised by LN severity outcome
| Publication | Study period | Remission | ESRD | Dialysis | Death |
|---|---|---|---|---|---|
| Qari, 2002 | January 2000–December 2001 | Yes | Yes | ||
| Al Arfaj, 2009 | 1980–2006 | Yes | Yes | Yes | Yes |
| Al Durahim, 2011 | 2001–2009 | Yes | Yes | ||
| Alsuwaida, 2012 | November 1996–April 2009 | Yes | |||
| Mitwalli, 2012 | January 1995–January 2008 | Yes | Yes | Yes | |
| Muzaffer, 2012 | 1997–2010 | Yes | Yes | Yes | |
| Alaiya, 2015 | 2003–2008 | Yes | Yes | ||
| Abdulla, 2016 | January 2006–December 2014 | Yes | |||
| Al-Mayouf, 2017 | January 2000–June 2015 | Yes | Yes | Yes | |
| Almalki, 2019 | January 2006–June 2017 | Yes | Yes | Yes | |
| Elbadawi, 2019 | June 2016–December 2018 | Yes | Yes | Yes | |
| Albirdisi, 2020 | 2014–2019 | Yes | Yes |
ESRD, end-stage renal disease; LN, lupus nephritis.
Remission status
A study conducted in the UAE found that most patients achieve remission at 6-month post treatment, with 56% and 37% achieving complete and partial responses, respectively; hypertension, diabetes mellitus and presence of antiphospholipid antibodies were associated with poorer outcomes in this cohort.48
Of patients with available follow-up data at 1 year in a Qatari observational study, 24% achieved complete remission, 16% partial remission and 22% had treatment failure; all patients achieving complete remission and 75% achieving partial remission were receiving MMF.49 A Saudi Arabian study showed that complete and partial remission occurred among 42% and 23% of patients, respectively, during 12–18 months of follow-up.35 In a separate Saudi Arabian observational study of patients with proliferative LN, complete or partial remission at 6 months was achieved in 39% and 28% of patients, respectively. Remission rates were higher in those patients treated with MMF than with CYC or other treatments, at the same timepoint.41 Another Saudi Arabian study showed that 76% of patients achieved disease remission at the time of their final follow-up.28
In two studies (one in each of the UAE and Saudi Arabia), patients with crescent LN were less likely to achieve remission compared with those without crescent LN.38,48
Incidence of ESRD, dialysis and death
Only 7% of patients from one cohort in the UAE required dialysis and 4% progressed to ESRD.48 Within a Saudi Arabian cohort, 28% of patients required dialysis and the 5-, 10- and 15-year survival rates were 92%, 77% and 77%, respectively; patients requiring dialysis were more likely to die during follow-up.37 A separate Saudi Arabian study showed that approximately 6% of patients with LN developed renal failure and required dialysis.30 In the 26-year Saudi Arabian observational study (1980–2006), 9% of patients with LN developed ESRD and required dialysis, whilst the 5- and 10-year survival rates were 96% and 95%, respectively.28
In a retrospective Saudi Arabian study (1995–2008) that included patients with biopsy-proven Class IV LN, 11% had concomitant HCV infection.65 These patients had poorer renal outcomes and reduced patient survival compared with those without HCV infection; specifically, 33% and 7% of HCV-positive and -negative patients died, respectively, during a mean follow-up period of 6.7 (±3) years; whilst 65% and 80%, respectively, remained dialysis-free after 10 years. Significantly more HCV-positive patients also developed ESRD compared with HCV-negative patients.65 A Saudi Arabian observational study showed that 12% of patients required dialysis and 11% required long-term dialysis beyond 6 months; at 48 months, the majority were alive (95%) and had functioning kidneys (84%).41
One study in Saudi Arabia found that there was no difference in patient survival or renal survival (requirement for dialysis or transplantation) between patients with Class IV global or Class IV segmental LN.36
Among 299 patients with LN in Saudi Arabia, risk factors associated with ESRD were older age of onset, elevated serum creatinine and presence of Class III/IV LN.28 Separate studies conducted in Saudi Arabia and the UAE showed that presence of crescent LN was also a risk factor for progression to ESRD compared with those without crescents;38,48 these crescents are most common in patients with Class IV LN.38
Paediatric outcomes
Within a Saudi Arabian paediatric cohort, 19% of patients progressed to ESRD and required dialysis (these patients generally had more severe disease), whilst the 5- and 10-year survival rates were 94% and 87%, respectively.50 Within another Saudi Arabian paediatric cohort, around 98% of patients with LN achieved remission, none required dialysis, and all were still alive during follow-up.51
Other outcomes
Three studies found that the most common ISN/RPS LN class across the Arabian Gulf region is Class IV,37,40,56 whereas four studies identified the most common WHO LN class also as Class IV.28,31,51,66 Four studies indicated that unspecified Class IV LN was most frequently observed in other studies.24,30,41,49 One study identified that Class IV LN occurred significantly more frequently among men compared with women, whilst there was no significant difference in prevalence of other classes of LN between the two sexes.67 The most frequently observed LN class in one study from the UAE was ISN/RPS Class III.48
Other studies have investigated the outcomes of patients with different subcategories of LN. One conducted in Saudi Arabia determined that necrotising lesions are more common in patients with Class IV LN than in those with Class III LN, and that these lesions are significantly associated with lower remission rates and higher doubling of creatinine.39
Pregnancies among patients with LN were at greater risk of still birth, eclampsia, intrauterine growth restriction and pregnancy-induced hypertension compared with those without LN in one Qatari study.68 Similarly, a Saudi Arabian study indicated that pregnant women with LN are at high risk of spontaneous miscarriage and perinatal mortality, especially among patients with active LN and pre-existing hypertension.69
One study in Kuwait investigated the outcomes of kidney transplants among patients with ESKD caused by SLE compared with those without SLE; patients with SLE had significantly higher rates of transplant rejection, higher prevalence of cytomegalovirus infection and required more anti-hypertensives.70
LN manifestations
Three Saudi Arabian studies have outlined frequently observed clinical manifestations. The most common extra-renal clinical manifestations were arthritis (79%) and skin rashes (78%) in one Saudi Arabian cohort.37 Approximately 22% of patients with Class II LN from another Saudi Arabian cohort had acute kidney injury.44 Elsewhere in Saudi Arabia, the most common manifestations were skin rash (65%), joint symptoms (62%) and hypertension (47%).66
In a Qatari study, the most frequent extra-renal manifestations at the time of LN diagnosis were arthralgia (50%), arthritis (34%), fever (34%) and serositis (22%).49
Summary
Many countries in the Arabian Gulf region generally follow the recommendations for treatment of LN as set out by APLAR, EULAR or KDIGO; despite this, many patients within the region fail to achieve complete or partial remission. Data from one Iranian study showed that 67% of patients achieved complete or partial remission during 566 patient-years of follow-up.58 Another Iranian study demonstrated that 68% and 18% of patients with LN achieve complete and partial remission, respectively, over at least 3 years of follow-up.60
Approximately 4%–28% of patients with LN required dialysis/developed ESRD across studies from the Arabian Gulf region; this compares with 5%–15% of patients in Iran,58–60,71 and 10%, 18% and 31% of Caucasians, Hispanics and African Americans, respectively.8 The range observed in these Arabian Gulf region studies may be due to different study designs, including variations in the duration of follow-up. Additionally, patients in some regions/centres may receive referrals later in their disease progression and so have poorer prognosis. Studies from the Arabian Gulf region reported a range of mortality rates, and between 0% and 23% of patients with LN in this region did not survive during their respective follow-up periods.
The majority of studies reported that the most frequently diagnosed class of LN was Class IV, occurring in approximately 37%–72% of patients with LN. Class IV LN was also most common amongst patients in Iran,58–60,71–75 and was shown to occur amongst 30%, 57% and 51% of Caucasians, Hispanics and African Americans with LN, respectively.8
Extra-renal manifestations that were frequently cited as occurring among patients with LN were arthritis, malar rash and hypertension; these manifestations were also commonly observed in several Iranian studies.59,73,74,76
Pathophysiology and biomarkers
Serum creatinine and proteinuria
Five studies have shown that patients with more severe LN have elevated levels of creatinine and proteinuria. In one Saudi Arabian study, patients with crescent LN had higher levels of creatinine and proteinuria than those without crescent LN.38 In a separate Saudi Arabian cohort, 32% of patients had elevated serum creatinine and 37% had nephrotic-range proteinuria at presentation of LN.41 Proteinuria was also a common laboratory manifestation in a paediatric patient cohort in Saudi Arabia.50 Elsewhere, there was a significant decrease from baseline in 24-h urine protein among patients in Saudi Arabia during an 8-year observational study (2001–2009); this study also showed that 95% and 36% of patients had proteinuria and nephrotic-range proteinuria, respectively.37 Another Saudi Arabian study also identified that elevated serum creatinine was a risk factor for ESRD.28 In a Qatari study, proteinuria and nephrotic-range proteinuria were seen in 72% and 34% of patients, respectively.49
Inflammatory markers
Low complement C3 and C4 levels were observed in 85% and 65% of patients with LN, respectively, in a Saudi Arabian cohort,41 and in 83% and 59% of patients at the time of LN diagnosis in a Qatari study.49 Lower baseline levels of C3 and C4 were associated with poorer survival in two separate Saudi Arabian studies.28,37 Elsewhere, patients who did not achieve remission following 6 months of therapy had lower levels of C3 than those who achieved complete or partial remission.35 However, levels of C4 were found to significantly increase among patients with LN over the course of an 8-year observational study (2001–2009) conducted in Saudi Arabia;37 in this study, baseline levels of C3 and C4 were significantly lower among patients who did not survive compared with those that did.37
One Saudi Arabian study used immunofluorescence to show that C3, C4 and C1q were negative in approximately 3%, 26% and 55% of biopsy samples from patients with LN, respectively.77 A study in Bahrain showed that anti-Smith and anti-Ro/SSA antibodies were significantly higher among patients with SLE without LN (43% and 20%, respectively) compared with patients with LN (18% and 5%, respectively); whilst anti–double-stranded DNA (anti-ds-DNA) antibodies were higher among patients with LN than in those without LN (84% vs 70%).78 One study conducted in the UAE compared the autoantibody profiles of patients with LN and those with SLE only; C3 hypocomplementaemia was significantly more frequent among patients with LN (60%) than in patients with SLE without LN (15.5%), whilst anti-RNP antibodies were significantly higher among patients with SLE only (33%) versus patients with LN (0%).79 Antiphospholipid antibody positivity has also been associated with poorer renal outcomes in the UAE.48 Anti-ds-DNA were positive in 90% of patients with LN in a Qatari study.49 High levels of immunoglobulin G among patients with LN have been observed among Saudi Arabian patients.77
Genomic and proteomic markers
Patients with global and segmental Class IV LN have similar proteomic signatures; however, expression of nine genes (including cytokeratins 18 and 19, and albumin) was significantly different compared with normal and antibody-associated vasculitis tissues in a study conducted in Saudi Arabia.36
Two studies conducted in Kuwait investigated the genomic and proteomic profiles of patients with LN and found that serum concentrations of both interleukin (IL)-17A and IL-17F were significantly higher among patients with LN compared with healthy controls,80 whilst expression of MMP9 was significantly lower, and expression of SLC4A1 and CEACAM3 was significantly higher among patients with LN compared with those with SLE only; altered genes were associated with pathways involved in apoptosis and inflammation.81
Other biomarkers
Haematuria was commonly observed in a paediatric cohort of patients with LN in Saudi Arabia.50 Elsewhere, significant rises in estimated glomerular filtration rate were seen among patients in the 8-year (2001–2009) observational Saudi Arabian study.37 A separate study in Saudi Arabia concluded that interstitial inflammation was associated with more severe LN and that patients who had resolved inflammation at repeat biopsy had more favourable outcomes.82
Summary
Levels of serum creatinine, proteinuria, haematuria, anti-ds-DNA antibodies and components of the complement system are considered as important biomarkers in LN and are commonly utilised by clinicians in the diagnosis and monitoring of patients with LN.83 Generally, these studies from the Arabian Gulf region indicate that sustained/increased levels of serum creatinine, proteinuria, and low levels of C3 and C4 are commonly seen in patients with LN across all Arabian Gulf countries.
Similarly, in several Iranian studies, sustained creatinine and proteinuria were associated with poor renal outcomes among patients with LN.59,60,71,73–75 Low serum C3 and C4 levels were observed in the majority of patients with class IV LN in one Iranian cohort.73
Elsewhere, in two separate European cohorts, proteinuria measurements were also shown to be robust predictors of long-term renal outcomes in patients with LN; lower levels at 1 year were associated with better outcomes.83–85 Additionally, one Italian study found that levels of C3 and C4 were low in 82% and 74% of patients, respectively.86
Conclusions
Our literature review suggests that patients with LN from the Arabian Gulf region have a more severe form of LN than other populations, with a high incidence of Class IV LN. It has been shown that LN is more severe among African American, Hispanic and Asian populations when compared with Caucasian populations;3 observations detailed here also suggest that patients from the Arabian Gulf region have more severe disease when compared with Caucasian populations.
Despite the availability of current treatments, many patients still progress to more severe disease and ESRD. This suggests that there is an unmet need for more effective treatments. The most widely prescribed induction therapy appeared to be CYC. Observations from some studies detailed here show that the survival rate of patients with LN remains low in the Arabian Gulf region. Therefore, the introduction of novel and more effective treatment regimens may help to improve patient outcomes in this region. Increased use of biologics in the Arabian Gulf region may be beneficial for patient outcomes; for example, rituximab and belimumab have been shown to improve renal outcomes in patients with LN.87,88
Additionally, more specific treatment guidelines are needed for patients with LN from the Arabian Gulf region. Currently, centres within this region follow a mixture of the APLAR, EULAR or KDIGO guidelines for treating patients with LN. This is perhaps due to many healthcare providers within the Arabian Gulf region originating from other parts of the world and following treatment recommendations specific to the region they have come from. However, LN appears to manifest differently in patients from the Arabian Gulf region compared with patients from other regions, and treatment guidelines specifically targeting this patient population may help to optimise treatments and improve patient outcomes in this region.
To develop more specific treatment guidelines, more structured research and long-term multicentre observational studies are required to help develop a greater understanding of LN within the Arabian Gulf region. Currently, a multicentre study, LUNELORD (A Descriptive, Prospective Study to Assess Demographic, Pharmacologic, Biomarker, Clinical Features and QoL of Patients With LUpus NEphritis and Long-term ORgan Damage, NCT04971590), is ongoing within the region. This study is investigating the current standard of care, healthcare resource utilisation and associated costs of LN, clinical characteristics and quality of life of patients with LN.
This review has some limitations. Some of the included studies did not exclusively include patients from the Arabian Gulf region. Inclusion of a small number of patients from outside this region may have affected the findings, and so they may not be entirely specific to patients from the Arabian Gulf region. Additionally, several studies were published more than 15 years ago, and so would not have considered more recently developed therapies for LN. For example, novel treatments such as belimumab and voclosporin (approved in the United States in 2011 and 2021, respectively) have been shown to significantly improve renal response over placebo in the treatment of LN;88–91 some of the large-scale trials included here that investigated the efficacy of LN treatments in the Arabian Gulf region did not include either of these treatments. Moreover, the publications used in this review were obtained by manually filtering results from a literature search. Whilst the results were cross-checked independently by two researchers, there still may have been an element of selection bias and some relevant publications may have been omitted from the final list. Only one database (EMBASE) was used to conduct the literature search. Whilst this was a narrative review, use of two separate databases may have made the results more robust, and decreased the likelihood of missing relevant publications. Finally, as with all literature reviews, the findings reported here are subject to publication and reporting bias and therefore do not account for unpublished outcomes from each of the reported studies.92
In conclusion, studies conducted in the Arabian Gulf suggest that LN often manifests severely in this region, and the most common form of LN is class IV. A greater understanding of the clinical characteristics of patients with LN and treatment patterns in the Arabian Gulf region, along with more targeted treatment recommendations, could help improve patient outcomes.
Supplemental Material
Supplemental Material for Literature review of lupus nephritis From the Arabian Gulf region (9/50 words max) by Arwa Al-Shujairi, Faisal Elbadawi, Jamal Al-Saleh, Mohamed Hamouda, Averyan Vasylyev and Munther Khamashta in Lupus
Acknowledgements
Editorial support (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating authors’ comments, grammatical editing and referencing) was provided by Robert Bloxham, PhD, of Fishawack Indicia Ltd, part of Fishawack Health, and was funded by GSK. The manuscript was submitted by Heather Dowling of Fishawack Indicia Ltd, part of Fishawack Health, on behalf of the authors.
AA-S and MH are employees of GSK. FE has participated in an advisory board meeting for GSK. JA-S has no conflicts to disclose. AV and MK are employees of GSK and hold stocks and shares in the company.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by GSK.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD
Munther Khamashta https://orcid.org/0000-0002-8927-6024
References
- 1.Mahajan A, Amelio J, Gairy K, et al. Systemic lupus erythematosus, lupus nephritis and end-stage renal disease: a pragmatic review mapping disease severity and progression. Lupus 2020; 29: 1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tektonidou MG, Dasgupta A, Ward MM. Risk of end-stage renal disease in patients with lupus nephritis. Arthritis & Rheumatol (Hoboken, NJ) 1971; 68: 1432–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanly JG, O'Keeffe AG, Su L, et al. The frequency and outcome of lupus nephritis: results from an international inception cohort study. Rheumatology (Oxford) 2016; 55: 252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Arfaj AS, Al-Balla SR, Al-Dalaan AN, et al. Prevalence of systemic lupus erythematosus in central Saudi Arabia. Saudi Med J 2002; 23: 87–89. [PubMed] [Google Scholar]
- 5.Al Dhanhani AM, Agarwal M, Othman YS, et al. Incidence and prevalence of systemic lupus erythematosus among the native Arab population in UAE. Lupus 2017; 26: 664–669. [DOI] [PubMed] [Google Scholar]
- 6.Gachpazan M, Akhlaghipour I, Rahimi HR, et al. Genetic and molecular biology of systemic lupus erythematosus among Iranian patients: an overview. Auto- Immun highlights 2021; 12: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus 2006; 15: 308–318. [DOI] [PubMed] [Google Scholar]
- 8.Contreras G, Lenz O, Pardo V, et al. Outcomes in African Americans and Hispanics with lupus nephritis. Kidney Int 2006; 69: 1846–1851. [DOI] [PubMed] [Google Scholar]
- 9.AlSaleh J, Jassim V, ElSayed M, et al. Clinical and immunological manifestations in 151 SLE patients living in Dubai. Lupus 2008; 17: 62–66. [DOI] [PubMed] [Google Scholar]
- 10.Fanouriakis A, Kostopoulou M, Alunno A, et al. Update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis 2019; 78: 736–745. [DOI] [PubMed] [Google Scholar]
- 11.Fanouriakis A, Kostopoulou M, Cheema K, et al. Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis 2019; 79: 713–723. [DOI] [PubMed] [Google Scholar]
- 12.Hahn BH, McMahon MA, Wilkinson A, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care & Res 2012; 64: 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mok CC. A consensus for the management of systemic lupus erythematosus in Asia. J Clin Rheumatol Immunol 2021; 21: 1–6. [Google Scholar]
- 14.Rovin BH, Adler SG, Barratt J, et al. Executive summary of the KDIGO 2021 guideline for the management of glomerular diseases. Kidney Int 2021; 100: 753–779. [DOI] [PubMed] [Google Scholar]
- 15.Al Arrayed A, George SM, Malik AK, et al. The spectrum of glomerular diseases in the kingdom of Bahrain: an epidemiological study based on renal biopsy interpretation. Transplant Proc 2004; 36: 1792–1795. [DOI] [PubMed] [Google Scholar]
- 16.Al Arrayed AA, Shariff S, Maamari MM. Kidney disease in Bahrain: a biopsy-based epidemiological study. Transplant Proc 2007; 39: 875–878. [DOI] [PubMed] [Google Scholar]
- 17.Al-Homrany M, Alghamdi S, Al-Hwiesh A, et al. Pattern of renal diseases and the need for establishment of renal biopsy registry in Saudi Arabia. Saudi Journal Kidney Diseases Transplantation: Official Publication Saudi Cent Organ Transplant Saudi Arabia 2019; 30: 628–633. [DOI] [PubMed] [Google Scholar]
- 18.Alyousef A, AlSahow A, Alhelal B, et al. SAT-379 Glomerulonephritis histopathological pattern change. Kidney Int Rep 2020; 5: S159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohan D, Siddika A, Al Alawi F, et al. SAT-416 Pattern of biopsy proven renal disease in adults: single-centre experience from a tertiary care hospital in dubai, UAE. Kidney Int Rep 2020; 5: S174. [Google Scholar]
- 20.Nawaz Z, Mushtaq F, Mousa D, et al. Pattern of glomerular disease in the Saudi population: a single-center, five-year retrospective study. Saudi J Kidney Dis Transpl, 2013, 24. 1265–1270. [DOI] [PubMed] [Google Scholar]
- 21.Shawarby M, Al Tamimi D, Al Mueilo S, et al. A clinicopathologic study of glomerular disease: experience of the King Fahd Hospital of the University, Eastern Province, Saudi Arabia. Hong Kong J Nephrol 2010; 12: 20–30. [Google Scholar]
- 22.Abu-Romeh SH, van der Meulen J, Cozma MC, et al. Renal diseases in Kuwait. Experience with 244 renal biopsies. Int Urol Nephrol 1989; 21: 25–29. [DOI] [PubMed] [Google Scholar]
- 23.Al Riyami D. The spectrum of glomerular diseases on renal biopsy: data from a single tertiary center in Oman. Oman Med J 2013; 28: 213–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alkhfaji D, Nabi Z, Abdelsalam M, et al. A clinicopathology correlation of lupus nephritis: a retrospective analysis of renal biopsies. Nephrology 2010; 15: 44–45. [Google Scholar]
- 25.Alkindi F, Salih F, Salih M, et al. SUN-433 Spectrum of biopsy proven renal pathologies over 10 years. Kidney Int Rep 2020; 5: S375–S376. [Google Scholar]
- 26.Alwahaibi NY, Alhabsi TA, Alrawahi SA. Pattern of glomerular diseases in Oman: a study based on light microscopy and immunofluorescence. Saudi J Kidney Dis Transpl, 2013, 387–391. [DOI] [PubMed] [Google Scholar]
- 27.Alkhunaizi AM. Pattern of renal pathology among renal biopsy specimens in Eastern Saudi Arabia. Saudi Med J 2007; 28: 1676–1681. [PubMed] [Google Scholar]
- 28.Al Arfaj AS, Khalil N, Al Saleh S. Lupus nephritis among 624 cases of systemic lupus erythematosus in Riyadh, Saudi Arabia. Rheumatol Int 2009; 29: 1057–1067. [DOI] [PubMed] [Google Scholar]
- 29.Al-Rayes H, Al-Swailem R, Arfin M, et al. Systemic lupus erythematosus and infections: a retrospective study in Saudis. Lupus 2007; 16: 755–763. [DOI] [PubMed] [Google Scholar]
- 30.Qari FA. Clinical pattern of systemic lupus erythematosus in Western Saudi Arabia. Saudi Med J 2002; 23: 1247–1250. [PubMed] [Google Scholar]
- 31.Al Rasbi A, Abdalla E, Sultan R, et al. Spectrum of systemic lupus erythematosus in Oman: from childhood to adulthood. Rheumatol Int 2018; 38: 1691–1698. [DOI] [PubMed] [Google Scholar]
- 32.Ale'ed AA, Al-Mayouf SM. Systemic lupus erythematosus in Arab children. Differences and similarities with different ethnicities. Saudi Med J 2014; 35: 566–571. [PubMed] [Google Scholar]
- 33.Akbarian M, Faezi ST, Akhlaghkhah M, et al. Lupus nephritis: a 33-year experience. Lupus 2010; 19: 60. [Google Scholar]
- 34.Fatemi A, Matinfar M, Maracy M, et al. Outcome of systemic lupus erythematosus in Iran. Lupus 2014; 23: 463. [DOI] [PubMed] [Google Scholar]
- 35.Alsuwaida A, Husain S, Alghonaim M, et al. Strategy for second kidney biopsy in patients with lupus nephritis. Nephrol Dial Transpl 2012; 27: 1472–1478. [DOI] [PubMed] [Google Scholar]
- 36.Alaiya A, Assad L, Alkhafaji D, et al. Proteomic analysis of Class IV lupus nephritis. Nephrol Dial Transplant, 2015, 62–70. [DOI] [PubMed] [Google Scholar]
- 37.Al Durahim H, Al Ghamdi G, Al Seraya A, et al. Predictors of mortality and end stage renal disease in Saudi patients with lupus nephritis. Lupus 2011; 20: 1329–1335. [DOI] [PubMed] [Google Scholar]
- 38.Alrowaie F, AlTurki M, Alhusani A, et al. MP206 Clinical features and outcome of lupus nephritis patients with crescent in renal biopsy. Nephrol Dial Transpl 2017; 32. [Google Scholar]
- 39.Alsuwaida A, Husain S, Al Ghonaim M, et al. Glomerular necrotic lesions and long-term outcomes among patients with proliferative lupus nephritis. Int J Clin Exp Pathol 2015; 8: 5787–5792. [PMC free article] [PubMed] [Google Scholar]
- 40.Albirdisi MR, Al-Homood IA. Characteristics of lupus nephritis in Saudi lupus patients: a retrospective observational study. Lupus 2020; 29: 1638–1643. [DOI] [PubMed] [Google Scholar]
- 41.Almalki AH, Alrowaie FA, Alhozali HM, et al. Remission and long-term outcomes of proliferative lupus nephritis: retrospective study of 96 patients from Saudi Arabia. Lupus 2019; 28: 1082–1090. [DOI] [PubMed] [Google Scholar]
- 42.Alhijji AA, Alswaida A, Kfouri H, et al. Lupus nephritis induction in Saudi Arabia; a multicenter retrospective study. Int J Rheum Dis 2017; 20: 64. [Google Scholar]
- 43.Mitwalli AH, Al Wakeel JS, Hurraib S, et al. Comparison of high and low dose of cyclophosphamide in lupus nephritis patients: a long-term randomized controlled trial. Saudi J Kidney Dis Transpl, 2011, 935–940. [PubMed] [Google Scholar]
- 44.Alsuwaida AO, Bakhit AA, Alsuwaida FA, et al. The long-term outcomes and histological transformation in class II lupus nephritis. Saudi Med J 2018; 39: 990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al Sahow A. SP197 Rituximab use for glomerular disorders in Kuwait. Nephrol Dial Transpl 2019; 34. [Google Scholar]
- 46.Gheith O, Alotaibi T, Nair P, et al. Systemic lupus erythematosus among renal transplant recipients: single center experience. Transpl Int 2017; 30: 522. [Google Scholar]
- 47.El-Reshaid K, El-Reshaid W, Al-Bader S, et al. New protocols for treatment of class IV lupus nephritis with emphasis on Rituximab as the sole maintenance therapy. Kuwait Med J 2018; 50: 343–350. [Google Scholar]
- 48.Elbadawi F, Khamashta M, Elsidig N, et al. AB0504 clinicopathological characteristics as possible predictor of renal outcome in patients with active lupus nephritis following the induction of remission phase. Ann Rheum Dis 2019; 78: 1715. [Google Scholar]
- 49.Abdulla N, Fituri O, Alam F, et al. Lupus Nephritis Qatar. Clin Exp Rheumatol 2016; 34: S94. [Google Scholar]
- 50.Al-Mayouf SM, AlAmeer A, Alfattani A, et al. Outcome of childhood lupus nephritis in Saudi children. Saudi J Kidney Dis Transpl 2017; 28, 1015–1020. [DOI] [PubMed] [Google Scholar]
- 51.Muzaffer M, Sawan A. Long term outcome of lupus nephritis in Saudi children. Intern Med J 2012; 42: 21. [Google Scholar]
- 52.Hayat SJ, Uppal SS, Narayanan Nampoory MR, et al. Safety and efficacy of infliximab in a patient with active WHO class IV lupus nephritis. Clin Rheumatol 2007; 26: 973–975. [DOI] [PubMed] [Google Scholar]
- 53.Dileep KS, Juma Alalawi F, Mohamed Hussei R, et al. SUN-273 Patients treated with therapeutic plasma exchange: a single center experience. Kidney Int Rep 2020; 5: S313. [Google Scholar]
- 54.Gupta RK, Nampoory MRN, Johny KV, et al. Intravenous immunoglobulins in lupus nephritis. Med Principles Pract 2001; 10: 197–203. [Google Scholar]
- 55.Hussein MM, Mooij JM, Al Malki N, et al. Late response to rituximab in a dialysis patient with severe lupus nephritis. Saudi J Kidney Dis Transpl 2013; 24: 976–980. [DOI] [PubMed] [Google Scholar]
- 56.Alsuwaida AO. The clinical significance of serial kidney biopsies in lupus nephritis. Mod Rheumatol 2014; 24: 453–456. [DOI] [PubMed] [Google Scholar]
- 57.Ahmadzadeh A, Derakhshan A, Ahmadzadeh A. A clinicopathological study of lupus nephritis in children. Saudi J Kidney Dis Transpl, 2008, 756–760. [PubMed] [Google Scholar]
- 58.Fatemi A, Kazemi M, Sayedbonakdar Z, et al. Long-term outcome of biopsy-proven lupus nephritis in Iran. Int J Rheum Dis 2013; 16: 739–746. [DOI] [PubMed] [Google Scholar]
- 59.Taheri S, Beiraghdar F. Lupus nephritis in Iranian children: a review of 60 patients. Renal failure 2011; 33: 499–505. [DOI] [PubMed] [Google Scholar]
- 60.Pakfetrat M, Malekmakan L, Kamranpour M, et al. A five consecutive years' study of renal function outcome among biopsy proven lupus nephritis patients in Southern Iran. Lupus 2017; 26: 1082–1088. [DOI] [PubMed] [Google Scholar]
- 61.Bartels-Peculis L, Sharma A, Edwards AM, et al. Treatment patterns and health care costs of lupus nephritis in a United States Payer population. Open access Rheumatol: Res Rev 2020; 12: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aldarmaki R, Khogali H, AlDhanhani A. Cause and rate of hospitalization of lupus patients. Arthritis Rheumatol 2018; 70: 1303. [Google Scholar]
- 63.Aldarmaki R, Al Khogali HI, Al Dhanhani AM. Hospitalization in patients with systemic lupus erythematosus at Tawam Hospital, United Arab Emirates (UAE): rates, causes, and factors associated with length of stay. Lupus 2021; 30: 845–851. [DOI] [PubMed] [Google Scholar]
- 64.Alalawi F, Ahmed M, AlNour H, et al. Epidemiology of end-stage renal disease in Dubai: single-center data. Saudi J Kidney Dis Transpl, 2017, 1119–1125. [DOI] [PubMed] [Google Scholar]
- 65.Mitwalli AH, Hayat A, Alwakeel J, et al. Effects of concomitant hepatitis C virus infection in patients with underlying lupus nephritis on long-term renal outcome. Nephrol Dial Transpl 2012; 27: 627–632. [DOI] [PubMed] [Google Scholar]
- 66.Al-Zahrani IH, Qayyum A. Lupus Nephritis Clinicopathological Correlation. Saudi Medical Journal 2007; 28: 1503–1505. [PubMed] [Google Scholar]
- 67.Faezi ST, Hosseini Almodarresi M, Akbarian M, et al. Clinical and immunological pattern of systemic lupus erythematosus in men in a cohort of 2355 patients. Int J Rheum Dis 2014; 17: 394–399. [DOI] [PubMed] [Google Scholar]
- 68.Poil AR, Hammoudeh M, Chandra P, et al. Pregnancy outcome in 69 pregnancies of multinational population in Qatar with systemic lupus erythematosus. Arthritis Rheum 2013; 65: S1079. [Google Scholar]
- 69.Rahman FZ, Rahman J, Al-Suleiman SA, et al. Pregnancy outcome in lupus nephropathy. Arch Gynecol Obstet 2005; 271: 222–226. [DOI] [PubMed] [Google Scholar]
- 70.Nampoory MRN, Al Otaibi T, Gheith O, et al. Impact of systemic lupus erythematosus on the outcome of renal transplant recipients: single center experience. Exp Clin Transplant 2014; 12: 308. [Google Scholar]
- 71.Shahgholi N, Faezi ST, Hakemi M, et al. Short and long term outcome in lupus nephritis. Int J Rheum Dis 2021; 24. [Google Scholar]
- 72.Faezi ST. Clinical picture of lupus nephritis in Persian patients with systemic lupus erythematosus (SLE): results of a large survey. Lupus Sci Med 2017; 4: A184. [Google Scholar]
- 73.Nejati MR, Asgari M, Ossareh S, et al. Lupus Nephritis Iran. Lab Invest 2010; 90: 343A–344A. [Google Scholar]
- 74.Nezhad ST, Sepaskhah R. Correlation of clinical and pathological findings in patients with lupus nephritis: a five-year experience in Iran. Saudi J Kidney Dis Transpl 2008; 19: 32–40. [PubMed] [Google Scholar]
- 75.Shariati-Sarabi Z, Ranjbar A, Monzavi SM, et al. Analysis of clinicopathologic correlations in Iranian patients with lupus nephritis. Int J Rheum Dis 2013; 16: 731–738. [DOI] [PubMed] [Google Scholar]
- 76.Shariati-Sarabi Z, Monzavi SM, Ranjbar A, et al. High disease activity is associated with high disease damage in an Iranian inception cohort of patients with lupus nephritis. Clin Exp Rheumatol 2013; 31: 69–75. [PubMed] [Google Scholar]
- 77.Qayyum A, Nagy AA. Immuno-histological changes in lupus nephritis in female patients: a four-year study. Saudi J Kidney Dis Transpl 2008; 19: 658–663. [PubMed] [Google Scholar]
- 78.Farid EM, Hassan AB, Abalkhail AA, et al. Immunological aspects of biopsy-proven lupus nephritis in Bahraini patients with systemic lupus erythematosus. Saudi J Kidney Dis Transpl 2013; 24: 1271–1279. [DOI] [PubMed] [Google Scholar]
- 79.Al Attia HM, Al Ahmed YH, Chandani AU. Serological markers in Arabs with lupus nephritis. Lupus 1998; 7: 198–201. [DOI] [PubMed] [Google Scholar]
- 80.Alfadhli S, Alfailakawi A, Ghanem AAM. Th-17 related regulatory network in the pathogenesis of Arab patients with systemic lupus erythematosus and lupus nephritis. Int J Rheum Dis 2016; 19: 512–520. [DOI] [PubMed] [Google Scholar]
- 81.Alfadhli S, Ghanem AAM, Nizam R. Genome-wide differential expression reveals candidate genes involved in the pathogenesis of lupus and lupus nephritis. Int J Rheum Dis 2016; 19: 55–64. [DOI] [PubMed] [Google Scholar]
- 82.Alsuwaida AO. Interstitial inflammation and long-term renal outcomes in lupus nephritis. Lupus 2013; 22: 1446–1454. [DOI] [PubMed] [Google Scholar]
- 83.Caster DJ, Powell DW. Utilization of biomarkers in lupus nephritis. Adv chronic kidney Dis 2019; 26: 351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dall'Era M, Cisternas MG, Smilek DE, et al. Predictors of long-term renal outcome in lupus nephritis trials: lessons learned from the Euro-Lupus Nephritis cohort. Arthritis & Rheumatol (Hoboken, NJ) 2015; 67: 1305–1313. [DOI] [PubMed] [Google Scholar]
- 85.Tamirou F, D'Cruz D, Sangle S, et al. Long-term follow-up of the MAINTAIN Nephritis Trial, comparing azathioprine and mycophenolate mofetil as maintenance therapy of lupus nephritis. Ann Rheum Dis 2016; 75: 526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Moroni G, Quaglini S, Radice A, et al. The value of a panel of autoantibodies for predicting the activity of lupus nephritis at time of renal biopsy. J Immunol Res 2015; 2015: 106904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Venuturupalli S. Rethinking biologics in lupus nephritis. Lupus 2016; 25: 1102–1110. [DOI] [PubMed] [Google Scholar]
- 88.Furie R, Rovin BH, Houssiau F, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. New Engl J Med 2020; 383: 1117–1128. [DOI] [PubMed] [Google Scholar]
- 89.Rovin BH, Teng YKO, Ginzler EM, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet (London, England) 2021; 397: 2070–2080. [DOI] [PubMed] [Google Scholar]
- 90.The Food and Drug Administration . LUPKYNIS (voclosporin); Highlights of prescribing information 2021. Food & Drug Administration Online. [Google Scholar]
- 91.The Food and Drug Administration . BENLYSTA (belimumab); Highlights of prescribing information 2017. Food & Drug Administration Online. [Google Scholar]
- 92.Ayorinde AA, Williams I, Mannion R, et al. Assessment of publication bias and outcome reporting bias in systematic reviews of health services and delivery research: a meta-epidemiological study. PloS one 2020; 15: e0227580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Literature review of lupus nephritis From the Arabian Gulf region (9/50 words max) by Arwa Al-Shujairi, Faisal Elbadawi, Jamal Al-Saleh, Mohamed Hamouda, Averyan Vasylyev and Munther Khamashta in Lupus