Abstract
Traditionally, the management of type B aortic dissection has been the domain of the vascular surgeons. Timing and type of intervention still generate debate. We sought to review our early experience with the treatment of this condition based on a hybrid approach following an aortic multi‐disciplinary team meeting involving close cooperation between cardiac surgeons, vascular surgeons, interventional radiologists, vascular anesthetists, and cardiac anesthetists. Four patients (age 41–56 years; 3 males; 1 female) with type B aortic dissection underwent aortic arch surgery through a hybrid approach: one elective procedure consisting of ascending aorta and hemi‐arch replacement with debranching followed by thoracic endovascular aortic repair (TEVAR); one redo procedure requiring aortic arch replacement with hybrid frozen elephant trunk; two acute presentations (aortic arch replacement and debranching followed by TEVAR; AVR with ascending aorta, arch, and proximal descending thoracic aorta replacement with conventional elephant trunk and debranching). Deep hypothermic circulatory arrest was required in three patients. Despite respiratory complications and slightly prolonged postoperative course, all patients survived without onset of stroke, paraplegia, malperfusion, endoleak, or need for re‐exploration. Follow‐up remains satisfactory. Different factors may affect outcome following complex aortic procedures. Nevertheless, close cooperation between cardiac surgeons, vascular surgeons, and interventional radiologists may reduce potential for complications and address aspects that may not be completely within the domain of individual specialists.
Keywords: aortic arch, aortic team, hybrid approach, PETTYCOAT and STABILIZE technique, surgery, TEVAR, type B aortic dissection
Surgical treatment for aortic disease remains challenging and demanding. Treatment often needs to be tailored to the patient or the group of patients being referred. A hybrid approach may have a role to play as an additional option to the surgical armamentarium. Cooperation between different specialists is essential.
1. INTRODUCTION
Thoracic endovascular aortic repair (TEVAR) has witnessed an increase in its indications following the advancement of graft technology. Initially considered for the treatment of thoracic aortic aneurysm disease, TEVAR has evolved to treat other aortic conditions such as penetrating atherosclerotic ulcer and intramural hematoma, aortic pseudoaneurysm, type B aortic dissection, blunt trauma, thoracic aortic injury, aorto‐esophageal, and aorto‐bronchial fistula. Nevertheless, an appropriate landing zone remains the key element for the success of this approach. Traditionally, the management of type B aortic dissection has been the domain of the vascular surgeons. The availability of combined vascular and endovascular techniques has allowed treatment in patients with previous surgery and those with multiple co‐morbidities. 1 Conservative management still remains the initial treatment for uncomplicated type B aortic dissection while an endovascular approach is considered the main treatment for patients with complicated disease. 2 , 3 The aim of endovascular treatment is to address the primary entry tear and reduce blood pressure in the false lumen, which may lead to its thrombosis, aortic remodeling, and wall stabilization. 4 , 5 , 6 , 7 , 8 There is an increasing evidence supporting the role of an interventional approach for the treatment of complicated type B aortic dissection 9 , 10 , 11 , 12 , 13 , 14 where endovascular intervention (TEVAR) has been more favorable compared to surgical treatment in terms of early mortality. 13 , 15 , 16 , 17 , 18 , 19 Nevertheless, TEVAR‐related complications may require surgery as definitive treatment. 20 TEVAR has also been advocated for the treatment of uncomplicated acute type B aortic dissection with reduced mortality at 5 years compared to medical treatment 21 and potentially for less physiologically fit patients. 22 The outcome of surgical treatment for acute type B aortic dissection is significantly affected by preoperative conditions 23 with high complications and mortality rate 24 , 25 , 26 although results have improved over the years. 24 , 26 , 27 , 28 Open repair is usually indicated following failure of endovascular treatment (persistent endoleak with or without contained rupture, retrograde dissection, stent migration) or for those patients who are not suitable for a TEVAR approach. 29 Nevertheless, open repair remains the treatment of choice in patients with connective tissue disease such as Marfan, Ehlers‐Danlos, and Loeys‐Dietz. 2 , 30 In view of these considerations, we sought to review our early experience based on a hybrid approach requiring a close cooperation between cardiac surgeons, vascular surgeons, and interventional radiologists.
2. PATIENTS AND METHODS
Four patients with type B aortic dissection are presented with details of the surgical approach used for their management. Further consent was waived due to the retrospective nature of the study.
2.1. Case 1
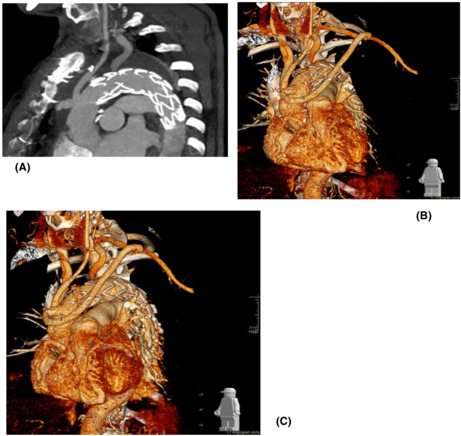
A 56‐year‐old female patient who presented with expanding distal aortic arch and proximal descending thoracic aorta (6 cm in diameter) following conservative management of type B aortic dissection with thrombosed false lumen (Figure 1A). Additional features included concomitant dilatation of the ascending aorta (5 cm in diameter), mild to moderate aortic regurgitation, and preserved left ventricular systolic function and also previous repair of abdominal aortic aneurysm 13 years earlier; right hemicolectomy with permanent ileostomy for colonic carcinoma 3 years earlier; hysterectomy and bilateral oophorectomy for recurrent cancer 2 months earlier. Additional comorbidities included COPD and rheumatoid arthritis with limited mobility. Given the absence of a suitable proximal landing zone for TEVAR, the outcome of the aortic MDT was in favor of a hybrid approach consisting of aortic arch debranching and ascending aorta replacement followed by TEVAR. Invasive arterial and venous monitoring was obtained through the left and right radial artery and the right internal jugular vein. The chest was entered through a median sternotomy. A Gelweave Dacron trifurcated arch graft (Vascutek) was used for the anastomosis of each epiaortic vessel in an end‐to‐end fashion leading to complete debranching of the aortic arch. Subsequently, cardiopulmonary bypass was established through right atrial cannulation and arterial return to the side arm of the trifurcated Dacron graft. A left ventricular vent was inserted through the right superior pulmonary vein. Antegrade and retrograde cold blood cardioplegia was delivered for myocardial protection. Body temperature was reduced to 30°C. Non‐invasive monitoring of cerebral oxygen saturation was maintained with near‐infrared spectroscopy (NIRS) using INVOS™ 5100C oximeter monitoring system. Following aortic cross‐clamping between the innominate artery and left common carotid artery with added vascular clamping to the base of the innominate artery, the ascending aortic aneurysm was excised including the proximal portion of the aortic arch. A 28 mm Gelweave interposition Dacron graft (Vascutek) was used without circulatory arrest. Following de‐airing and removal of the aortic cross‐clamp, the circulation was rewarmed. Weaning off cardiopulmonary bypass was uneventful. The proximal end of the trifurcated graft was connected to the ascending aortic graft in an end‐to‐side fashion and was marked using Ligaclips to aid subsequent TEVAR procedure. The epi‐aortic vessels were tied and clipped at their aortic origin. Trans‐esophageal echocardiographic assessment showed mild residual aortic regurgitation and preserved biventricular systolic function. Protamine was administered, and hemostasis was achieved. Four drains were inserted, and the chest was closed in layers. Finally, the patient was transferred to the intensive care unit for continuity of care. A TEVAR procedure was performed uneventfully 12 days postoperatively. A ProGlide preclose technique was used to allow a 22Fr percutaneous right common femoral artery access. A stiff support wire (Meier wire) was introduced into the proximal ascending aorta. Angiography demonstrated the neo‐landing zone within the graft (Figure 2). A Gore cTAG 37 × 20 was implanted into the ascending graft and extended with a further Gore cTAG. Finally, the seal zones were balloon molded. Angiography confirmed exclusion of false lumen with widely patent true lumen, bypass grafts, and visceral vessels (Figure 2).
FIGURE 1.
Surface shading reconstruction from CT angiogram. (A) Thoracic aortic aneurysms of the ascending and proximal descending thoracic aorta. (B) Repair with interposition graft to ascending aorta and proximally anastomosed bypass grafts to brachiocephalic, left common carotid, and left subclavian arteries. Remnant stumps of tied‐off great vessels are seen on the superior border of the aortic arch. The proximal and distal suture lines of the interposition graft are seen with a neo‐proximal landing zone created for completion of TEVAR. (C) Post‐TEVAR CT angiogram with successful exclusion of aortic aneurysm and patent bypass grafts
FIGURE 2.
Angiography in steep LAO pre‐ and post‐TEVAR. Pre‐graft implantation shows no suitable native landing zone, but suitable seal zone within the ascending graft. Completion angiography shows successful exclusion of the proximal descending thoracic aortic aneurysm with patent bypass grafts
2.2. Case 2
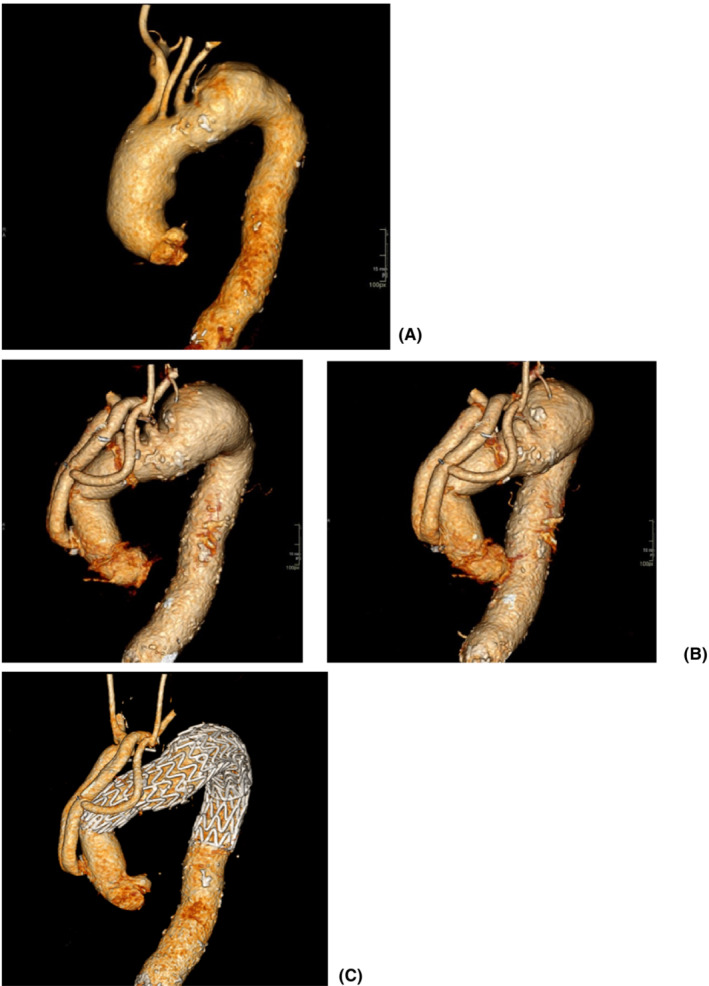
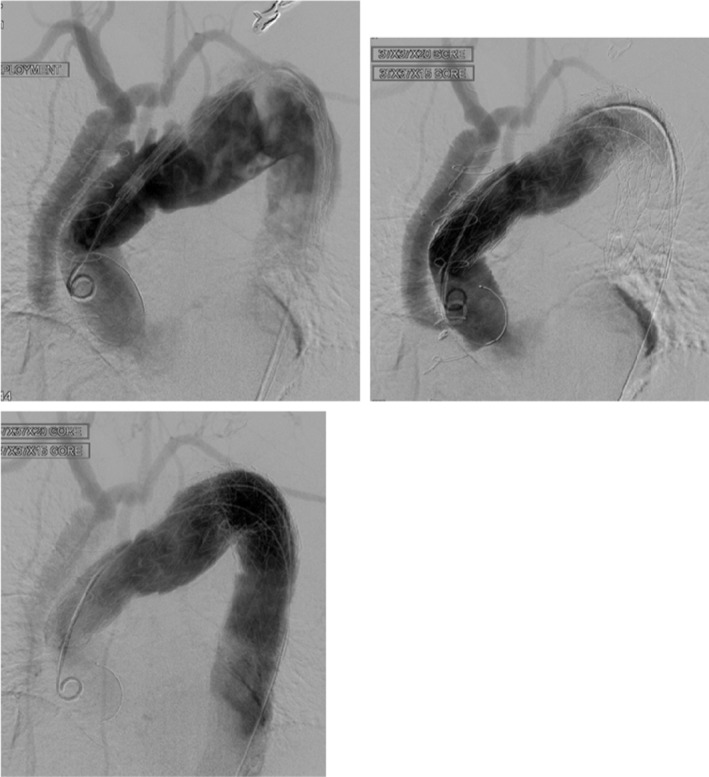
A 54‐year‐old male patient presented with sudden onset of central chest pain radiating to the back and transient right leg paresthesia. Imaging investigation showed the presence of an intramural hematoma in the distal aortic arch and a type B aortic dissection (Figure 3A). Comorbidities included long‐standing partially controlled hypertension, acute renal failure, and thrombocytopenia. The dissection affected the renal artery, and during imaging review, we felt the “shotgun” renal artery to be driving the hypertension and the reduced renal function. Therefore, the initial agreed approach following the aortic MDT was to proceed with renal stenting/fenestration to improve renal function and better control of his hypertension. Subsequently, the retrograde progression of the intramural hematoma into the arch required earlier surgical treatment given the absence of landing zone for TEVAR. Invasive arterial and venous monitoring was obtained through the left and right radial artery and the right internal jugular vein. The right axillary artery was exposed and anastomosed to 8 mm Dacron tubular graft in an end‐to‐side fashion. The chest was entered through a median sternotomy. The epi‐aortic vessels were identified and looped. Cardiopulmonary bypass was established through right atrial cannulation and arterial return to the right axillary artery previously connected to a 22Fr EOPA cannula and direct cannulation of the ascending aorta with a 24Fr EOPA cannula using a Seldinger technique. A left ventricular vent was inserted through the right superior pulmonary vein. Antegrade and retrograde cold blood cardioplegia was delivered for myocardial protection. Body temperature was reduced to 18°C. Non‐ invasive monitoring of cerebral oxygen saturation was maintained with near‐infrared spectroscopy (NIRS) using INVOS™ 5100C oximeter monitoring system. The epi‐aortic vessels were divided and anastomosed in an end‐to‐end fashion to a Gelweave Dacron trifurcated arch graft (Vascutek) according to a “branch‐first” technique during the cooling phase. Then, circulatory arrest was initiated, the ascending aortic cannula was removed, and antegrade cerebral perfusion delivered through the previously cannulated right axillary artery and subsequently through the trifurcated graft maintaining continuous bilateral cerebral perfusion. The ascending aorta was opened, and the incision extended to the aortic arch. A 28 mm side‐branched Gelweave Dacron graft (Vascutek) was anastomosed distally at the origin of the left common carotid artery (Zone 1). Following completion of the anastomosis, distal antegrade perfusion was restarted through the side branch of the graft and rewarming initiated. Then, the graft was clamped, the aortic valve leaflets were re‐suspended, and the proximal anastomosis was completed. Air was evacuated, and the clamp was removed with spontaneous return of the heart into sinus rhythm. Finally, the proximal end of the trifurcated graft was connected to the newly created ascending aorta in an end‐to‐side fashion and was marked using Ligaclips to aid subsequent TEVAR procedure. Weaning off cardiopulmonary bypass was uneventful. Trans‐esophageal echocardiographic assessment showed preserved biventricular systolic function and absence of aortic regurgitation. Protamine was administered, and hemostasis was achieved. Two chest drains were inserted, and the chest was closed in layers. Finally, the patient was transferred to the intensive care unit for continuity of care. A TEVAR procedure was performed uneventfully 2 months later based on a PETTICOAT and STABILIZE technique. 31 A ProGlide preclose technique was used to allow percutaneous 24Fr right common femoral artery access. A Gore cTAG was partially deployed immediately beyond the great vessel bypass graft. 100% inner curve angulation was utilized and the graft fully deployed. A completion TEVAR was achieved just proximally to the coeliac axis, followed by Cook Zenith dissection stent to aortic bifurcation (PETTICOAT technique) prior to STABILIZE technique. A catheter was placed in the false lumen prior to aortic balloon molding to provide landmark for true lumen expansion; potentially aid target vessel catheterization in the event of occlusion post‐molding; confirm false lumen obliteration; and finally provide access for false lumen coil/plug embolization if needed. Balloon molding of the whole stented aorta was performed from proximal graft to aortic bifurcation with visible disruption of the aortic flap. Angiography confirmed exclusion of false lumen with widely patent true lumen, bypass grafts, and visceral vessels (Figure 4).
FIGURE 3.
CT aortic angiogram. (A) Type B aortic dissection extending to the aortic bifurcation; additional retrograde extension with intramural hematoma extending to the aortic valve; absence of genuine proximal landing zone for TEVAR; evidence of end‐organ ischemia with shotgun right renal artery and delayed right nephrogram in the context of deteriorating renal function. (B) Surface shading reconstruction after surgical procedure with creation of a suitable landing zone for TEVAR and persistent dissection beyond the distal anastomosis. (C) Surface shading reconstruction 1‐year post‐intervention with widely patent true lumen and bypass grafts; total aortic remodeling and complete obliteration of the false lumen
FIGURE 4.
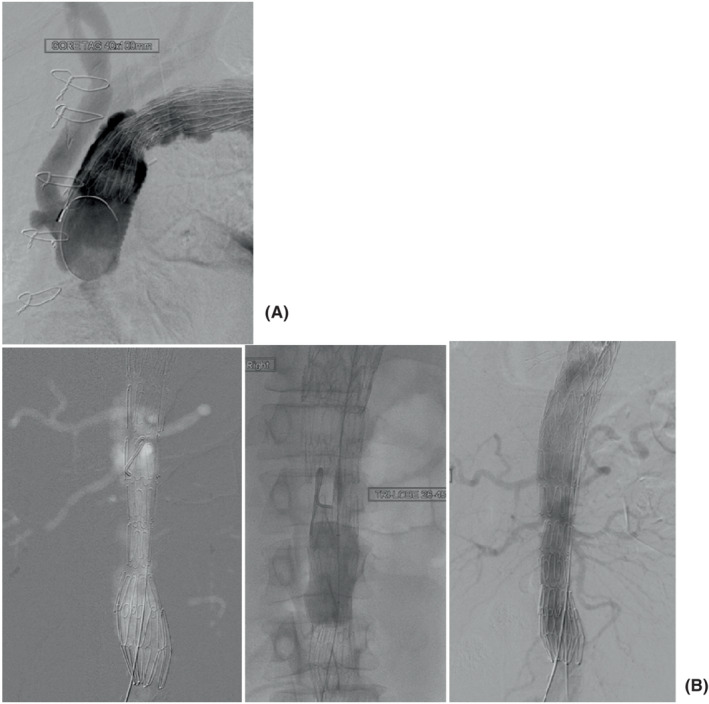
(A) Angiography demonstrates suitable proximal landing zone in the ascending aortic interposition graft with patent bypass grafts. Gore cTAG thoracic graft partially deployed with 100% angulation applied to maximize inner curve wall apposition to prevent “bird‐beaking.” (B) CO2 angiography to minimize nephrotoxic iodinated contrast shows implanted Cook Zenith dissection stent through the abdominal aorta (PETTICOAT technique), but with compression in the true lumen and persistent flow in the false lumen. Middle fluoroscopic grab image shows molding balloon dilatation of true lumen (STABILIZE technique). Final angiogram shows widely patent true lumen and visceral vessels with obliteration of false lumen
2.3. Case 3
A 41‐year‐old male patient presented with acute abdominal pain to the Emergency Department. Imaging investigation revealed the presence of non‐A non‐B distal aortic arch dissection with large entry tear and compression of the true lumen, dilatation of the aortic root (4.2 cm in diameter) and the ascending aorta (5 cm in diameter), and bovine aortic arch anatomy with separate origin of the left vertebral artery (Figure 5). Echocardiographic assessment showed a bicuspid aortic valve with moderate to severe regurgitation and preserved left ventricular systolic function. Comorbidities included renal impairment and refractory hypertension despite full medical treatment with multiple agents. The outcome of the aortic MDT was in favor of total aortic arch replacement and TEVAR if required to address further tear in the proximal descending thoracic aorta to avoid expansion of the false lumen. Invasive arterial and venous monitoring was obtained through the left and right radial artery, the right femoral artery, and the right internal jugular vein. The right axillary artery was exposed and anastomosed to 8 mm Dacron tubular graft in an end‐to‐side fashion. The chest was entered through a median sternotomy. The epi‐aortic vessels were identified and looped. Anatomical features were consistent with a bovine aortic arch with separate origin of the left vertebral artery from the aortic arch. Cardiopulmonary bypass was established through right atrial cannulation and arterial return to the right axillary artery and ascending aorta. A left ventricular vent was inserted through the right superior pulmonary vein. Antegrade cold blood cardioplegia was delivered directly through the coronary ostia for myocardial protection. Body temperature was reduced to 18°C. Non‐invasive monitoring of cerebral oxygen saturation was maintained with NIRS using INVOS™ 5100C oximeter monitoring system. The epi‐aortic vessels were divided and anastomosed in an end‐to‐end fashion to a Gelweave Dacron trifurcated arch graft (Vascutek) previously connected to the right axillary artery, according to the “branch‐first” technique. Then, circulatory arrest was initiated, the ascending aortic cannula was removed, and antegrade cerebral perfusion was delivered through the previously cannulated right axillary artery and subsequently through the trifurcated graft maintaining continuous bilateral cerebral perfusion. The ascending aorta was opened and the incision extended to the aortic arch followed by complete excision of the aneurysmal ascending aorta and aortic arch. The left subclavian artery and the aberrant left vertebral artery were divided on a common aortic wall button for anastomosis at a later stage. The distal anastomosis was performed in zone 3 with two separate Dacron tubular grafts: an internal 26 mm Dacron graft (4 cm in length) as an elephant trunk with an external Teflon strip to address the proximal descending thoracic aorta; a 28 mm side‐branched Gelweave Dacron tubular graft (Vascutek) to replace the aortic arch and the ascending aorta. Following completion of the anastomosis, distal antegrade perfusion was restarted through the side branch of the graft which was clamped proximally. The island including the left subclavian and the aberrant left vertebral artery was connected to the trifurcated graft in an end‐to‐side fashion and perfused followed by rewarming. Then, the native aortic valve was excised and replaced with a 25 mm On‐X mechanical prosthesis in the supra‐annular position using interrupted pledgeted sutures. The proximal anastomosis between the native aortic root and the newly created ascending aorta was completed. Finally, the proximal end of the trifurcated graft was anastomosed to the ascending aortic graft in an end‐to‐side fashion and was marked using Ligaclips to aid subsequent TEVAR procedure. The aortic cross‐clamp was released following de‐airing. Two atrial and two ventricular pacing wires were placed. Weaning off cardiopulmonary bypass was uneventful with satisfactory trans‐esophageal echocardiographic assessment. Hemostasis was achieved with blood products and surgical sealants. Four chest drains were inserted and the chest closed in layers. Subsequently, the patient was transferred to the intensive care unit for continuity of care.
FIGURE 5.
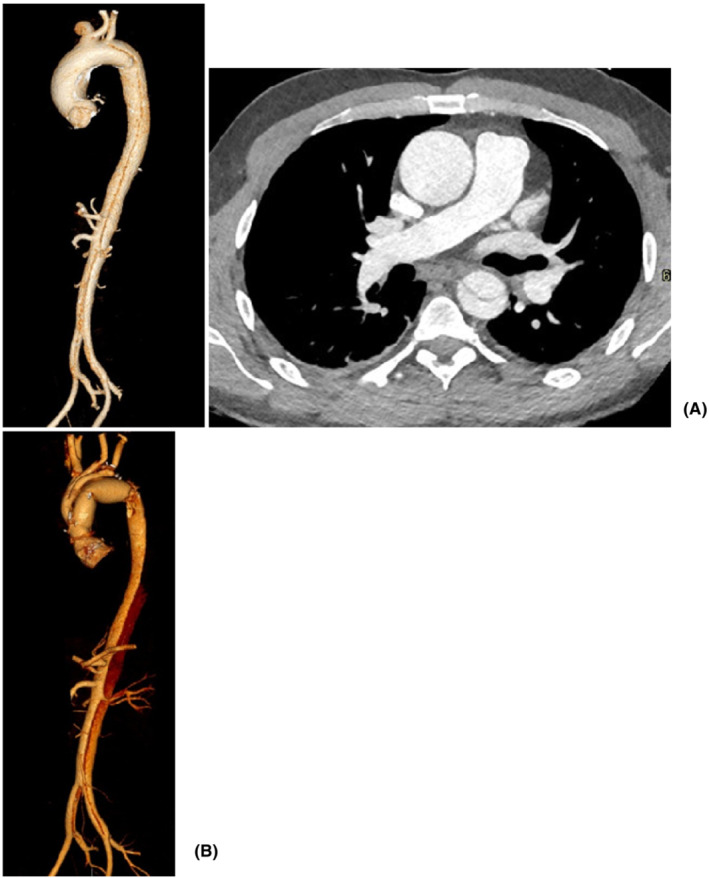
CT aortic angiogram. (A) Type B aortic dissection with the entry tear at the level of the left subclavian artery and concurrent ascending aortic aneurysm; (B) surface shading reconstruction following surgery
Satisfactory expansion of the true lumen (TL) was achieved as confirmed by imaging investigation postoperatively. The MDT decision was for surveillance with a view to TEVAR, or further surgery in the future should expansion of the false lumen occur.
2.4. Case 4
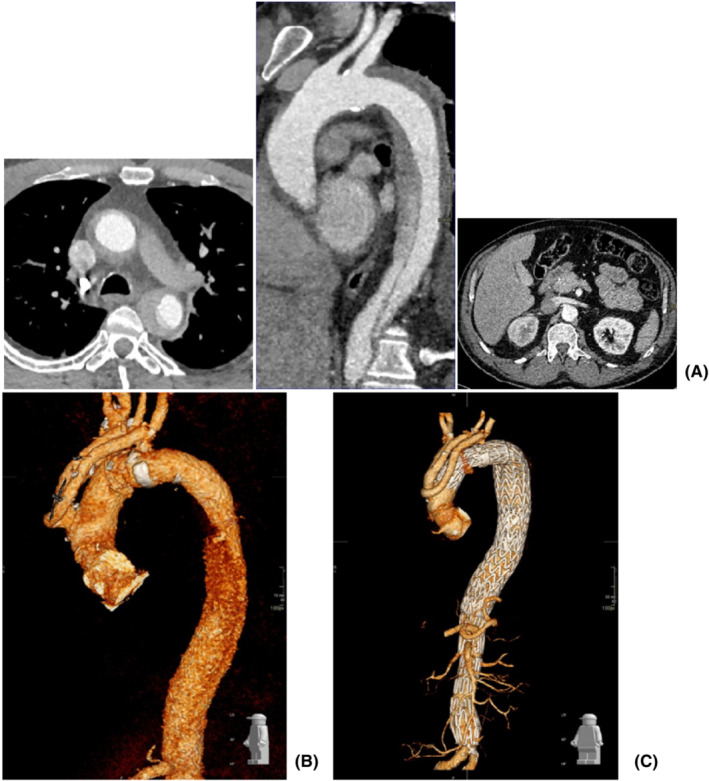
A 55‐year‐old male patient was referred to our unit for expanding false lumen in the aortic arch following repair of type A aortic dissection with interposition Dacron graft to the ascending aorta 7 months earlier in a different hospital (Figure 6). Imaging investigation confirmed increased dimension of the false lumen from 4 cm to 5.5 cm. The outcome of the aortic MDT was to proceed to elective aortic arch replacement with a hybrid frozen elephant trunk. Subsequently, he was admitted to his local hospital with further onset of chest pain and imaging investigation suspicious for new penetrating ulcer of the aortic arch distal to the origin of the left subclavian artery without active extravasation or mediastinal hematoma. Therefore, the patient was transferred to our intensive care unit for aggressive blood pressure control and expedition of the surgical procedure. Further CT aortic angiogram revealed anatomical stability but incompleteness of the circle of Willis, which played a key role for the choice of surgical strategy. Invasive arterial and venous monitoring was obtained through the left and right radial artery, and the right internal jugular vein. The left and right axillary arteries were exposed and anastomosed to 8 mm Dacron tubular graft in an end‐to‐side fashion. A guide wire was inserted through a 6Fr left common femoral artery access and directed into the true lumen of the aorta under fluoroscopic imaging to guide stent insertion at a later stage of the procedure. Cardiopulmonary bypass was established through percutaneous cannulation of the right femoral vein and arterial return to the axillary artery grafts bilaterally. The chest was entered through the previous median sternotomy. Extensive dissection was required due to strong adhesions from previous surgery. Body temperature was reduced to 18°C. Non‐invasive monitoring of cerebral oxygen saturation was maintained with NIRS using INVOS™ 5100C oximeter monitoring system. The innominate vein was doubly ligated and divided to facilitate access to the epi‐aortic vessels which were identified, dissected, and subsequently clamped to initiate antegrade cerebral perfusion through the previously cannulated right axillary artery. The innominate and the left common carotid arteries were de‐branched and anastomosed in an end‐to‐end fashion to a separate trifurcated Dacron graft. Following circulatory arrest, the ascending aortic Dacron graft was opened and the incision extended to the aortic arch. Antegrade cold blood cardioplegia was delivered directly through the coronary ostia for myocardial protection. The origin of the left subclavian artery was closed from the inside with pledgeted sutures in view of previous difficult access from the outside. A 34 mm Valiant Navion aortic Coveredseal stent graft (Medtronic) was advanced antegradely over the previously inserted wire and placed across the distal aortic arch and the proximal descending thoracic aorta. Balloon molding ensured further expansion and adhesion to the aortic wall. The proximal end of the stent was secured to the aortic wall at the origin of the left common carotid artery (zone 1) with double Teflon buttress using two layers of continuous Prolene sutures. A 32 mm side‐branched Gelweave Dacron tubular graft (Vascutek) was sutured to the stent to replace the aortic arch as a hybrid frozen elephant trunk. After completion of the distal anastomosis, the aortic arch graft was clamped and the systemic circulation was resumed. The proximal anastomosis between the newly created aortic arch and the previous ascending aortic graft was completed, and the clamp was removed. The proximal end of the trifurcated graft was anastomosed to the aortic arch graft in end‐to‐side fashion. Then, the left axillary artery graft was transposed into the mediastinum via tunneling through the intercostal space and sutured to the side arm of the aortic graft in end‐to‐end fashion. Following rewarming, cardiopulmonary bypass was weaned off uneventfully. Two atrial and two ventricular pacing wires were placed. Trans‐esophageal echocardiographic assessment showed preserved biventricular systolic function with appropriate placement and expansion of the stent in the proximal descending thoracic aorta. Hemostasis was achieved with packing, blood products, and surgical sealants. Four drains were inserted and the chest closed in layers. Aortic angiogram confirmed the satisfactory position of the endovascular stent without leakage and patency of the grafts to the epiaortic vessels. The patient was transferred to the intensive care unit for continuity of care. The postoperative CT‐scan showed persistent satisfactory outcome of the TEVAR procedure (Figure 7).
FIGURE 6.
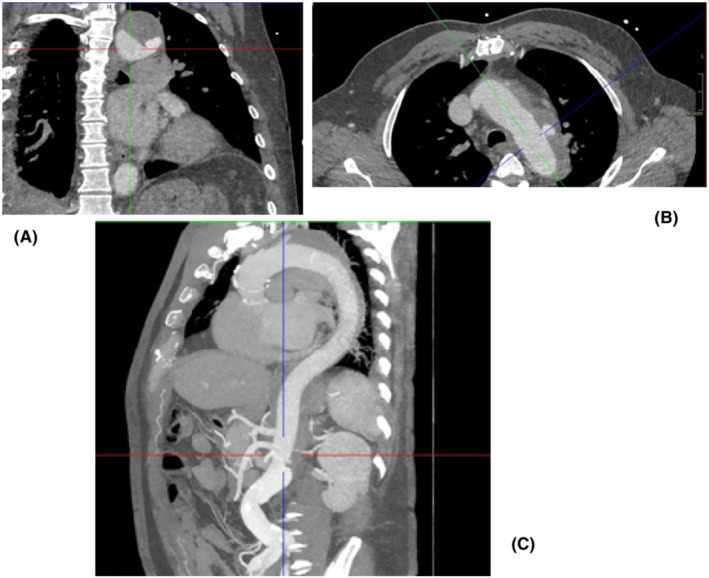
CT aortic angiogram showing frontal (A), transverse (B), and longitudinal (C) views of the expanding lesion
FIGURE 7.
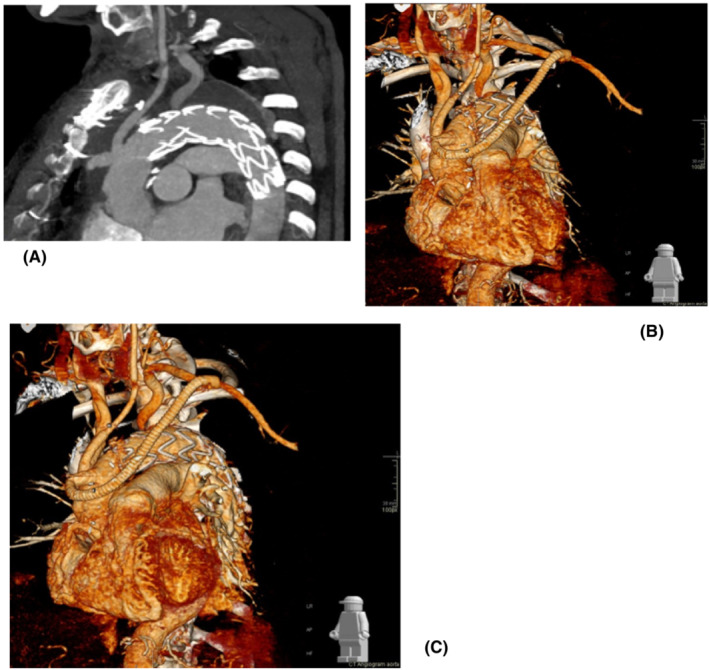
(A) Postoperative CT aortic angiogram showing the position of the stent in the proximal descending thoracic aorta. (B) Surface shading reconstruction of CT angiogram with emphasis on the graft from the left subclavian sutured to the branch of the ascending aortic graft. (C) Further 3D reconstructed view showing a more complete overview of the anatomical relationships
3. RESULTS
Table 1 gives key perioperative and postoperative data. Case complexity required longer cardiopulmonary bypass time for patient 3 and 4. Complications following surgery are listed in Table 2. No endoleak was observed following endovascular treatment early postoperatively.
TABLE 1.
Perioperative and postoperative data
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| X‐clamp (min) | 47 min | 137 | 246 | 113 |
| CPB (min) | 87 min | 278 | 342 | 327 |
| DHCA (min) | – | 81 | 87 | 113 |
| Cerebral ischemic time (min) | – | 0 | 0 | 0 |
| CICU stay (days) | 5 | 7 | 5 | 3 |
| Postop stay (days) | 16 | 14 | 13 | 6 |
Abbreviations: CICU, cardiac intensive care unit; CPB, cardiopulmonary bypass; DHCA, deep hypothermic circulatory arrest; Postop stay, postoperative stay; X‐clamp, aortic cross‐clamp.
TABLE 2.
Postoperative complications
| Complications | |
|---|---|
| Patient 1 | Transient delirium |
| Patient 2 | Drainage of pleural effusion |
| Patient 3 | Complete atrio‐ventricular block |
| Patient 4 | None |
At latest follow‐up, a CT‐aortic angiogram has shown stable anatomical features for patient 1 and patient 2, respectively 18 and 16 months postoperatively. The latest CT‐aortic angiogram for patient 3 has shown stable anatomical features with residual flap in the distal thoracic aorta and no thrombosis of the false lumen at 10 months postoperatively. Although no endovascular leak was detected early postoperatively, a follow‐up CT‐aortic angiogram for patient 4 has revealed a small type 2 endoleak in proximity of the origin of the left Subclavian artery for which he underwent a successful embolization.
4. DISCUSSION
Aggressive anti‐hypertensive treatment remains widely accepted as the initial approach for uncomplicated acute type B aortic dissection. 2 , 3 Nevertheless, medical treatment alone is not completely effective in preventing further progression of the disease with only 41% 6 year intervention‐free survival, 32 associated morbidity of 30% 33 and higher annual aortic growth rate in the presence of partial thrombosis of the false lumen. 34 Endovascular intervention is considered for type B aortic dissection complicated by aortic rupture, end‐organ ischemia, persistent pain, and hypertension despite full medical treatment, early false lumen expansion, and large single entry. 35 , 36 Endovascular intervention for uncomplicated acute type B aortic dissection is emerging as an attractive option in view of the suboptimal long‐term outcome of medical treatment alone, with up to 50% mortality at 5 years and up to 50% delayed expansion of the false lumen at 4 years. 37 Although the INSTEAD 38 and the ADSORB 39 , 40 trials have confirmed a higher rate of favorable aortic remodeling compared to medical treatment alone, there is still controversy whether these findings may lead to reduced long‐term mortality sufficient to balance the early perioperative hazards of endovascular intervention. 41 Nevertheless, extended follow‐up of the INSTEAD trial (INSTEAD‐XL) has shown that aorta‐related mortality and disease progression were significantly lower after 5 years in TEVAR patients compared to those receiving only medical treatment. 42 Age and dilatation of the thoracic aorta are associated with a higher risk of death, whereas false lumen thickness and concomitant abdominal aortic dilatation increase the risk for aortic events during follow‐up of patients with uncomplicated type B aortic dissection. 43 In addition, more recent evidence confirms an independent survival advantage for TEVAR over medical treatment adding further input for a paradigm shift in the acute management of type B aortic dissection in favor of early TEVAR. 44 The concept of “time until treatment equipoise” (TUTE) has been developed as an attempt to offer more detailed risk quantification for different treatments. 45 Patient's prognosis is significantly influenced by false lumen diameter, location of the primary entry site, and retrograde progression of the dissection into the aortic arch. 46 Therefore, the ultimate question is to identify those patients with type B aortic dissection who may benefit from early intervention regardless of whether they are complicated or not. Although TEVAR remains the treatment of choice in acute complicated type B aortic dissection, 47 , 48 peripheral arterial disease, severe tortuosity of the iliac arteries, a sharp angle of the aortic arch, and the absence of a proximal landing zone for the stent graft are indications for open surgery. The cases presented in this context are examples of how aortic changes or complications may benefit from a hybrid approach, which may become the way forward at an earlier stage as an attempt to reduce the onset of false lumen expansion frequently observed in patients on anti‐hypertensive treatment, in the absence of a feasible standalone TEVAR solution. Besides, the onset of type B aortic dissection in the context of aneurysmatic ascending aorta and/or aortic arch may well benefit from a hybrid approach. Combining the advantages of surgery and stenting may lead to a more versatile treatment option. Our strategy includes the use of a PETTICOAT and STABILIZE technique when appropriate and safe to do so. STABILIZE results in higher abdominal aortic remodeling with total false lumen thrombosis although the risk of aortic rupture from balloon molding needs to be taken into account. 31 The frozen elephant trunk technique may have a role to play in the absence of a proximal landing zone as it eliminates the risk of retrograde progression of the dissection. 49 The benefit of a multidisciplinary approach to high surgical risk patients has been acknowledged and previously described. 50 , 51 , 52 , 53 More specifically, in our case, the key element for the management of patients with complex aortic disease remains a true multidisciplinary approach based on close cooperation between cardiac and vascular surgeons and interventional radiologists. The value of this approach has also been experienced by one of the authors in a different centre 54 , 55 , 56 although still not completely and widely practiced. We did experience few postoperative complications but it is reassuring that none of the patients developed cerebrovascular accidents, required re‐exploration for bleeding, developed malperfusion or any type of endoleak early postoperatively. Patient 3 developed late onset of paresthesia in the right upper limb on follow‐up with negative imaging investigation. Patient 4 underwent successful embolization of the left Subclavian artery type 2 endoleak. The clinical conditions of all patients remain satisfactory at further follow‐up.
We cannot make claims based on four cases only. Nevertheless, these are examples of how treatment may evolve toward a more aggressive hybrid approach based on close cooperation between specialists with a common aim. At the time of the study, the Thoraflex and Evita devices were not readily available to our group. In addition, the use of an alternative approach based on graft deployment under direct vision was considered an appropriate choice. Given the unfavorable anatomy of the cases, the use of composite devices such as Thoraflex and Evita with inferior radial force compared to Endovascular stents may potentially lead to incomplete expansion requiring balloon molding peri‐operatively. As a group, we prefer to reduce the use of balloon dilatation unless strictly necessary. It is worth bearing in mind that bovine anatomy findings in patient 3 are not uncommon as highlighted in previous studies, 57 although there is no mention about this aspect in current guidelines. It is also noteworthy that current guidelines put more emphasis on the significance of family history of aortic dissection, overlooking the crucial role of “individual” history of aortic dissection, in the same patient in a different aortic segment, on decision‐making and aortic size threshold for early intervention. Each of the four patients presented in this context has a unique presentation that deserves specific attention by a team of specialists during a MDT meeting, which confirms its value and rationale. Besides, a more personalized approach based on patient‐specific modeling may add a different dimension. 58 , 59 , 60 , 61 , 62 The ability to model and simulate the effect of device implantation with reference to key elements and discuss the findings during a MDT meeting would allow visualization of the intended therapeutic approach and help with preoperative planning in relation to treatment optimization and outcome prediction. 63 , 64 , 65 , 66 , 67 The mapping of certain patterns of flow behavior may also help identify potential problems during patients' follow‐up and become an aid for decision‐making should further treatment be required. 68 , 69 Although experience and clinical judgment still remain essential for this purpose where the clinician is the ultimate decision‐maker, the ability to make a final decision on a difficult case following a simulation approach would be an attractive prospect. Evidence‐based practice (EBP) advocates that everything has to follow the outcome of rigorously designed randomized controlled clinical trials (RCTs). Nevertheless, there will always be gray areas without clear answers or treatments that have shown their beneficial effect despite the absence of the required evidence. EBP is meant to provide a secure ground for clinical decision‐making which may not be realistic. EBP seeks to address uncertainty but it cannot eliminate it. The results of trials are applicable in general but may not be completely suitable for a specific group of patients or even for the single patient, bringing to mind Heraclitus words, “No man ever steps in the same river twice, for it is not the same river and he is not the same man.” A limitation of the interpretation of RCTs is the apparent willingness to disregard the importance of the context and minimize the complexity of the individual patients involved. 70 As clinicians, we would like to have a solid ground for decision‐making but the reality is that this ground in some areas of practice is controversial, still evolving or does not exist. A more personalized approach to treatment may add a different dimension with some potential advantages as long as it is considered in the appropriate context and not as a “magic bullet.” Nevertheless, willingness, initiative, and common sense may well lead to the development of a common language that may ease fears and help build more bridges toward better cooperation between clinicians and scientists.
5. CONCLUSION
Different factors may affect outcome following complex aortic procedures. Nevertheless, the role of the aortic team based on a close cooperation between cardiac surgeons, vascular surgeons, and interventional radiologists may reduce potential for complications and address aspects that may not be completely within the domain of individual specialists. Endovascular intervention has contributed to reduce morbidity and mortality in complex disease and remains the gold standard in acute complicated type B aortic dissection. Nevertheless, its use remains controversial in the context of uncomplicated type B aortic dissection as primary approach. Further developments should hopefully continue to improve outcome and reduce current controversy. Patient‐specific modeling based on a multi‐disciplinary cooperation involving clinicians and engineering scientists has the potential to become an established approach in the context of a multi‐disciplinary meeting for clinical decision‐making. This is an aspect we may consider to address in a future study.
AUTHOR CONTRIBUTIONS
Massimo Capoccia: Conceptualization; data curation; formal analysis; methodology; resources; validation; writing – original draft; writing – review and editing. Mohamed Ashur Sherif: Formal analysis; resources; writing – review and editing. Nassef A: Writing – review and editing. Shaw D: Software; writing – review and editing. Walker P: Software; writing – review and editing. Evans B: Writing – review and editing. Pankaj Kaul: Writing – review and editing. Walid Elmahdy: Conceptualization; project administration; supervision; writing – review and editing.
FUNDING INFORMATION
This research received no external funding.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
CONSENT
Written informed consent was obtained from each patient at the time of surgery to publish this report in accordance with the journal's patient consent policy.
INTERNATIONAL REVIEW BOARD
Nope.
CLINICAL TRIAL REGISTRATION
None.
ACKNOWLEDGMENT
None.
Capoccia M, Sherif MA, Nassef A, et al. Aortic arch surgery for type B aortic dissection: How far should we go? The value of a hybrid approach. Clin Case Rep. 2023;11:e06742. doi: 10.1002/ccr3.6742
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.
REFERENCES
- 1. Czerny M, Schmidli J, Adler S, et al. Editor's choice – current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus Document of the European Association for Cardio‐Thoracic Surgery (EACTS) & the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2019;57:165‐198. [DOI] [PubMed] [Google Scholar]
- 2. Erbel R, Aboyans V, Boileau C, et al. The task force for the diagnosis and treatment of aortic diseases of the European Society of Cardiology (ESC). Eur Heart J. 2014;35(41):2873‐2926. [DOI] [PubMed] [Google Scholar]
- 3. Riambau V, Böckler D, Brunkwall J, et al. Editor's choice e management of descending thoracic aorta diseases. clinical practice guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53:4‐52. [DOI] [PubMed] [Google Scholar]
- 4. Nienaber CA, Fattori R, Lund G, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent graft placement. N Engl J Med. 1999;340:1539‐1545. [DOI] [PubMed] [Google Scholar]
- 5. Dake MD, Kato N, Mitchell RS, Semba CP, Razavi MK, Shimono T. Endovascular stent graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340:1546‐1552. [DOI] [PubMed] [Google Scholar]
- 6. Sayer D, Bratby M, Brooks M, Loftus I, Morgan R, Thompson M. Aortic morphology following endovascular repair of acute and chronic type B aortic dissection: implications for management. Eur J Vasc Endovasc Surg. 2008;36:522‐529. [DOI] [PubMed] [Google Scholar]
- 7. Andacheh ID, Donayre C, Othman F, Walot I, Kopchok G, White R. Patient outcomes and thoracic aortic volume and morphologic changes following thoracic endovascular aortic repair in patients with complicated chronic type B aortic dissection. J Vasc Surg. 2012;56(3):644‐650. [DOI] [PubMed] [Google Scholar]
- 8. Mani K, Clough RE, Lyons OTA, et al. Predictors of outcome after endovascular repair for chronic type B dissection. Eur J Vasc Endovasc Surg. 2012;43(4):386‐391. [DOI] [PubMed] [Google Scholar]
- 9. Hagan PG, Nienaber CA, Isselbacher EM, et al. The international registry of acute aortic dissection (IRAD): new insights into an old disease. Jama. 2000;283:897‐903. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki T, Mehta RH, Ince H, et al. Clinical profiles and outcomes of acute type B aortic dissection in the current era: lessons from the international registry of aortic dissection (IRAD). Circulation. 2003;108(Suppl. 1):II312‐II317. [DOI] [PubMed] [Google Scholar]
- 11. Cambria RP, Clouse WD, Davison JK, et al. A multicenter clinical trial of endovascular stent graft repair of acute catastrophes of the descending thoracic aorta. J Vasc Surg. 2009;50:1255‐1264. [DOI] [PubMed] [Google Scholar]
- 12. Trimarchi S, Eagle KA, Nienaber CA, et al. Importance of refractory pain and hypertension in acute type B aortic dissection: insights from the international registry of acute aortic dissection (IRAD). Circulation. 2010;122:1283‐1289. [DOI] [PubMed] [Google Scholar]
- 13. Zeeshan A, Woo EY, Bavaria JE, et al. Thoracic endovascular aortic repair for acute complicated type B aortic dissection: superiority relative to conventional OS and medical therapy. J Thorac Cardiovasc Surg. 2010;140(Suppl):S109‐S115. [DOI] [PubMed] [Google Scholar]
- 14. Hanna JM, Andersen ND, Ganapathi AM, McCann RL, Hughes GC. Five‐year results for endovascular repair of acute complicated type B aortic dissection. J Vasc Surg. 2014;59:96‐106. [DOI] [PubMed] [Google Scholar]
- 15. Umana JP, Lai DT, Mitchell RS, et al. What is the best treatment for patients with acute type B aortic dissections e medical, surgical, or endovascular stent grafting? Ann Thorac Surg. 2002;74:S1840‐S1843. [DOI] [PubMed] [Google Scholar]
- 16. Svensson LG, Kouchoukos NT, Miller DC, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent grafts. Ann Thorac Surg. 2008;85:S1‐S41. [DOI] [PubMed] [Google Scholar]
- 17. Verhoye JP, Miller DC, Sze D, Dake MD, Mitchell RS. Complicated acute type B aortic dissection: midterm results of emergency endovascular stent grafting. J Thorac Cardiovasc Surg. 2008;136:424‐430. [DOI] [PubMed] [Google Scholar]
- 18. Fattori R, Tsai TT, Myrmel T, et al. Complicated acute type B dissection: is surgery still the best option?: a report from the international registry of acute aortic dissection. JACC Cardiovasc Interv. 2008;1:395‐402. [DOI] [PubMed] [Google Scholar]
- 19. Steuer J, Eriksson MO, Nyman R, Bjorck M, Wanhainen A. Early and long‐term outcome after thoracic endovascular aortic repair (TEVAR) for acute complicated type B aortic dissection. Eur J Vasc Endovasc Surg. 2011;41:318‐323. [DOI] [PubMed] [Google Scholar]
- 20. Bockler D, Schumacher H, Ganten M, et al. Complications after endovascular repair of acute symptomatic and chronic expanding Stanford type B aortic dissections. J Thorac Cardiovasc Surg. 2006;132:361‐368. [DOI] [PubMed] [Google Scholar]
- 21. Fattori R, Montgomery D, Lovato L, et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the international registry of acute aortic dissection (IRAD). JACC Cardiovasc Interv. 2013;6(8):876‐882. [DOI] [PubMed] [Google Scholar]
- 22. Nakamura K, Uchida T, Hamasaki A, Sadahiro M. How should we treat uncomplicated subacute type B aortic dissection in octogenarians? J Cardiothorac Surg. 2019;14:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trimarchi S, Nienaber CA, Rampoldi V, et al. Role and results of surgery in acute type B aortic dissection: insights from the international registry of acute aortic dissection (IRAD). Circulation. 2006;114(Suppl.1):I357‐I364. [DOI] [PubMed] [Google Scholar]
- 24. Lansman SL, Hagl C, Fink D, et al. Acute type B aortic dissection: surgical therapy. Ann Thorac Surg. 2002;74:S1833‐S1835. [DOI] [PubMed] [Google Scholar]
- 25. Estrera AL, Miller CC, Goodrick J, et al. Update on outcomes of acute type B aortic dissection. Ann Thorac Surg. 2007;83:S842‐S845. [DOI] [PubMed] [Google Scholar]
- 26. Bozinovski J, Coselli JS. Outcomes and survival in surgical treatment of descending thoracic aorta with acute dissection. Ann Thorac Surg. 2008;85:965‐970. [DOI] [PubMed] [Google Scholar]
- 27. Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: part I: from etiology to diagnostic strategies. Circulation. 2003;108:628‐635. [DOI] [PubMed] [Google Scholar]
- 28. Coselli JS, LeMaire SA, Conklin LD, Adams GJ. Left heart bypass during descending thoracic aortic aneurysm repair does not reduce the incidence of paraplegia. Ann Thorac Surg. 2004;77:1298‐1303. [DOI] [PubMed] [Google Scholar]
- 29. Alfson DB, Ham SW. Type B aortic dissections:Current Guidelines for Treatment. Cardiol Clin. 2017;35:387‐410. [DOI] [PubMed] [Google Scholar]
- 30. Hiratzka LF, Bakris GL, Beckman JA, et al. A report of the American College of Cardiology Foundation/American Heart Association task force on practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and society for vascular medicine. J Am Coll Cardiol. 2010;55(14):e27‐e129. [DOI] [PubMed] [Google Scholar]
- 31. Zhong J, Osman A, Tingerides C, et al. Technique‐based evaluation of clinical outcomes and aortic Remodelling following TEVAR in acute and subacute type B aortic dissection. Cardiovasc Intervent Radiol. 2021;44:537‐547. [DOI] [PubMed] [Google Scholar]
- 32. Durham CA, Cambria RP, Wang LJ, et al. The natural history of medically managed acute type B aortic dissection. J Vasc Surg. 2015;61:1192‐1199. [DOI] [PubMed] [Google Scholar]
- 33. Cooper M, Hicks C, Ratchford EV, Salameh MJ, Malas M. Diagnosis and treatment of uncomplicated type B aortic dissection. Vasc Med. 2016;21(6):547‐552. [DOI] [PubMed] [Google Scholar]
- 34. Trimarchi S, Tolenaar JL, Jonker FHW, et al. Importance of false lumen thrombosis in type B aortic dissection prognosis. J Thorac Cardiovasc Surg. 2013;145:S208‐S212. [DOI] [PubMed] [Google Scholar]
- 35. Akin I, Kische S, Rehders TC, Ince H, Nienaber CA. Acute aortic syndromes. Medicine. 2010;38(8):450‐455. [Google Scholar]
- 36. Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet. 2015;385:800‐811. [DOI] [PubMed] [Google Scholar]
- 37. Qin Y‐L, Wang F, Li T‐X, et al. Endovascular repair compared with medical management of patients with uncomplicated type B acute aortic dissection. J Am Coll Cardiol. 2016;67:2835‐2842. [DOI] [PubMed] [Google Scholar]
- 38. Nienaber CA, Rousseau H, Eggebrecht H, et al. Randomized comparison of strategies for type B aortic dissection: the INvestigation of STEnt grafts in aortic dissection (INSTEAD) trial. Circulation. 2009;120:2519‐2528. [DOI] [PubMed] [Google Scholar]
- 39. Brunkwall J, Lammer J, Verhoeven E, Taylor P. ADSORB: a study on the efficacy of endovascular grafting in uncomplicated acute dissection of the descending aorta. Eur J Vasc Endovasc Surg. 2012;44:31‐36. [DOI] [PubMed] [Google Scholar]
- 40. Brunkwall J, Kasprzak P, Verhoeven E, Heijmen R, Taylor P, the ADSORB Trialists . Endovascular repair of acute uncomplicated aortic type B dissection promotes aortic Remodelling: 1 year results of the ADSORB trial. Eur J Vasc Endovasc Surg. 2014;48(3):285‐291. [DOI] [PubMed] [Google Scholar]
- 41. Scott AJ, Bicknell CD. Contemporary management of acute type B dissection. Eur J Vasc Endovasc Surg. 2016;51:452‐459. [DOI] [PubMed] [Google Scholar]
- 42. Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long‐term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. 2013;6:407‐416. [DOI] [PubMed] [Google Scholar]
- 43. Shimamoto T, Komiya T, Tsuneyoshi H. Fate of uncomplicated acute type B aortic dissection and impact of concurrent aortic dilatation on remote aortic events. J Thorac Cardiovasc Surg. 2019;157:854‐863. [DOI] [PubMed] [Google Scholar]
- 44. Iannuzzi JC, Stapleton SM, Bababekov YJ, et al. Favourable impact of thoracic endovascular aortic repair on survival of patients with acute uncomplicated type B aortic dissection. J Vasc Surg. 2018;68:1649‐1655. [DOI] [PubMed] [Google Scholar]
- 45. Kamman AV, de Beaufort HWL, van Bogerijen GHW, et al. Contemporary management strategies for chronic type B aortic dissections: a systematic review. PLoS ONE. 2016;11(5):e0154930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weiss G, Wolner I, Folkmann S, et al. The location of the primary entry tear in acute type B aortic dissection affects early outcome. Eur J Cardiothorac Surg. 2012;42:571‐576. [DOI] [PubMed] [Google Scholar]
- 47. Grabenwoger M, Alfonso F, Bachet J, et al. Thoracic endovascular aortic repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio‐Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J. 2012;33:1558‐1563. [DOI] [PubMed] [Google Scholar]
- 48. Heijmen RH, Thompson MM, Fattori R, Goktay Y, Teebken OE, Orend KH. Valiant thoracic stent‐graft deployed with the new captivia delivery system: procedural and 30‐day results of the valiant Captivia registry. J Endovasc Ther. 2012;19:213‐225. [DOI] [PubMed] [Google Scholar]
- 49. Weiss G, Tsagakis K, Jakob H, et al. The frozen elephant trunk technique for the treatment of complicated type B aortic dissection with involvement of the aortic arch: multicentre early experience. Eur J Cardiothorac Surg. 2015;47(1):106‐114. [DOI] [PubMed] [Google Scholar]
- 50. Whiteman AR, Dhesi JK, Walker D. The high‐risk surgical patient: a role for a multi‐disciplinary team approach? Br J Anaesth. 2016;116:311‐314. [DOI] [PubMed] [Google Scholar]
- 51. Ziabari Y, Wigmore T, Kasivisvanathan R. The multidisciplinary team approach for high‐risk and major cancer surgery. BJA Education. 2017;17(8):255‐261. [Google Scholar]
- 52. Selwood A, Senthuran S, Blakely B, Lane P, North J, Clay‐Williams R. Improving outcomes from high‐risk surgery: a multimethod evaluation of a patient‐centred advanced care planning intervention. BMJ Open. 2017;7:e014906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koike M, YoshimuraM MY, Uezono S. The effects of a preoperative multidisciplinary conference on outcomes for high‐risk patients with challenging surgical treatment options: a retrospective study. BMC Anesthesiol. 2021;21:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Capoccia M, Mireskandari M, Cheshire NJ, Rosendahl UP. Delayed repair of aortic dissection in sickle cell anaemia as a combined cardiac and vascular surgical approach. J Saudi Heart Assoc. 2020;32(2):208‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Capoccia M, Pal S, Murphy M, et al. Cardiac and vascular surgeons for the treatment of aortic disease: a successful Partnership for Decision‐Making and Management of complex cases. J Investig Med High Impact Case Rep. 2021;9:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Capoccia M, Nienaber CA, Mireskandari M, et al. Alternative approach for cerebral protection during complex aortic arch and redo surgery. J Cardiovasc Dev Dis. 2021;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ziganshin BA, Elefteriades JA. Guilt by association: a paradigm for detection of silent aortic disease. Ann Cardiothorac Surg. 2016;5(3):174‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen D, Müller‐Eschner M, Kotelis D, Böckler D, Ventikos Y, von Tengg‐Kobligk H. A longitudinal study of type B aortic dissection and endovascular repair scenarios: computational analyses. Med Eng Phys. 2013;35:1321‐1330. [DOI] [PubMed] [Google Scholar]
- 59. Cheng Z, Juli C, Wood NB, Gibbs RGJ, Xu XY. Predicting flow in aortic dissection: comparison of computational model with PC‐MRI velocity measurements. Med Eng Phys. 2014;36:1176‐1184. [DOI] [PubMed] [Google Scholar]
- 60. Bonfanti M, Franzetti G, Maritati G, Homer‐Vanniasinkam S, Balabani S, Díaz‐Zuccarini V. Patient‐specific haemodynamic simulations of complex aortic dissections informed by commonly available clinical datasets. Med Eng Phys. 2019;71:45‐55. [DOI] [PubMed] [Google Scholar]
- 61. Bonfanti M, Franzetti G, Homer‐Vanniasinkam S, Díaz‐Zuccarini V, Balabani S. A combined In vivo, In vitro, In silico approach for patient‐specific Haemodynamic studies of aortic dissection. Ann Biomed Eng. 2020;48(12):2950‐2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Abazari MA, Rafieianzab D, Soltani M, Alimohammadi M. The effect of beta‐blockers on haemodynamic parameters in patient‐specific blood flow simulations of type‐B aortic dissection: a virtual study. Sci Rep. 2021;11:16058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tse KM, Chiu P, Lee HP, Ho P. Investigation of hemodynamics in the development of dissecting aneurysm within patient‐specific dissecting aneurismal aortas using computational fluid dynamics (CFD) simulations. J Biomech. 2011;44:827‐836. [DOI] [PubMed] [Google Scholar]
- 64. Tang AYS, Fan Y, Cheng SWK, Chow KW. Biomechanical factors influencing type B thoracic aortic dissection: computational fluid dynamics study. Eng Appl Comput Fluid Mech. 2012;6:622‐632. [Google Scholar]
- 65. Wan Ab Naim WN, Ganesan PB, Sun Z, Osman K, Lim E. The impact of the number of tears in patient‐specific Stanford type B aortic dissecting aneurysm: CFD simulation. J Mech Med Biol. 2013;14:1450017. [Google Scholar]
- 66. Shang EK, Nathan DP, Fairman RM, et al. Use of computational fluid dynamics studies in predicting aneurysmal degeneration of acute type B aortic dissections. J Vasc Surg. 2015;62:279‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cheng Z, Wood NB, Gibbs RGJ, Xu XY. Geometric and flow features of type B aortic dissection: initial findings and comparison of medically treated and stented cases. Ann Biomed Eng. 2015;43:177‐189. [DOI] [PubMed] [Google Scholar]
- 68. Simon CH, Liu W, Wong RH, Underwood M, Wang D. The potential of computational fluid dynamics simulation on serial monitoring of hemodynamic change in type B aortic dissection. Cardiovasc Interv Radiol. 2016;39:1090‐1098. [DOI] [PubMed] [Google Scholar]
- 69. Xu H, Li Z, Dong H, et al. Hemodynamic parameters that may predict false‐lumen growth in type B aortic dissection after endovascular repair: a preliminary study on long‐term multiple follow‐ups. Med Eng Phys. 2017;50:12‐21. [DOI] [PubMed] [Google Scholar]
- 70. Baumann SL. The limitations of evidence‐based practice. Nurs Sci Q. 2010;23(3):226‐230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.


