Abstract
Tuberculosis (TB), the leading infectious cause of death worldwide, like coronavirus disease 2019 (COVID-19), is mainly transmitted through the respiratory route and affects the lungs. Though TB-COVID co-infection is not common, but might be missed due to similar clinical presentation. Therefore, a high index of suspicion of co-infections is needed so that there is prompt diagnosis and appropriate treatment. A higher mortality of 13% in cases of co infections is alarming. Here we are reporting a case series of SARS-CoV-2 – TB co-infection from Eastern India.
Keywords: COVID-19, diagnosis, mortality, tuberculosis
Background
The emergence of coronavirus disease 2019 (COVID-19) has multiplied the problem of tuberculosis (TB), particularly in a country like India, where TB is endemic.[1,2] TB and COVID-19 co-infection cases usually belong to one of the three categories: patients with TB occurring before COVID-19, those with COVID-19 followed by TB, and those in whom these two infections occurred in the same week.[3] While this co-infection might be purely incidental, higher mortality of 13% in the cases with apparent co-infections is alarming, especially in patients with other co morbidities.[4] Respiratory manifestations are predominant in both TB and COVID-19 disease and patients suffering from both active as well as latent tuberculosis are more susceptible to COVID-19 with higher severity of the disease.[5] Due to similar clinical manifestations, the clinicians should consider the presence of COVID-19 while treating tuberculosis and vice-versa, especially in the elderly age group and patients with other co-morbidities like diabetes and hypertension. Herein, we report a series of four cases of TB and COVID-19 co-infection from a tertiary care hospital in eastern India.
Case 1
A 50-year-old man presented to us with complaints of intermittent fever for three months, cough with expectoration and shortness of breath on exertion for one month. His cough was productive in nature, with a postural variation. There was also a history of night sweats, loss of appetite and weight loss. There was no history of hemoptysis, chest pain, or palpitations. He was a known case of Type 2 diabetes, coronary artery disease, and a living donor renal transplant for native autosomal dominant polycystic kidney disease (ADPKD). Physical examination was normal. Computed tomography (CT) of thorax showed thick-walled cavity with air-fluid levels and centrilobular nodules in the right lower lobe. SARS-CoV-2 RT–PCR was positive, and the patient was isolated for three weeks; he was treated with doxycycline, ivermectin, prophylactic anticoagulation, and multivitamins. After completion of isolation, he was shifted to a non-COVID ward. The patient again developed fever and had a fall in oxygen saturation. Repeat CT scan of thorax showed new areas of consolidations in the left lung. Antibiotics were escalated to meropenem, linezolid, and liposomal amphotericin B. Sputum was positive for acid-fast bacilli (AFB), cartridge based nucleic acid amplification test (CBNAAT) was positive for M.tuberculosis (MTB), and rifampicin resistance was not detected. The patient was started on antitubercular therapy (ATT). Sputum cultures revealed MDR Acinetobacter spp. Blood culture revealed Staph aureus (MSSA). Antibiotics were modified to intravenous colistin, and nebulized colistin and liposomal amphotericin B were stopped. The patient improved with treatment, and was discharged.
Case 2
A 30-year-old woman who was on ATT for the last five months (for pulmonary tuberculosis) presented with severe pain abdomen associated with two episodes of vomiting and non-passage of stool for the previous two days. She had a history of similar complaints three months back. Abdomen examination showed the presence of guarding and diffuse tenderness. Ultrasonography of the abdomen showed moderate amount of free fluid with few pockets of septate collection noted in the pelvis. Blood levels of CRP, D-dimer and LDH were raised. Peritoneal fluid culture grew Eschericia coli and Enterobacter spp. (Sensitive to all antibiotics). Fungal culture and sensitivity of peritoneal fluid showed Candida tropicalis (Sensitive to fluconazole, voriconazole, caspofungin, micafungin, amphotericin B. Intermediate susceptibility to flucytosine). Drain fluid culture showed growth of Enterococcus avium. Urine culture was sterile. Blood culture showed growth of methicillin resistant Staphylococcus aureus (MRSA). Intraoperative findings showed that the abdomen was grossly contaminated with bilio-purulent fluid and two perforations, the proximal one of size 1 × 1 cm and the distal one of size 2 × 2 cm, found within 7 cm of ileal segment 40 cm away from the ileocaecal junction. Ileal resection was done. She was tested COVID-19 positive and shifted to COVID ICU. Once RT–PCR was tested negative, she was shifted to the surgery ward. The pelvic drain was removed on post operative day 17. Later, she was discharged in a haemodynamically stable state.
Case 3
A 65-year-old female, referred from another tertiary care hospital in the same city, as a suspected case of COVID-19 pneumonia, came to our hospital with chief complaints of fever, cough, and shortness of breath for 10 days. Her oxygen saturation was 72% on room air. RT–PCR for COVID-19 was positive. Chest radiograph showed bilateral heterogeneous opacities involving all zones along with a cavitary lesion in the left midzone [Figure 1]. The patient received inj. Remdesivir, Methylprednisolone, Piperacillin-Tazobactam, and non-invasive mechanical ventilation. Antibiotics were later upscaled to meropenem, linezolid, and subsequently to colistin and voriconazole in view of persistent septic shock. Xpert MTB/RIF (cartridge-based nucleic acid amplification test for M. Tuberculosis) of endotracheal secretion revealed Mycobacterium tuberculosis with rifampicin resistance. Antitubercular therapy started as per the weight band. In due course, she also developed acute kidney injury (AKI) and hence haemodialysis was done. But eventually, the patient died in the hospital course.
Figure 1.
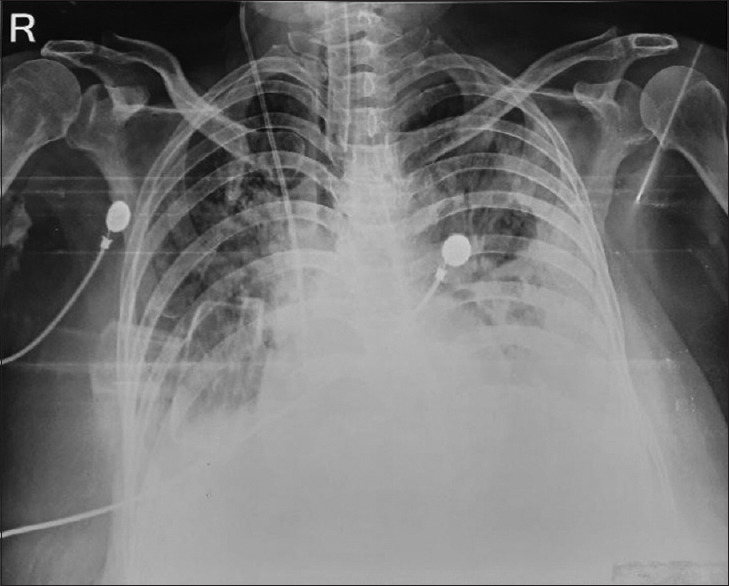
Chest radiograph showing homogenous opacity in the lower zone of both lungs and more on left side, airspace consolidation seen in bilateral midzone
Case 4
A 51-year-old diabetic and hypertensive male presented with shortness of breath and loss of appetite for five days. He was a known case of left-sided tubercular pleural effusion and was on antitubercular treatment for the last 15 days. There was no history of travel or contact with any person with COVID disease. On physical examination, the patient was tachypneic with decreased breath sounds on the left infra-scapular area and crepitations over right infra-scapular area. He was also found to have type-1 respiratory failure. He was started on intravenous Pipercillin-Tazobactam along with other supportive management. Chest X-ray showed bilateral lower zone heterogenous opacity with blunting of left costophrenic angle [Figure 2]. The patient’s condition worsened rapidly, and he was shifted to the intensive care unit. He was put on oxygen with high-flow mask, and arterial blood gas analysis showed metabolic acidosis. Antibiotics were escalated to meropenem. COVID-19 RT–PCR was sent. But on the same-day patient had a cardiac arrest. He was revived after cardiopulmonary resuscitation and was put on mechanical ventilation. His COVID RT–PCR came positive. He had a high vasopressor requirement and signs of multiorgan failure despite optimal medical management. Unfortunately, the patient passed away on the third day of hospital admission.
Figure 2.
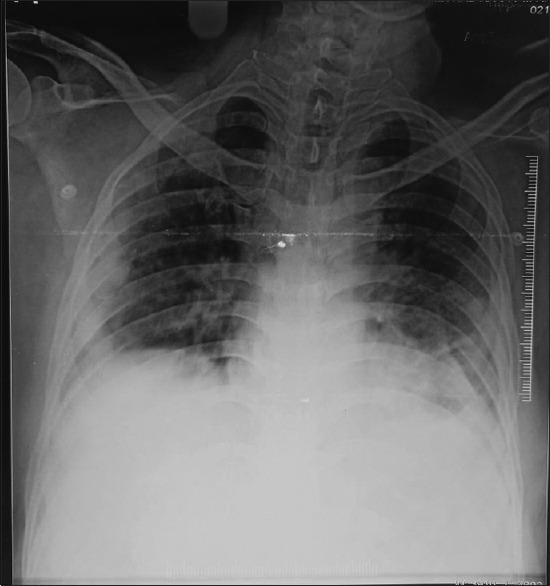
Chest radiograph showing bilateral lower zone subpleural airspace consolidation
Discussion
We discussed the clinical courses of a case series of TB with COVID-19 coinfection, with varied clinical presentations and mixed outcomes [Table 1]. Two of our patients died of dual infections. Three of our patients were admitted with severe COVID, later diagnosed as having tuberculosis due to a history of non-improvement and radiological shadows, not compatible with COVID. TB is an infectious disease spread by droplet infection, whereas COVID-19 is known to spread primarily by aerosols, and both disorders primarily involve the lungs. COVID-19 is characterized by several clinical features ranging from an asymptomatic state to complaints of fever, cough, shortness of breath over a short incubation period; less common symptoms of loss of sense of taste and smell and severe forms with immune dysregulation.[6] As for TB, the lung is the most frequently affected organ. In addition to the respiratory symptoms, patients might present with weight loss and night sweats; in a minority of patients, it may lead to rapid respiratory failure and death.[7] As the clinical manifestations of the diseases are similar to some extent, it is important to have a high index of suspicion for the development of new symptoms in patients of TB or COVID-19 for timely diagnosis of coinfection in order to prevent further complications. TB and COVID-19 coinfection has been reported across both genders and different age groups.[8] A recent report from a tertiary care hospital in India among 22 patients with TB and COVID-19 coinfection reported an overall mortality rate of 27.3%.[9]
Table 1.
Characteristics of tuberculosis patients with a recent history of COVID-19
| Demography | Case-1 | Case-2 | Case-3 | Case-4 |
|---|---|---|---|---|
| Age in years, Sex | 50 Male | 30 Female | 65 Female | 51 male |
| COVID-19 history | 4 days | 2 days | 10 days | 1 day |
| Timing prior to TB diagnosis | 3 months | 5 months | At hospital | 15 days |
| TB diagnosis | On admission | 5 months | - At hospital | - |
| CBNAAT (Xpert MTB/Rif) | Positive | Positive | Positive, Rifampicin Resistance detected. | Positive |
| TB risk factors and other medical conditions | Yes, CAD, ADPKD | MRSA | AKI | DM, Hypertension, pleural effusion |
| Drug resistance | Nil | Nil | Rifampicin Resistance | Nil |
| HIV | Non-reactive | Non-reactive | Non-reactive | Non-reactive |
| Smoking | nil | nil | Nil | Nil |
| Alcohol excess and illicit drugs | nil | nil | Nil | Nil |
| Type 2 diabetes | yes | nil | Nil | Yes |
| Outcome | Improved | improved | died | Died |
A change in the radiological picture and a sharp rise in the inflammatory markers in tuberculosis patients should also raise suspicion of coinfection with COVID-19. Two out of four patients in this case series developed COVID-19 infection during the course of treatment with ATT and the other two patients were diagnosed to have TB during the course of hospitalization for COVID-19. Two of the patients had severe COVID-19 disease and both of them did not survive. As India is a TB endemic nation, the use of immunomodulators (IL-6 inhibitors) and corticosteroids in moderate to severe COVID-19 may lead to reactivation of TB.[10] The other association might be the occurrence of drug interaction between rifampicin and lopinavir/ritonavir (if used to treat co-infection). There could be additional hepatotoxicity due to available COVID-19 therapeutics like Remdesivir in addition to antitubercular drugs.[8] Presence of other factors like poverty, overcrowding and undernourished populations further aid the spread of both the diseases.[11] Likewise, patients with active TB or structural lung disease secondary to healed pulmonary TB are probably at a greater risk of developing COVID-19. The interactions between the two micro-organisms have been reported in a meta-analysis in which overlapping of genetic signatures between TB and COVID-19 were identified.[12]
In the present series of cases, two of the four patients died. The mortality in COVID-19 alone has been estimated to be up to 2% with higher mortality in the elderly, patients with co-morbidities like diabetes, hypertension, chronic obstructive pulmonary disease, and ischaemic heart disease, higher D-dimer levels.[13,14] A mortality rate of 10.2% with COVID-19 and pulmonary TB co-infection, which was higher in elderly patients with multiple co-morbidities has been reported by Tandolini et al.[3] Coinfection led to more deaths, according to a study conducted by Motta et al.[7] among two cohorts of migrants (14.3% and 10.2%, respectively). However, Stochino et al. described a lower case fatality rate (CFR) of 5%.[15] In a study in South Africa, around 2128 patients out of 22308 patients studied, had TB and COVID-19 coinfection, with worse outcomes in patients with COVID-TB coinfection.[16] In a Philippines-based study, 1% of the patients infected with COVID-19 had coinfection with TB, and patients with TB had 2.17 times higher risk of death when compared to those without TB. Additionally, those with TB and COVID-19 coinfection had 25% less recovery risk than patients without TB.[7]
As a precautionary measure, the Government had imposed massive country-wide lockdowns due to the COVID-19 pandemic occurring in two waves. With the nation bracing for a third wave, the diagnosis of new cases and follow-up of previous cases in TB clinics has been hampered greatly due to fear of exposure to COVID-19. This had led to reduced access to ATT. Decreased adherence to treatment regimens can increase drug-resistant cases and increase deaths, which might go unnoticed and unnotified. The lockdown due to the COVID-19 pandemic has been the reason behind the missing of TB as well as COVID-19 cases who have not attended the healthcare clinics.
The COVID-19 pandemic has put immense pressure on the Indian healthcare system. The healthcare logistics assigned for various services (almost about 94%) were diverted towards COVID-19 diagnostics and patient management.[17] This has led to a disruption of patient diagnostics and follow-up of other infectious diseases like tuberculosis and non-communicable diseases like diabetes, diabetes-related complications, cardiovascular diseases, and cancers. A WHO survey conducted in 155 countries in 3 weeks in May 2020, stated that the impact is global, with low-income countries being the worst affected.[18] The CFR of tuberculosis in India is about 5%, according to a recent review[19] whereas the CFR for COVID-19 for the world is 4.8%, and for India, it is 2.92% only.[20] Hence, immediate steps need to be taken amidst the pandemic, as a catch-up effort to diagnose actively, trace, treat and prevent tuberculosis, particularly in high-TB burden nations.
A significant positive impact of the COVID-19 pandemic is the use of face masks, cough etiquettes, and social distancing, which if continued to be practised after the pandemic, will help in TB control also. As both the diseases have respiratory symptoms predominantly, it is important to screen patients with TB for COVID-19, and it is important not to miss the possibility of co-existence of both diseases. Hence, COVID-19 RT–PCR might be coupled with Xpert MTB Rif assay for the earlier detection of coinfections, especially in high-risk individuals.
The WHO report titled ‘Global Tuberculosis Report 2021’, released recently, shows that the most significant contribution to the decrease in TB notifications in 2020 was by India (a 41% decline in TB notifications), with a total global drop of 1.3 million.[21] There have been reversals in the progress, the global TB targets are off-track, and an arduous task to bring back the TB control to the pre-COVID era.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. Coronavirus disease (COVID-19) Pandemic –Emergency Use Listing Procedure (EUL) open for in vitro diagnostics. Geneva: World Health Organization; [Last accessed on 2020 Jun 15]. Available from: https://www.who.int/diagnostics_laboratory/EUL/en/ [Google Scholar]
- 2.WHO. Global Tuberculosis Report 2020. 2020. Available from: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf .
- 3.Tadolini M, Codecasa LK, García-García JM, Blanc FX, Borisov S, Alffenaar JW, et al. Active tuberculosis, sequelae and COVID-19 co-infection:First cohort of 49 cases. Eur Respir J. 2020;56:2001398. doi: 10.1183/13993003.01398-2020. doi:10.1183/13993003.01398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koupaei M, Naimi A, Moafi N, Mohammadi P, Tabatabaei FS, Ghazizadeh S, et al. Clinical Characteristics, Diagnosis, Treatment, and Mortality Rate of TB/COVID-19 Coinfected Patients:A Systematic Review. Front Med (Lausanne) 2021;8:740593. doi: 10.3389/fmed.2021.740593. doi:10.3389/fmed.2021.740593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Wang Y, Fleming J, Yu Y, Gu Y, Liu C, et al. Active or latent tuberculosis increases susceptibility to COVID-19 and disease severity. MedRxiv. 2020 doi:10.1101/2020.03.10.20033795. [Google Scholar]
- 6.Falasca L, Nardacci R, Colombo D, Lalle E, Di Caro A, Nicastri E, et al. Postmortem findings in Italian patients with COVID-19:A descriptive full autopsy study of cases with and without co-morbidities. J Infect Dis. 2020;222:1807–15. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motta I, Centis R, D'Ambrosio L, García-García JM, Goletti D, Gualano G, et al. Tuberculosis, COVID-19 and migrants:Preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. 2020;26:233–40. doi: 10.1016/j.pulmoe.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra AK, George AA, Sahu KK, Lal A, Abraham G. Review of clinical profile, risk factors, and outcomin patients with tuberculosis and covid-19. Acta Biomedica. 2021;92 doi:10.23750/abm.v92i1.10738. [Google Scholar]
- 9.Gupta N, Ish P, Gupta A, Malhotra N, Caminero JA, Singla R, et al. A profile of a retrospective cohort of 22 patients of COVID-19-19 with active/treated tuberculosis. Eur Respir J. 2020;56:2003408. doi: 10.1183/13993003.03408-2020. doi:10.1183/13993003.03408-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pathak L, Gayan S, Pal B, Talukdar J, Bhuyan S, Sandhya S, et al. Corona virus activates a stem cell mediated defense mechanism that accelerates activation of dormant tuberculosis:Implications for the COVID-19 pandemic. bioRxiv. 2020 doi:10.1101/2020.05.06.077883. [Google Scholar]
- 11.Wingfield T, Tovar MA, Datta S, Saunders MJ, Evans CA. Addressing social determinants to end tuberculosis. Lancet. 2018;391:1129–32. doi: 10.1016/S0140-6736(18)30484-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheerin D, Abhimanyu Wang X, Johnson WE, Coussens A. Systematic evaluation of transcriptomic disease risk and diagnostic biomarker overlap between COVID-19 and tuberculosis:A patient-level meta-analysis. medRxiv. 2020 2020.11.25.20236646. doi:10.1101/2020.11.25.20236646. [Google Scholar]
- 13.Shabrawishi M, AlQarni A, Ghazawi M, Melibari B, Baljoon T, Alwafi H, et al. New disease and old threats:A case series of COVID-19 and tuberculosis coinfection in Saudi Arabia. Clin Case Rep. 2021;9:e04233. doi: 10.1002/ccr3.4233. doi:10.1002/ccr3.4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China:A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stochino C, Villa S, Zucchi P, Parravicini P, Gori A, Raviglione MC, et al. Clinical characteristics of COVID-19 and active tuberculosis coinfection in an Italian reference hospital. Eur Respir J. 2020;56:2001708. doi: 10.1183/13993003.01708-2020. doi:10.1183/13993003.01708-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies M-A. HIV and risk of COVID-19 death:A population cohort study from the western cape province, South Africa. MedRxiv. 2020:1–21. doi:10.1101/2020.07.02.20145185. [Google Scholar]
- 17.Behera D. TB control in India in the COVID era. Indian J Tuberc. 2021;68:128–33. doi: 10.1016/j.ijtb.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.COVID-19 Significantly Impacts Health Services for Noncommunicable Diseases. June 1, 2020. Available from:www.who.int. [Google Scholar]
- 19.Huddart S, Svadzian A, Nafade V, Satyanarayana S, Pai M. Tuberculosis case fatality in India:A systematic review and meta-analysis. BMJ Global Health. 2020;5:e002080. doi: 10.1136/bmjgh-2019-002080. doi:10.1136/bmjgh-2019-002080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Center for Disease Prevention and Control (ECDC) Available from: https://github.com/owid/covid-19-data/tree/master/public/data .
- 21.WHO Global Tuberculosis Report 2021. Available from: https://www.who.int/publications/i/item/9789240037021 .


