Abstract
PNETs (pancreatic neuroendocrine tumors) are a rare sub-type of pancreatic tumors, with the majority of them being insulinomas. The vast majority of insulinomas (90%) are benign and solitary, with only 10% being malignant. It has a wide range of clinical manifestations and requires a high level of suspicion to diagnose. Surgical excision has long been the gold standard for treating localized PNET and is still the therapy of choice. Recurrent hypoglycemia is usual in diabetic patients, but this is a rare finding in non-diabetic individuals. Here, we are presenting a rare case of insulinoma who was non-diabetic and presented with recurrent hypoglycemic episodes. A 61-year-old non-diabetic male presented with multiple episodes of hypoglycemia in the past. On thorough workup, there was an increased fasting insulin level with the fasting blood glucose level ranging from 60 to 90 mg/dl. His C-peptide and proinsulin were markedly elevated. His abdominal ultrasound failed to pick up any abnormality. His DOTANOC scan revealed a 2 × 2 cm sized lesion in the distal pancreas suggestive of neuroendocrine pathology. He subsequently underwent spleen preserving distal pancreatectomy, following which his blood sugar levels remained normal, and continued to be free of symptoms on follow-up. Our instance emphasizes the need for evaluating insulinoma as a cause of recurrent hypoglycemia in people who are not diabetic. A high index of suspicion in hypoglycemic individuals who do not respond to standard treatment or whose symptom pattern changes will lower the likelihood of insulinoma diagnosis being delayed.
Keywords: DOTANOC scan, hypoglycemia, insulinoma, pancreatic neuroendocrine tumor
Introduction
Pancreatic neuroendocrine tumors (PNETs) are an uncommon sub-type of pancreatic tumors with a reported frequency of 4 occurrences per million per year, with the majority being insulinomas.[1] The vast majority of insulinomas (90%) are benign and solitary, with only 10% being malignant. Insulinomas are sporadic in 90% of cases, whereas 10% of them are linked to the MEN-1 disease.[2] These are uniformly dispersed throughout the pancreatic head, body, and tail.
At the time of diagnosis, more than 90% of insulinomas are small solitary lesions, although numerous tumors can develop, particularly in patients with the multiple endocrine neoplasia 1 syndrome. Insulinomas that are 0.5 cm or more are considered malignant, just like other PNETs.[3]
Neuroglycopenic symptoms such as anxiety, dizziness, light-headedness, personality changes, confusion, incoherence, blurred vision, seizures, and coma as well as sympathoadrenal signs and symptoms such as palpitations, tremulousness, diaphoresis, and tachycardia, which are caused by catecholamine release in response to hypoglycemia, are all common in insulinomas.[4]
Surgical excision has been the mainstay of treatment for localized PNET.[5] Unresectable disease was treated with streptozocin alone or in combination with doxorubicin; however, the efficacy of these medications was questioned, and patients still had a dismal prognosis.[6]
Here, we present a rare case of insulinoma who showed typical Whipple’s Triad and was successfully surgically treated with spleen preserving distal pancreatectomy.
Case Presentation
Clinical features
A 61-year-old non-diabetic man presented to the emergency department with complaints of sweating and palpitations after a fasting period. He had a few episodes of hypoglycemia in the past 2 years. His random blood sugar level was found to be 47 mg/dl, after which he was given IV dextrose in the emergency. The patient was admitted for further evaluation. He denied having any co-morbidities and was not on any medications. During his stay in the hospital, his fasting blood glucose levels ranged from 50 to 80 mg/dl.
Lab values
On the basis of his history, keeping insulinoma as a possibility, the patient was subjected to laboratory investigations, which revealed an insulin level of 56.14 ng/ml. His C-peptide level was 1361 pmol/L and the pro-insulin level was 37.3 pmol/L, both of which were markedly elevated. His serum cortisol level was normal, suggesting an intact pituitary adrenal axis. Abdominal sonography failed to pick any abnormality.
Imaging studies
With high suspicion of insulinoma, DOTANOC scan was performed, which revealed evidence of a somatostatin receptor expressing lesion, measuring approximately 1.6 × 2.0 × 2.3 cm in size, with an suv max of 58.7, involving the tail of pancreas, suggesting neoplastic disease of neuroendocrine etiology. Figure 1 is 68Ga-DOTATOC imaging showing the neuroendocrine lesion of the pancreas.
Figure 1.
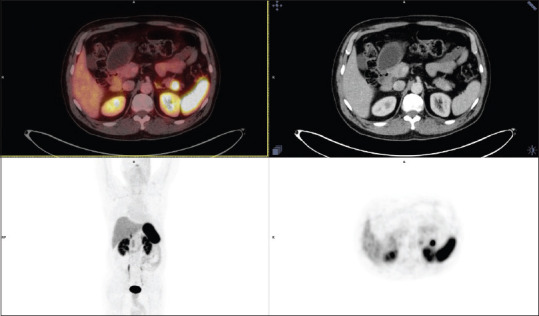
68 Ga – DOTATOC scan showing insulinoma
Tri-phasic computed tomography (CT) scan of the whole abdomen revealed a tiny avidly contrast enhancing (on the arterial phase) lesion measuring 13 mm × 10 mm showing persistent enhancement on a delayed series. The pancreatic duct was not dilated. Peri-pancreatic fat plains and vascular structures were normal.
Treatment
The patient was taken up for elective distal pancreatectomy. The abdomen was explored with mid-line laparotomy. Liver metastasis and peritoneal metastasis were excluded. The gastrocolic ligament was then divided, and the lesser sac was entered. Figure 2 shows the intra-operative picture of the pancreas with the neuroendocrine tumor, insulinoma. Figure 2 shows a marked pancreatic lesion in the distal pancreatectomy specimen. On palpation of the pancreas, a 2 × 2 cm firm, solitary round mass was found at its tail. Branches of splenic vessels supplying the pancreatic tail distal to the lesion were secured and ligated. Then, spleen preserving distal pancreatectomy was performed.
Figure 2.

Intraoperative picture and resected specimen showing insulinoma
On histopathological examination, the section showed 1 × 1 cm of the lesion, which was a well-differentiated grade 1 neuroendocrine tumor, and the margins were free of tumor. Figure 3 shows the histology of the lesion where the normal pancreatic tissue is separated by from insulinoma by the fibrous capsule. The pancreatic tumor has the classic neuroendocrine nesting, trabecular, and solid pattern of growth.
Figure 3.

Histology image of the pancreatic lesion
Post-operative treatment and follow-up
Post-operatively, blood glucose levels normalized without requiring any additional dextrose. The patient was discharged after 8 days. Two weeks after his discharge, the patient continued to show improvement and there were no episodes of hypoglycemic attack.
Discussion
Two case series from the Mayo Clinic provided the two main bodies of data on insulinoma. Their two datasets span the years 1927–1986 and 1987–2007.[7]
Although insulinomas are uncommon, any patient who presents with non-diabetic hypoglycemia should be evaluated for this possibility. From 1987 to 2007, a Mayo Clinic case series from Olmstead County found that 73% of patients exclusively had hypoglycemia when fasting, whereas 21% had symptoms both fasting and post-prandially. Another 6% with confirmed insulinoma cases reported only post-prandial hypoglycemia as the only presentation.[8]
Whipple and Franz documented a triad of clinical features that were specific to insulinoma patients in 1935: hypoglycemia symptoms, a plasma glucose level of 45 mg/dl (2.5 mmol/liter) or below when hypoglycemia symptoms occurred, and alleviation of symptoms with glucose infusion.[9] More recently, in the absence of renal insufficiency, the following bio-chemical criteria were recommended by Service et al.[10]: an insulin level of 36 pmol/liter or more as measured by Radioimmunoassay (RIA) or 18 pmol/liter or more as measured by an immune-chemiluminescence assay, a C peptide level of 200 pmol/liter or more, and a pro-insulin level of 5 pmol/liter or more in a patient with a serum glucose level of 45 mg/dl (2.5 mmol/liter) or less.
Somatostatin receptors 2 are expressed in around 80% of insulinomas, and the Ga-68 DOTATOC scan has a high affinity for these receptors, resulting in great sensitivity in the detection of these tumors, especially low-grade and well-differentiated ones. When assessing insulinomas, this study has a sensitivity of up to 90%.
Insulinomas are best treated with surgical excision.[11] During the procedure, the entire abdomen must be examined, and liver metastases must be ruled out. The pancreas should be mobilized by dividing the gastrocolic ligament and entering the lesser sac. The second part of the duodenum should be mobilized too (Kocher’s maneuver). The head of the pancreas should be carefully palpated and inspected anteriorly and posteriorly, as well as the body and tail of the pancreas. After locating the tumor and ruling out any signs of metastasis, it is enucleated (because 90% of tumors are benign),[12] especially when located in the head of the pancreas. For the distal pancreatic tumor, it is best localized and distal pancreatectomy with or with spleen preservation is performed. Our case had a tumor located in the tail of the pancreas with a size of 2 × 2 cm and was away from the splenic hilum, so spleen preserving distal pancreatectomy was performed.
Conclusion
Our instance emphasizes the need for evaluating insulinoma as a cause of recurrent hypoglycemia in people who are not diabetic. Patients with insulinoma have hypoglycemic episodes, more characteristically as recurrent fasting hypoglycemia. Insulinoma is usually diagnosed by bio-chemical testing when there is high clinical suspicion.
Therefore, having a high index of suspicion in hypoglycemic individuals who do not respond to standard treatment or whose symptom pattern changes will lower the likelihood of insulinoma diagnosis being delayed and treated effectively with surgical resection as most are benign.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zhang X, Jia H, Li F, Fang C, Zhen J, He Q, et al. Ectopic insulinoma diagnosed by 68Ga-Exendin-4-PET/CT. Medicine (Baltimore) 2021;100:e25076. doi: 10.1097/MD.0000000000025076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Saigh TH. Insulinoma:Rare yet important. Case Rep. 2014;2014:bcr2013202395. doi: 10.1136/bcr-2013-202395. doi:10.1136/bcr-2013-202395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schott M, Klöppel G, Raffel A, Saleh A, Knoefel WT, Scherbaum WA. Neuroendocrine neoplasms of the gastrointestinal tract. Dtsch Aerzteblatt Online. 2011. [Last accessed on 2021 Nov 30]. Available from: https://www.aerzteblatt.de/10.3238/arztebl. 2011.0305 . [DOI] [PMC free article] [PubMed]
- 4.Callacondo D, Arenas JL, Ganoza AJ, Rojas-Camayo J, Quesada-Olarte J, Robledo H. Giant insulinoma:A report of 3 cases and review of the literature. Pancreas. 2013;42:1323–32. doi: 10.1097/MPA.0b013e318292006a. [DOI] [PubMed] [Google Scholar]
- 5.Uwagawa T, Misawa T, Fujiwara Y, Furukawa K, Tsutsui N, Kitamura H, et al. Erlotinib-induced thrombocytosis in patients with recurrence of pancreatic cancer after distal pancreatectomy. Pancreas. 2013;42:1196–7. doi: 10.1097/MPA.0b013e31828cf976. [DOI] [PubMed] [Google Scholar]
- 6.Oberstein PE, Remotti H, Saif MW, Libutti SK. Pancreatic neuroendocrine tumors:Entering a new era. JOP J Pancreas. 2012;13:169–73. [PubMed] [Google Scholar]
- 7.Wolfenden T, Dashora U, Carroll P. Hypoglycaemia in a patient who is non-diabetic. Case Rep. 2014;2014:bcr2013203260. doi: 10.1136/bcr-2013-203260. doi:10.1136/bcr-2013-203260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Placzkowski KA, Vella A, Thompson GB, Grant CS, Reading CC, Charboneau JW, et al. Secular trends in the presentation and management of functioning insulinoma at the Mayo Clinic, 1987–2007. J Clin Endocrinol Metab. 2009;94:1069–73. doi: 10.1210/jc.2008-2031. [DOI] [PubMed] [Google Scholar]
- 9.Mittendorf EA, Liu YC, McHenry CR. Giant insulinoma:Case report and review of the literature. J Clin Endocrinol Metab. 2005;90:575–80. doi: 10.1210/jc.2004-0825. [DOI] [PubMed] [Google Scholar]
- 10.Service FJ. Diagnostic approach to adults with hypoglycemic disorders. Endocrinol Metab Clin North Am. 1999;28:519–32, vi. doi: 10.1016/s0889-8529(05)70086-4. [DOI] [PubMed] [Google Scholar]
- 11.Zhang X, Jia H, Li F, Fang C, Zhen J, He Q, et al. Ectopic insulinoma diagnosed by 68Ga-Exendin-4-PET/CT:A case report and review of literature. Medicine (Baltimore) 2021;100:e25076. doi: 10.1097/MD.0000000000025076. doi:10.1097/MD.0000000000025076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furihata M, Tagaya N, Kubota K. Laparoscopic enucleation of insulinoma in the pancreas:Case report and review of the literature. Surg Laparosc Endosc Percutan Tech. 2001;11:279–83. doi: 10.1097/00129689-200108000-00011. [DOI] [PubMed] [Google Scholar]


