Abstract
Background:
Diabetes, is known to have a bilateral relationship with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Precise mechanism of diabetes onset in COVID-19 patients remains unclear.
Aim:
To analyse the incidence of new onset diabetes (NODM) among COVID-19 patients, as well as the effect of body mass index (BMI), family history, and steroid use on the incidence of the disease.
Methods:
Adult, not known diabetic patients, tested positive with Rapid Antigen Test or RT-PCR admitted to a tertiary care hospital and research institute were included in the present prospective observational study. The patients who developed NODM and NOPD (New Onset Pre-diabetes) during the three months follow-up and the risk factors associated were assessed. Patients with HbA1c >6.4% were diagnosed with NODM. An HbA1c of 5.7% to 6.4% was used to characterize NOPD.
Results:
Out of 273 previously not known diabetic COVID-19 infected individuals, a total of 100 were studied for three months after consent. Mean age of the patients 48.31 ± 19.07 years with male predominance (67%). Among these, 58% were non-diabetics and 42% were pre-diabetics. 6 (10.3%) of the 58 non-diabetics developed NOPD, and 8 (13.8%) developed NODM. 6 (14.2%) of the 42 pre-diabetics became non-diabetic, and 16.6% (7) developed NODM. Family history of DM (P < 0.001), severity at admission (P < 0.006), diabetic ketoacidosis (P < 0.0275), and persistent symptoms were associated significantly with NODM. Those with NODM had significantly greater BMI, O2 duration, steroid duration, FBS, and PPBS (P < 0.001 for all). Nearly 67% of the patients who developed NOPD had shortness of breath as the common symptom at time of admission (P = 0.0165).
Conclusion:
The incidence of NODM was strongly influenced by positive family history of DM, higher BMI, steroid dosage, and its duration. Hence, patients with COVID-19 need to be under surveillance for blood glucose screening.
Keywords: BMI, family history of DM, HbA1c, NODM, NOPD, SARS-CoV-2, steroids
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that causes coronavirus disease 2019 (COVID-19) times.[1] SARS-CoV-2 causes an increase in mortality and morbidity in the elderly individuals, particularly in those with comorbidities, such as diabetes mellitus (DM). Diabetes and SARS-CoV-2, on the other hand, are known to have a bidirectional relationship.[2] It is well-known that viral infections can trigger diabetes.[3] Previously, diabetes has been linked to infections with influenza and dengue viruses in India. There is also mounting evidence that SARS-CoV-2 can cause diabetes in people who have never had diabetes or taken glucocorticoids.[2]
COVID-19 associated DM and hyperglycemia were found in 19.70% and 25.23% of patients, respectively,[2] but the incidence of complications such as need for ICU and intubation, varies between studies, with some showing an increase and others no difference when compared to normoglycemic patients.[4] However, the exact mechanisms causing new onset diabetes in COVID-19 patients are unknown.[5] Several complex interrelated processes, such as previously undetected diabetes, stress hyperglycemia, steroid-induced hyperglycemia, and direct or indirect effects of SARS-CoV-2 on the β-cell are likely to be implicated.[5] DM and reactive hyperglycemia have been identified as predictors of severity in SARS-CoV-2 infected patients.[5]
Several research studies reported an association between COVID-19 pandemic and hyperglycemia in people with and without known diabetes.[6,7,8] However, in the Indian context, there is little data on new-onset diabetes and the long-term impact of COVID-19 infection on glycemic management. To identify management modalities for patients presenting with new-onset diabetes during this pandemic and those with hyperglycemia triggered by severe COVID-19, it is recommended to practice a rigorous and close long-term follow-up.
As a result, the study aims to analyse the incidence of new onset diabetes in Covid-19 patients and the effect of BMI, family history, and steroid use on the incidence of the disease.
Materials and Methods
Study design and settings
We conducted a prospective observational study over a period of six months from March 2021 to August 2021 at a tertiary care hospital and research institute. Informed consent of all participants was taken verbally after reading out the form in their native language and institutional scientific review committee permission was sought before the study commencement. 273 not known diabetic patients >18 years who tested positive with Rapid Antigen Test or RT-PCR and treated at our centre were included. After verbal consent, study was carried out in 100 individuals as 173 patients withdrew consent for follow-up. Treatment of all patients was done as per ICMR protocol. Scientific Research Committee approval number TIMS/2020-21/08 obtained on 12/03/2021.
All patients were followed up at regular intervals for one month for steroid dosage tapering and compliance, and after three months for HbA1c sampling. Patients who lost to follow-up, unwilling for the study and who are on long-term steroid treatment were exempted.
Data collection
Demographic data, clinical severity (according to ICMR grading[9]), duration of hospital stay, comorbidities, day of illness and day of admission to hospital, systemic corticosteroid dose during hospitalization of all patients were documented. Any oxygen support or need for higher mode of ventilator support was noted. Laboratory parameters – fasting blood glucose (FBS), post-breakfast blood sugar (PBFS), glycosylated hemoglobin (HbA1c) at admission and at follow-up using immunoturbidometry method were done.
Definitions
Non-diabetic patients were defined as having an HbA1C<5.7%.[10] Pre-diabetes and NOPD was defined by an HbA1c of 5.7% to 6.4%.[10] A diagnosis of NODM was made in patients with HbA1c >6.4%.[10] A requirement of 40-80 mg/day, >80 mg/day, and >125 mg/day of methylprednisolone was taken as low, high, and pulse dosage, respectively.[11,12] Steroid induced hyperglycemia was identified as rise in fasting blood glucose >125 mg/dL in a previously normal individual who required steroids during hospitalization.[13] Stress induced hyperglycemia was identified as rise in fasting blood glucose >125 mg/dL in a previously normal individual who did not require steroids during hospitalization.
Follow-up
Patients were followed up telephonically for one month for steroid dosage tapering, compliance, persisting symptoms and HbA1c after three months (if no steroids were given) and four months (if low dose steroid was given for treatment) of tapering steroids.
Statistical analysis
Data was analyzed using Rv4.1.1. Categorical variables were shown in the form of frequency table. Continuous variables were shown as Mean ± SD/Median (Min, Max) form. Two sample t test/Welch’s t test was used to compare means of variables over NODM. Mann—Whitney U test was used to compare the distributions of variables over NODM. Chi-square test was used to check the association between attributes. P ≤ 0.05 indicates statistical significance.
Results
Of 100 patients at admission, 58 were non-diabetics and 42 were pre-diabetics. Subjects were within the age range of 18 to 84 years with mean age of 48.31 ± 19.07 years and male predominance (67%) [Figure 1].
Figure 1.
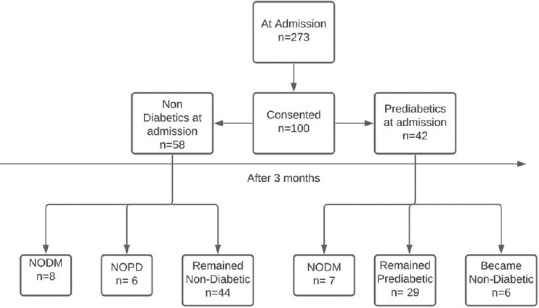
Flow chart depicting the findings of this study. (Note – Pre-diabetics at admission were diagnosed Denovo)
Of 58 non-diabetics, 6 (10.3%) developed NOPD, 8 (13.7%) developed NODM after three months. Of 42 pre-diabetics, 6 (14.3%) became non-diabetic, 29 (69%) remained pre-diabetic and 7 (16.67%) developed NODM after three months. Altogether, from HBA1c at admission and three months, 15 subjects had NODM.
39 (67.2%) non-diabetics did not require steroids yet 20 (51.3%) had impaired fasting glucose levels (stress induced hyperglycaemia) of which 4 (20%) developed NODM.
Out of 15 NODM, 3 (20%) non-diabetics at admission and 1 (6.66%) pre-diabetic at admission did not require steroids and did not have impaired fasting sugars and yet developed NODM after three months.
Table 1 shows baseline characteristics of non-diabetic COVID-19 patients who developed NODM after three months. Family history of DM was present in 9 (60%) patients who developed NODM (P < 0.001). Fever (10; 66.67%) and cough (9; 60%) were the most common symptoms in patients who developed NODM. DKA was found in only two (13.33%) patients with NODM. Hypertension (33%) was the common comorbidity found in patients with NODM. 9 (60%) of the newly diagnosed DM patients were overweight or obese. Nearly 75% of the patients who developed NODM took steroids during COVID-19 treatment (P < 0.005). There was a statistically significant association between family history of DM, severity at admission, DKA, persistent symptoms, and incidence of NODM. There was a significant difference between BMI (25 ± 4.39 vs. 21.66 ± 2.76; P < 0.0117), O2 duration (3.2 ± 3.03 vs. 1.16 ± 2.57; P < 0.0015), steroid treatment duration (24.07 ± 16.65 vs. 7.31 ± 14.34; P < 0.001) in patients who developed NODM and those who did not. Out of 15 patients who developed NODM, 8 (53.33%) had moderate severity at the time of admission.
Table 1.
Demographic, clinical, and biochemical characteristics of the patients by incidence of NODM after three months
| Variables | NODM after 3 months | P | |
|---|---|---|---|
|
| |||
| No=85 | Yes=15 | ||
| Age (years) | |||
| 18-35 | 28 (32.94%) | 6 (40%) | 0.6787MC |
| 36-65 | 36 (42.35%) | 7 (46.67%) | |
| >65 | 21 (24.71%) | 2 (13.33%) | |
| Mean±SD | 49.49±19.53 | 41.6±15.02 | 0.0871WT |
| Gender | |||
| Female | 25 (29.41%) | 8 (53.33%) | 0.087MC |
| Male | 60 (70.59%) | 7 (46.67%) | |
| Body Mass Index | |||
| <18.5 (Underweight) | 10 (11.76%) | 1 (6.67%) | <0.001MC* |
| 18.5-24.9 (Normal) | 63 (74.12%) | 5 (33.33%) | |
| 25-29.9 (Overweight) | 12 (14.12%) | 5 (33.33%) | |
| ≥30 (Obese) | 0 | 4 (26.67%) | |
| Mean±SD | 21.66±2.76 | 25±4.39 | 0.0117WT* |
| Family history of diabetes mellitus | |||
| No | 80 (94.12%) | 6 (40%) | <0.001MC* |
| Yes | 5 (5.88%) | 9 (60%) | |
| Day of illness (from symptom onset) at admission | |||
| Mean±SD, | 5.8±2.05 | 5.93±2.12 | 0.9337MW |
| Median (minimum, maximum) | 5 (2, 10) | 6 (3, 11) | |
| Severity at admission | |||
| Mild | 68 (80%) | 6 (40%) | 0.006MC* |
| Moderate | 12 (14.12%) | 8 (53.33%) | |
| Severe | 5 (5.88%) | 1 (6.67%) | |
| Severity progression | |||
| No progression | 79 (92.94%) | 13 (86.67%) | |
| Mild to moderate | 3 (3.53%) | 1 (6.67%) | 1MC |
| Moderate to severe | 3 (3.53%) | 1 (6.67%) | |
| Oxygen duration | |||
| Mean±SD, | 1.16±2.57 | 3.2±3.03 | 0.0015MW* |
| Median (minimum, maximum) | 0 (0, 10) | 3 (0, 7) | |
| Steroid duration | |||
| Mean±SD, | 7.31±14.34 | 24.07±16.65 | <0.001MW* |
| Median (minimum, maximum) | 0 (0, 56) | 25 (0, 46) | |
| ICU requirement | |||
| No | 79 (92.9%) | 11 (73.33%) | 0.0645MC |
| Yes | 6 (7.05%) | 4 (26.67%) | |
| Steroid dosage | |||
| No steroid | 65 (76.47%) | 4 (26.67%) | |
| Low dose (40-60 mg/day) | 12 (14.12%) | 5 (33.33%) | 0.005MC* |
| High dose (60-80 mg/day) | 4 (4.71%) | 3 (20%) | |
| Pulse therapy (125 mg/day) | 4 (4.71%) | 3 (20%) | |
| Diabetic Ketoacidosis | |||
| No | 85 (100%) | 13 (86.67%) | 0.0275MC* |
| Yes | 0 | 2 (13.33%) | |
| C-Reactive Protein | |||
| Normal (0-6) | 40 (47.06%) | 6 (40%) | |
| Mild (6-26) | 27 (31.76%) | 4 (26.67%) | 0.6287MC |
| Moderate (26-100) | 18 (21.18%) | 5 (33.33%) | |
| Symptoms at admission | |||
| Fever | 50 (58.82%) | 10 (66.67%) | 0.5676C |
| Cough | 34 (40%) | 9 (60%) | 0.1492C |
| Shortness of breath | 19 (22.35%) | 0 | 0.0755MC |
| Cold | 9 (10.59%) | 2 (13.33%) | 1MC |
| Headache | 12 (14.12%) | 3 (20%) | 0.7036MC |
| Generalized Malaise | 31 (36.47%) | 5 (33.33%) | 0.8155C |
| Myalgia | 15 (17.65%) | 0 | 0.1299MC |
| Comorbidity | |||
| Hypertension | 27 (31.76%) | 5 (33.33%) | 1MC |
| Respiratory disease | 6 (7.06%) | 2 (13.33%) | 0.6022MC |
| Persisting symptoms after three months | |||
| Polydipsia | 3 (3.53%) | 8 (53.33%) | <0.001MC* |
| Hair loss | 12 (14.12%) | 6 (40%) | 0.029MC* |
| Polyphagia | 6 (7.06%) | 6 (40%) | 0.005MC* |
| Weight gain | 4 (4.71%) | 3 (20%) | 0.0745MC |
| Weight loss | 0 | 3 (20%) | 0.005MC* |
| Weakness | 10 (11.76%) | 5 (33.33%) | 0.056MC |
| Body pains | 28 (32.94%) | 5 (33.33%) | 1MC |
| Cough | 19 (22.35%) | 2 (13.33%) | 0.5262MC |
| Shortness of breath | 15 (17.65%) | 3 (20%) | 1MC |
C – Chi-square test, MC - Chi-square test with Monte Carlo simulation, WT – Welch’s t-test, MW – Mann–Whitney U-test, * indicates statistical significance
Box plot shows that of the 15 NODM, 7 pre-diabetic and 8 non-diabetic COVID-19 patients before the study turned diabetic after three months [Figure 2].
Figure 2.
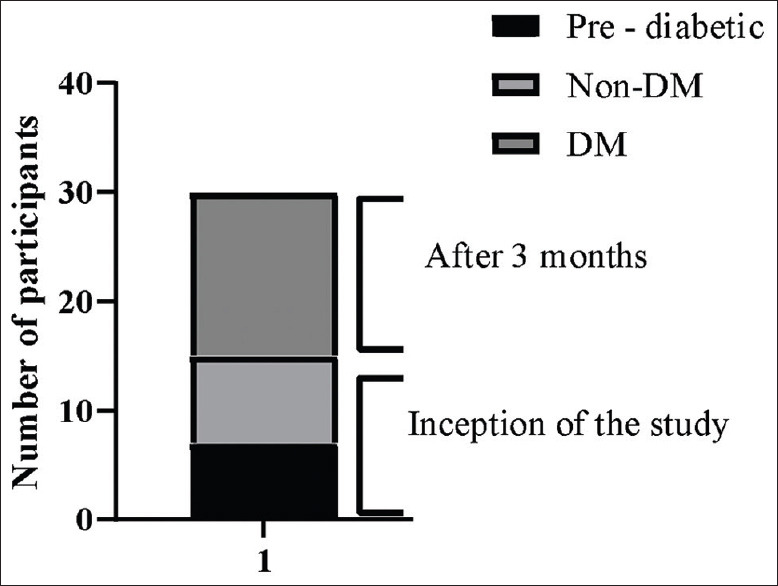
Boxplot showing number of participants. Non-DM: Non-Diabetes Mellitus, DM: Diabetes Mellitus
The Box-violin plots of FBS and PPBS levels in patients who developed NODM or NOPD and patients who did not develop are shown in 3. FBS (113.33 ± 8.34 vs. 101.52 ± 28.88; P = 0.001) and PPBS (174.4 ± 29.03 vs. 144.92 ± 48.39; P = 0.001) after initiating steroid were found to be significantly higher among those who developed NODM than those who didn’t develop NODM. PPBS (194.33 ± 40.19 vs. 146.47 ± 46.21; P = 0.008) after initiating steroid were found to be significantly higher among those who developed NOPD than those who didn’t develop. Although, FBS (106.83 ± 7.76 vs. 103.06 ± 27.9; P = 0.2307) after initiating steroid was found to be higher among those who developed NOPD than those who did not develop, however difference was statistically insignificant [Figure 3].
Figure 3.
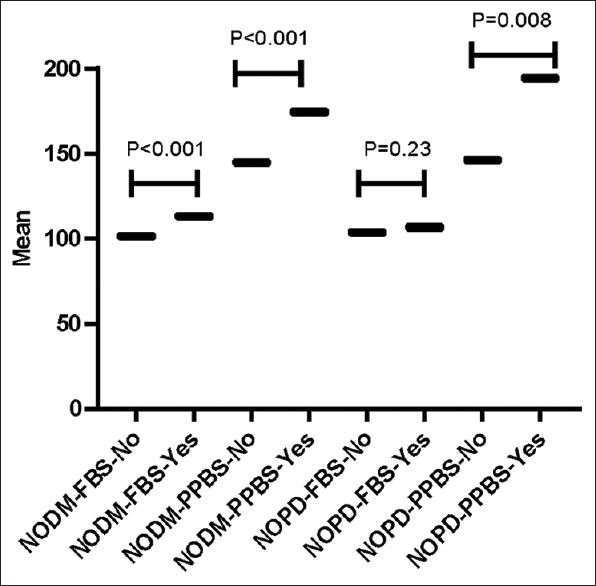
Box Violin plot for NODM and NOPD FBS: Fasting Blood Sugar, PPBS: Post-prandial blood sugar, NODM: New onset diabetics NOPD: New onset pre-diabetic
Discussion
SARS-CoV-2 infection can cause severe systemic inflammatory response syndrome (SIRS), acute respiratory distress syndrome (ARDS), multiorgan involvement (MODS), and shock. COVID-19 puts individuals with co-existing DM at higher risk of severe disease and mortality.[14,15] According to a recent pooled meta-analysis study, COVID-19-associated NODM patients had the highest mortality rate (24.96%), followed by patients with pre-existing DM (16.03%), and non-diabetic patients (9.29%). COVID-19 associated NODM patients had the highest adverse effects, followed by patients with pre-existing DM, COVID-19 associated hyperglycemia, and non-diabetic patients.[2]
In our study, 15 (15%) patients with NODM were found among the 100 patients admitted with COVID-19. In another study, COVID-19-associated DM and hyperglycemia were found in 19.70% and 25.23% of patients, respectively.[2] In a separate retrospective analysis, 2 (5.7%) of 35 COVID-19 patients had recently been diagnosed diabetes.[16] Patients under 60 years old had similar incidence of DM and pre-existing DM as those over 60 years old.[10] However, the patients under 60 years old exhibited a substantially higher mean HbA1C level and had higher BMI than older individuals (8.0% vs 6.9%; P = 0.003).[10] In our study, most (86.6%) of the patients who had dysregulated glucose levels were under 65 years of age suggesting that younger individuals are more likely to have aberrant glucose metabolism because of obesity, putting them at a higher risk of having severe COVID-19. In our study, it was found that severity of admission was significantly associated with NODM. Similarly, in another study conducted by Fadini et al.[17] after adjusting for age and sex, diabetes (pre-existing and newly diagnosed altogether) and COVID-19 severity remained significant (RR 1.49; 95% C.I. 1.07–2.09; P = 0.019). The study stated that COVID-19 severity was significantly stronger for patients with NODM than for those with pre-existing DM.[16] Altogether, our study states that COVID-19 severity can predict NODM.
In our study, 14 (14%) patients had positive family history of diabetes. It was reported that people with family history of DM develop an early endothelial dysfunction, which would increase the susceptibility and severity of infection and damage to the endothelium, in the patient infected with the SARS-CoV-2.[18] Hence, this indicates that endothelial dysfunction might lead to NODM indicating positive family history is a strong risk factor.[18] Likewise, in our study, 60% of the patients who developed NODM had positive family history of DM. Also, a recent case study reported that out of the three cases studies two cases had positive family history of DM.[19] While in another Indian study, out of 273 NODM patients, 39% had family history of DM, however the association was statistically insignificant.[20] In our study, COVID-19-associated NODM may predispose patients for higher demand for oxygen support and steroid treatment which eventually led to prolonged hospital stay and worse clinical outcome. Similarly, Montefusco et al.,[6] reported that patients with NODM required a longer in-hospital stay, higher proportion requiring oxygen support and ventilation as compared to patients with normoglycaemia.
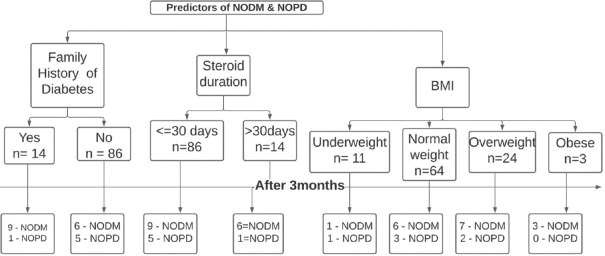
In our study, two (2%) patients developed DKA, both had positive family history, of whom one was non-diabetic at admission and other was pre-diabetic at admission [Figure 4]. In a Chinese study, 27 out of 42 COVID-19 patients who had never been diagnosed with diabetes experienced ketoacidosis.[21] According to an U.K. study, children with DKA presented more frequently during the pandemic period (10% severe pre-pandemic vs. 47% during the first wave of the pandemic) and had higher HbA1c (13% vs. 10.4%).[22] Insulinopenia is likely to cause DKA in COVID-19 patients.[16] In another large U.S. study, COVID-19 patients had higher BMI, greater insulin requirement, and protracted time to resolution of DKA than those without COVID-19.[23] Therefore, high degree of suspicion is essential to improve the timely prognosis of COVID-19-related DKA.
Figure 4.
Flowchart showing the strong predictors of NODM. BMI, Body mass index; NODM, New onset of diabetes mellitus, NOPD, New onset pre-diabetes
In our study, when compared to pre-diabetics or non-diabetics, most symptoms (polydipsia, hair loss, polyphagia, and weight loss) persisted even after three months in NODM patients. In a case series from Bihar, young, non-diabetic individuals presented to hospital within one month post-COVID infection, with fatigue, polydipsia, polyuria and weight loss and were diagnosed with NODM and DKA.[24] Also, ADA states that polyuria, polydipsia, weight loss, polyphagia are symptoms of diabetes.[10]
It was known that although steroids are safe and effective, high-dose steroids causes serious side effects especially altered blood glucose levels in COVD-19 patients.[25] Previous studies showed that 53–70% of individuals without diabetes develop steroid-induced hyperglycemia.[26] A meta-analysis of 13 studies showed that overall, 32.3% of people developed glucocorticoid-induced hyperglycemia and 18.6% developed diabetes.[27] In this study, 31 required steroids, of which 11 (35.5%) developed NODM after three months. This indicates that using steroids in patients admitted to the hospital with COVID-19 may therefore be associated with an increased risk of developing diabetes, which could be attributed to delayed recovery of β cell function.[28]
In our study, 39 out of 58 (67.2%) non-diabetics did not require steroids yet 20 of those 39 patients (51.3%) developed impaired fasting glucose levels (stress induced hyperglycemia). Of these 20, 4 (20%) developed NODM (Ref Supplementary Table 1). However, 4 out of 15 NODM (26.66%) did not require steroids and did not have impaired fasting sugars (i.e., FBS >125 mg/dl) yet developed diabetes after three months. This indicates that COVID-19 can be an independent risk factor for developing new onset diabetes. A recent China study also reported NODM in 3.3% of 1,733 people at 6 months following discharge from hospital with COVID-19.[29] Another UK-based study also reported that 47,780 people discharged from hospital following admission for COVID-19 showed 4.9% developed diabetes at a mean follow-up of 140 days.[30] In another Italian-based cohort study conducted among 551 patients hospitalized for COVID-19 showed altered glycometabolic control, with insulin resistance and an abnormal cytokine profile, in 27% of normoglycaemic patients for at least two months in patients. Altogether the aberrant glycometabolic control persisting even after recovery mandates further investigation of metabolic abnormalities in long run.[6]
Supplementary Table 1.
Risk factors and predictors for NODM and NOPD
| RISK FACTORS AND PREDICTORS FOR NON-DIABETIC TO NODM/NOPD | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| SEVERITY AT ADMN | S NO | AGE | GENDER | BMI | FAMILY HISTORY | OTHER COMORBS | Severity at Admission | Severity Progression | STEROID DOSAGE | ICU REQUIREMENT | Impaired Glycemic control during hospitalization | NODM/NOPD | DKA |
| MILD | 1 | 32 | M | 30 | Nil | None | 1 | 0 | LD | No | Yes | DM | No |
| 2 | 49 | F | 26 | + | Htn | 1 | 0 | No | No | Yes | DM | No | |
| 3 | 27 | M | 19 | +++ | None | 1 | 0 | No | No | Yes | DM | No | |
| 4 | 40 | F | 30 | Nil | None | 1 | 0 | No | No | Yes | DM | No | |
| 5 | 37 | M | 26 | Nil | None | 1 | 0 | No | No | Yes | DM | No | |
| 6 | 30 | M | 20 | Nil | None | 1 | Mild to Mod | HD | Yes | Yes | DM | No | |
| MODERATE | 1 | 70 | F | 27 | ++ | Htn, Asthma | 2 | 0 | HD | Yes | Yes | DM | No |
| 2 | 29 | M | 30 | Nil | None | 2 | 0 | LD | No | Yes | DM | No | |
| 3 | 32 | F | 30 | ++ | Asthma | 2 | Mod to severe | Pulse | Yes | Yes | DM | No | |
| 4 | 41 | M | 18 | ++ | None | 2 | 0 | LD | No | Yes | DM | Yes | |
| 5 | 22 | F | 21 | + | Denovo htn | 2 | 0 | LD | No | Yes | DM | No | |
| 6 | 68 | F | 27 | + | Htn | 2 | 0 | HD | No | Yes | DM | No | |
| 7 | 49 | F | 28 | + | Htn | 2 | 0 | LD | No | Yes | DM | Yes | |
| 8 | 62 | F | 22 | Nil | None | 2 | 0 | Pulse | Yes | Yes | DM | No | |
| SEVERE | 1 | 36 | M | 21 | + | None | 3 | 0 | Pulse | Yes | Yes | DM | No |
| NON-DIABETICS TO NOPD | 1 | 33 | M | 19 | Nil | None | 2 | 0 | LD | No | Yes | PD | No |
| 2 | 82 | F | 18 | Nil | Htn | 2 | 0 | LD | No | Yes | PD | No | |
| 3 | 26 | M | 20 | Nil | None | 3 | 0 | Pulse | Yes | Yes | PD | No | |
| 4 | 31 | M | 25 | Nil | None | 1 | 0 | No | No | Yes | PD | No | |
| 5 | 26 | M | 20 | + | None | 1 | 2 | LD | No | Yes | PD | No | |
| 6 | 61 | F | 26 | Nil | Asthma | 1 | 0 | No | No | Yes | PD | No | |
Abbreviations M – male, F - female, BMI, Body mass index, *,**,*** one, two, and three members, respectively, in family with history of diabetes mellitus, Htn – hypertension, LD - low dose, HD - high dose, DM - new onset diabetes mellitus, PD - new onset pre-diabetes
The findings of this study should alarm clinicians that new onset diabetes is a serious prognostic factor for COVID-19.
Limitations
This was a single institution study and convenient sampling was done from patients admitted to a government hospital which makes generalization difficult. This study was conducted during peak of Delta variant of SARS-CoV-2 in India, thus these findings cannot be extrapolated to all SARS-CoV-2 variants. Also, ours being a government center, majority of lower and middle socioeconomic patients seek care at our center, thus, data of patients belonging to high socioeconomic stratum being admitted in private hospitals in India with acute COVID-19 is missing. Our study did not distinguish between Type 1 and Type 2 DM. Finally, a thorough understanding of the exercise and dietary patterns would have been preferable. These data could be extremely useful in determining the risk factors for NODM in this population.
Conclusion
Positive family history, higher BMI, higher steroid dosage, and duration in non-diabetics are strongly associated with and probable predictors of NODM. Thus, we recommend HbA1c at follow up to screen for NODM. Also, severe COVID-19 should probably be considered as an independent risk factor of NODM. Because of the limited follow-up of these individuals, it is unclear whether NODM will be permanent. Therefore, further larger studies and more prospective metabolic investigations are needed to determine a definitive correlation, etiology, possible risk factors, prognosis, and treatment for NODM post-COVID-19 infection.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We sincerely thank all our patients to consent for analysing their data. We appreciate Sister Shaili for her support during the study. We thank Dr. Vini Mehta and her team for statistical analysis of the data.
References
- 1.Metwally AA, Mehta P, Johnson BS, Nagarjuna A, Snyder MP. COVID-19-Induced new-onset diabetes:Trends and technologies. Diabetes. 2021;70:2733–44. doi: 10.2337/dbi21-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shrestha DB, Budhathoki P, Raut S, Adhikari S, Ghimire P, Thapaliya S, et al. New-onset diabetes in COVID-19 and clinical outcomes:A systematic review and meta-analysis. World J Virol. 2021;10:275–87. doi: 10.5501/wjv.v10.i5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filippi CM, von Herrath MG. Viral trigger for type 1 diabetes:Pros and cons. Diabetes. 2008;57:2863–71. doi: 10.2337/db07-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michalakis K, Ilias I. COVID-19 and hyperglycemia/diabetes. World J Diabetes. 2021;12:642–50. doi: 10.4239/wjd.v12.i5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khunti K, Prato SD, Mathieu C, Kahn SE, Gabbay RA, Buse JB. COVID-19, hyperglycemia, and new-onset diabetes. Diabetes Care. 2021;44:2645–55. doi: 10.2337/dc21-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montefusco L, Ben Nasr M, D'Addio F, Loretelli C, Rossi A, Pastore I, et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat Metab. 2021;3:774–85. doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813–21. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H, Tian S, Chen T, Cui Z, Shi N, Zhong X, et al. Newly diagnosed diabetes is associated with a higher risk of mortality than known diabetes in hospitalized patients with COVID-19. Diabetes Obes Metab. 2020;22:1897–906. doi: 10.1111/dom.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Preetam M, Anurag A. MuLBSTA score in COVID-19 pneumonia and prediction of 14-day mortality risk:A study in an Indian cohort. J Fam Med Prim Care. 2021;10:223–27. doi: 10.4103/jfmpc.jfmpc_1766_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith SM, Boppana A, Traupman JA, Unson E, Maddock DA, Chao K, et al. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID-19. J Med Virol. 2021;93:409–15. doi: 10.1002/jmv.26227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng F, Gao D, Ma X, Guo Y, Wang R, Jiang W, et al. Corticosteroids in diabetes patients infected with COVID-19. Ir J Med Sci. 2021;190:29–31. doi: 10.1007/s11845-020-02287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edalatifard M, Akhtari M, Salehi M, Naderi Z, Jamshidi A, Mostafaei S, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients:Results from a randomised controlled clinical trial. Eur Respir J. 2020;56:2002808. doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hwang JL, Weiss RE. Steroid-induced diabetes:A clinical and molecular approach to understanding and treatment. Diabetes Metab Res Rev. 2014;30:96–102. doi: 10.1002/dmrr.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang JK, Lin SS, Ji XJ, Guo LM. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–9. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed F, Raal FJ, Mbelle M, Zamparini J, Venturas J, Shaddock E, et al. Glycaemic characteristics and outcomes of COVID-19 patients admitted to a tertiary hospital in Johannesburg. Wits J Clin Med. 2020;2:123. [Google Scholar]
- 16.Armeni E, Aziz U, Qamar S, Nasir S, Nethaji C, Negus R, et al. Protracted ketonaemia in hyperglycaemic emergencies in COVID-19:A retrospective case series. Lancet Diabetes Endocrinol. 2020;8:660–3. doi: 10.1016/S2213-8587(20)30221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fadini GP, Morieri ML, Boscari F, Fioretto P, Maran A, Busetto L, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020;168:108374. doi: 10.1016/j.diabres.2020.108374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarado-Vasquez N. Could a family history of type 2 diabetes be a risk factor to the endothelial damage in the patient with COVID-19? Med Hypotheses. 2021;146:110378. doi: 10.1016/j.mehy.2020.110378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suwanwongse K, Shabarek N. Newly diagnosed diabetes mellitus, DKA, and COVID-19:Causality or coincidence? A report of three cases. J Med Virol. 2021;93:1150–3. doi: 10.1002/jmv.26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh A, Anjana RM, Shanthi Rani CS, Rani SJ, Gupta R, Jha A, et al. Glycemic parameters in patients with new-onset diabetes during COVID-19 pandemic are more severe than in patients with new-onset diabetes before the pandemic:NOD COVID India study. Diabetes Metab Syndr. 2021;15:215–20. doi: 10.1016/j.dsx.2020.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22:1935–41. doi: 10.1111/dom.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGlacken-Byrne SM, Drew SEV, Turner K, Peters C, Amin R. The SARS-CoV-2 pandemic is associated with increased severity of presentation of childhood onset type 1 diabetes mellitus:A multi-centre study of the first COVID-19 wave. Diabet Med. 2021;38:e14640. doi: 10.1111/dme.14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pasquel FJ, Messler J, Booth R, Kubacka B, Mumpower A, Umpierrez G, et al. Characteristics of and mortality associated with diabetic ketoacidosis among US patients hospitalized with or without COVID-19. JAMA Netw Open. 2021;4:e211091. doi: 10.1001/jamanetworkopen.2021.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarwani A, Al Saeed M, Taha H, Al Fardan RM. New-onset diabetes mellitus presenting as diabetic ketoacidosis in patients with COVID-19:A case series. Cureus. 2021;13:e16290. doi: 10.7759/cureus.16290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piccica M, Lagi F, Trotta M, Spinicci M, Zammarchi L, Bartoloni A. High-dose steroids for the treatment of severe COVID-19. Intern Emerg Med. 2021;16:1395–9. doi: 10.1007/s11739-021-02707-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung NW. Steroid-induced hyperglycaemia in hospitalised patients:Does it matter? Diabetologia. 2016;59:2507–9. doi: 10.1007/s00125-016-4116-z. [DOI] [PubMed] [Google Scholar]
- 27.Liu X-x, Zhu X-m, Miao Q, Ye H-y, Zhang Z-y, Li Y-M. Hyperglycemia induced by glucocorticoids in nondiabetic patients:A meta-analysis. Ann Nutr Metab. 2014;65:324–32. doi: 10.1159/000365892. [DOI] [PubMed] [Google Scholar]
- 28.Accili D. Can COVID-19 cause diabetes? Nat Metab. 2021;3:123–5. doi: 10.1038/s42255-020-00339-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital:A cohort study. Lancet Lond Engl. 2021;397:220–32. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ayoubkhani D, Khunti K, Nafilyan V, Humberstone B, Diamond I, Banerjee A. Post-covid syndrome in individuals admitted to hospital with covid-19:Retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]