Abstract
We have previously shown that a Pasteurella multocida cexA mutant (PBA875) was impaired in capsule export and highly attenuated in virulence for mice (J. D. Boyce and B. Adler, Infect. Immun. 68:3463–3468, 2000). In this study we show that immunization with high, but not low, doses of PBA875 can confer significant protection against wild-type challenge. We have also constructed a genetically defined acapsular P. multocida strain (AL18) by inactivation of bcbH, a gene predicted to be involved in polysaccharide biosynthesis. AL18 failed to produce immunoreactive polysaccharide as determined by immunofluorescence and Western immunoblot. Immunization of mice with live AL18 conferred significant protection against wild-type challenge, while immunization with similar doses of either killed wild-type or killed AL18 failed to confer protection.
The gram-negative bacterium Pasteurella multocida is the etiological agent of hemorrhagic septicemia in cattle, fowl cholera in birds, and atrophic rhinitis in pigs (18). P. multocida strains can be separated into serogroups A, B, D, E, and F based on the antigenicity of their capsule (6, 26) and serotypes 1 to 16 based on lipopolysaccharide (LPS) antigens (12). The capsular serogroup is generally related to disease predilection, with hemorrhagic septicemia strains belonging to serogroup B or E (30) and the majority of fowl cholera strains belonging to serogroup A (7).
Hemorrhagic septicemia is endemic in most parts of tropical Asia, Africa, and India and causes high mortality in livestock (2). It is considered to be the most economically important disease of livestock in South East Asia and causes significant economic losses in India and Africa (2, 30). Cattle and buffalo are the most common hosts, but pigs, sheep, goats, deer, and camels are also susceptible to infection and disease (3, 9). Vaccination with undefined, killed vaccines is practiced in areas where the disease is endemic and has reduced the incidence of disease, but the duration of immunity is short and significant outbreaks still occur (2, 30). Little is known about why vaccination procedures sometimes fail.
Current P. multocida vaccines contain either inactivated bacteria (bacterins) or live attenuated bacteria (10, 20, 30). Bacterins are inexpensive to produce but must be injected, often cause severe tissue reactions, and give very limited protection against heterologous serotypes (8, 25). Furthermore, it is not uncommon for groups vaccinated with these bacterins to suffer disease outbreaks (28). Immunization with live attenuated bacteria or bacterins derived from in vivo-grown cells elicits the production of antibodies which give protection against a range of serotypes (14, 25–27). This suggests that antigens expressed solely in vivo play an important role in cross-protective immunity. However, the live attenuated strains currently in use as vaccines are undefined, and there have been pasteurellosis outbreaks attributed to the vaccine strains (8, 13, 15, 30). Therefore, there is significant interest in producing rationally attenuated P. multocida mutants for use as live attenuated vaccine strains.
In gram-negative bacteria such as Neisseria meningitidis and Haemophilus influenzae, capsule is the major protective antigen and forms the basis of licensed vaccines (11). In P. multocida serogroup B there has been conflicting evidence on whether the capsule is a protective antigen (19, 21, 22), but the most recent work (19) indicates that the capsule is unlikely to be a protective antigen. However, no recombinant single antigens have been shown to stimulate protection against P. multocida serogroup B, and only limited protection in the absence of capsule has been demonstrated by passive administration of anti-LPS monoclonal antibodies (1). In contrast, rationally attenuated acapsular mutants of other species have shown promise as vaccine candidates (16, 29), suggesting that a similar approach to the development of a Pasteurella vaccine may be successful. Thus, the main aim of this work was to construct defined acapsular mutants of P. multocida and to test them for their ability to stimulate protective immunity against wild-type challenge.
We have shown previously that the P. multocida cexA mutant PBA875 was acapsular due to its inability to export capsule and was highly attenuated in virulence for mice (4). This acapsular strain was significantly more susceptible to macrophage uptake than wild-type bacteria and was cleared from the blood of infected mice (>99.98% of bacteria removed) in less than 4 h, while wild-type organisms multiplied rapidly. In order to determine if PBA875 could stimulate protective immunity against wild-type challenge, female 8- to 10-week-old BALB/c mice were vaccinated intraperitoneally (i.p.) with either one or two doses of live PBA875 given 14 days apart. Mice were challenged by i.p. injection of 3 × 103 wild-type P. multocida M1404 (>300 50% infective dose [ID50] [4]) 1 month after the primary vaccination (Table 1). No protection was observed with immunization doses of less than 105 CFU or when only a single immunization dose was given. However, significant protection (P < 0.001, Fisher's exact test) was observed with an immunization regime comprising two doses of either 8 × 105 or 8 × 106 CFU of live PBA875 (Table 1). These data indicated that live acapsular P. multocida could stimulate protective immunity at high immunization doses. We suggest that this dependence on dose was due to the rapid clearance of the acapsular strain from the host (4).
TABLE 1.
Protection conferred by immunization with live P. multocida capsule-deficient mutant
| Immunization dose (CFU)a | Booster dose | % Survival (no. of animals in group)b |
|---|---|---|
| None (BHIB) | No | 0 (11) |
| 8 × 102 | No | 0 (10) |
| 8 × 102 | Yes | 0 (10) |
| 8 × 104 | Yes | 0 (6) |
| 8 × 105 | Yes | 77 (9)c |
| 8 × 106 | No | 0 (10) |
| 8 × 106 | Yes | 88 (8)c |
Mice were immunized with live acapsular P. multocida (PBA875) cexA::tet (M) mutant in 0.1 ml of brain heart infusion broth (BHIB).
Mice were challenged with 3 × 103 CFU of wild type P. multocida M1404, observed for signs of disease, and killed by cervical dislocation when deemed moribund, in accordance with animal ethics requirements. Sensitive mice usually succumbed within 36 h, and mice which survived for at least 1 week after challenge were deemed immune. There was no significant difference observed in time to death for sensitive mice.
Significant protection compared to nonimmunized controls (P < 0.001, Fisher's exact test).
The acapsular strain PBA875 produced immunoreactive polysaccharide but failed to export this polysaccharide to the cell surface (4). Thus, immunity conferred by this strain could be due in part to the stimulation of antibody production by polysaccharide released from lysed cells. To investigate this possibility, we constructed a second acapsular P. multocida strain by inactivation of bcbH, located within region 2 of the cap locus (5).
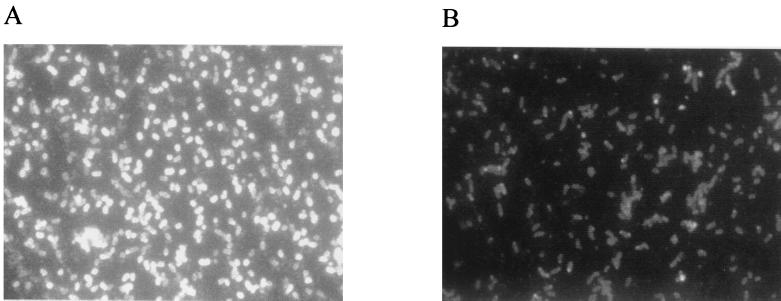
A P. multocida bcbH::tet(M) mutant was constructed by allelic exchange as described previously for construction of PBA875 (4). The tet(M) casette was inserted at the unique EcoRI site within bcbH, resulting in a predicted truncation of the BcbH protein at amino acid 105 of 720. The phenotype of the P. multocida strains was investigated by immunofluorescence (Fig. 1) and Western blotting (data not shown) using P. multocida serogroup B typing serum as the primary antibody. Wild-type P. multocida showed strong fluorescence completely encircling the bacteria, while the mutant strain (AL18) showed only very weak fluorescence (Fig. 1). These data indicated that AL18 failed to produce immunoreactive polysaccharide.
FIG. 1.
Phenotypic characterization of P. multocida capsule expression. Digital photomicrographs of methanol-fixed P. multocida 1404 (wild-type) (A) and AL18 acapsular mutant (B) visualized by immunofluorescence, with P. multocida serogroup B typing antiserum as the primary antibody and fluorescein isothiocyanate-labeled anti-rabbit immunoglobulin as the secondary antibody. Both charge-coupled device images were acquired using identical integration times.
The ID50 of the acapsular strain AL18 was approximately 106 CFU as determined by i.p. challenge of female BALB/c mice. Thus, like PBA875, AL18 was highly attenuated in virulence for mice. To investigate the protection conferred by vaccination with AL18 and to determine whether protection was dependent on the presence of live bacteria, female 8- to 10-week-old BALB/c mice were injected with between 4 × 105 and 2 × 106 CFU of either live or killed P. multocida M1404 or AL18. Where appropriate, bacteria were killed by incubation at 55°C for 30 min and suspensions were checked for surviving bacteria by direct plating onto nutrient agar (Difco). Mice were vaccinated either i.p. or intramuscularly (i.m.) into the upper thigh muscle at day zero, given a second dose, identical to the first 14 days later, and challenged by i.p. injection of 120 CFU of wild-type P. multocida M1404 (>12 ID50 [4]) 15 days after the second vaccination. Mice were observed for signs of disease and were killed by cervical dislocation when deemed moribund, in accordance with animal ethics reqirements. Sensitive mice usually succumbed within 36 h, and mice which survived for at least 1 week after challenge, when the experiment was terminated, were deemed immune.
No statistically significant protection (P > 0.05, Fisher's exact test) was observed in mice immunized either i.m. or i.p. with brain heart infusion broth or with bacteria that had been heat killed prior to vaccination (Table 2). Significant protection (90%; P < 0.001) was observed when mice were immunized i.p. with live AL18, and statistically significant, but low, protection (50%; P = 0.03) was observed when mice were immunized i.m. with live AL18. The antibody responses of a sample of the mice from each immunized group were examined by Western blot of P. multocida whole-cell extracts. Sera from all mice reacted with a range of P. multocida antigens irrespective of whether the mice were protected against challenge (data not shown). There was no correlation between the response to specific antigens, as determined by Western immunoblot, and the protection status.
TABLE 2.
Protection conferred by immunization with live or killed P. multocida strains
| Straina | Immunization route | % Survival (no. of animals in group)b |
|---|---|---|
| None (BHIB) | i.p. | 0 (5) |
| None (BHIB) | i.m. | 0 (5) |
| AL18 (live) | i.p. | 90 (10)c |
| AL18 (live) | i.m. | 50 (10)c |
| AL18 (killed) | i.p. | 0 (5) |
| AL18 (killed) | i.m. | 40 (5) |
| M1404 (killed) | i.p. | 40 (5) |
| M1404 (killed) | i.m. | 0 (5) |
Mice were immunized with either live or killed P. multocida AL18 (acapsular mutant) or M1404 (wild type) in 0.05 ml of brain heart infusion broth (BHIB).
Mice were challenged with 120 CFU of wild-type P. multocida M1404, observed for signs of disease, and killed by cervical dislocation when deemed moribund, in accordance with animal ethics requirements. Sensitive mice usually succumbed within 36 h, and mice which survived for at least 1 week after challenge were deemed immune. There was no significant difference observed in time to death for sensitive mice.
Significant protection compared to nonimmunized controls (P < 0.05; Fisher's exact test).
The capsule forms the basis of licensed vaccines against a number of organisms including H. influenzae, N. meningitidis, and Streptococcus pneumoniae (11). However, in the closely related organism Actinobacillus pleuropneumoniae acapsular organisms can stimulate significant protection (16). We have constructed two rationally attenuated P. multocida strains that were acapsular due to either an inability to synthesize immunoreactive polysaccharide or an inability to export capsule to the cell surface. Although our results do not preclude the possibility of capsule being a protective antigen in P. multocida B:2, the bcbH mutant could stimulate significant protective immunity in mice, demonstrating that protection against pasteurellosis could be provided in the total absence of capsule or capsular polysaccharide. Taken together with the results of Muniandy et al. (19), this suggests that capsule is not an important protective antigen in P. multocida. The protection afforded by PBA875 and AL18 was less than 100%, and we believe that this is due to the rapid removal of acapsular bacteria from the blood (4), a view which is supported by the strong dependence of the level of protection on vaccine dose.
Previous work has indicated that protection against P. multocida type B is due mainly to an antibody response but has indicated only a minor role in protection for anti-LPS antibodies (24). Protection in the total absence of capsule has been shown for a very limited number of antigens from other P. multocida serotypes. Synthetic OmpH peptides have been shown to provide protection against P. multocida serotype A (17), and recombinant P. multocida toxin gives protection against P. multocida serotype D toxin (23). The present study suggests no more than a minor role, if any, for anti-capsule antibodies. Thus, further work is required to identify the major protective antigens in P. multocida serogroup B.
Acknowledgments
We thank Vicki Vallance and Ian McPherson for excellent technical assistance and Harry Sakellaris and Jing Chung for critical reading of the manuscript. We thank the late Rick Rimler for providing the type B capsular antiserum.
This work was funded in part by a grant from the Australian Research Council, Canberra, Australia.
REFERENCES
- 1.Adler B, Chancellor R, Homchampa P, Hunt M, Ruffolo C, Strugnell R, Wapling D. Immunity and vaccine development in Pasteurella multocida infections. J Biotechnol. 1996;44:139–144. doi: 10.1016/0168-1656(95)00113-1. [DOI] [PubMed] [Google Scholar]
- 2.Bain R V S, De Alwis M C L, Carter G R, Gupta B K. FAO Animal Production and Health Paper 33. Rome, Italy: Food and Agriculture Organization of the United Nations; 1982. Haemorrhagic septicaemia; pp. 11–33. [Google Scholar]
- 3.Blackall P J, Fegan N, Pahoff J L, Storie G J, McIntosh G B, Cameron R D A, O'Boyle D, Frost A J, Bara M R, Marr G, Holder J. The molecular epidemiology of four outbreaks of porcine pasteurellosis. Vet Microbiol. 2000;72:111–120. doi: 10.1016/s0378-1135(99)00192-3. [DOI] [PubMed] [Google Scholar]
- 4.Boyce J D, Adler B. The capsule is a virulence determinant in the pathogenesis of Pasteurella multocida M1404 (B:2) Infect Immun. 2000;68:3463–3468. doi: 10.1128/iai.68.6.3463-3468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce J D, Chung J Y, Adler B. Genetic organisation of the capsule biosynthetic locus of Pasteurella multocida M1404 (B:2) Vet Microbiol. 2000;72:121–134. doi: 10.1016/s0378-1135(99)00193-5. [DOI] [PubMed] [Google Scholar]
- 6.Carter G R. Pasteurellosis: Pasteurella multocida and Pasteurella hemolytica. Adv Vet Sci. 1967;11:321–379. [PubMed] [Google Scholar]
- 7.Christensen J P, Bisgaard M. Avian pasteurellosis: taxonomy of the organisms involved and aspects of pathogenesis. Avian Pathol. 1997;26:461–483. doi: 10.1080/03079459708419228. [DOI] [PubMed] [Google Scholar]
- 8.Davis R B. Cholera and broiler breeders. Poult Dig. 1987;Oct:430–434. [Google Scholar]
- 9.Dawkins H J S, Ramdani, Johnson R B, Spencer T L. Haemorrhagic septicaemia: correlation of vaccinal antibody responses in mice with protection against Pasteurella multocida strain M1404. Vet Microbiol. 1991;27:309–326. doi: 10.1016/0378-1135(91)90157-b. [DOI] [PubMed] [Google Scholar]
- 10.Derieux W T. Response of broiler-type chickens to live Pasteurella multocida-duration of immunity and minimum dose. Avian Dis. 1984;28:281–284. [PubMed] [Google Scholar]
- 11.Goldblatt D. Recent developments in bacterial conjugate vaccines. J Med Microbiol. 1998;47:563–7. doi: 10.1099/00222615-47-7-563. [DOI] [PubMed] [Google Scholar]
- 12.Heddleston K L, Gallagher J E, Rebers P A. Fowl cholera: gel diffusion precipitin test for serotyping Pasteruella multocida from avian species. Avian Dis. 1972;16:925–936. [PubMed] [Google Scholar]
- 13.Hofacre C L, Glisson J R. A serotypic survey of Pasteurella multocida isolated from poultry. Avian Dis. 1986;30:632–633. [PubMed] [Google Scholar]
- 14.Homchampa P, Strugnell R A, Adler B. Cross-protective immunity conferred by a marker-free aroA mutant of Pasteurella multocida. Vaccine. 1997;15:203–208. doi: 10.1016/s0264-410x(96)00139-9. [DOI] [PubMed] [Google Scholar]
- 15.Hopkins B A, Huang T H M, Olson L D. Differentiating turkey postvaccination isolants of Pasteurella multocida using arbitrarily primed polymerase chain reaction. Avian Dis. 1998;42:265–274. [PubMed] [Google Scholar]
- 16.Inzana T J, Todd J, Veit H P. Safety, stability, and efficacy of noncapsulated mutants of Actinobacillus pleuropneumoniae for use in live vaccines. Infect Immun. 1993;61:1682–1686. doi: 10.1128/iai.61.5.1682-1686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Y G, Zeng Q D, Glisson J R, Jackwood M W, Cheng I H N, Wang C L. Sequence analysis of Pasteurella multocida major outer membrane protein (OmpH) and application of synthetic peptides in vaccination of chickens against homologous strain challenge. Vaccine. 1999;17:821–831. doi: 10.1016/s0264-410x(98)00266-7. [DOI] [PubMed] [Google Scholar]
- 18.Mannheim W. Family III: Pasteurellaceae. In: Krieg N R, Kolt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 550–575. [Google Scholar]
- 19.Muniandy N, Edgar J, Woolcock J B, Mukkur T K S. Virulence, purification, structure, and protective potential of the putative capsular polysaccharide of Pasteurella multocida type 6:B. In: Patten B E, Spencer T L, Johnson R B, Hoffman H D, Lehane L, editors. Pasteurellosis in production animals. The International Workshop on Pasteurellosis in Production Animals. Bali, Indonesia: Australian Centre for International and Agricultural Research; 1992. pp. 47–54. [Google Scholar]
- 20.Myint A, Carter G R, Jones T O. Prevention of experimental haemorrhagic septicaemia with a live vaccine. Vet Rec. 1987;120:500–501. doi: 10.1136/vr.120.21.500. [DOI] [PubMed] [Google Scholar]
- 21.Nagy L K, Penn C W. Protection of cattle against experimental haemorrhagic septicaemia by the capsular antigens of Pasteurella multocida, types B and E. Res Vet Sci. 1976;20:249–253. [PubMed] [Google Scholar]
- 22.Penn C W, Nagy L K. Isolation of a protective, non-toxic capsular antigen from Pasteurella multocida, types B and E. Res Vet Sci. 1976;20:90–96. [PubMed] [Google Scholar]
- 23.Petersen S K, Foged N T, Bording A, Nielsen J P, Riemann H K, Frandsen P L. Recombinant derivatives of Pasteurella multocida toxin: candidates for a vaccine against progressive atrophic rhinitis. Infect Immun. 1991;59:1387–1393. doi: 10.1128/iai.59.4.1387-1393.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramdani B, Adler Opsonic monoclonal antibodies against lipopolysaccharide (LPS) antigens of Pasteurella multocida and the role of LPS in immunity. Vet Microbiol. 1991;26:335–347. doi: 10.1016/0378-1135(91)90027-d. [DOI] [PubMed] [Google Scholar]
- 25.Rebers P A, Heddleston K L. Fowl cholera: induction of cross-protection in turkeys with bacterins prepared from host-passaged Pasteurella multocida. Avian Dis. 1977;21:50–56. [PubMed] [Google Scholar]
- 26.Rimler R B. Cross-protection factor(s) of Pasteurella multocida: passive immunization of turkeys against fowl cholera caused by different serotypes. Avian Dis. 1987;31:884–887. [PubMed] [Google Scholar]
- 27.Rimler R B, Rhoades K R. Lysates of turkey-grown Pasteurella multocida: protection against homologous and heterologous serotype challenge exposures. Am J Vet Res. 1981;42:2117–2121. [PubMed] [Google Scholar]
- 28.Sheikh M A, Anzam M, Shakoori A R. Observations on haemorrhagic septicaemia in pakistan livestock. Zentbl Veterinarmed B. 1996;43:293–304. doi: 10.1111/j.1439-0450.1996.tb00317.x. [DOI] [PubMed] [Google Scholar]
- 29.Shimoji Y, Mori Y, Sekizaki T, Shibahara T, Yokomizo Y. Construction and vaccine potential of acapsular mutants of Erysipelothrix rhusiopathiae: use of excision of Tn916 to inactivate a target gene. Infect Immun. 1998;66:3250–3254. doi: 10.1128/iai.66.7.3250-3254.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verma R, Jaiswal T N. Haemorrhagic septicaemia vaccines. Vaccine. 1998;16:1184–1192. doi: 10.1016/s0264-410x(98)80118-7. [DOI] [PubMed] [Google Scholar]