Abstract
Aim:
To delineate and analyze the mortality from COVID -19 in our institute during the devastating second wave of pandemic.
Settings and Design:
A retrospective cohort analysis.
Methods and Materials:
A comprehensive mortality analysis of 142 laboratory-confirmed severe acute respiratory syndrome coronavirus 2-infected deceased patients from our hospital’s medical records was done. These patients presented with severe disease at the time of admission and were managed in intensive care units.
Statistical Analysis Used:
Statistical Package for Social Sciences software, IBM manufacturer, Chicago, USA, version 21.0 was used.
Results:
The number of deceased males (82, 62.6%) was higher than females (53, 37.3%). Median age of deceased patient was 57 (44.25–69.75) years. Most frequent comorbidities were diabetes mellitus (42, 29.6%) and hypertension (41, 28.9%). Most common symptoms being shortness of breath (137, 96.5%), fever (94, 66.2%) and cough (73, 51.4%). Median peripheral capillary oxygen saturation (SpO2) at time of admission was 86% (77.25–90). Median time interval from symptom onset to admission in hospital was 3 (2.25–5) days. Neutrophil lymphocyte ratio was more than 5 in 117 (90.7%) patients. Complications seen were acute respiratory distress syndrome in 82.3%, acute liver injury in 58.4%, acute kidney injury in 26.7%, sepsis in 13.3% and acute cardiac injury in 12% patients. The median high-resolution computed tomography score was 20 (17–22).
Conclusions:
Male and elderly patients with underlying comorbidities had poorer outcome and involvement of multiple organ systems was common. A short time interval between symptom onset and admission/mortality, particularly encountered was worrisome.
Keywords: COVID-19, mortality, SARS-CoV-2
Introduction
The origin and progression of Coronavirus disease 2019 (COVID-19) has been associated with an unending quest to decipher its pathophysiology, clinical manifestations, laboratory and radiological findings. Globally, there have been 513,955,910 confirmed cases of COVID-19, including 6,249,700 deaths, uptil 6 May 2022.[1] Though there is no dearth of data on COVID-19-related morbidity and mortality, yet the developing countries still lag behind in the same. Various studies conducted so far, prospectively as well as retrospectively, have thrown light on the course and factors that could predict the disease outcome.[2,3,4]
The aim of our study was to look into the characteristic features including demographics, clinical, laboratory, radiological and comorbidities along with assessment of various time intervals such as between symptom onset, hospital admission and death of the deceased patients. It is crucial that a genuine comparison of the results of a particular geographical region with the worldwide data should be made so that better understanding of pitfalls can be made to reduce the burden at primary care level.
Materials and Methods
During the second wave of COVID-19 in April and May 2021, India faced a sudden overwhelming rise in the number of COVID19 cases. Our hospital served as one of the level 3 COVID facility during that time where critically ill patients were referred from all nearby areas.
Ethical clearance: The study commenced after prior approval of the Institutional Scientific Research Committee and Ethics Committee.
Study design: We did a retrospective analysis of mortality in COVID-19 patients admitted in our hospital during the second wave.
A retrospective review of medical records of death cases due to COVID-19 from 1 April 2021 to 31 May 2021 was done.
Inclusion criteria: All deceased patients who tested positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by use of reverse transcription– polymerase chain reaction (RT-PCR) on samples from their respiratory tract were included in the study.
Exclusion criteria: The deceased patients who had negative RT-PCR were not included in our study. Also, pregnant females and patients less than 18 years of age were excluded.
Data collection: The clinical symptoms and signs, laboratory findings and radiologic assessments including chest X-ray or computed tomography (CT) was extracted from medical records. An assessment and analysis of the distribution and pattern of lung abnormalities on high-resolution computed tomography (HRCT) chest was done. Data was systematically entered by a trained team of doctors and verified by two treating physicians. The clinicians who were in charge of the COVID ICU patients were contacted if any core data was found to be missing.
Statistical methods: The presentation of categorical variables was done in the form of number and percentage (%). On the other hand, the quantitative data was presented as the means ± SD and as median with 25th and 75th percentiles (interquartile range). The data normality was checked by using Kolmogorov–Smirnov test. The cases in which the data was not normal, we used nonparametric tests. The following statistical tests were applied for the results:
The association of the variables which were quantitative and not normally distributed in nature were analyzed using Mann–Whitney Test (for two groups) and Kruskal–Wallis test (for more than two groups).
The association of the variables which were qualitative in nature were analyzed using Chi-square test. If any cell had an expected value of less than 5, then Fisher’s exact test was used.
The data entry was done in the Microsoft EXCEL spreadsheet and the final analysis was done with the use of Statistical Package for Social Sciences software, IBM manufacturer, Chicago, USA, version 21.0.
For statistical significance, P value of less than 0.05 was considered statistically significant.
Results
From 1st April 2021 to 31 May 2021, a total of 142 patients succumbed due to COVID-19 in our hospital. The main presenting characteristics of deceased patients including most common symptoms prevalent at the onset of disease are summarized in Table 1. The number of males (82, 62.6%) was higher than that of females (53, 37.3%) in deceased patients. The median age of deceased patient was 57 (44.25–69.75) years. Diabetes mellitus (42, 29.6%) and hypertension (41, 28.9%) were the most frequent comorbidities. Most of the deceased COVID-19 patients required noninvasive and/or invasive mechanical ventilation in the ICU.
Table 1.
Presenting characteristics of deceased patients (Original)
| Characteristics | Range | Total (n=142) |
|---|---|---|
| Age (in years) | <30 | 7 (4.93%) |
| 30-45 | 31 (21.83%) | |
| 46-60 | 42 (29.58%) | |
| >60 | 62 (43.66%) | |
| Median (25th to 75th percentile) | 57 (44.25-69.75) | |
| Sex | Male | 53 (37.32%) |
| Female | 89 (62.68%) | |
| Comorbidities | Diabetes mellitus | 42 (29.58%) |
| Hypertension | 41 (28.87%) | |
| Obesity | 14 (9.86%) | |
| Coronary artery disease | 11 (7.75%) | |
| Hypothyroidism | 10 (7.04%) | |
| Chronic kidney disease | 05 (3.52%) | |
| COPD/Bronchial asthma | 04 (2.82%) | |
| Malignancy | 02 (1.41%) | |
| Chronic liver disease | 01 (0.70%) | |
| Diabetes mellitus + hypertension | 21 (14.79%) | |
| Symptoms | Shortness of breath | 137 (96.48%) |
| Fever | 94 (66.20%) | |
| Cough | 73 (51.41%) | |
| Diarrhoea | 19 (13.38%) | |
| Sore throat | 5 (3.52%) | |
| Time interval from symptom onset to admission (in days) | Median (25th-75th percentile) | 3 (2.25-5) |
| Duration of stay (in days) | <1 day | 22 (15.49%) |
| 2-7 days | 82 (57.75%) | |
| >7 days | 38 (26.76%) | |
| Peripheral capillary Oxygen saturation (SpO2 in % on room air) | <70 | 17 (11.97%) |
| 70-79 | 23 (16.20%) | |
| 80-89 | 52 (36.62%) | |
| ≥90 | 50 (35.21%) | |
| Median (25th-75th percentile) | 86 (77.25-90) |
The median time interval from symptom onset to admission in hospital and duration of hospital stay are mentioned in Table 1.
The results of biochemical and other laboratory parameters, including hemograms and inflammatory marker levels during the stay of deceased patients are summarized in Table 2.
Table 2.
Laboratory findings of deceased patients (original)
| Laboratory findings (normal range) | Ranges in our study | Number of patients (percentage) | Median value (25th-75th percentile) |
|---|---|---|---|
| Total leukocyte count (TLC) (4-10×109/L) | <4 | 0 | 14.3 (10.3-18.14) |
| 4-10 | 30 (23.26%) | ||
| >10 | 99 (76.74%) | ||
| Neutrophil lymphocyte ratio (NLR) | ≤ 5 | 12 (9.3%) | 1.6 (8-19.8) |
| >5 | 117 (90.7%) | ||
| Haemoglobin (12-17.5 g/L) | <8 | 2 (2.52%) | 12.6 (11.21-14.09) |
| 8-12 | 23 (23.53%) | ||
| >12 | 89 (73.95%) | ||
| Platelets (150-400×109/L) | <100 | 23 (18.85%) | 160 (111.25-235.75) |
| 100-149 | 29 (23.77%) | ||
| >=150 | 70 (57.38%) | ||
| Creatinine (0.7-1.3 mg/dL) | <1.3 | 80 (62.50%) | 1.27 (1.05-1.785) |
| ≥1.3 | 48 (37.50%) | ||
| Aspartate transaminase (AST) (<45 U/L) | ≤45 | 44 (33.59%) | 66 (36-121) |
| >45 to 100 | 48 (36.64%) | ||
| >100 | 39 (29.77%) | ||
| Alanine transaminase (ALT) (<45 U/L) | ≤45 | 52 (40.00%) | 57.5 (35.125-104.75) |
| >45-100 | 42 (32.31%) | ||
| >100 | 36 (27.69%) | ||
| Albumin (35-52 g/L) | <35 | 50 (37.88%) | 3.6 (3.45-3.86) |
| >35 | 82 (62.12%) | ||
| C reactive protein (CRP) (<1 mg/L) | <10 | 02 (1.96%) | 101.85 (69.825-129.325) |
| 10 to<50 | 11 (10.78%) | ||
| 50 to<100 | 35 (34.31%) | ||
| ≥100 | 54 (52.94%) | ||
| Lactate dehydrogenase (LDH) (140-280 U/L) | <280 | 3 (2.24%) | 879.95 (666.2-1163.875) |
| ≥280 | 131 (97.76%) | ||
| D dimer (<0.5 µgm/mL) | <0.5 | 13 (9.70%) | 0.93 (0.655-2.035) |
| 0.5 to 1 | 57 (42.54%) | ||
| >1 | 64 (47.76%) | ||
| Ferritin (20-300 µgm/L) | <300 | 5 (3.73%) | 873.95 (650.225-1332.25) |
| ≥300 | 129 (96.27%) | ||
| Interleukin-6 (IL-6) (<7 pg/mL) | ≥7 | 57 (100%) | 190.44 (76-312) |
| Procalcitonin (<0.1 ng/mL) | <0.5 | 5 (19.23%) | 2.05 (0.8-4.45) |
| 0.5 to 1 | 2 (7.69%) | ||
| >1 | 19 (73.08%) |
A progressive deterioration of the laboratory parameters was noted among the deceased patients. Fifty-eight (48.3%) patients were found to have leukocytosis (white blood cell count ≥10 × 109/L) in their initial reports after admission. More significant and frequent leukocytosis in 99 (76.7%) patients was seen in the last 24 h of the deceased patients with a median leukocyte count of 14.3 × 109/L (10.3–18.14). A high neutrophil lymphocyte ratio (NLR) of more than 5 was found in 117 (90.7%) patients with a median NLR of 11.6 (8–19.8).
Elevation in the levels of hepatic transaminases with 87 (61%) patients having abnormal aspartate aminotransferase and 78 (55%) patients with abnormal alanine aminotransferase concentrations (>45 U/L) was seen in deceased patients. Fifty patients (37.9%) had lower serum albumin levels.
Deceased patients displayed higher levels of C-reactive protein (CRP), lactate dehydrogenase (LDH), interleukin-6 (IL-6) and ferritin. The median initial and peak CRP value was 93.7 and 101.8 in our subjects. Median peak levels of ferritin (873.95 ng/mL), D-dimer (0.93 μg/mL), LDH (879.95 U/L) and IL-6 (>190.44 pg/mL) were raised. Though procalcitonin levels were not available for a large number of patients, 19 patients had high procalcitonin levels of >1, which suggested secondary bacterial infection.
The complications are seen in deceased patients, as illustrated in Figure 1. Association of SpO2 on admission with duration of stay was found to be statistically significant (P-value < 0.0001), as depicted in Figure 2.
Figure 1.
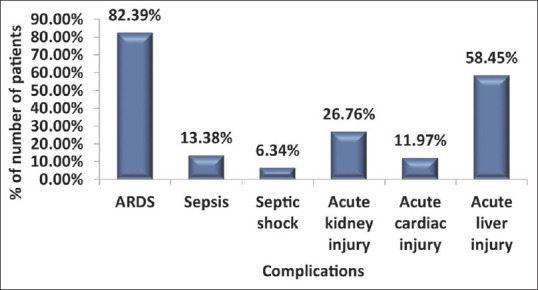
Distribution of complications in study subjects
Figure 2.
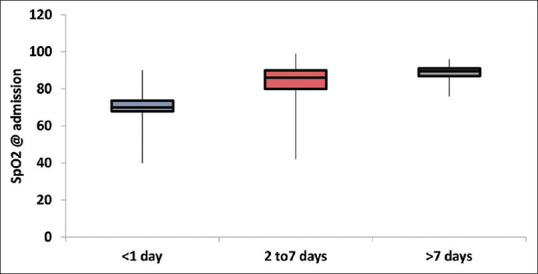
Association of SpO2 on admission with duration of stay (nonparametric variable, Box-whisker plot)
Chest radiographs had abnormal findings with bilateral involvement in all the deceased patients. HRCT score was available for only 68 patients as critical condition of patients in ICU made it difficult to shift the patients for CT scan. Distribution of HRCT score in our study patients is shown in Table 3. Median HRCT score in the deceased patients was found to be 20 (17–22).
Table 3.
Distribution of HRCT score of study subjects (Original)
| HRCT Score | Frequency (Percentage) |
|---|---|
| 8-16{Moderate} | 13 (19.12%) |
| 17-25{Severe} | 55 (80.88%) |
| Mean±SD | 19.49±3.43 |
| Median (25th-75th percentile) | 20 (17-22) |
| Range | 10-24 |
Discussion
The variation in mortality rates in COVID-19 pandemic over different geographical regions of the world is a well-known fact, but the factors responsible for this variation are not so very clear. We tried to do a detailed study and analyse the summaries of deceased patient.
The preponderance of male sex among the deceased patients (82, 62.6%) in our study was comparable to various other studies worldwide.[2] There is a possible role of some form of innate immunity in females probably because of sex hormones and genetic chromosomal makeup resulting in less mortality among them.[3]
The median age of deceased patient in our study was 57 (44.25–69.75) years. Though age of around 62 (43%) patients was over 60 years, we had many middle-aged patients (41–50 years old) too who succumbed. The existing evidence has found age to be as one of the significant risk factor associated with mortality in COVID-19. Multiple factors such as increased prevalence of underlying comorbid illnesses, limited reserve of organ function and reduced immunity levels with rising age may be responsible for this association.[4,5] The studies in initial months of 2020 worldwide primarily report median age of nonsurvivors between 68 and 72 years.[2,4] A lower median age of deceased patients in our study can be attributed to the fact the vaccination drive started in January 2021 in our country with healthcare staff, frontline workers followed by residents over the age of 60 years in the second phase in March 2021.[6] So when the second wave struck in April and May, many of our elderly population above 60 years were already vaccinated. This could have protected this vulnerable population from falling prey to this deadly virus in our study to some extent.
Comorbidities have been indicated as an important predictor of severity of disease in COVID-19 by the various studies conducted so far.[2,3,4] A significant proportion of patients who died had underlying chronic conditions such as diabetes mellitus (42, 29.6%) and hypertension (41, 28.9%) followed by obesity (14, 9.8%) in our study as well.
The most common symptoms prevalent at the onset of disease in our deceased study group were shortness of breath, fever and cough. Majority of the studies have also reported the same findings.[2,3,7] A large study of 24,410 cases across nine countries reported fever in 78% and cough in 58% patients.[8] Median SpO2 of the patients at the time of admission was 86% (77.25–90) which itself indicated critical condition of the deceased patients on admission. The association of SpO2 with duration of stay was seeked, but the magnitude of association was found to be small and unlikely significant.
A median time interval of 3 days was found between symptom onset and hospital admission in our study, which was remarkably shorter than the interval reported by some studies earlier in the pandemic but similar to some current reported analyses.[3,9] This observation may be following the phasic changes in the natural history of any epidemic when with time, awareness and knowledge regarding any outbreak increases, more access to health care is achieved. Some other factors such as fear and panic among the masses may enhance the health-seeking behaviour leading to early admissions may also play substantial role. The study from South India was in line with our study with a mean of 4 days between symptom onset and admission to hospital,[3] while a deceased cohort from Wuhan, China, reported it to be 10 days.[2]
Though majority of studies worldwide have so far reported a slightly higher interval range between hospital admission and death,[2,10] we found it to be short. A majority of our patients died within 2–7 days of hospital stay. Considering these facts in our study we observed a rapid course of poor outcome which was comparable to few studies.[3,9] In contrast, a time interval between disease onset and death of 17.8 and 18.5 days was reported in two different studies.[11,12]
Majority of patients in our study group had neutrophilia and lymphopenia on admission and thereafter. Neutrophils being the predominant source of inflammatory cytokines and chemokines play significant role in generating cytokine storm and consequent lung injury. Lymphopenia has been found to be an important reflector of deficient cellular immunity, which was associated with poor prognosis. A study from Wuhan, China, including 113 deceased patients reported persistent and more severe lymphopenia in deceased subjects 44 (39%) than recovered patients.[2] Lymphopenia, neutrophilia and high NLR were continuously present and were found in all deceased patients in another study.[4]A high NLR was seen in 90% of our patients, which was consistent with studies in other parts of the world and reflected the perturbed immune system because of SARS-CoV-2.[2,4] Some studies report lower platelets, and lower haemoglobin in deceased patients.[2,4] Though we had reduced platelet count in 44 (36%) patients, median haemoglobin level was found in normal range.
Elevation of inflammatory markers most notably CRP, LDH and ferritin was seen in deceased patients which was in accordance with the data studied worldwide.[2,4,13]
As mentioned earlier that procalcitonin levels were not available for all patients in our study group. We had 19 (13.3%) patients who had high procalcitonin levels of > 1 ng/mL which suggested secondary bacterial infection and sepsis. A study from Wuhan, China found elevated procalcitonin levels in around one third of patients thereby suggesting secondary bacterial infection in large proportion of deceased patients and thereby indicating strong association with mortality.[2] Similarly, a study from North India reported significant high levels of procalcitonin in their death cohort.[13]
Multiple organ involvement, particularly of the kidney, liver and myocardium has been widely reported in nonsurvivors until so far.[2,13,14] In our study, 58.4% patients had acute liver injury, 26.7% had acute kidney injury and 12% acute cardiac injury.
A study of 300 cases in China investigated the relationship between acute liver injury particularly steatosis and NLR and showed their association with severity of illness in COVID-19.[15] Though in our study we could not comment on the histologic pattern of liver injury in deceased patients, while trying to assess an association between organ injury and NLR, only acute liver injury showed better association with high NLR, though not statistically significant (P-value 0.085, Table 4). Another study reported that abnormal liver enzymes with mild hepatitis, steatosis and viral RNA in liver were often found in autopsy of COVID-19 nonsurvivors.[16] The mechanisms of liver injury are probably multifactorial and demand further evaluation.
Table 4.
Association of complications with neutrophil lymphocyte ratio (NLR) (original)
| Complications | <=5 (n=12) | >5 (n=117) | Total | P |
|---|---|---|---|---|
| ARDS | 8 (66.67%) | 98 (83.76%) | 106 (82.17%) | 0.225† |
| Sepsis | 0 (0%) | 19 (16.24%) | 19 (14.73%) | 0.212† |
| Septic shock | 0 (0%) | 9 (7.69%) | 9 (6.98%) | 1† |
| Acute kidney injury | 3 (25%) | 35 (29.91%) | 38 (29.46%) | 1† |
| Acute cardiac injury | 2 (16.67%) | 13 (11.11%) | 15 (11.63%) | 0.631† |
| Acute liver injury | 5 (41.67%) | 78 (66.67%) | 83 (64.34%) | 0.085‡ |
† Fisher’s exact test, ‡ Chi-square test
Hyperglycaemia is associated with altered immunity with impairment of phagocytosis and opsonisation capabilities with disturbed regulation of immune system. Among the various complications seen in our deceased patients, acute kidney injury (P-value 0.048) and acute cardiac injury (P-value 0.005) showed statistically significant difference between diabetics and nondiabetics. Zhu et al.[17] reported that uncontrolled blood glucose levels were associated with increased prevalence of acute respiratory distress syndrome (ARDS), acute cardiac injury and all-cause mortality from SARS-CoV-2.
HRCT chest was done in 68 (48%) patients. The rest of the patients being on one or the other modes of assisted ventilation in ICU could not be shifted to the CT unit before they succumbed and were followed up with chest radiographs. CT images keeping up with COVID-19 pneumonitis include peripheral distribution of patches of ground-glass opacities and consolidation, more so in the lower lobes [Figures 3 and 4]. Subsequent findings with advanced stages of the disease include fibrosis, septal thickening and architectural distortion.[18,19] A high median CT severity score[20] of 20 (17–22) showing greater extent of lung parenchymal involvement was found in the deceased patients with no sex predilection. Typical imaging features of ARDS including bilateral extensive confluent opacities with septal thickening were observed in critically ill patients who later succumbed. The findings corroborated with those in other studies[21] and were even cited in a systematic review of literature in 919 patients in South Carolina, USA.[22]
Figure 3.
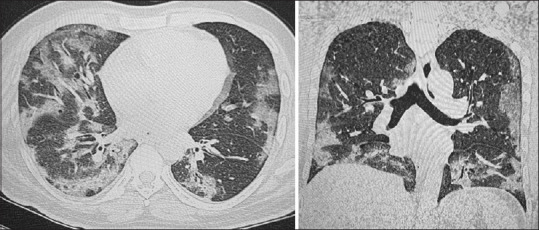
Axial and coronal images from the HRCT Chest of a 44-year-old male patient. There is moderate lung involvement with multiple, peripheral patchy areas of ground glass attenuation and septal thickening. The findings were more prominent in the lower lobes bilaterally. The CT severity score was 18/25
Figure 4.
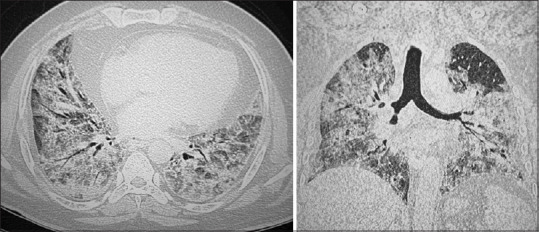
Axial and coronal images of HRCT chest of a 61–year-old male patient showing extensive, confluent areas of ground glass attenuation and consolidations diffusely involving both lungs interspersed with septal thickening. Reported as ARDS and the CT severity score was 24/25
Our study had few limitations. Some laboratory reports were missing from the records and few investigations such as IL6, cardiac enzymes and procalcitonin could not be sent in all patients. This missing data can be a reason for the bias in our results. Similarly, dynamic changes in laboratory profile could not be assessed for all parameters and in all patients. Additionally, patients being in the different stages of the illness when they were admitted, the investigation data could not represent temporal uniformity. This study analysed only the nonsurvivors; hence, a comparison between nonsurvivors and survivors could not be made. Lastly some deceased patients who were RT-PCR negative were excluded from our study per our inclusion criteria. These patients who met clinical criteria of the illness could be false negative cases and may alter the actual mortality rates of our study population. Autopsy could have been the answer in such cases but was not available.
Conclusion
The worldwide surge of COVID-19 cases, the mortality and adverse outcomes associated with it has put unprecedented challenge on medical science with far reaching repercussions.
With the flashing speculations and reemerging threats of mutations in SARS-CoV-2, it seems that medical science is still inexperienced in dealing with this virus. The various characteristics of our deceased patients, including demographics, clinical manifestations, laboratory and radiological parameters, may be significant predictors of poorer outcome in COVID-19, but at the same time, this needs further evaluation and analysis on a wider scale to stratify the risk and understand the exact mechanism behind the enormous mortality.
Key message: Multiorgan dysfunction with both pulmonary and systemic inflammation with rapid deterioration can be seen in COVID-19 highlighting the significance of intensive monitoring and supportive care at early stage.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.World Health Organization. Coronavirus disease (COVID19):Data as received by WHO from national authorities, as of 04 October 2020, 10 am CEST. 2020. Available from: https://www.who.int/docs/defaultsource/coronaviruse/situationreports/20201005 weeklyepiupdate8.pdf .
- 2.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019:Retrospective study. BMJ. 2020;368:m1295. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asirvatham ES, Sarman CJ, Saravanamurthy SP, Mahalingam P, Maduraipandian S, Lakshmanan J. Who is dying from COVID-19 and when? An Analysis of fatalities in Tamil Nadu, India. Clin Epidemiol Glob Health. 2021;9:275–9. doi: 10.1016/j.cegh.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang B, Zhou X, Qiu Y, Song Y, Feng F, Feng J, et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. doi: 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choudhary OP, Choudhary P, Singh I. India's COVID-19 vaccination drive:Key challenges and resolutions. Lancet Infect Dis. 2021;21:1483–4. doi: 10.1016/S1473-3099(21)00567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanyaolu A, Okorie C, Marinkovic A, Patidar R, Younis K, Desai P, et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020;2:1069–76. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant MC, Geoghegan L, Arbyn M, Mohammed Z, McGuinness L, Clarke EL, et al. The prevalence of symptoms in 24,410 adults infected by the novel coronavirus (SARS-CoV-2;COVID-19):A systematic review and meta-analysis of 148 studies from 9 countries. PLoS One. 2020;15:e0234765. doi: 10.1371/journal.pone.0234765. doi:10.1371/journal.pone.0234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pung R, Chiew CJ, Young BE, Chin S, Chen MI, Clapham HE, et al. Investigation of three clusters of COVID-19 in Singapore:Implications for surveillance and response measures. Lancet. 2020;395:1039–46. doi: 10.1016/S0140-6736(20)30528-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2:A prospective cohort study. Eur Respir J. 2020;55:2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verity R, Okell LC, Dorigatti I, Winskill P, Whittaker C, Imai N, et al. Estimates of the severity of coronavirus disease 2019:A model-based analysis. Lancet Infect Dis. 2020;20:669–77. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China:A retrospective cohort study. Lancet. 2020;395:1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bairwa M, Kumar R, Beniwal K, Kalita D, Bahurupi Y. Hematological profile and biochemical markers of COVID-19 non-survivors:A retrospective analysis. Clin Epidemiol Glob Health. 2021;11:100770. doi: 10.1016/j.cegh.2021.100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Liu S, Tian J, Pan H, Liu Y, Hu J, et al. Disease progression patterns and risk factors associated with mortality in deceased patients with COVID-19 in Hubei Province, China. Immun Inflamm Dis. 2020;8:584–94. doi: 10.1002/iid3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, et al. Detrimental effects of metabolic dysfunction-associated fatty liver disease and increased neutrophil-to-lymphocyte ratio on severity of COVID-19. Diabetes Metab. 2020;46:505–7. doi: 10.1016/j.diabet.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagana SM, Kudose S, Iuga AC, Lee MJ, Fazlollahi L, Remotti HE, et al. Hepatic pathology in patients dying of COVID-19:A series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33:2147–55. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–77.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carotti M, Salaffi F, Sarzi-Puttini P, Agostini A, Borgheresi A, Minorati D, et al. Chest CT features of coronavirus disease 2019 (COVID-19) pneumonia:Key points for radiologists. Radiol Med. 2020;125:636–46. doi: 10.1007/s11547-020-01237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caruso D, Polidori T, Guido G, Nicolai M, Bracci B, Cremona A, et al. Typical and atypical COVID-19 computed tomography findings. World J Clin Cases. 2020;8:3177–87. doi: 10.12998/wjcc.v8.i15.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–7. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller NL, Ooi GC, Khong PL, Zhou LJ, Tsang KWT, Nicolaou S. High-resolution CT findings of severe acute respiratory syndrome at presentation and after admission. AJR Am J Roentgenol. 2004;182:39–44. doi: 10.2214/ajr.182.1.1820039. [DOI] [PubMed] [Google Scholar]
- 22.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19):A systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]


