Abstract
Purpose:
Coronavirus disease 2019 (COVID-19) is purely a viral illness which is not affected by the usage of antibiotics, but the risk of development of secondary bacterial infections during the course of respiratory illness or hospitalisation has led to a surge of antibiotic use. Anti-microbial resistance has taken an upward trend to some of the commonly used or over-used antibiotics. The present study was planned to focus on the trends of resistance rates noticed for the common antibiotics, namely, doxycycline, azithromycin, and so on, before and after the advent of this pandemic.
Material and Methods:
The study was conducted at a tertiary care hospital of North India with 2000 samples, 1000 samples between March 2019 and March 2020 before the COVID pandemic and 1000 samples between April 2020 and April 2021 after the advent of the pandemic. Identification and zones for doxycycline and erythromycin were interpreted as per Clinical and Laboratory Standards Institute guidelines.
Results:
Among the various samples, pus/aspirated fluids were in majority (47%), followed by blood (29%), respiratory specimens (18%), and urine (6%). On stratifying the various pathogens associated with the treatment of doxycycline and erythromycin, Staphylococcus species were the predominant ones in almost 82% of the cases, followed by Enterococcus (12%) and Streptococcus (6%) species. For doxycycline, the overall sensitivity was noted to be 46% in the year 2019–20 and 31% in the year 2020–21, whereas for erythromycin, the sensitivity was seen as 39% in 2019–20 and dropped down to 26% in 2020–21.
Conclusions:
The authors noted a dip in the overall sensitivity towards doxycycline and azithromycin. This finding clearly indicates the increasing rates of antibiotic resistance in a developing country such as India during these COVID times. A proper anti-microbial stewardship programme during these times will help to de-escalate the increasing resistance rates and will prove to be of great help to the primary care physicians.
Keywords: Antibiotics, anti-microbial, azithromycin, bacterial infections, doxycycline, resistance, pandemic
Introduction
Last year, in December 2019, China reported an outbreak of a respiratory illness affecting the human population.[1] The World Health Organisation (WHO) identified the causal agent of this illness as “Corona virus disease 2019 (COVID 19)” by January 2020. The human race till now has witnessed the emergence of four severe viral outbreaks in the past 2 decades: the 2002 severe acute respiratory syndrome coronavirus (SARS-CoV) epidemic, the 2009 influenza A,
H1N1 pandemic, the 2012 Middle East respiratory syndrome (MERS) outbreak, and the most recent COVID-19 pandemic.[2] SARS CoV-2 belongs to the family Coronaviridae and the order Nidovirales. It is an enveloped virus with a single-stranded positive sense RNA.[3] Globally, as on 29 April 2021, there had been 149,216,984 confirmed cases of COVID-19, including 3,144,028 deaths, reported to WHO. Developing Asian countries such as ours are experiencing the major brunt of this second wave of the pandemic.
COVID-19 is a complex disease, and a myriad of symptoms has been seen in the past one and a half years. The respiratory manifestations seen most commonly are cough, sputum, shortness of breath, fever, and so on, which may progress to ARDS (acute respiratory distress syndrome), shock, and death. Musculoskeletal: myalgia, joint pain, headache, fatigue; enteric: abdominal pain, vomiting, diarrhea; and mucocutaneous symptoms are encountered less commonly.[4]
There is a great deal of uncertainty about the disease process, and very less is known about the exact pathogenesis of COVID-19. Much research is underway regarding the treatment options including vaccines for this deadly virus. Different studies advocate the beneficial effects of antibiotics such as azithromycin with hydroxychloroquine, doxycycline, and other drugs such as ivermectin, tocilizumab, and favipiravir. The viral replicative cycle and immune mechanisms are the various target options of these drugs. Favipiravir is a selective and potent inhibitor of RNA-dependent RNA polymerase (RdRp) of RNA viruses. Favipiravir is incorporated into the nascent viral RNA by error-prone viral RdRp, which leads to chain termination and viral mutagenesis.[5] Tocilizumab is an anti-interleukin-6 receptor monoclonal antibody, which has been approved for the treatment of multiple inflammatory diseases and has recently been studied to improve outcomes in patients with COVID-19 pneumonia in observational studies in the United States and globally.[6] However, the definitive and curative role is not 100% established in any of these treatment options.
COVID-19 is purely a viral illness which is not affected by the usage of antibiotics, but the risk of development of secondary bacterial infections during the course of respiratory illness or hospitalisation has led to a surge of antibiotic use. COVID-19 leads to a longer hospital stay as compared to other influenza-like illnesses (ILIs) and hence a higher risk of acquiring nosocomial infections. The situation is more grime because of the unmeasured but possibly huge number of people taking antibiotics on their own or with the encouragement of some local practitioners in misguided attempts to protect themselves from this pandemic. Well before the advent of this deadly pandemic in 2019, the world was already facing an emerging threat of anti-microbial resistance and many research papers as well as media reports have very well sounded the alarm of its further escalation during these COVID times.[7] Anti-microbial resistance has taken an upward trend because of the increased and rapidly evolving resistance mechanisms of the pathogens to the commonly used or over-used antibiotics. A New York-based study reflected that approx. 71% COVID-positive patients were administered antibiotics, whereas less than 4% of these had documented bacterial coinfections.[8]
The present study was planned to focus on the trends of resistance rates noticed for the common antibiotics, namely, doxycycline, azithromycin, and so on, before the advent of this pandemic (2019–2020) and after this pandemic (2020–2021).
Methods
Place and duration of study
The study was conducted at a tertiary care hospital of North India between March 2019 and March 2020 before the COVID pandemic and between April 2020 and April 2021 after the advent of the pandemic, that is, 1 year before and 1 year after the pandemic.
Study design: Cross-sectional study
Sample size: A total of 2000 samples including pus, urine, respiratory fluids, and blood were analysed in the study, 1000 before the pandemic and 1000 after the advent of the pandemic.
Ethical clearance: Institutional ethics committee (IEC) permission was taken before the study.
Patient selection
Bacterial culture and processing: All the specimens (blood, respiratory fluids, urine, or pus/aspiration) were cultured on both MacConkey and blood agar plates according to standard microbiological techniques. Further, colonies were isolated and sub-cultures were performed accordingly.
-
Identification:
-
Conventional method using biochemical tests:The bacterial isolates were first identified using routine staining and biochemical tests as are being followed in our laboratory. [9]
- Automated methods: The identity of bacteria was confirmed using a MALDI TOF MS and Vitek 2 system (Biomerieux, France), an automated identification, and a susceptibility testing system.[10]
-
Antibiotic susceptibility testing: This was performed by Kirby–Bauer’s disk diffusion method on Muller Hinton agar and interpreted based on Clinical and Laboratory Standards Institute (CLSI) guidelines.[11] Erythromycin was used as an equivalent agent to test for azithromycin. Zones for doxycycline and erythromycin were interpreted as per these guidelines.
Statistical analysis
The results were analysed using the SPSS version 22 software (SPSS Inc., Chicago, IL, USA). The frequencies are shown with 95% confidence intervals (95%CI). The Chi-square and Mann–Whitney U tests were used to analyse the statistically significant variables. The statistically significant values were considered as P value <0.05.
Results
A total of 2000 samples were analysed in the study including pus, blood, cerebrospinal fluid (CSF), urine, and respiratory specimens. The mean age of the patients was 63 years, and the range was 10–72 years. Males comprised the majority of the cases, 57%, as compared to females. Among the various samples collected from the patients, pus/aspirated fluids were in majority (47%). Blood (29%), respiratory specimens (18%), and urine (6%) contributed for the other bacteriology samples. On stratifying the various pathogens associated with the treatment of doxycycline and erythromycin, Staphylococcus species were the predominant ones in almost 82% of the cases, followed by Enterococcus (12%) and Streptococcus (6%) species.
Sensitivity of these pathogens was studied individually for these two mentioned drugs. For doxycycline, the overall sensitivity was noted to be 46% in the year 2019–20 and 31% in the year 2020–21, whereas for erythromycin, the sensitivity was seen as 39% in 2019–20 and dropped down to 26% in 2020-21 [Figure 1]. Organism-wise, the sensitivity for doxycycline in the years 2019–20 and 2020–21 was as follows: Staphylococcus: 48 versus 35%, Enterococcus: 42 versus 30%, and Streptococcus: 47 versus 28%. The erythromycin sensitivity noted for various groups was as follows: Staphylococcus: 42 versus 31%, Enterococcus: 37 versus 27%, and Streptococcus: 38 versus 20%.
Figure 1.
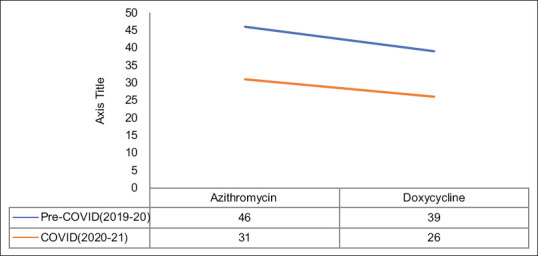
Resistance trends of azithromycin and doxycycline
Further on assessing the clinical outcomes in these two group of patients, a significant difference was noted in the days of hospital stay (p < 0.001) and clearance of infection (p < 0.01), whereas no significant difference was noted in in-patient mortality [Table 1].
Table 1.
Comparison of co-morbidities and clinical outcomes in the two groups before and during the pandemic
| Clinical parameters | Cases before the COVID pandemic (2019-2020) (%) | Cases during the COVID pandemic (2020-2021) (%) | P |
|---|---|---|---|
| Age (Mean, Range in years) | 63 (8-75) | 54 (3-71) | 0.007 |
| Gender | |||
| Males | 61 | 63 | 0.019 |
| Females | 39 | 37 | |
| Co-morbidities | |||
| Hypertension | 67 | 65 | 0.622 |
| Diabetes Mellitus | 61 | 63 | 0.588 |
| COPD/Asthma | 32 | 30 | 0.712 |
| CKD | 23 | 18 | 0.421 |
| CLD | 19 | 21 | 0.399 |
| Heart diseases | 11 | 13 | 0.512 |
| Outcomes | |||
| In-patient mortality | 39 | 37 | <0.7 |
| In-hospital stay, in days | 42 (38-56) | 67 (42-89) | <0.001 |
| Infection clearance duration, in days | 21 (17-32) | 35 (29-48) | <0.001 |
Discussion
Doxycycline is commonly prescribed at a dose of 100mg twice daily to treat bacterial infections and dermatologic conditions (e.g., acne vulgaris and rosacea). Doxycycline has several potential mechanisms of action through which it may prevent or ameliorate the effects of COVID-19 infection.[12] It can inhibit metalloproteinases (MMPs), particularly MMP-9, which is likely required for initial viral entry into the cell. Doxycycline inhibits interleukin (IL)-6, with both IL-6 and MMP key regulators of the ‘cytokine storm’ often associated with severe viral pneumonitis.[12,13] Doxycycline inhibits nuclear factor (NF)-kB, which may lower the risk of viral entry because of direct inhibition of the DPP4 cell surface receptor and diminish the hyper-active immune response following infection. It is also an established ionophore, helping transport zinc intracellularly, with increased cellular concentrations of zinc shown in vitro to inhibit coronavirus replication. Structural analysis demonstrates that doxycycline has the potential to inhibit papain-like proteinase (PLpro) and 3C-like main protease (3CLpro), viral proteins which are both essential to viral replication and the life cycle.[13,14]
Macrolide antibiotics include azithromycin, clarithromycin, erythromycin, and spiramycin and are used to treat respiratory, gastro-intestinal, and skin infections. In an in vitro study reported in a pre-print, Poschet and colleagues[15] found that treatment with macrolides increased the pH of the recycling endosome. Both the Golgi network and recycling endosome play important roles in the packaging of proteins into vesicles and facilitate the replication and spread of viruses. Altering the pH of these organelles may therefore interfere with these intra-cellular viral activities. The authors also state that the raised pH of the trans-Golgi network may alter glycosylation of the angiotensin-converting enzyme 2 (ACE 2) receptor. Glycosylation of the receptor may therefore inhibit SARS-CoV-2 from binding to host cells.
SARS-CoV-2 is believed to possess a furin-like cleavage site in the spike protein, the protein that facilitates virus entry into host cells. It is possible that azithromycin interferes with cleavage of the spike protein, preventing viral entry into host cells. Macrolide antibiotics are believed to reduce the production of pro-inflammatory cytokines, such as Interleukin-6 and TNF-alpha and therefore abate the pro-inflammatory state induced by SARS-CoV-2 infection, which can ultimately lead to acute respiratory distress syndrome.[16]
Previous literature search and Centres for Disease Control and Prevention (CDC) reports reveal the high rates of antibiotic resistance in developing countries such as India. The CDC map suggests overall resistance rates for doxycycline to be 82–86% and for erythromycin to be 44–75%.[17,18] In the present study, the authors noted a dip in the overall sensitivity towards doxycycline and azithromycin. For doxycycline, the overall sensitivity was noted to be 46% in the year 2019–20 and 31% in the year 2020–21, whereas for erythromycin, the sensitivity was seen as 39% in 2019–20 and dropped down to 26% in 2020–21. This finding clearly indicates the increasing rates of antibiotic resistance in a developing country such as India during these COVID times.
A study from North India reported a bacterial co-infection rate of 5.2% in COVID-positive patients and stressed the higher prevalence of co-infections among the elderly age group (>65 years).[19] Colistin, fosfomycin, and vancomycin proved to be some very effective drugs in treating bacterial infections in COVID-positive cases in this study. Commonly used antibiotics such as doxycycline, azithromycin, and so on are usually advocated in these co-infection cases of COVID-19.
Administration of antibiotics in these co-infection sub-groups is vital to combat the ongoing bacterial infection in the form of blood steam and urinary and respiratory infections as well as to avoid the increased chances of acquiring secondary bacterial infections in such co-infection patients.
Discussing the clinical outcomes, the authors also noticed a much higher rate of in-patient mortality in the bacterial co-infection COVID group (33%) as compared to the no infection group (19%). However, no significant difference was noted in the mortality rates of the cases before and after the pandemic because of resistance of these drugs. Indiscriminate use of drugs has also led to an increase in many opportunistic bacterial and fungal infections during these pandemic times.[20]
The injudicious use of these over-the-counter drugs also caused a significant increase in the days of hospital stay and also led to a longer duration in infection clearance.
Limitations
Some limitations of the present study were a shorter time span of study, a smaller sample size, and patient demographic parameters. A larger number of sample sizes will add further detailed information towards this much needed aspect.
Conclusion
Indiscriminate and injudicious use of the easily available antibiotics such as azithromycin and doxycycline has led to the increasing trends in the resistance to these drugs. This calls for a proper anti-microbial stewardship programme during these COVID times to de-escalate these increasing resistance rates, and further detailed research studies in this direction will throw light on this upcoming unavoidable threat. This document will also be of great help to the primary care physicians in making the right judgement on correct and judicious prescription of antibiotics in COVID patients to minimise the risk of anti-microbial resistance.
Ethics approval
Informed consent was obtained from all the patients and their legal guardians (in case of minors) regarding the publication of images and clinical information in the journal. They were informed of the confidentiality of the data, however, the anonymity cannot be guaranteed.
Author’s contribution
S.S., A.P, CS, SSP performed literature search, data analysis, and first draft of the manuscript and figures. USS and ZH helped in clinical and radiological diagnosis. CS and UG contributed with the final draft of the manuscript and editing.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–33. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarner J. Three emerging coronaviruses in two decades. The story of SARS, MERS, and now COVID-19. Am J Clin Pathol. 2020;153:420–1. doi: 10.1093/ajcp/aqaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannan S, Shaik Syed Ali P, Sheeza A, Hemalatha K. COVID-19 (Novel Coronavirus 2019) –recent trends. Eur Rev Med Pharmacol Sci. 2020;24:2006–11. doi: 10.26355/eurrev_202002_20378. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Transmission of SARS-CoV-2:Implications for infection prevention precautions. [Last accessed on 2021 Apr 30]. Available from: https://www.who.int/publications/i/item/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations .
- 5.Joshi S, Parkar J, Ansari A, Vora A, Talwar D, Tiwaskar M, et al. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis. 2021;102:501–8. doi: 10.1016/j.ijid.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Wang W, Hayek SS, Chan L, Mathews KS, Melamad ML, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181:41–51. doi: 10.1001/jamainternmed.2020.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ. 2020;369:m1983. doi: 10.1136/bmj.m1983. [DOI] [PubMed] [Google Scholar]
- 8.Nori P, Cowman K, Chen V, Bartash R, Szymczak W, Madaline T, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalizedduring the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021;42:84–8. doi: 10.1017/ice.2020.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collee JG, Miles RS, Watt B. Tests for the identification of bacteria. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie and McCartney Practical Medical Microbiology. 14th ed. New York: Churchill Livingstone; 1996. pp. 131–45. [Google Scholar]
- 10.Ling TK, Tam PC, Liu ZK, Cheng AF. Evaluation of VITEK 2 rapid identification and susceptibility testing system against gram-negative clinical isolates. J Clin Microbiol. 2001;39:2964–6. doi: 10.1128/JCM.39.8.2964-2966.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CLSI. Performance Standards for Antimicrobial Susceptibility Testing. CLSI supplement M100. 27th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 12.Conforti C, Giuffrida R, Zalaudek I, Meo ND. Doxycycline, a widely used antibiotic in dermatology with a possible anti-inflammatory action against IL-6 in COVID-19 outbreak. Dermatol Ther. 2020;33:e13437. doi: 10.1111/dth.13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santa-Cecília FV, Socias B, Ouidja MO, Diaz JES, Acuna L, Silva RL, et al. Doxycycline suppresses microglial activation by inhibiting the p38 MAPK and NF-kB signaling pathways. Neurotox Res. 2016;29:447–59. doi: 10.1007/s12640-015-9592-2. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Zheng KI, Liu S, Yan Z, Xu C, Qiao Z, et al. Plasma CRP level is positively associated with the severity of COVID-19. Ann Clin Microbiol Antimicrob. 2020;19:18. doi: 10.1186/s12941-020-00362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poschet J, Perkett E, Timmins G, Deretic V. Azithromycin and ciprofloxacin have a chloroquine-like effect on respiratory epithelial cells. bioRxiv. 2020 2020.03.29.008631. doi:10.1101/2020.03.29.008631. [Google Scholar]
- 16.Min JY, Jang YJ. Macrolide therapy in respiratory viral infections. Mediators Inflamm. 2012;2012:649570. doi: 10.1155/2012/649570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dharmapalan D, Shet A, Yewale V, Sharland M. High reported rates of antimicrobial resistance in indian neonatal and pediatric blood stream infections. J Pediatr Infect Dis Soc. 2017;6:e62–8. doi: 10.1093/jpids/piw092. [DOI] [PubMed] [Google Scholar]
- 18.The Center for Disease, Dynamics Economics &Policy. Resistance Map:Antibiotic resistance. 2021. [Last accessed on: 2021 Sept 08]. Available form: https://resistancemap.cddep.org/AntibioticResistance.php .
- 19.Sahu C, Singh S, Pathak A, Singh S, Patel SS, Ghoshal U, et al. Bacterial coinfections in COVID:Prevalence, antibiotic sensitivity patterns and clinical outcomes from a tertiary institute of Northern India. J Family Med Prim Care. 2022 doi: 10.4103/jfmpc.jfmpc_41_22. doi:10.4103/jfmpc.jfmpc_41_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh S, Sahu C, Patel SS, Garg A, Ghoshal U. Pandoraea apista bacteremia in a COVID-positive man:A rare coinfection case report from North India. J Lab Physicians. 2021;13:192–4. doi: 10.1055/s-0041-1730847. [DOI] [PMC free article] [PubMed] [Google Scholar]


