Abstract
Objective:
We conducted a meta-analysis in which the blood pressure (BP)-reducing effect of telmisartan was compared to losartan among hypertensive subjects and its association with ethnicity, age, and gender was investigated.
Materials and Methods:
PubMed, Google Scholar, and the Cochrane library were searched from inception to April 2021 to obtain relevant articles. Cochrane risk of bias assessment tool was used for assessment of bias risk. GRADE analysis was done for determining the certainty of evidence. Data was analyzed using Revman 5.4.2 software. The pooled mean difference with 95% confidence interval (CI) was computed using random-effects model. Heterogeneity was also assessed using meta-regression and subgroup analysis. This study has been registered in PROSPERO with registration no. CRD42021245122.
Results:
Fifteen randomized controlled trials (RCTs) with 1926 subjects were selected from various countries. Both systolic BP (SBP) and diastolic BP (DBP) were found to be significantly reduced among telmisartan-treated groups (weighted mean difference [WMD] = 2.69, 95% CI: 1.38–4.00 and WMD = 1.26, 95% CI: 0.45–2.08 respectively). One subgroup analysis noted better reduction in both SBP and DBP among Asian population compared to Caucasians.
Conclusion:
Telmisartan was found to be a better hypertensive drug compared to losartan in patients with mild to moderate hypertension. Its efficacy was higher in Asian population compared to Caucasian population.
Keywords: Blood Pressure, hypertension, losartan, meta-analysis, telmisartan
Introduction
Hypertension (HTN) could be controlled with appropriate treatment.[1] It is one of the leading modifiable risk factors which may lead to early death.[2]
Hypertension is also a risk factor for ischemic heart disease, stroke and other cardiovascular diseases.[3] Among renin angiotensin aldosterone system (RAAS) inhibiting medicines, angiotensin receptor blockers (ARBs) are highly efficacious drugs as shown in numerous randomized controlled trials (RCTs).[4] The most often prescribed ARBs are telmisartan and losartan. According to the World Health Organization, Losartan is an essential drug.[5]
Telmisartan is an ARB that selectively inhibits the angiotensin II receptor. It has a partial peroxisome proliferator-activated receptor-gamma (PPAR-g) agonistic activity.[6] PPAR-g belongs to the nuclear hormone receptor superfamily.[7] Inhibition by losartan is transient and readily reversible, while that of telmisartan is insurmountable, which causes prolonged and irreversible inhibition of angiotensin 1 (AT1).[8]
A previous meta-analysis conducted in 2008 using 11 studies[9] highlighted that telmisartan shows better blood pressure (BP)-reducing potential, with similar adverse effects. In 2003[10] and 2013,[11] metaanalyes were done, where reduction in ambulatory BP was compared. But whether the antihypertensive efficacy of the drugs is associated with ethnicity, age, or gender has not been explored in these meta-analyses.
Our primary care physicians taking care of hypertensive patients are always in search of optimal antihypertensive drugs which are cost-effective, have higher efficacy, and show minimal side effects for better management of HTN. Inconsistent evidence is available for use of both telmisartan and losartan for better management of patients with HTN. Earlier meta-analysis (published in year 2013) has shown the beneficial effects of telmisartan compared to losartan for the management of HTN. After the publication of earlier meta-analysis, several studies appeared in the literature, and to obtain the precise evidence, updated meta-analysis is necessary. Therefore, we conducted an updated meta-analysis to obtain precise evidence for comparative effectiveness of both the drugs for treatment of HTN and to understand the variation across different age groups, genders, and ethnicities.
Materials and Methods
Study selection process
PubMed, Google Scholar, and the Cochrane library were searched by two independent authors to obtain eligible randomized controlled studies for this current meta-analysis published between 1966 and April 2021. The key words for the search strategies were (losartan OR losar) OR (telmisartan OR telma) AND Hypertension. Reference list used in the previous meta-analysis on the same topic or similar topic was also searched.
Research question
P-Patient with mild to moderate HTN with age more than 18 years of age
I-Telmisartan-treated subjects
C-Losartan-treated subjects
O-Reduction in systolic BP (SBP) and diastolic BP (DBP) measured with the help of automatic or mercury sphygmomanometer.
Eligibility criteria
Inclusion criteria: (1) All RCTs in which the antihypertensive effect of telmisartan was compared with losartan in subjects with mild to moderate HTN, (2) age more than 18 years, and (3) intervention varied between 4 weeks and 1 year.
Exclusion criteria: (1) All studies other than the RCTs, (2) RCTs in which fixed-dose combination (FDC) has been used, (3) published in a language other than English, and (4) those studies that have not reported sufficient data to compare pooled effect size.
Data extraction
Data extraction was done by the two authors independently and was cross-checked by another reviewer to obtain accurate data. Any disagreement between the two was sorted out by rechecking and discussion. Data was collected under the following headings: author’s name, study duration, sample size, study design, mean age of participants, dose of the drug used, characteristics of study population, location, associated comorbidity, mean SBP and DBP values with standard deviation (SD), adverse effects, and outcome.
Statistical analyses
We computed standardized mean difference with 95% confidence interval (CI) for the continuous outcome to determine the pooled effect size using random-effects model in case of heterogeneity (I2) more than 50%; otherwise, fixed-effect model was used. If multiple readings had been taken, then the last reading was taken as the end point BP. BP was written along with SD. In case SD was not available, then it was calculated using formulas as in the previous meta-analysis.[10]
Risk ratio with 95% CI was computed for the binary outcome data. Statistical analysis was done using software Revman 5.4.2. Subgroup analysis was done according to the study design, dose of the drugs, and on the basis of ethnicity of the population. Meta-regression analysis was also done to explore the source of heterogeneity using STATA software.
Bias assessment tool
We used Cochrane risk of bias assessment tool. All the six components of this tool were used. Two independent reviewers assessed the risk of different biases in each study. If there was any disagreement between the two assessors, then it was rechecked for correction. Each bias has been characterized into low, unclear, and high risk. In attrition bias, if less than 10% of the study population has loss to follow-up, it is low risk of bias and if more than 10% has loss to follow-up, then it is high risk of bias. In reporting bias assessment, if all outcomes have been reported, it is low risk; if incomplete outcome data has been given, then it is high risk.
Reporting of bias was done in the form of risk of bias summary and graph. It was done using Revman 5.4.2 Software. Low risk of bias is shown by green color, unclear risk by yellow color, and high risk by red color in the risk of bias graph. Risk of bias summary is in the form of table in which rows show individual studies and columns show their status of risk. Publication bias was also assessed using funnel plot figure and Begg and Egger test.
This study has been registered in PROSPERO with registration no. CRD42021245122.
Results
Study selection
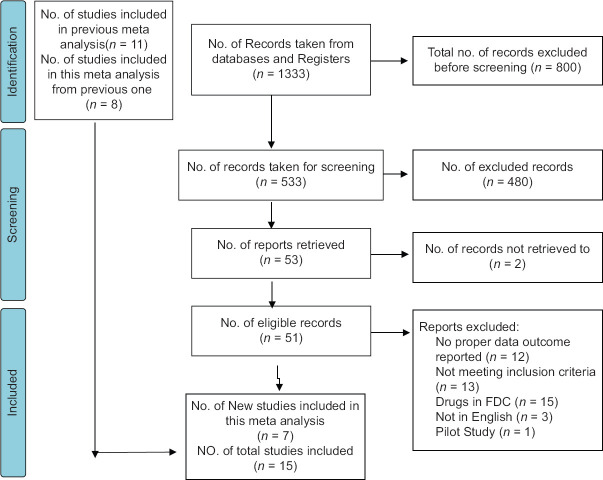
Our search strategy identified 1333 studies. Before screening, 800 studies were excluded due to being irrelevant in nature in terms of study objective. Therefore, 533 were screened for obtaining relevant information. After screening titles and abstracts, 482 were excluded due to them not being relevant. Fifty-one potentially eligible full-text records were identified. Among these, 44 were excluded. The reasons are mentioned in the study flow diagram [Figure 1]. Finally, 15 studies were included for our meta-analysis.[4,10,12,13,24]
Figure 1.
Study selection process
Study characteristics
All RCTs which were conducted in different countries of Asia, Europe, and America were included in this meta-analysis. Among these, five studies were multicentered RCTs, five were open-label, parallel-group studies, one was double-blind crossover study, one was single-blinded study, and eight were double-blinded parallel studies, as shown in Table 1. The study population consisted of hypertensive patients with metabolic syndrome in two studies, hypertensive obese patients in one study, and patients of HTN with cognitive impairment in one study.
Table 1.
Characteristics of the included studies
| Author | Year | Duration | Sample size | Study design | Patient | Location | Mean age (years) | Dose of telmisartan (mg) | Dose of losartan (mg) | Comorbidity | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mallion[12] | 1999 | 6 weeks | 223 | DB-P | HTN | France, Canada | 57 | 40-80 | 50 | No | BP reduction |
| Bahadir[13] | 2006 | 8 weeks | 42 | O-P | HTN | Turkey | 50 | 80 | 50 | Metabolic syndrome | Insulin resistance |
| Lee[14] | 2004 | 8 weeks | 180 | DB-P | HTN | Taiwan | 53.2 | 40-80 | 50-100 | No | BP reduction |
| Jhu[15] | 2004 | 8 weeks | 330 | DB-P | HTN | China | 50.8 | 40-80 | 50-100 | No | BP reduction |
| Smith[10] | 2003 | 8 weeks | 720 | DB-P | HTN | USA, Europe | 53.8 | 40-80 | 50-100 | No | Efficacy |
| Fogari[16] | 2001 | 4 weeks | 30 | DB-C | HTN | Italy | 52 | 40 | 50 | No | Efficacy |
| Ding[17] | 2004 | 6 weeks | 61 | DB-P | HTN | Taiwan | 51.6 | 40 | 50 | No | Efficacy |
| Vitale[18] | 2005 | 3 months | 40 | DB-P | HTN | Italy | 55.75 | 80 | 50 | Metabolic syndrome | Metabolic effect |
| Hasegawa[4] | 2011 | 1 year | 58 | O-P | HTN | Japan | 47.1 | 40 | 50 | No | Cardio protection |
| Nedogoda[19] | 2013 | 24 weeks | 120 | SB-P | HTN | Russia | 49.1 | 80 | 100 | Obesity | CV risk factors |
| Kalikar[20] | 2017 | 12 weeks | 60 | O-P | HTN | India | 60.25 | 40 | 50 | No | Efficacy |
| Bakris[21] | 2008 | 52 weeks | 860 | DB-P | HTN | USA | 58.8 | 80 | 100 | Diabetics | Proteinuria |
| Kyvelou[22] | 2005 | 6 months | 2438 | O-P | HTN | Greece | 60.3 | 80 | 50 | No | Metabolic effect |
| Puram[23] | 2016 | 24 weeks | 51 | SB-P | HTN | India | 60.93 | 20 | 50 | Cognition defect | BP reduction + cognition |
| Chandrashekhar[24] | 2021 | 24 weeks | 100 | O-P | HTN | India | 46.95 | 40 | 50 | No | Efficacy + CV parameter |
BP=blood pressure, CV=cardiovascular, DB-P=double blind-parallel, HTN=hypertension, O-P=open-parallel, SB-P=single blind-parallel
Primary outcome
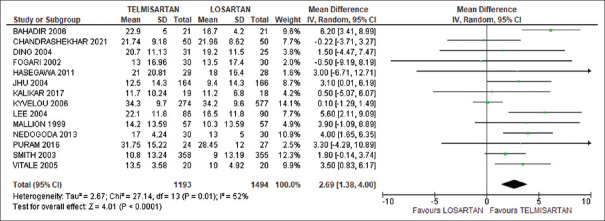
Our meta-analysis showed a significant reduction in SBP among telmisartan-treated subjects compared to losartan-treated subjects (weighted mean difference [WMD] 2.69, 95% CI: 1.39–4.00). There was significant heterogeneity in this analysis (χ2 = 27.14, l2 = 52%, P = 0.0001), as shown in Figure 2. In the sensitivity analysis, after removal of Kyvelou et al.’s[22] study, the heterogeneity was reduced to 17% with more reduction of SBP (WMD = 3.16, 95% CI: 2.21–4.10).
Figure 2.
Comparison of systolic blood pressure between telmisartan and losartan groups
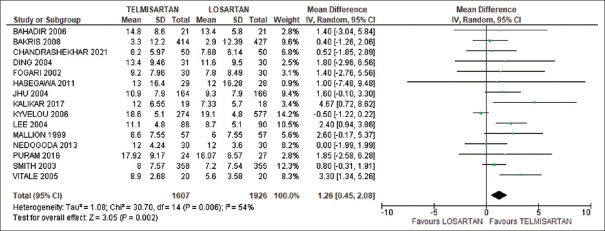
DBP was also significantly reduced in telmisartan group compared to losartan group with WMD = 1.26 and 95% CI: 0.45–2.08. There was a significant heterogeneity in this analysis (χ2 = 30.70, l2 = 54%, P = 0.006). Test for overall effect was Z = 3.00, P = 0.003, as shown in Figure 3. In the sensitivity analysis, if Kyvelou et al.’s[22] study was excluded, heterogeneity became 6% (Z = 4.90, P < 0.00001) and DBP was significantly reduced with WMD = 1.42 and 95% CI: 0.85–1.99.
Figure 3.
Comparison of diastolic blood pressure between telmisartan and losartan groups
Subgroup analyses
Subgroup analysis was based on ethnicity (Asian and Caucasian populations). Among Asian population (eight studies), homogeneous finding was noted with statistically significant reduction of SBP and DBP in telmisartan-treated group compared to losartan group (WMD = 3.66) with 95% CI = 2.43–4.90; (WMD = 1.59) with 95% CI = 0.76 to 2.4 respectively however, no significant difference in DBP was noted among Caucasian population (seven studies), as shown in Table 2.
Table 2.
Subgroup analysis
| Subgroup characteristics | BP parameter | No. of studies | WMD (95% CI) | Test for heterogeneity | Overall effect |
|---|---|---|---|---|---|
| Asian | SBP | 8 | WMD 3.66 with 95% CI (2.43, 4.90) | χ2=11.07, I2=37% | Z=5.82, P<0.00001 |
| DBP | 8 | WMD 1.59 with 95% CI (0.76, 2.4) | χ2=6.77, I2=0% | Z=3.76, P=0.0002 | |
| Caucasian | SBP | 6 | WMD 1.22 with 95% CI (0.21, 2.23) | χ2=7.03, I2=29% | Z=2.37, P=0.02 |
| DBP | 7 | WMD 0.31 with 95% CI (−0.21, 0.84) | χ2=17.48, I2=66% | Z=1.16, P=0.24 | |
| Telmisartan 40 mg versus losartan 50 mg | SBP | 5 | 0.97 (−1.29, 3.23) | χ2=1.94, I2=0% | Z=0.84, P=0.40 |
| DBP | 5 | 1.88 (0.43, 3.33) | χ2=3.49, I2=0% | Z=2.54, P=0.01 | |
| Telmisartan 40-80 mg versus losartan 50-100 mg | SBP | 4 | 2.80 (1.33, 4.26) | χ2=3.53, I2=15% | Z=3.73, P=0.0002 |
| DBP | 4 | 1.43 (0.64, 2.21) | χ2=2.98 | Z=3.58, P=0.0003 | |
| Open-label study | SBP | 4 | 1.17 (0.00, 2.33) | χ2=15.48, I2=81% | Z=1.96, P=0.05 |
| DBP | 5 | −31.12 (−31.75, −30.49) | χ2=−55,279.31, I2=100% | Z=97.11, P<0.00001 | |
| Blind label study | SBP | 9 | 3.13 (2.06, 4.20) | χ2=5.38 I2=0% | Z=5.73, P<0.00001 |
| DBP | 10 | 1.41 (0.81, 2.01) | χ2=10.66 I2=16% | Z=4.61, P<0.00001 |
BP = blood pressure, CI = confidence interval, DBP = diastolic blood pressure, SBP = systolic blood pressure, WMD = weighted mean difference
Subgroup analysis was also done to evaluate the effect of different doses of drugs. In the subgroup comparison between telmisartan 40 mg and losartan 50 mg, a significant reduction in DBP was observed among telmisartan 40 mg-treated subjects when compared with losartan 50 mg (five trials) (WMD = 1.88, 95% CI: 0.43–3.33, χ2 = 3.49, I2 = 0%, Z = 2.54, P = 0.01). However, no significant reduction in SBP was observed in telmisartan 40 mg-treated group when compared with 50 mg losartan-treated subjects, as in Table 2. In another subgroup analysis, comparison was done between trials using 40–80 mg telmisartan and 50–100 mg losartan. We observed that both SBP and DBP were significantly reduced in telmisartan group compared to losartan group. No significant heterogeneity was seen.
Sensitivity analysis
The subgroup analysis based on blinded design (nine studies for SBP and 10 studies for DBP) heterogeneity was reduced to zero, and pooled results showed significant reduction in SBP (WMD = 3.13, 95% CI: 2.06–4.20, Z = 5.73, P < 0.0000) and DBP (WMD = 1.41, 95% CI: 0.81–2.01, χ2 = 10.66, l2 = 16%, Z = 4.61, P < 0.00001), as evident from Table 2. As expected, the heterogeneity was considerable in the subgroup analysis based on open-label studies.
Secondary outcome
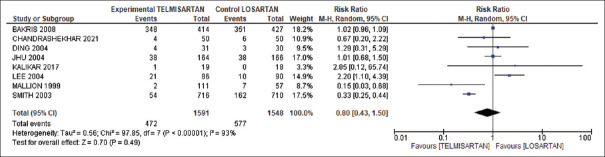
Our pooled analysis on adverse events showed 17% less adverse events in telmisartan group compared to losartan group (risk ratio 0.80, 95% CI: 0.43–1.50). However, heterogeneity was significant between the studies (I2 = 93%), as evident from Figure 4.
Figure 4.
Comparison of adverse events between telmisartan and losartan groups
Meta-regression analysis
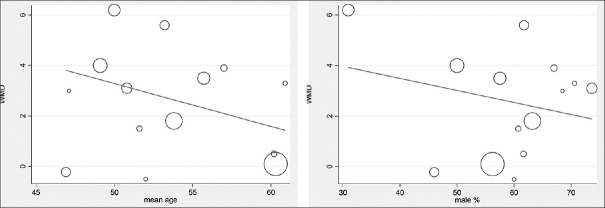
Meta-regression analysis gave the preliminary evidence that antihypertensive effect declines with increase in age, but this moderate effect of age was not statistically significant, as evident from Figure 5.
Figure 5.
Meta-regression analysis
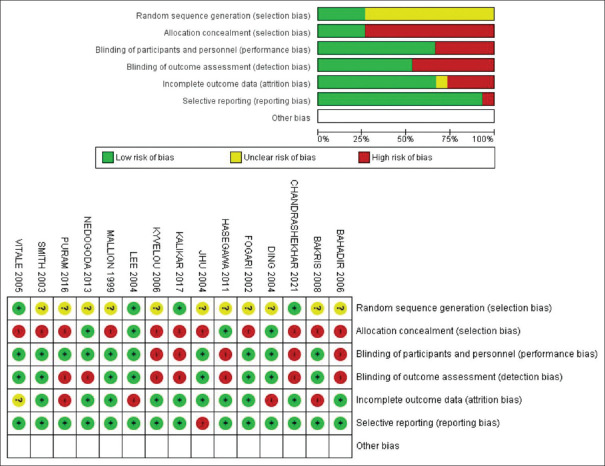
Risk of bias assessment
Our meta-analysis showed that 25% of our studies had low risk in randomization method and allocation concealment, 75% showed unclear risk in randomization method, and 75% showed high risk in allocation concealment. About 70% of studies had low risk of blinding of participants, while the rest of them showed high risk. In blinding of outcome assessment, about 50% showed high risk and 50% showed low risk. In attrition bias, 70% showed low risk, 5% showed unclear risk, and 25% had high risk. Also, 90% showed low risk of reporting bias, while 5% showed high risk, as evident from Figure 6. Risk of bias analysis suggested only 25% of the studies showed low risk of selection bias, while 75% showed high risk of selection bias. Performance bias was found to be low in almost 70% studies, while detection bias was low in 50% studies. Also, 25% had high risk of attrition bias, while 95% showed low risk of reporting bias.
Figure 6.
Risk of bias analysis. BP = blood pressure, CI = confidence interval, DBP = diastolic blood pressure, SBP = systolic blood pressure, WMD = weighted mean difference CI = confidence interval, MD = mean difference
Discussion
This meta-analysis, which included a total of 15 RCTs involving 1926 subjects suffering from mild to moderate essential HTN, showed that telmisartan had better efficacy compared to losartan. This finding is similar to the findings reported in the meta-analysis conducted in 2008.[9] This meta-analysis included a total of 11 studies and demonstrated that telmisartan was more effective with similar adverse effects in both the groups. Our pooled analysis indicated that telmisartan group had fewer side effects compared to losartan. Both drugs are ARBs, however, studies have shown that telmisartan shows more selective affinity for AT1.[7] This could be the reason for less adverse effects in telmisartan-treated patients. On the other hand, losartan is a prodrug that needs to be metabolized in the liver to become active. Therefore, it has slower onset of action compared to telmisartan. Additional advantage of telmisartan is its prolonged action due to longer half-life. Besides this, telmisartan has PPAR-g activity also, which is responsible for its other cardiovascular protective effects.
In the current meta-analysis, we noted significant heterogeneity for our primary outcomes in telmisartan-treated group compared with losartan-treated group (I2 = 52% and 54%, respectively). However, in the sensitivity analysis, after exclusion of Kyvelou et al.’s[22] study, heterogeneity turned out to be 17% and 6%, respectively. Interestingly, in the subgroup analysis, according to the design of study (open-label and blinded study), we did not find considerable heterogeneity. We can conclude that probable cause for the high heterogeneity in our meta-analysis was open-label studies which have more chances of all kinds of biases.
We compared the drug effects in Asian and Caucasian populations also on the basis of racial differences. Surprisingly, we found that in the Caucasian population, telmisartan only lowers SBP, while in the Asian population, it reduces both SBP and DBP considerably. The probable cause of this difference might be due to the difference in salt sensitivity, which is ultimately related with RAAS, as evident from one meta-analysis.[25]
Subgroup analysis according to dose suggested similar reduction of DBP in telmisartan group, as observed in the previous meta-analysis.[9] In contrast to the previous meta-analysis finding we noted no significant difference in SBP when treated with telmisartan 40 mg compared to losartan 50 mg.
We also observed that increase in age had a downward trend in the BP-reducing potential of telmisartan, but the difference was not statistically significant. It is obvious that, increase in age is associated with higher risk for atherosclerosis, which further increases BP in hypertensive patients. Power of this analysis was not sufficient to exclude the type II error, which demands further well-designed study to obtain more reliable evidence in this regard.
In the gender-wise subgroup analysis, we observed negative relation of efficacy of the telmisartan drug with male gender population, as evident from Figure 5, but the difference was not statistically significant. This may be due to the difference in sex hormone level, mostly estrogen, which is evident from one of the animal studies.[26] Our result showed a downward trend, but it was not significant. It may be due to inadequate power, and further well-designed studies may provide add-on evidence in this direction.
Although we observed significant difference in BP reduction between both the groups, the risk of bias in the included studies had some concern. In our meta-analysis, we found 25% studies had low risk of selection bias due to lack of information about random sequence generation and allocation concealment. Method of randomization also had not been mentioned in most of the studies, which is a very important step in an RCT. In almost all studies that had reported blinding, their process of blinding was also not clearly mentioned. We found lowest risk of reporting bias in the studies, while high risk of performance, detection, and attrition bias were seen in less than 50% studies.
Our updated meta-analysis strengthens the evidence that telmisartan drug response could be better in terms of reducing BP compared to losartan with low certainty of evidence, as shown in Table 3. For the first time up to best of our knowledge, we have shown the preliminary evidence that Asian population might have higher therapeutic response compared to Caucasian population.
Table 3.
GRADE analysis
| Certainty assessment | №. of patients | Effect | Certainty | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| №. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Telmisartan | Losartan | Relative (95% CI) | Absolute (95% CI) | ||
| Reduction in systolic blood pressure | ||||||||||||
|
| ||||||||||||
| 14 | Randomized trials | Serious | Serious | Not serious | Not serious | None | 1193 | 1494 | - | MD 2.69 higher (1.38 higher to 4 higher) | ⨁⨁◯◯ Low |
|
|
| ||||||||||||
| Reduction in diastolic blood pressure | ||||||||||||
|
| ||||||||||||
| 15 | Randomized trials | Serious | Serious | Not serious | Not serious | None | 1607 | 1926 | - | MD 1.26 higher (0.45 higher to 2.08 higher) | ⨁⨁◯◯ Low |
|
CI=confidence interval, MD=mean difference
Findings of the present meta-analysis have important clinical implications in terms of deciding better antihypertensive drugs by primary health care and family physicians in the following manner.
Our study observed superior effects of telmisartan compared to losartan, with similar adverse effects found between both the drugs. The evidence of PPAR-γ activity for telmisartan was observed, which improves the insulin sensitivity and controls the plasma glucose and dyslipidemia better in patients with metabolic syndrome.[18]
The half-life of telmisartan is approximately 24 h. It provides additional benefit for trough BP control, which provides the maximum efficacy of telmisartan at the end of dosing interval, leading to reduction in the incidence of cardiovascular events.[8]
Apart from these additional benefits, treatment with telmisartan for HTN requires fewer uptitrations as compared to losartan to achieve target DBP provide ease for the primary health care physicians for management and treatment of HTN. Telmisartan is also an effective BP-lowering agent when given alone or in combination during day-to-day practice for Asian hypertensive patients with comorbidities like diabetes or dyslipidemia.[27]
Effectiveness of telmisartan in diabetics is also supported by one animal study which shows that telmisartan works through voltage-dependent potassium channel also.[28]
The power of this analysis was not adequate, which demands further prospective validation studies and multicentric RCTs to validate the findings observed in the current meta-analysis.
Limitations
This meta-analysis had some limitations. The included studies had different methodology as they included open-label studies, single-blind studies, double-blind studies, parallel studies, and crossover studies. The differences in the population, clinical characteristics, and unaccounted confounding factors might have led to heterogeneity among studies. Another limitation of the review process is that only studies which were published in English have been included. Therefore, some studies published in languages other than English have not been included here. Our results are also confined to monotherapy, whereas many patients receive combination therapy in their real life. Regarding outcome, our limitation is that this meta-analysis did not measure many outcomes like cardiovascular and cerebrovascular incidence.
Despite the limitations described above, our study highlights many points like telmisartan is a drug with better efficacy than losartan in terms of reduction of BP. But it may warrant further investigation for building more evidence.
Conclusion
The present meta-analysis has demonstrated that telmisartan has more BP-reducing potential with lesser adverse effects than losartan and better effects in the Asian population. The certainty of evidence was low in the pooled analysis, which demands well-designed, methodologically sound studies to build upon evidence in this direction.
Key points
The present updated meta-analysis including 15 RCTs provides precise evidence regarding the comparative efficacy of telmisartan and losartan among mild to moderate essential hypertensive adult subjects.
For the first time, we did GRADE analysis, which showed certainty of evidence for telmisartan for treatment of HTN.
One subgroup analysis noted better reduction in both SBP and DBP among Asian population compared to Caucasians.
Key take home message
Telmisartan could be used with ease by the primary health-care physicians for treatment of HTN, which requires fewer uptitrations. It has additional benefit in terms of controlling blood glucose, better insulin sensitivity, and control of dyslipidemia.
Novelty
For the first time, we have shown a comparison between telmisartan and losartan using GRADE analysis for certainty of evidence. Our study showed updated evidence that effects of telmisartan are higher in the Asian population compared to Caucasian population.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults:Report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–20. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 2.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37. doi: 10.1038/s41581-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control:A systematic analysis of population-based studies from 90 countries. Circulation. 2016;134:441–50. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasegawa H, Takano H, Narumi H, Ohtsuka M, Mizuguchi T, Namiki T, et al. Effects of telmisartan and losartan on cardiovascular protection in Japanese hypertensive patients. Hypertens Res Off J Jpn Soc Hypertens. 2011;34:1179–84. doi: 10.1038/hr.2011.114. [DOI] [PubMed] [Google Scholar]
- 5.WHO. WHO Model List of Essential Medicine. Geneva: WHO; 2019. 21st List 2019. [Google Scholar]
- 6.Ayza MA, Zewdie KA, Tesfaye BA, Gebrekirstos ST, Berhe DF. Anti-diabetic effect of telmisartan through its partial PPARg-agonistic activity. Diabetes Metab Syndr Obes Targets Ther. 2020;13:3627–35. doi: 10.2147/DMSO.S265399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rakhshandehroo M, Knoch B, Müller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010;2010:612089. doi: 10.1155/2010/612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Liefde I, Vauquelin G. Sartan-AT1 receptor interactions: In vitro evidence for insurmountable antagonism and inverse agonism. Mol Cell Endocrinol. 2009;302:237–43. doi: 10.1016/j.mce.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Xi G-L, Cheng J-W, Lu G-C. Meta-analysis of randomized controlled trials comparing telmisartan with losartan in the treatment of patients with hypertension. Am J Hypertens. 2008;21:546–52. doi: 10.1038/ajh.2008.30. [DOI] [PubMed] [Google Scholar]
- 10.Smith DHG, Cramer M-JM, Neutel JM, Hettiarachchi R, Koval S. Comparison of telmisartan versus losartan:Meta-analysis of titration-to-response studies. Blood Press Monit. 2003;8:111–7. doi: 10.1097/00126097-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Takagi H, Niwa M, Mizuno Y, Goto S, Umemoto T All-Literature Investigation of Cardiovascular Evidence Group. A meta-analysis of randomized trials of telmisartan versus losartan for reduction of ambulatory blood pressure. Hypertens Res Off J Jpn Soc Hypertens. 2013;36:959–66. doi: 10.1038/hr.2013.78. [DOI] [PubMed] [Google Scholar]
- 12.Mallion J, Siche J, Lacourcière Y. ABPM comparison of the antihypertensive profiles of the selective angiotensin II receptor antagonists telmisartan and losartan in patients with mild-to-moderate hypertension. J Hum Hypertens. 1999;13:657–64. doi: 10.1038/sj.jhh.1000925. [DOI] [PubMed] [Google Scholar]
- 13.Bahadir O, Uzunlulu M, Oguz A, Bahadir MA. Effects of telmisartan and losartan on insulin resistance in hypertensive patients with metabolic syndrome. Hypertens Res Off J Jpn Soc Hypertens. 2007;30:49–53. doi: 10.1291/hypres.30.49. [DOI] [PubMed] [Google Scholar]
- 14.Lee YT, Lee CM, Lin CS, Sheu SH, Kuo WK, Tsai CW, et al. A double-blind comparison of the efficacy and tolerability of telmisartan 40-80 mg vs. losartan 50-100 mg in Taiwanese hypertensive patients. Int J Clin Pract Suppl. 2004;40:5. doi: 10.1111/j.1742-1241.2004.00409.x. doi:10.1111/j. 1742-1241.2004.00409.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhu JR, Bai J, Cai NS, Tang B, Fan WH, Guo JZ, et al. Efficacy and safety of telmisartan vs. losartan in control of mild-to-moderate hypertension: A multicentre, randomised, double-blind study. Int J Clin Pract Suppl. 2004;46:9. doi: 10.1111/j.1742-1241.2004.00410.x. doi:10.1111/j. 1742-1241.2004.00410.x. [DOI] [PubMed] [Google Scholar]
- 16.Fogari R, Mugellini A, Zoppi A, Derosa G, Rinaldi A, Fogari E, et al. Efficacy of losartan, valsartan, and telmisartan in patients with mild to moderate hypertension:A double-blind, placebo-controlled, crossover study using ambulatory blood pressure monitoring. Curr Ther Res Clin Exp. 2002;63:1–14. [Google Scholar]
- 17.Ding PYA, Chu KM, Chiang HT, Shu KH. A double-blind ambulatory blood pressure monitoring study of the efficacy and tolerability of once-daily telmisartan 40 mg in comparison with losartan 50 mg in the treatment of mild-to-moderate hypertension in Taiwanese patients. Int J Clin Pract Suppl. 2004:16–22. doi: 10.1111/j.1742-1241.2004.00405.x. doi:10.1111/j. 1742-1241.2004.00405.x. [DOI] [PubMed] [Google Scholar]
- 18.Vitale C, Mercuro G, Castiglioni C, Cornoldi A, Tulli A, Fini M, et al. Metabolic effect of telmisartan and losartan in hypertensive patients with metabolic syndrome. Cardiovasc Diabetol. 2005;4:6. doi: 10.1186/1475-2840-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nedogoda SV, Ledyaeva AA, Chumachok EV, Tsoma VV, Mazina G, Salasyuk AS, et al. Randomized trial of perindopril, enalapril, losartan and telmisartan in overweight or obese patients with hypertension. Clin Drug Investig. 2013;33:553–61. doi: 10.1007/s40261-013-0094-9. [DOI] [PubMed] [Google Scholar]
- 20.Kalikar M, Nivangune KS, Dakhale GN, Bajait CS, Sontakke SD, Motghare VM, et al. Efficacy and tolerability of olmesartan, telmisartan, and losartan in patients of stage i hypertension:A randomized, open-label study. J Pharmacol Pharmacother. 2017;8:106–11. doi: 10.4103/jpp.JPP_39_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakris G, Burgess E, Weir M, Davidai G, Koval S AMADEO Study Investigators. Telmisartan is more effective than losartan in reducing proteinuria in patients with diabetic nephropathy. Kidney Int. 2008;74:364–9. doi: 10.1038/ki.2008.204. [DOI] [PubMed] [Google Scholar]
- 22.Kyvelou S-MG, Vyssoulis GP, Karpanou EA, Adamopoulos DN, Zervoudaki AI, Pietri PG, et al. Effects of antihypertensive treatment with angiotensin II receptor blockers on lipid profile:An open multi-drug comparison trial. Hell J Cardiol HJC Hell Kardiologike Epitheorese. 2006;47:21–8. [PubMed] [Google Scholar]
- 23.Puram NN, Karande VB, Ramanand JB, Ramanand SJ, Halasawadekar NR, Bhosale RR. Comparison of efficacy of telmisartan with losartan in patients of essential hypertension with cognitive impairment. Int J Basic Clin Pharmacol. 2016;5:702–6. [Google Scholar]
- 24.Chandrasekar T, Muniappan M, Muthiah NS. Comparison on safety and efficacy of telmisartan and losartan with their effects on cardiovascular –A single centre study. J Pharm Res Int. 2021;33:1–12. [Google Scholar]
- 25.Kato N. Ethnic differences in genetic predisposition to hypertension. Hypertens Res Off J Jpn Soc Hypertens. 2012;35:574–81. doi: 10.1038/hr.2012.44. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertens Dallas Tex 1979. 1999;33:323–8. doi: 10.1161/01.hyp.33.1.323. [DOI] [PubMed] [Google Scholar]
- 27.Khan MY, Pandit S, Abdulkutty J, Navasundi G, Hazra PK, Phadke U, et al. Effectiveness of telmisartan on blood pressure control in hypertensive patients in India:A real-world retrospective study from electronic medical records. Cardiol Ther. 2021;10:255–69. doi: 10.1007/s40119-021-00217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu T, Cui L, Xue H, Yang X, Liu M, Zhi L, et al. Telmisartan potentiates insulin secretion via ion channels, independent of the AT1 receptor and PPARg. Front Pharmacol. 2021;12:739637. doi: 10.3389/fphar.2021.739637. doi:10.3389/fphar.2021.739637. [DOI] [PMC free article] [PubMed] [Google Scholar]