Introduction
The creation of an iatrogenic atrial septal defect (iASD) with transseptal puncture (TSP) is an inherently accepted risk for patients undergoing most left-sided catheter ablations and structural heart interventions. After TSP, 20%–37% of patients undergoing pulmonary vein isolation have a persistent iASD after 1 year of follow-up.1,2 A small percentage of patients with iASDs require percutaneous or surgical closure. Acute hemodynamic changes from intracardiac shunting following TSP are even less well described, and there have been no documented cases of iASD with right-to-left (R-L) intracardiac shunting following TSP for catheter ablation of ventricular tachycardia (VT). We aim to highlight a dangerous complication of TSP for VT ablation in patients with a left ventricular assist device (LVAD) and a history of progressive right ventricular (RV) failure.
Key Teaching Points.
-
•
Right-to-left shunting across iatrogenic atrial septal defects following transseptal puncture are a known but uncommon complication of catheter ablation procedures.
-
•
Increased size of septal puncture, prolonged duration of catheter ablation, and elevated preprocedural right-sided pressures are risk factors for complications from transseptal puncture.
-
•
Patients with left ventricular assist devices undergoing catheter ablation for ventricular tachycardia are at inherently higher risk for complications from transseptal puncture.
-
•
Significant shunting across iatrogenic atrial septal defects is challenging to recognize and intervene against complications unless the atrial septum undergoes dedicated imaging following catheter ablation.
Case report
A 74-year-old man with a history of end-stage nonischemic cardiomyopathy and biventricular failure that required HeartMate 3 LVAD (Abbott, Chicago, IL) implantation, paroxysmal atrial fibrillation, and VT with previous endocardial/epicardial ablation presented with recurrent VT resulting in implantable cardioverter-defibrillator (ICD) shock while he was taking oral amiodarone, quinidine, and propranolol. While admitted for electrical storm, he had additional VT while receiving intravenous amiodarone, lidocaine, and procainamide (Figure 1). Transthoracic echocardiogram demonstrated a dilated left ventricle (LV) with severely reduced systolic function, an apically placed LVAD cannula, RV dilation with mildly reduced systolic function, a midline interatrial septum, no intracardiac shunting, and no pericardial effusion. The decision was made to attempt a salvage endocardial ablation. Before undergoing catheter ablation, the patient was receiving no vasopressor/inotropic support, having mean arterial pressure of 88 mm Hg and Spo2 of 96% on room air. He underwent right heart catheterization, with right atrial pressure of 12 mm Hg, pulmonary artery systolic pressure 32 mm Hg, pulmonary artery diastolic pressure 8 mm Hg, mean pulmonary artery pressure 18 mm Hg, pulmonary capillary wedge pressure 9 mm Hg, cardiac index using Fick principle of 1.8 L/min/m2, and calculated pulmonary artery pulsatility index of 1.9.
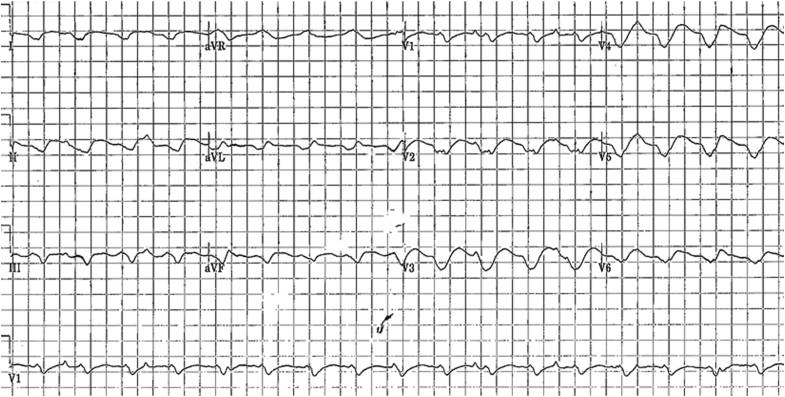
Figure 1.
Twelve-lead electrocardiogram showing the patient’s presenting wide complex tachycardia with a ventricular rate of 125 bpm, atrioventricular (AV) dissociation, and QRS morphology consistent with the patient’s dominant ventricular tachycardia.
With the patient general anesthesia, his femoral veins were accessed with a 9F long sheath, 7F short sheath, and 8.5F guiding sheath (VersaCross, Baylis Medical, Montreal, Canada). An intracardiac echocardiography catheter (SoundStar 3D diagnostic ultrasound catheter, Biosense Webster) was placed through the 9F long sheath, and ultrasound electroanatomic mapping of the left atrium, LV cavity, papillary muscles, aortic valve leaflets, and LVAD cannula insertion site was performed (CARTO 3-Sound, Biosense Webster) (Figure 2). A VersaCross Access solution with pigtail radiofrequency (RF) wire (180 cm) was used to perform TSP under hemodynamic, fluoroscopic, and intracardiac echocardiographic guidance. There was no bowing of the septum at the time of TSP. Heparin was infused to maintain an activated clotting time continuously >300 seconds. The guiding sheath was then exchanged over the long pigtail RF wire, which was anchored in the left atrial appendage, for a 11.5F deflectable sheath (CARTO Vizigo Bi-Directional, Biosense Webster). A PentaRay NAV ECO high-density mapping catheter (Biosense Webster) was then advanced into the LV, and electroanatomic mapping was performed (CARTO 3D, Biosense Webster). Bipolar voltage mapping of the LV in sinus rhythm revealed an extensive amount of scar tissue, with limited viable LV tissue in the basal to mid septum. The segments of scar adjacent to the LVAD inflow cannula insertion site had multiple sites of slow conduction, with isolated late potentials and low-amplitude, long-fractionated signals that were tagged.
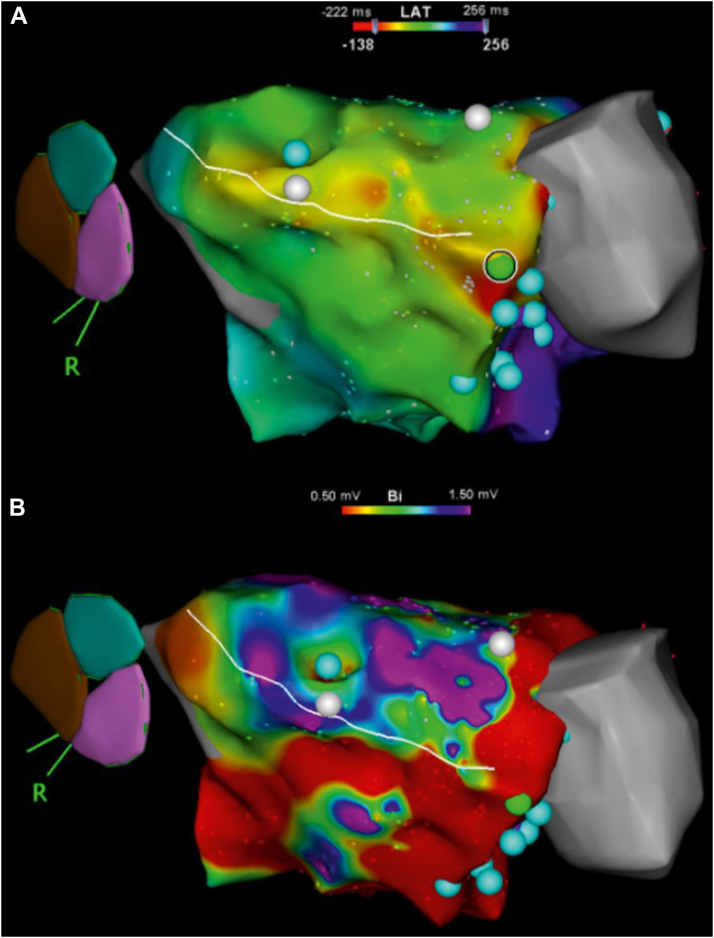
Figure 2.
Right anterior oblique projection of 3-dimensional bipolar activation (A) and voltage (B) electroanatomic map of the left ventricular substrate. A: Activation mapping of the inducible ventricular tachycardia demonstrates multiple sites of early activation in the anterior left ventricle near the left ventricular assist device (LVAD) cannula (gray area). B: Voltage mapping demonstrates a large burden of low voltage around the anterior, apical, and inferior areas of the left ventricle and the LVAD cannula. Cyan dots represent complex fractionated electrograms. Green dot identifies the location of the earliest potential. White design line identifies the location of a planned ablation line running from the LVAD cannula to the mitral annulus.
While the patient was in sinus rhythm, electrical stimulation was performed with double extrastimuli that readily induced VT having a variable cycle length between 400 and 450 ms. Electrocardiographic localization of the VT was limited by significant LVAD artifact and prevented entrainment maneuvers from being reliably performed. Although the VT cycle length was variable, the VT morphology remained unchanged throughout the case. Activation mapping was able to localize the entire cycle length within the LV, with an earliest meet late site next to the LVAD inflow cannula confirming the reentrant nature of the VT (Figure 2). A 3.5-mm, irrigated-tip RF ablation catheter (Thermocool Smarttouch SF, Biosense Webster) was next advanced to the LV. RF ablation was performed at 35 W with utilization of impedance drop to monitor the quality of lesions. Ablation was performed until the local electrograms were reduced or eliminated. Despite aggressive catheter ablation with completion of a circumferential ablation lesion set around the cannula, the VT could not be terminated. At this time, the decision was made to perform a substrate-based ablation with scar homogenization to create an ablation line from the base of the ventricle at the level mitral valve to the LVAD cannula with the aim of disrupting the reentrant circuit. During the ablation, the cycle length of the VT prolonged to ∼580 ms without termination. Because of the extensive ablation time and tenuous patient hemodynamics, the procedure was terminated. After an unsuccessful attempt at pace terminating the VT, the patient was successfully externally cardioverted with 360 J of energy. At the conclusion of the procedure, intracardiac echocardiography was used to confirm that there was no pericardial effusion, worsening RV dilation, and/or marked interatrial shunting. The patient then underwent venous vascular closure (VASCADE MVP, Cardiva Medical, Santa Clara, CA). He was extubated easily, weaned off all vasopressor/inotropic support, and transferred to the intensive care unit.
Thirty-six hours after undergoing ablation, the patient developed recurrent VT requiring multiple ICD shocks. A Swan-Ganz catheter was urgently placed, which revealed the patient had a dramatic decline in RV function. He had elevated right atrial pressures to 20 mm Hg, mean pulmonary artery pressure 27 mm Hg, pulmonary capillary wedge pressure 22 mm Hg, cardiac index using Fick principle of 1.9 L/min/m2, and calculated pulmonary artery pulsatility index of 0.5. Transthoracic echocardiogram demonstrated severely reduced LV ejection fraction, RV dilation with moderately reduced systolic function, bowed interatrial septum toward to the left atria, bowed interventricular septum toward the LV, no intracardiac shunting, and no pericardial effusion. At the time, his mean arterial pressure was 90 mm Hg, and SpO2 was 99% on 2-L nasal cannula.
Over the next 12 hours, the patient developed progressive, refractory hypoxemia despite intubation and mechanical ventilation. He ultimately was found to have Spo2 of 85% with Pao2 41 mm Hg and Svo2 52% despite mechanical ventilation with Fio2 of 100% and positive end-expiratory pressure of 10 cm H20. On chest radiograph-ray, the patient’s lungs were clear without evidence of infiltrate or pulmonary edema. Transesophageal echocardiogram (TEE) demonstrated an 8-mm atrial septal defect with near-continuous R-L shunting that was not seen on the transthoracic echocardiogram following ablation (Figure 3). Transient changes to LVAD pump speed did not make any appreciable difference to the degree of shunt. The patient was deemed not to be a candidate for surgical or percutaneous closure of the iASD, and ultimately he was transitioned to comfort care.
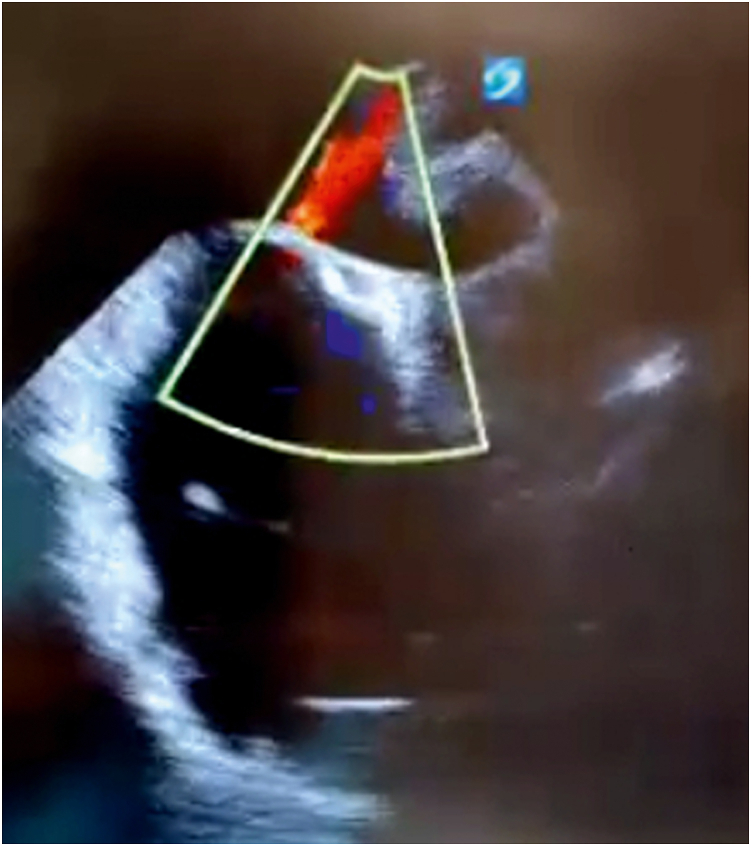
Figure 3.
Bicaval transesophageal echocardiographic view during clinical decompensation showing continuous right-to-left shunting through an iatrogenic atrial septal defect.
Discussion
Ventricular arrhythmias occur in 27%–37% of patients following LVAD implantation and have been associated with increased mortality.3,4 The development of electrical storm, defined as ≥3 episodes of VA in a period <24 hours, is even more strongly associated with increased mortality in this population.5 Catheter ablation for treatment of VT is a particularly important tool for treating patients with LVADs, yet there are a variety of unique anatomic obstacles to achieving safe and effective ablation. Retrograde access can be challenging because of the position of the LVAD outflow cannula, periaortic thrombosis is not uncommon, and the LVAD outflow cannula can generate a suction effect that will distort left atrial size.6 In our patient, we were unable to perform diagnostic entrainment maneuvers because of significant artifact from the LVAD and epicardial access was not a procedural option because of prior sternotomies with LVAD placement. One underappreciated complication of VT ablation in a patient with advanced heart failure and an LVAD is the potential hemodynamic effects of TSP.
Classically, iASDs from TSP were thought to close spontaneously. This is true for the majority of patients; however, one-fifth to one-third of patients have persistent iASDs after short-term follow-up. One illustrative cohort of patients with iASDs and serial follow-up imaging demonstrated that 22% of patients with iASD after 1-year follow-up had spontaneous closure at 2 years, and no spontaneous closure was seen in patients with iASD after 3 years of follow-up.7 Persistent iASD after pulmonary vein isolation has been associated with elevated preprocedural pulmonary artery pressures, longer ablation time, use of cryoballoon catheters, and lower left atrial appendage flow velocity.2,7, 8, 9
Although persistent iASDs following TSP are not uncommon, only 5% of intraprocedural iASDs are complicated by R-L intracardiac shunting.10 The risk of developing R-L shunting is associated with severe tricuspid regurgitation, elevated mean pulmonary artery pressures, and elevated right atrial pressures. A small minority of cases of iASDs with R-L shunting have demonstrated a need for percutaneous iASD closure following demonstration of symptomatic hypoxemia, and only a handful of cases of emergent percutaneous iASD closure following TSP due to refractory hypoxemia from R-L shunting have been described.7,10, 11, 12, 13 This is the first reported case of a complication from TSP described in a patient undergoing VT ablation or with an LVAD.
The risk of developing hemodynamically significant R-L intracardiac shunt following TSP is associated with increased size of TSP and elevated right-sided pressures.10 Our patient, who had an LVAD and severe RV failure, was at elevated risk for developing R-L shunt following TSP due to poor RV compliance with elevated right-sided pressures and continuous left-sided suction from the LVAD. After presenting with recurrent VT resulting in electrical storm, the patient was at even higher risk for complication due to additional injury to the RV from prolonged tachycardia and ICD shocks. In our case, the patient clinically tolerated TSP intraprocedurally and iASD immediately following catheter ablation. He did not have any noted desaturation and was easily extubated following catheter ablation. The patient did not undergo dedicated TEE immediately following the ablation, but the transthoracic echocardiogram did not demonstrate any significant shunt across the atrial septum. It was not until the patient developed worsening RV function with recurrent VT that he also began to develop clinical evidence of intracardiac shunt with refractory hypoxemia requiring intubation. It is likely that ventilation–perfusion mismatch, including from pulmonary edema and inhibition of hypoxic pulmonary vasoconstriction, also contributed to our patient’s profound hypoxemia. We believe that this case illustrates the dynamic challenges of R-L shunting and highlights an important complication of VT ablation.
The burden of ventricular arrhythmias is expected to grow in patients with LVADs as the proportion of patients receiving LVAD implantation for destination therapy expands and survival after LVAD implantation continues to improve.14 Therefore, it is important to recognize potential challenges inherent to pursuing catheter ablation in patients with LVADs. The risk of R-L shunting following TSP is an uncommon complication, and elevated right-sided pressures place patients at increased risk of morbidity and mortality. While the percentage of patients with severe RV failure receiving LVAD implantation has significantly increased, right heart function should be characterized and medically optimized before catheter ablation is performed.14 It is also best practice to perform dedicated imaging of the interatrial septum to characterize an iASD after completion of any procedure involving TSP. Additional study is needed to determine the optimal TSP technique to minimize the risk of persistent iASD.15
Conclusion
It is important to recognize the risks of hemodynamic changes following TSP in all patients and even more critical to recognize those patients at highest risk for complications. TEE is an important tool to characterize the iASD after TSP and should be routinely performed in all patients at high risk for hemodynamic changes from iASD, including patients with RV dysfunction and an LVAD.
Footnotes
Funding Sources: None. Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Sieira J., Chierchia G.B., Di Giovanni G., et al. One year incidence of iatrogenic atrial septal defect after cryoballoon ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25:11–15. doi: 10.1111/jce.12279. [DOI] [PubMed] [Google Scholar]
- 2.Linhart M., Werner J.T., Stöckigt F., et al. High rate of persistent iatrogenic atrial septal defect after single transseptal puncture for cryoballoon pulmonary vein isolation. J Interv Card Electrophysiol. 2018;52:141–148. doi: 10.1007/s10840-018-0352-0. [DOI] [PubMed] [Google Scholar]
- 3.Galand V., Flécher E., Auffret V., et al. ASSIST-ICD Investigators Predictors and clinical impact of late ventricular arrhythmias in patients with continuous-flow left ventricular assist devices. JACC Clin Electrophysiol. 2018;4:1166–1175. doi: 10.1016/j.jacep.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Gordon J.S., Maynes E.J., Choi J.H., et al. Ventricular arrhythmias following continuous-flow left ventricular assist device implantation: a systematic review. Artif Organs. 2020;44:E313–E325. doi: 10.1111/aor.13665. [DOI] [PubMed] [Google Scholar]
- 5.Rehorn M.R., Black-Maier E., Loungani R., et al. Electrical storm in patients with left ventricular assist devices: risk factors, incidence, and impact on survival. Heart Rhythm. 2021;18:1263–1271. doi: 10.1016/j.hrthm.2021.03.047. [DOI] [PubMed] [Google Scholar]
- 6.Sisti N., Santoro A., Carreras G., et al. Ablation therapy for ventricular arrhythmias in patients with LVAD: multiple faces of an electrophysiological challenge. J Arrhythm. 2021;37:535–543. doi: 10.1002/joa3.12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan N.Y., Choy C.C., Yuen H.C., Chow H.F., Fong H.F. A very long-term longitudinal study on the evolution and clinical outcomes of persistent iatrogenic atrial septal defect after cryoballoon ablation. Can J Cardiol. 2019;35:396–404. doi: 10.1016/j.cjca.2018.12.028. [DOI] [PubMed] [Google Scholar]
- 8.Hammerstingl C., Lickfett L., Jeong K.M., et al. Persistence of iatrogenic atrial septal defect after pulmonary vein isolation—an underestimated risk? Am Heart J. 2006;152:362. doi: 10.1016/j.ahj.2006.04.034. e1–e5. [DOI] [PubMed] [Google Scholar]
- 9.Mugnai G., Sieira J., Ciconte G., et al. One year incidence of atrial septal defect after PV isolation: a comparison between conventional radiofrequency and cryoballoon ablation. Pacing Clin Electrophysiol. 2015;38:1049–1057. doi: 10.1111/pace.12663. [DOI] [PubMed] [Google Scholar]
- 10.Morikawa T., Miyasaka M., Flint N., et al. Right-to-left shunt through iatrogenic atrial septal defect after MitraClip procedure. JACC Cardiovasc Interv. 2020;13:1544–1553. doi: 10.1016/j.jcin.2020.03.056. [DOI] [PubMed] [Google Scholar]
- 11.Lee A., Mahadevan V.S., Gerstenfeld E.P. Iatrogenic atrial septal defect with right-to-left shunt following atrial fibrillation ablation in a patient with arrhythmogenic right ventricular cardiomyopathy. HeartRhythm Case Rep. 2018;4:159–162. doi: 10.1016/j.hrcr.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aznaouridis K., Hobson N., Rigg C., Bragadeesh T. Emergency percutaneous closure of an iatrogenic atrial septal defect causing right-to-left shunt and severe refractory hypoxemia after pulmonary vein isolation. JACC Cardiovasc Interv. 2015;8:e179–e181. doi: 10.1016/j.jcin.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 13.Chandraprakasam S., Satpathy R. When to close iatrogenic atrial septal defect after percutaneous edge to edge repair of mitral valve regurgitation. Cardiovasc Revasc Med. 2016;17:421–423. doi: 10.1016/j.carrev.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Teuteberg J.J., Cleveland J.C., Jr., Cowger J., et al. The Society of Thoracic Surgeons Intermacs 2019 annual report: the changing landscape of devices and indications. Ann Thorac Surg. 2020;109:649–660. doi: 10.1016/j.athoracsur.2019.12.005. [DOI] [PubMed] [Google Scholar]
- 15.Rich M.E., Tseng A., Lim H.W., Wang P.J., Su W.W. Reduction of iatrogenic atrial septal defects with an anterior and inferior transseptal puncture site when operating the cryoballoon ablation catheter. J Vis Exp. 2015:e52811. doi: 10.3791/52811. [DOI] [PMC free article] [PubMed] [Google Scholar]