Abstract
Objectives
Evaluate risk of major adverse cardiovascular events (MACE) with tofacitinib versus tumour necrosis factor inhibitors (TNFi) in patients with rheumatoid arthritis (RA) with or without a history of atherosclerotic cardiovascular disease (ASCVD) in ORAL Surveillance.
Methods
Patients with RA aged ≥50 years with ≥1 additional CV risk factor received tofacitinib 5 mg or 10 mg two times per day or TNFi. Hazard rations (HRs) were evaluated for the overall population and by history of ASCVD (exploratory analysis).
Results
Risk of MACE, myocardial infarction and sudden cardiac death were increased with tofacitinib versus TNFi in ORAL Surveillance. In patients with history of ASCVD (14.7%; 640/4362), MACE incidence was higher with tofacitinib 5 mg two times per day (8.3%; 17/204) and 10 mg two times per day (7.7%; 17/222) versus TNFi (4.2%; 9/214). HR (combined tofacitinib doses vs TNFi) was 1.98 (95% confidence interval (CI) 0.95 to 4.14; interaction p values: 0.196 (for HR)/0.059 (for incidence rate difference)). In patients without history of ASCVD, MACE HRs for tofacitinib 5 mg two times per day (2.4%; 30/1251) and 10 mg two times per day (2.8%; 34/1234) versus TNFi (2.3%; 28/1237) were, respectively, 1.03 (0.62 to 1.73) and 1.25 (0.76 to 2.07).
Conclusions
This post hoc analysis observed higher MACE risk with tofacitinib versus TNFi in patients with RA and history of ASCVD. Among patients without history of ASCVD, all with prevalent CV risk factors, MACE risk did not appear different with tofacitinib 5 mg two times per day versus TNFi. Due to the exploratory nature of this analysis and low statistical power, we cannot exclude differential MACE risk for tofacitinib 5 mg two times per day versus TNFi among patients without history of ASCVD, but any absolute risk excess is likely low.
Trial registration number
Keywords: Antirheumatic Agents; Arthritis, Rheumatoid; Cardiovascular Diseases; Therapeutics
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
ORAL Surveillance, which included patients with rheumatoid arthritis (RA) aged ≥50 years with ≥1 additional cardiovascular (CV) risk factor, was the first study to evaluate the safety of Janus kinase inhibitors in a CV risk-enriched RA population.
Primary findings indicated an increased risk of major adverse cardiovascular events (MACE) with tofacitinib versus tumour necrosis factor inhibitors (TNFi) (hazard ratio=1.33; 95% confidence interval (CI) 0.91 to 1.94). The non-inferiority criterion was not met (upper limit of 95% CI was >1.80).8
The increased risk of MACE with tofacitinib versus TNFi was more pronounced in patients aged ≥65 years than in patients aged <65 years.8
Risk of malignancies (excluding non-melanoma skin cancer) and infections was also higher with tofacitinib versus TNFi in ORAL Surveillance.8 26
WHAT THIS STUDY ADDS
This post hoc analysis of ORAL Surveillance shows an increased risk of MACE with tofacitinib 5 mg and 10 mg two times per day versus TNFi that was primarily observed in patients with a history of atherosclerotic cardiovascular disease (ASCVD; ie, history of coronary artery disease, cerebrovascular disease or peripheral artery disease) at baseline.
Risk of MACE did not appear different with tofacitinib 5 mg two times per day versus TNFi in patients without a history of ASCVD; but, given the exploratory nature of the analysis and the low event rate, we cannot rule out an increased risk of MACE in patients with several CV risk factors.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
This exploratory analysis of MACE in ORAL Surveillance underscores the value of including patients with a history of ASCVD for appropriate risk enhancement when investigating CV safety of RA treatments.
Our findings emphasise the importance of rheumatologists assessing overall CV risk, including medical history of ASCVD, when considering tofacitinib as a treatment for patients with RA.
Introduction
Compared with the general population, individuals with rheumatoid arthritis (RA) have a greater risk of cardiovascular (CV) disease.1 2 This is attributed to RA-associated systemic inflammation and traditional CV risk factors,2–7 and both require effective control to mitigate the risk. The European Alliance of Associations for Rheumatology (EULAR) recommends regular CV risk assessments in patients with RA using validated risk prediction models.2
ORAL Surveillance was a post-authorisation safety study conducted, in part, due to observations of increased serum lipid levels with the Janus kinase inhibitor, tofacitinib.8–10 The study was the first to evaluate the relative risk of adjudicated major adverse cardiovascular events (MACE) and malignancies with tofacitinib versus tumour necrosis factor inhibitors (TNFi) in patients with RA aged ≥50 years with ≥1 additional CV risk factor. For combined tofacitinib doses (5 mg and 10 mg two times per day) versus TNFi, non-inferiority was not shown for adjudicated MACE (incidence rate (IR) of 0.98 per 100 patient-years, 95% CI 0.79 to 1.19, versus IR of 0.73 per 100 patient-years, 95% CI 0.52 to 1.01; hazard ratio (HR)=1.33, 95% CI 0.91 to 1.94).8 For context, in the ENTRACTE study of patients with RA aged ≥50 years with ≥1 CV risk factor, rates of MACE per 100 patient-years were 1.70 with etanercept and 1.82 with tocilizumab.11
ORAL Surveillance included patients with RA and other risk factors that impact absolute risk of MACE,8 and this CV-risk enriched population likely reflected a spectrum of CV risk. Guidelines on CV disease prevention distinguish between patients with or without atherosclerotic CV disease (ASCVD).12 ASCVD includes a history of coronary artery disease (CAD), which was one of the eligibility criteria for the study, but also cerebrovascular disease (CeVD) and peripheral artery disease (PAD) (table 1).12 13 Patients with ASCVD are generally considered to have high to very high absolute risk of MACE.12 In recent CV outcome trials of patients with type 2 diabetes, MACE IRs in placebo-treated patients with ASCVD were 4.0–6.5 per 100 patient-years, compared with 1.3–3.3 per 100 patient-years, in patients without ASCVD but with multiple CV risk factors.14 Here, we further evaluate risk of MACE with tofacitinib versus TNFi in the ORAL Surveillance overall population, and in patients with or without a history of ASCVD.
Table 1.
ASCVD is defined based on events, diagnoses and procedures associated with atherosclerosis in arteries of the heart, head and neck and the periphery
| Group | Events | Diagnoses | Procedures |
| CAD | MI; unstable angina | CHD; stable angina pectoris | Coronary artery revascularisation; coronary artery bypass grafting |
| CeVD | Ischaemic stroke; transient ischaemic attack |
Carotid artery stenosis; carotid atherosclerosis | Carotid endarterectomy |
| PAD | Peripheral artery thrombosis | Aortic atherosclerosis; intermittent claudication | Peripheral artery angioplasty |
Events, diagnoses and procedures mentioned are examples, the list is not exhaustive. A complete list of terms used to define history of ASCVD in the present study is shown in online supplemental table 1.
ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; CeVD, cerebrovascular disease; CHD, coronary heart disease; MI, myocardial infarction; PAD, peripheral artery disease.
ard-2022-222259supp001.pdf (856.4KB, pdf)
Methods
Study design and patients
ORAL Surveillance (NCT02092467) was a phase IIIb/IV randomised, open-label, non-inferiority, safety endpoint study conducted from March 2014 to July 2020 in patients with active moderate-to-severe RA despite methotrexate treatment who were aged ≥50 years with ≥1 additional CV risk factor (current smoking, hypertension, high-density lipoprotein cholesterol (HDL-c) <40 mg/dL, diabetes mellitus, family history of premature coronary heart disease (CHD), RA-associated extra-articular disease and/or history of CAD).8
Patients were randomised 1:1:1 to receive oral tofacitinib 5 mg or 10 mg two times per day, or subcutaneous TNFi (adalimumab 40 mg every 2 weeks (North America) or etanercept 50 mg once weekly (rest of the world)). All patients continued their prestudy stable dose of methotrexate unless modification was clinically indicated. In February 2019, the tofacitinib 10 mg two-times-per-day dose was reduced to 5 mg two times per day after the Data Safety Monitoring Board noted an increased frequency of pulmonary embolism in patients receiving tofacitinib 10 mg two times per day versus TNFi and an increase in overall mortality with tofacitinib 10 mg versus 5 mg two times per day and TNFi.
Evaluation of history of ASCVD and baseline CV risk
A history of ASCVD was defined as the composite of history of CAD, CeVD and PAD. A history of CAD was an eligibility criterion in ORAL Surveillance (reported as ≥one of history of myocardial infarction (MI), unstable angina, stable angina pectoris, coronary artery procedures or other CHD).8 A history of CeVD (including ischaemic stroke and transient ischaemic attack) and PAD was identified in patients’ general medical history through Medical Dictionary for Regulatory Activities’ preferred terms (online supplemental table 1).
In patients without a history of ASCVD, 10-year risk of events associated with ASCVD (ie, MACE) was calculated by ASCVD-Pooled Cohort Equations (ASCVD-PCE).15 Scores werecalculated based on patients’ baseline age, sex, race (white/black/other), smoking status (yes/no), systolic blood pressure, antihypertensive treatment (yes/no), total cholesterol, HDL-c and diabetes (yes/no). In line with EULAR recommendations, a 1.5 multiplier was applied to all ASCVD-PCE scores.2 Based on the resulting scores, and as suggested by the American College of Cardiology/American Heart Association,16 patients without a history of ASCVD were assigned to the following 10-year risk categories: high (≥20%), intermediate (≥7.5–<20%), borderline (≥5–<7.5%) and low (<5%).
Outcomes
MACE and its components were based on adjudicated events assessed by an external, independent adjudication committee. MACE was defined as the composite of CV death (ie, death due to MI, stroke, sudden cardiac death, heart failure, CV procedures, CV haemorrhage and other CV causes, but not death due to pulmonary embolism), non-fatal MI and non-fatal stroke (including reversible focal neurological defects with imaging evidence of a new cerebral lesion consistent with ischaemia or haemorrhage).
Statistical analyses
Outcomes were analysed using the safety analysis set, which included all randomised patients receiving ≥1 dose of study drug. For patients randomised to tofacitinib 10 mg two times per day who had their dose reduced to 5 mg two times per day in February 2019, the data collected after the dose switch were counted in the tofacitinib 10 mg two-times-per-day group.
CV events were counted within the predefined risk period, based on 60-day on-treatment time, defined as time from first to last study dose +60 days or to last contact date (if a patient died, last contact date was death date), whichever was earliest. Patients without events were censored at the end of the risk period.
Crude IRs were expressed as the number of patients with first events per 100 patient-years, along with two-sided 95% CIs using the exact Poisson method.17 HRs and two-sided 95% CIs for pairwise comparisons among treatment groups (tofacitinib doses vs TNFi) were estimated using Cox proportional hazard regression models.
Subgroup analyses were conducted to assess for an association between history of ASCVD or baseline risk of MACE (ie, categories of CV risk in patients without history of ASCVD) with risk (HRs and IRs) of MACE, MI and stroke with tofacitinib versus TNFi. Across these exploratory analyses, no multiplicity adjustments were applied. Statistical analyses of treatment by history of ASCVD interactions are described in the online supplemental material.
The number needed to harm (NNH) was calculated as the reciprocal of the difference in IRs between tofacitinib and TNFi.18 Positive NNH was defined as patient-years of tofacitinib exposure needed for one more patient to report an additional event versus TNFi. Negative NNH was defined as the reverse. When the 95% CI of the IR difference includes 0, the 95% CI of the NNH has 2 disjoint (positive and negative) intervals, implying harm in either tofacitinib versus TNFi (positive) or TNFi versus tofacitinib (negative). NNH for patients exposed for 5 years was calculated by dividing the number of patient-years needed to harm by 5.
Patient and public involvement
Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Results
Patients
In total, 4362 patients were randomised and treated(tofacitinib 5 mg two times per day, n=1455; tofacitinib 10 mg two times per day, n=1456; TNFi, n=1451). Median follow-up was 4.0 years; 3111/4362 (71.3%) patients completed the trial and 2745/4362 (62.9%) completed trial treatment.8 Full patient demographics and baseline disease characteristics are described elsewhere.8 Table 2 summarises CV risk factors and the CV risk profile (online supplemental figure 1) of the study population versus patients with and without a history of ASCVD. These were well-balanced across treatment groups in ORAL Surveillance; 14.7% (640/4362) of patients had a history of ASCVD. Patients with a history of ASCVD were more likely to be ≥65 years, male, past smokers and have a history of diabetes mellitus, hypertension or hyperlipidaemia, compared with those with no history of ASCVD (table 2).
Table 2.
Demographic and baseline disease characteristics in the ORAL Surveillance overall population and in patients with and without a history of ASCVD
| Overall | History of ASCVD | No history of ASCVD | |||||||
| Tofacitinib 5 mg two times per day (N=1455) |
Tofacitinib 10 mg two times per day (N=1456) |
TNFi (N=1451) |
Tofacitinib 5 mg two times per day (N=204) |
Tofacitinib 10 mg two times per day (N=222) |
TNFi (N=214) |
Tofacitinib 5 mg two times per day (N=1251) |
Tofacitinib 10 mg two times per day (N=1234) |
TNFi (N=1237) |
|
| Age (years), mean (SD) | 60.8 (6.8) | 61.4 (7.1) | 61.3 (7.5) | 63.2 (7.1) | 64.7 (7.5) | 65.6 (7.8) | 60.4 (6.7) | 60.8 (6.8) | 60.6 (7.2) |
| Median (range) | 60 (50–86) | 61 (50–85) | 60 (50–88) | 62 (50–83) | 64 (50–82) | 66 (50–88) | 60 (50–86) | 60 (50–85) | 60 (50–87) |
| ≥65 years, n (%) | 413 (28.4) | 478 (32.8) | 462 (31.8) | 84 (41.2) | 109 (49.1) | 113 (52.8) | 329 (26.3) | 369 (29.9) | 349 (28.2) |
| Female sex, n (%) | 1169 (80.3) | 1124 (77.2) | 1117 (77.0) | 141 (69.1) | 143 (64.4) | 139 (65.0) | 1028 (82.2) | 981 (79.5) | 978 (79.1) |
| History of ASCVD, n (%) | 204 (14.0) | 222 (15.2) | 214 (14.7) | 204 (100) | 222 (100) | 214 (100) | – | – | – |
| History of CAD | 161 (11.1) | 172 (11.8) | 164 (11.3) | 161 (78.9) | 172 (77.5) | 164 (76.6) | – | – | – |
| History of CeVD | 41 (2.8) | 49 (3.4) | 31 (2.1) | 41 (20.1) | 49 (22.1) | 31 (14.5) | – | – | – |
| History of PAD | 15 (1.0) | 20 (1.4) | 35 (2.4) | 15 (7.4) | 20 (9.0) | 35 (16.4) | – | – | – |
| 10-year risk of MACE, n (%)* | |||||||||
| High (≥20%) | 258 (17.7) | 289 (19.8) | 278 (19.2) | – | – | – | 258 (20.6) | 289 (23.4) | 278 (22.5) |
| Intermediate (≥7.5–<20%) | 472 (32.4) | 490 (33.7) | 483 (33.3) | – | – | – | 472 (37.7) | 490 (39.7) | 483 (39.0) |
| Borderline (≥5–<7.5%) | 198 (13.6) | 169 (11.6) | 153 (10.5) | – | – | – | 198 (15.8) | 169 (13.7) | 153 (12.4) |
| Low (<5%) | 306 (21.0) | 268 (18.4) | 308 (21.2) | – | – | – | 306 (24.5) | 268 (21.7) | 308 (24.9) |
| Smoking status, n (%) | |||||||||
| Current smoker | 411 (28.2) | 402 (27.6) | 353 (24.3) | 54 (26.5) | 58 (26.1) | 56 (26.2) | 357 (28.5) | 344 (27.9) | 297 (24.0) |
| Past smoker | 309 (21.2) | 302 (20.7) | 326 (22.5) | 71 (34.8) | 77 (34.7) | 78 (36.4) | 238 (19.0) | 225 (18.2) | 248 (20.0) |
| Never smoked | 735 (50.5) | 752 (51.6) | 772 (53.2) | 79 (38.7) | 87 (39.2) | 80 (37.4) | 656 (52.4) | 665 (53.9) | 692 (55.9) |
| History of diabetes mellitus, n (%) | 243 (16.7) | 261 (17.9) | 255 (17.6) | 53 (26.0) | 51 (23.0) | 52 (24.3) | 190 (15.2) | 210 (17.0) | 203 (16.4) |
| History of hypertension, n (%) | 955 (65.6) | 954 (65.5) | 969 (66.8) | 156 (76.5) | 181 (81.5) | 168 (78.5) | 799 (63.9) | 773 (62.6) | 801 (64.8) |
| History of hyperlipidaemia, n (%) | 525 (36.1) | 518 (35.6) | 491 (33.8) | 120 (58.8) | 143 (64.4) | 117 (54.7) | 405 (32.4) | 375 (30.4) | 374 (30.2) |
| Family history of CHD, n (%) | |||||||||
| First-degree male relative <55 years | 154 (10.6) | 132 (9.1) | 151 (10.4) | 27 (13.2) | 29 (13.1) | 25 (11.7) | 127 (10.2) | 103 (8.3) | 126 (10.2) |
| First-degree female relative <65 years | 115 (7.9) | 107 (7.3) | 100 (6.9) | 19 (9.3) | 23 (10.4) | 19 (8.9) | 96 (7.7) | 84 (6.8) | 81 (6.5) |
| Baseline corticosteroids, n (%)† | 836 (57.5) | 829 (56.9) | 830 (57.2) | 106 (52.0) | 137 (61.7) | 116 (54.2) | 730 (58.4) | 692 (56.1) | 714 (57.7) |
| Baseline antiplatelets including aspirin, n (%)† | 226 (15.5) | 244 (16.8) | 237 (16.3) | 110 (53.9) | 124 (55.9) | 107 (50.0) | 116 (9.3) | 120 (9.7) | 130 (10.5) |
| Baseline statins, n (%)† | 349 (24.0) | 350 (24.0) | 321 (22.1) | 107 (52.5) | 127 (57.2) | 105 (49.1) | 242 (19.3) | 223 (18.1) | 216 (17.5) |
| Prior use of TNFi, n (%) | 115 (7.9) | 110 (7.6) | 105 (7.2) | 15 (7.4) | 15 (6.8) | 14 (6.5) | 100 (8.0) | 95 (7.7) | 91 (7.4) |
For patients randomised to the tofacitinib 10 mg two-times-per-day group who had their dose of tofacitinib reduced to 5 mg two times per day, the data collected after patients were switched to tofacitinib 5 mg two times per day were counted in the tofacitinib 10 mg two-times-per-day group.
*A 10-year risk of MACE was calculated with the ASCVD-PCE calculator and a 1.5 multiplier was applied for RA, as recommended by EULAR.2 15 In the tofacitinib 5 mg two-times-per-day, tofacitinib 10 mg two-times-per-day and TNFi groups, there were 17 patients (1.2%), 18 patients (1.2%) and 15 patients (1.0%) without a history of ASCVD who had missing ASCVD-PCE scores due to missing components.
†Based on day 1 of treatment with tofacitinib or TNFi in ORAL Surveillance.
ASCVD, atherosclerotic cardiovascular disease; ASCVD-PCE, atherosclerotic cardiovascular disease-Pooled Cohort Equations; CAD, coronary artery disease; CeVD, cerebrovascular disease; CHD, coronary heart disease; EULAR, European Alliance of Associations for Rheumatology; MACE, major adverse cardiovascular events; n, number of patients with characteristic; N, number of patients in the safety population; PAD, peripheral artery disease; RA, rheumatoid arthritis; SD, standard deviation; TNFi, tumour necrosis factor inhibitor.
Risk of adjudicated MACE outcomes with tofacitinib versus TNFi in ORAL Surveillance
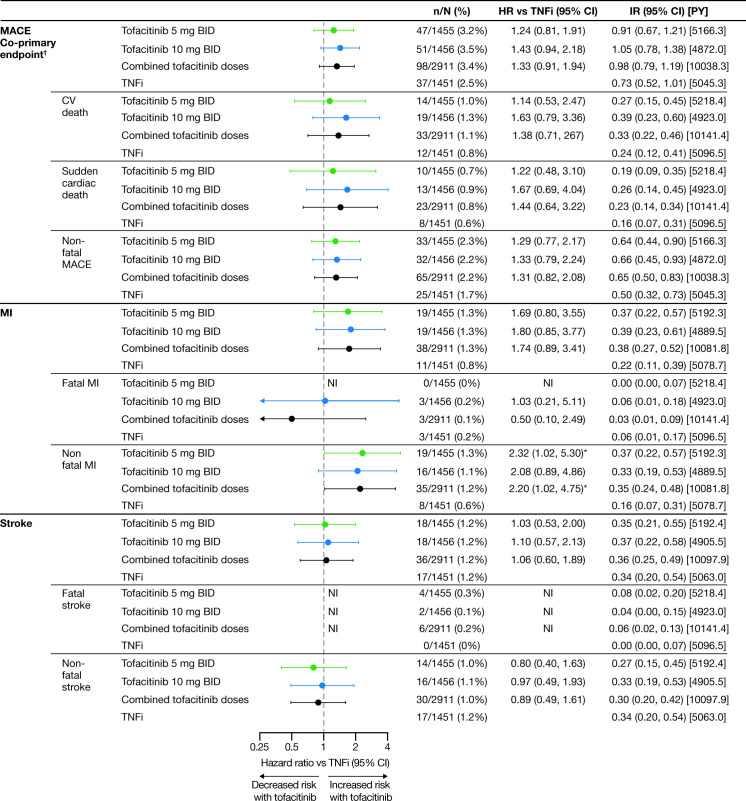
Risk of MACE, MI and sudden cardiac death were increased with both tofacitinib doses versus TNFi as reflected by HRs >1.0 and higher IRs (figure 1 and online supplemental figure 2). Risk of non-fatal MI with tofacitinib 5 mg two times per day versus TNFi was noticeably increased (HR=2.32; 95% CI 1.02 to 5.30; figure 1). Stroke HRs and IRs across treatment groups are shown in figure 1 and online supplemental figure 2.
Figure 1.
Adjudicated MACE outcomes with tofacitinib versus TNFi in ORAL Surveillance. HRs are shown on a logarithmic scale. Arrows indicate that the CI extends beyond the graph axis. For patients randomised to the tofacitinib 10 mg two-times-per-day group who had their dose of tofacitinib reduced to 5 mg two times per day, the data collected after patients were switched to tofacitinib 5 mg two times per day were counted in the tofacitinib 10 mg two-times-per-day group. HRs (95% CIs) are based on two simple Cox proportional hazard models (one for comparing combined tofacitinib doses vs TNFi, and the other for comparing tofacitinib 5 mg and 10 mg two times per day vs TNFi), with treatment as the only covariate. HRs and 95% CIs were NI when the total number of patients with events was ≤2 for the corresponding pair of treatments in the comparison or when one of the treatments in the comparison had 0 events. IRs express number of patients with first events per 100 PY. †Results reported in Ytterberg et al 8 and included for reference. *HR 95% CI excludes 1. BID, two times per day; CI, confidence interval; CV, cardiovascular; HR, hazard ratio; IR, incidence rate; MACE, major adverse cardiovascular events; MI, myocardial infarction; n, number of patients with events; N, number of evaluable patients; NI, non-informative; PY, patient-years; TNFi, tumour necrosis factor inhibitor.
Across treatment groups, the most frequent cause of CV death was sudden cardiac death (figure 1). One patient had fatal heart failure (tofacitinib 10 mg two times per day), and one died of other CV causes (TNFi).
HRs for MACE with tofacitinib versus TNFi in a total time analysis, including all events up to last contact date regardless of when study drug was discontinued, were consistent with the primary analysis (online supplemental figure 3). MACE IRs by 6-month intervals are shown in online supplemental figure 4.
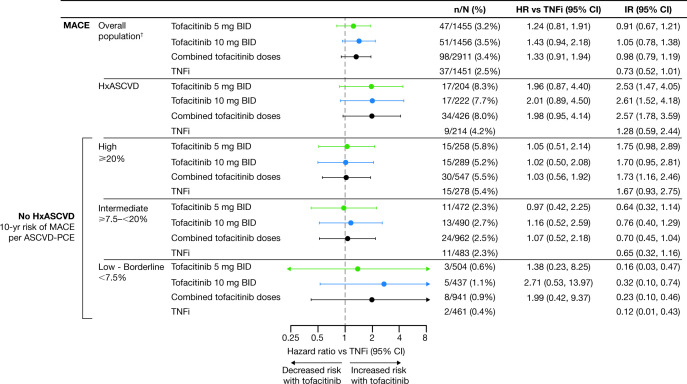
Risk of MACE with tofacitinib versus TNFi according to a history of ASCVD
Among patients with a history of ASCVD, MACE was reported in 17/204 (8.3%), 17/222 (7.7%) and 9/214 (4.2%) of patients in the tofacitinib 5 mg two-times-per-day, tofacitinib 10 mg two-times-per-day and TNFi treatment groups, respectively. MACE HRs (95% CI) were 1.96 (0.87 to 4.40) for tofacitinib 5 mg two times per day versus TNFi, 2.01 (0.89 to 4.50) fortofacitinib 10 mg two times per day versus TNFi and 1.98 (0.95 to 4.14) for combined tofacitinib doses versus TNFi (figure 2A and online supplemental figure 5). Based on the IR differences, this corresponds to NNH of 16 (95% CI −∞ to −91 and 7 to ∞) and 15 (95% CI −∞ to −117 and 7 to ∞) patients who would need to be treated with tofacitinib 5 mg and 10 mg two times per day, respectively, versus TNFi, over 5 years to have 1 additional MACE (figure 2A; online supplemental table 2).
Figure 2.
Risk of MACE with tofacitinib versus TNFi by history of ASCVD. (A) HRs (95% CIs) are based on two simple Cox proportional hazard models (one for comparing combined tofacitinib doses versus TNFi, and the other for comparing tofacitinib 5 mg and 10 mg two times per day vs TNFi), with treatment as the only covariate. IRs express number of patients with first events per 100 PY. NNH (PY) should be interpreted as the number of PY of exposure to tofacitinib required to have one additional MACE versus TNFi. NNH (5-year) should be interpreted as the number of patients who would need to be treated for that duration with tofacitinib rather than with a TNFi to result in one additional MACE. *IRD 95% CI excluded 0. †Results reported in Ytterberg et al 8 and included for reference. ‡NNH 95% CIs are reported in online supplemental table 2. (B) Treatment-by-HxASCVD interaction p values for HRs (χ2 test with 1 degree of freedom) and IRD (2-sided, normal approximation of difference in IR). See supplementary material for details. (C) Cumulative probability of patients with adjudicated MACE events, calculated based on the Kaplan-Meier estimate, in patients with history of ASCVD (left panel) and without history of ASCVD (right panel). HRs are shown on a logarithmic scale. Arrows indicate that the CI extends beyond the graph axis. For patients randomised to the tofacitinib 10 mg two-times-per-day group who had their dose of tofacitinib reduced to 5 mg two times per day, the data collected after patients were switched to tofacitinib 5 mg two times per day were counted in the tofacitinib 10 mg two-times-per-day group. BID, two times per day; CI, confidence interval; CV RF, cardiovascular risk factor; HR, hazard ratio; HxASCVD, history of atherosclerotic cardiovascular disease; IR, incidence rate; IRD, incidence rate difference; MACE, major adverse cardiovascular events; n, number of patients with events; N, number of evaluable patients; NNH, number needed to harm; PY, patient-years; TNFi, tumour necrosis factor inhibitor.
In patients without a history of ASCVD but with CV risk factors, MACE was reported in 30/1251 (2.4%), 34/1234 (2.8%) and 28/1237 (2.3%), in the tofacitinib 5 mg two-times-per-day, tofacitinib 10 mg two-times-per-day and TNFi treatment groups, respectively (figure 2A). HRs (95% CI) for MACE were 1.03 (0.62 to 1.73) for tofacitinib 5 mg two times per day versus TNFi, 1.25 (0.76 to 2.07) for tofacitinib 10 mg two times per day versus TNFi and 1.14 (0.73 to 1.78) for combined tofacitinib doses versus TNFi (figure 2A and online supplemental figure 5). Based on the IR differences, this corresponds to NNH of 869 (95% CI −∞ to −64 and 55 to ∞) and 124 (95% CI −∞ to −100 and 38 to ∞) patients who would need to be treated with tofacitinib5 mg and 10 mg two times per day, respectively, versus TNFi, over 5 years to have 1 additional MACE (figure 2A; online supplemental table 2). P values for the treatment by history of ASCVD interaction (combined tofacitinib doses vs TNFi) for MACE were 0.196 for the HRs and 0.059 for the IR difference (figure 2B).
Kaplan-Meier curves for MACE (figure 2C) indicated separation between the tofacitinib and TNFi groups by month 3 in patients with history of ASCVD, and no separation between treatment groups in patients without history of ASCVD.
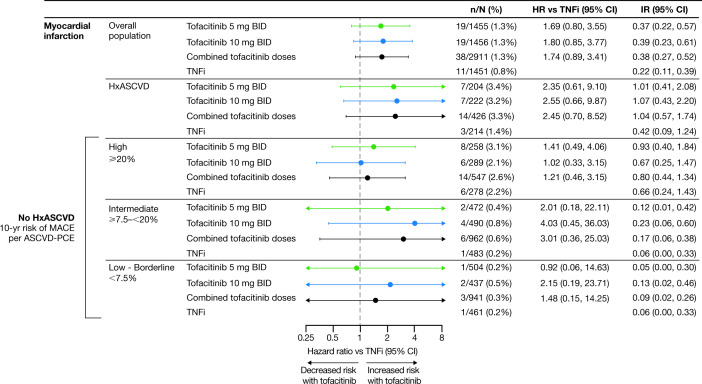
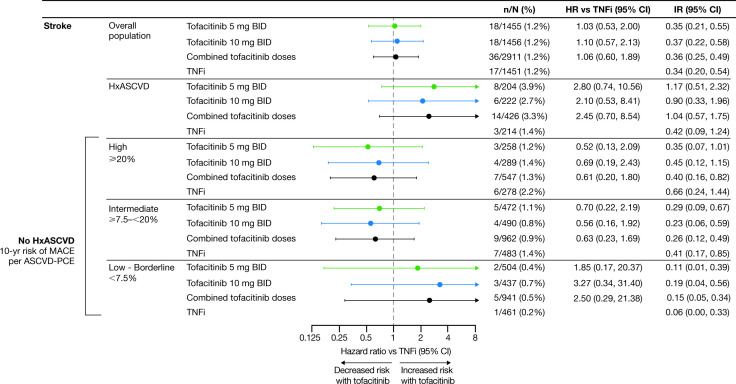
Risk of MI and stroke with tofacitinib versus TNFi according to history of ASCVD
In patients with a history of ASCVD, treatment with tofacitinib5 mg or 10 mg two times per day was associated with increased risk of MI and stroke versus TNFi (figure 3 and online supplemental figure 5). Risk of MI was also increased with tofacitinib versus TNFi in patients without a history of ASCVD (figure 3 and online supplemental figure 5). In the assessment of MI and stroke according to history of ASCVD, the number of events overall was low, and these results should be interpreted with caution.
Figure 3.
Risk of MI and stroke with tofacitinib versus TNFi by history of ASCVD. (A) HRs are shown on a logarithmic scale. Arrows indicate that the CI extends beyond the graph axis. For patients randomised to the tofacitinib 10 mg two-times-per-day group who had their dose of tofacitinib reduced to 5 mg two times per day, the data collected after patients were switched to tofacitinib 5 mg two times per day were counted in the tofacitinib 10 mg two-times-per-day group. HRs (95% CIs) are based on two simple Cox proportional hazard models (one for comparing combined tofacitinib doses vs TNFi, and the other for comparing tofacitinib 5 mg and 10 mg two times per day vs TNFi), with treatment as the only covariate. IRs express the number of patients with first events per 100 PY. (B) Treatment-by-HxASCVD interaction p values for HRs (χ2 test with 1 degree of freedom) and IRD (two-sided, normal approximation of difference in IR). See supplementary material for details. BID, two times per day; CI, confidence interval; CV RF, cardiovascular risk factor; HxASCVD, history of atherosclerotic cardiovascular disease; IR, incidence rate; IRD, incidence rate difference; MI, myocardial infarction; n, number of patients with events; N, number of evaluable patients; PY, patient-years; TNFi, tumour necrosis factor inhibitor.
Association between baseline CV risk scores and risk of MACE, MI and stroke with tofacitinib versus TNFi in patients without a history of ASCVD
Patients without a history of ASCVD were grouped by their 10-year risk of MACE.2 MACE IRs, regardless of treatment group, were highest in patients at high risk (ie, ≥20% 10-year risk of MACE) (figure 4 and online supplemental figure 6). There was no difference in risk of MACE with tofacitinib 5 mg or 10 mg two times per day versus TNFi in patients at high or intermediate risk. While HRs for tofacitinib versus TNFi were >1.0 in patients with low or borderline risk, the number of events was low.
Figure 4.
Risk of MACE with tofacitinib versus TNFi in patients without a history of ASCVD, according to CV risk categories. HRs are shown on a logarithmic scale. Arrows indicate that the CI extends beyond the graph axis. Patients without HxASCVD were categorised according to their 10-year risk of MACE, per the ASCVD-PCE risk calculator. In line with EULAR recommendations, a 1.5 multiplier was applied to all ASCVD-PCE scores.2 Because of missing ASCVD-PCE score, two MACE could not be associated with baseline CV risk (n=1 (MI) in the tofacitinib 5 mg two-times-per-day group and n=1 (stroke) in the tofacitinib 10 mg two-times-per-day group). For patients randomised to the tofacitinib 10 mg two-times-per-day group who had their dose of tofacitinib reduced to 5 mg two times per day, the data collected after patients were switched to tofacitinib 5 mg two times per day were counted in the tofacitinib 10 mg two-times-per-day group. HRs (95% CIs) are based on two simple Cox proportional hazard models (one for comparing combined tofacitinib doses vs TNFi, and the other for comparing tofacitinib 5 mg and 10 mg two times per day vs TNFi), with treatment as the only covariate. IRs express the number of patients with first events per 100 PY. †Results reported in Ytterberg et al 8 and included for reference. ASCVD-PCE, atherosclerotic cardiovascular disease-Pooled Cohort Equations; BID, two times per day; CI, confidence interval; CV, cardiovascular; EULAR, European Alliance of Associations for Rheumatology; HxASCVD, history of atherosclerotic cardiovascular disease; IR, incidence rate; MACE, major adverse cardiovascular events; n, number of patients with events; N, number of evaluable patients; PY, patient-years; TNFi, tumour necrosis factor inhibitor.
MI IRs were highest in patients with a high CV risk score (figure 5 and online supplemental figure 7). There was an increased risk of MI with tofacitinib 5 mg two times per day versus TNFi in patients with high 10-year risk of MACE. There were fewer MIs reported in the other risk categories.
Figure 5.
Risk of MI with tofacitinib versus TNFi in patients without history of ASCVD, according to CV risk categories. HRs are shown on a logarithmic scale. Arrows indicate that the CI extends beyond the graph axis. Patients without HxASCVD were categorised according to their 10-year risk of MACE, per the ASCVD-PCE risk calculator. In line with EULAR recommendations, a 1.5 multiplier was applied to all ASCVD-PCE scores.2 Because of missing ASCVD-PCE score, one MI in the tofacitinib 5 mg two-times-per-day group could not be associated with baseline CV risk. For patients randomised to the tofacitinib 10 mg two-times-per-day group who had their dose of tofacitinib reduced to 5 mg two times per day, the data collected after patients were switched to tofacitinib 5 mg two times per day were counted in the tofacitinib 10 mg two-times-per-day group. HRs (95% CIs) are based on two simple Cox proportional hazard models (one for comparing combined tofacitinib doses vs TNFi, and the other for comparing tofacitinib 5 and 10 mg two times per day vs TNFi), with treatment as the only covariate. IRs express the number of patients with first events per 100 PY. ASCVD-PCE, atherosclerotic cardiovascular disease-Pooled Cohort Equations; BID, two times per day; CI, confidence interval; CV, cardiovascular; EULAR, European Alliance of Associations for Rheumatology; HR, hazard ratio; HxASCVD, history of atherosclerotic cardiovascular disease; IR, incidence rate; MACE, major adverse cardiovascular events; MI, myocardial infarction; n, number of patients with events; N, number of evaluable patients; PY, patient-years; TNFi, tumour necrosis factor inhibitor.
The association between baseline CV risk and stroke IRs was less apparent than observed for MACE and MI (figure 6 and online supplemental file 8). Overall, event numbers in each risk category were low.
Figure 6.
Risk of stroke with tofacitinib versus TNFi in patients without a history of ASCVD, according to CV risk categories. HRs are shown on a logarithmic scale. Arrows indicate that the CI extends beyond the graph axis. Patients without history of ASCVD were categorised according to their 10-year risk of MACE, per the ASCVD-PCE risk calculator. In line with EULAR recommendations, a 1.5 multiplier was applied to all ASCVD-PCE scores.2 Because of missing ASCVD-PCE score, one stroke in the tofacitinib 10 mg two-times-per-day group could not be associated with baseline CV risk. For patients randomised to the tofacitinib 10 mg two-times-per-day group who had their dose of tofacitinib reduced to 5 mg two times per day, the data collected after patients were switched to tofacitinib 5 mg two times per day were counted in the tofacitinib 10 mg two-times-per-day group. HRs (95% CIs) are based on two simple Cox proportional hazard models (one for comparing combined tofacitinib doses versus TNFi, and the other for comparing tofacitinib 5 mg and 10 mg two times per day vs TNFi), with treatment as the only covariate. IRs express the number of patients with first events per 100 PY. ASCVD-PCE, atherosclerotic cardiovascular disease-Pooled Cohort Equations; BID, two times per day; CV, cardiovascular; EULAR, European Alliance of Associations for Rheumatology; HxASCVD, history of atherosclerotic cardiovascular disease; IR, incidence rate; MACE, major adverse cardiovascular events; n, number of patients with events; N, number of evaluable patients; PY, patient-years; TNFi, tumour necrosis factor inhibitor.
Discussion
Primary analyses of ORAL Surveillance, which included patients aged ≥50 years with ≥1 additional CV risk factor and was the first study to evaluate tofacitinib safety in a CV risk-enriched RA population, found an increased risk of MACE with tofacitinib versus TNFi.8 In this post hoc analysis, increased risk of MACE was primarily identified in patients with a history of ASCVD (ie, pre-existing CAD, CeVD or PAD). In patients without a history of ASCVD but with CV risk factors, there did not appear to be a detectable difference in risk of MACE with tofacitinib 5 mg two times per day or the combined tofacitinib doses versus TNFi.
ORAL Surveillance was powered to assess non-inferiority for risk of MACE with combined tofacitinib doses versus TNFi and not powered to compare individual MACE components across treatment groups.8 The exploratory analyses on CV outcomes and subgroup analyses we provide, therefore, need to be interpreted cautiously and as hypothesis-generating. Notwithstanding, our analysis of the overall study population supplements the primary analysis of the study and shows increased risk of MI and sudden cardiac death with tofacitinib versus TNFi in this CV risk-enriched population.
Almost 15% of the patients in ORAL Surveillance had a history of ASCVD. In this subgroup, we found increased risk of MACE, MI and stroke with tofacitinib versus TNFi. In the remaining 85% of patients without a history of ASCVD, who nevertheless had CV risk factors, we did not find increased relative risk of MACE with tofacitinib versus TNFi. This observation is supported by our assessment of relative risk across categories of predicted MACE risk; there was no clear difference in risk of MACE in patients without a history of ASCVD who had high (≥20%) or intermediate (≥7.5–<20%) predicted 10-year risk at baseline. Approximately one-third of the ORAL Surveillance population had low or borderline absolute risk of MACE, and the low number of MACE in this group makes assessment of relative risk less certain.
A large observational study that used USA claims data to assess risk of CV outcomes (composite of hospitalisation for MI or stroke) with tofacitinib versus TNFi in patients with RA (Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis; STAR-RA) was recently published.19 Evidence for an increased risk of CV outcomes with tofacitinib was not identified in this real-world evidence cohort. However, STAR-RA included a cohort that mirrored ORAL Surveillance inclusion and exclusion criteria (randomised controlled trial (RCT)-duplicate cohort). The primary outcome of the RCT-duplicate cohort aligned with the increased risk of MACE with tofacitinib versus TNFi observed in ORAL Surveillance (ie, approximately 25% relative risk increase with tofacitinib 5 mg two times per day vs TNFi).8 19 STAR-RA also prespecified subgroup analyses of patients with or without previous CV disease. These results were also similar to ORAL Surveillance; risk of CV outcomes appeared to be increased with tofacitinib versus TNFi in patients with, but not in those without, pre-existing CV disease.19
Our analysis of MACE in ORAL Surveillance underscores the importance of investigating the long-term safety of RA treatments in appropriately designed, prospective, randomised and comparative trials of sufficient size and duration to adequately evaluate safety events of interest, including CV adverse events. Consequently, for ORAL Surveillance to be declared complete, ≥1500 patients had to be followed for 3 years and the study was conducted in a CV risk-enriched population to ensure accumulation of a sufficient number of CV events. To the best of our knowledge, the only similar studies in RA are the ENTRACTE and PRECISION trials that assessed CV safety of tocilizumab versus etanercept and celecoxib versus naproxen versus ibuprofen, respectively.11 20 In ENTRACTE and PRECISION, 11% (347/3080) and 24% (584/2436) of patients with RA had previous CV disease diagnoses, events and procedures consistent with ASCVD.11 21 In contrast, the wider tofacitinib RA clinical trial programme included 1.3% (100/7964) of patients with a history of MI and 0.4% (30/7964) with a history of CHD, and 39% (3126/7964) of tofacitinib-treated patients met the CV risk-enrichment criteria of ORAL Surveillance.22 Similarly, a recent report on the baricitinib RA clinical trial programme found that 35% (1325/3770) of patients met ORAL Surveillance inclusion criteria, and 2.3% had a history of ASCVD.23 The non-CV risk-enriched wider tofacitinib clinical trial programme did not identify the increased risk of MACE with tofacitinib versus TNFi that was observed in ORAL Surveillance. Based on our data, future trials with objectives overlapping with ORAL Surveillance should include sufficient patients with high absolute CV risk and history of ASCVD, and even prespecify the analysis we present herein.
Overall, limitations of ORAL Surveillance have been published previously.8 The exploratory nature and the lack of statistical evidence (ie, nominally significant p values) of a treatment by history of ASCVD interaction limits our conclusions on this subgroup analysis. This analysis points to a need for more data on risk of MACE with tofacitinib versus other advanced RA treatments in patients with increased CV risk but no history of ASCVD. The subgroup distribution was also uneven (14.7% with vs 85.3% without history of ASCVD). In the history of ASCVD group, across treatment arms, there were relatively few patients (N=204–222) and patients with MACE events (n=9–17). Accordingly, IRs and HRs should be regarded as statistically uncertain, as reflected in the wider 95% CIs, and be interpreted with caution. In addition, substantial literature supports the atheroprotective effects of TNFi, effects that likely extend to other immunomodulators via their ability to modulate synovial and systemic inflammation.3 6 24 25 These treatment-associated effects cannot be assessed in ORAL Surveillance given the lack of an untreated control group, but the results we present should be interpreted in this context.
Conclusion
Our post hoc analysis of ORAL Surveillance showed that increased risk of MACE with tofacitinib 5 mg and 10 mg two times per day versus TNFi was found in patients with a history of ASCVD. Among patients without a history of ASCVD, who all had prevalent CV risk factors, risk of MACE did not appear to be different comparing tofacitinib 5 mg two times per day and TNFi. Due to the exploratory nature of this analysis and low statistical power, we cannot exclude any differential MACE risk for tofacitinib 5 mg two times per day versus TNFi among patients without HxASCVD, but any absolute risk excess is likely low.
ard-2022-222259supp002.pdf (294.9KB, pdf)
ard-2022-222259supp003.pdf (293.9KB, pdf)
ard-2022-222259supp004.pdf (294.9KB, pdf)
ard-2022-222259supp005.pdf (292.9KB, pdf)
ard-2022-222259supp006.pdf (294.2KB, pdf)
ard-2022-222259supp007.pdf (297.2KB, pdf)
ard-2022-222259supp008.pdf (293KB, pdf)
ard-2022-222259supp009.pdf (292.9KB, pdf)
ard-2022-222259supp010.pdf (292.9KB, pdf)
ard-2022-222259supp011.pdf (296.3KB, pdf)
ard-2022-222259supp012.pdf (293KB, pdf)
ard-2022-222259supp013.pdf (293.1KB, pdf)
ard-2022-222259supp014.pdf (293KB, pdf)
ard-2022-222259supp015.pdf (293.3KB, pdf)
ard-2022-222259supp016.pdf (293.1KB, pdf)
Acknowledgments
Select data in this manuscript were previously presented at ACR Convergence 2021.27 The authors would like to thank the patients, investigators and study teams involved in the study. The authors would like to thank Hyejin Jo and Annette Szumski from Syneos Health for their contribution to the statistical analyses. This study was sponsored by Pfizer Inc. Medical writing support, under the guidance of the authors, was provided by Kirsten Woollcott, MSc, CMC Connect, a division of IPG Health Medical Communications, and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015;163:461–4).
Footnotes
Handling editor: Josef S Smolen
Contributors: DLB, SRY, JW, CW, KK, AY and CAC conceived or designed the study and data analyses. KK acquired the data. CC-S, DLB, JTG, SRY, JW, CW, KK and AY analysed the data. All authors had access to the data, were involved in interpretation of data and reviewed and approved the manuscript’s content before submission. CC-S accepts final responsibility for this work and controlled the decision to publish.
Funding: This study was sponsored by Pfizer Inc.
Competing interests: CCS has acted as a consultant for AbbVie, Gilead Sciences, Pfizer Inc and Regeneron-Sanofi, and has received grant/research support from AbbVie, Bristol-Myers Squibb and Pfizer Inc. MHB has acted as a consultant for AbbVie, Eli Lilly, Gilead Sciences, MSD, Pfizer Inc and Roche, has received grant/research support from Pfizer Inc, Roche and UCB, and is a member of the speakers’ bureau for AbbVie (paid to host institution). MHB is supported by the National Institute for Health and Care Research (NIHR) Manchester Biomedical Research Centre and is in receipt of an NIHR Senior Investigator award. The views expressed in this article are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. MD has acted as a consultant for AbbVie, Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer Inc, Roche and UCB, and has received grant/research support from AbbVie, Bristol-Myers Squibb, Eli Lilly, Merck, Novartis, Pfizer Inc, Roche and UCB. DLB served as a member of the Steering Committee for ORAL Surveillance, with funding from Pfizer Inc paid to Brigham and Women’s Hospital. He is a member of the advisory board for AngioWave, Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; serves on the Board of Directors for AngioWave (stock options), Boston VA Research Institute, Bristol Myers Squibb (stock), DRS.LINQ (stock options), High Enroll (stock), the Society of Cardiovascular Patient Care, and TobeSoft; is the Inaugural Chair for the American Heart Association Quality Oversight Committee; is on the Data Monitoring Committees for Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute), for the PORTICO trial, (funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, the Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), for the ABILITY-DM trial (funded by Concept Medical), Novartis, the Population Health Research Institute, and Rutgers University (for the NIH-funded MINT trial); has received honoraria from the American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; the RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; the AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), the Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), the Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), the Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), and Wiley (steering committee); holds positions at Clinical Cardiology (Deputy Editor), the NCDR-ACTION Registry Steering Committee (Chair), and VA CART Research and Publications Committee (Chair); is named on a patent for sotagliflozin assigned to Brigham and Women's Hospital who assigned to Lexicon (neither DLB nor Brigham and Women's Hospital receive any income from this patent); has received research funding from Abbott, Acesion Pharma, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer Inc, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company and 89bio; has received royalties from Elsevier (Editor, Braunwald’s Heart Disease); is a site co-investigator for Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Phillips, SpectraWAVE, Svelte, and Vascular Solutions; is a trustee for the American College of Cardiology; and conducts unfunded research with FlowCo and Takeda.
JTG has acted as a consultant for AbbVie, Bristol-Myers Squibb, Eli Lilly, Genentech, Gilead Sciences and UCB, and has received grant/research support from Pfizer Inc. SRY has acted as a consultant for Corbus and Pfizer Inc, and has received remuneration from Pfizer Inc for their services as a member of the Steering Committee for ORAL Surveillance. GGK is a shareholder of IQVIA, has received grant/research support from AbbVie, Acceleron, Amgen, Arena, AstraZeneca, Cytokinetics, Eli Lilly, Gilead Sciences, GlaxoSmithKline, Huya Bioscience International, Johnson & Johnson, Landos Biopharma, Merck, Momentum, Novartis, Otsuka, Pfizer Inc, Sanofi and vTv Therapeutics, is an employee of the University of North Carolina at Chapel Hill. He served as a member of the Steering Committee for ORAL Surveillance, with funding from Pfizer Inc paid to the University of North Carolina at Chapel Hill. IV, JW, CW, KK, SM, JLR, and AY are employees and stockholders of Pfizer Inc. CAC was an employee and stockholder of Pfizer Inc at the time of this analysis. ZS has acted as an advisor for AbbVie, Eli Lilly, Gedeon Richter, Novartis, Pfizer Inc and Roche, a consultant for AbbVie, Eli Lilly, Novartis, Pfizer Inc, Roche and Sanofi, has received grant/research support from Pfizer Inc and UCB, and is a member of the speakers’ bureau for AbbVie, Eli Lilly, MSD, Novartis, Pfizer Inc, Roche, Sanofi and UCB.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
ORAL Surveillance was conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines of the International Council on Harmonisation, and local country regulations, and was approved by the institutional review board and/or independent ethics committee at each centre. Participants gave informed consent to participate in the study before taking part.
References
- 1. Aviña-Zubieta JA, Thomas J, Sadatsafavi M, et al. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis 2012;71:1524–9. 10.1136/annrheumdis-2011-200726 [DOI] [PubMed] [Google Scholar]
- 2. Agca R, Heslinga SC, Rollefstad S, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis 2017;76:17–28. 10.1136/annrheumdis-2016-209775 [DOI] [PubMed] [Google Scholar]
- 3. Choy E, Ganeshalingam K, Semb AG, et al. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology 2014;53:2143–54. 10.1093/rheumatology/keu224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kitas GD, Gabriel SE. Cardiovascular disease in rheumatoid arthritis: state of the art and future perspectives. Ann Rheum Dis 2011;70:8–14. 10.1136/ard.2010.142133 [DOI] [PubMed] [Google Scholar]
- 5. Zhang J, Chen L, Delzell E, et al. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis 2014;73:1301–8. 10.1136/annrheumdis-2013-204715 [DOI] [PubMed] [Google Scholar]
- 6. Atzeni F, Rodríguez-Carrio J, Popa CD, et al. Cardiovascular effects of approved drugs for rheumatoid arthritis. Nat Rev Rheumatol 2021;17:270–90. 10.1038/s41584-021-00593-3 [DOI] [PubMed] [Google Scholar]
- 7. Maksimowicz-McKinnon K, Bhatt DL, Calabrese LH. Recent advances in vascular inflammation: C-reactive protein and other inflammatory biomarkers. Curr Opin Rheumatol 2004;16:18–24. 10.1097/00002281-200401000-00005 [DOI] [PubMed] [Google Scholar]
- 8. Ytterberg SR, Bhatt DL, Mikuls TR, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med 2022;386:316–26. 10.1056/NEJMoa2109927 [DOI] [PubMed] [Google Scholar]
- 9. Charles-Schoeman C, Fleischmann R, Davignon J, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol 2015;67:616–25. 10.1002/art.38974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Charles-Schoeman C, DeMasi R, Valdez H, et al. Risk factors for major adverse cardiovascular events in phase III and long-term extension studies of tofacitinib in patients with rheumatoid arthritis. Arthritis Rheumatol 2019;71:1450–9. 10.1002/art.40911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giles JT, Sattar N, Gabriel S, et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol 2020;72:31–40. 10.1002/art.41095 [DOI] [PubMed] [Google Scholar]
- 12. Visseren FLJ, Mach F, Smulders YM, et al. 2021 ESC guidelines onn cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 13. Grundy SM, Stone NJ, Bailey AL. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2018;2019:3168. 10.1016/j.jacc.2018.11.002 [DOI] [Google Scholar]
- 14. McGuire DK, Shih WJ, Cosentino F, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol 2021;6:148–58. 10.1001/jamacardio.2020.4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation 2014;129:S49–73. 10.1161/01.cir.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 16. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019;140:e596–646. 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daly L. Simple SAS macros for the calculation of exact binomial and Poisson confidence limits. Comput Biol Med 1992;22:351–61. 10.1016/0010-4825(92)90023-G [DOI] [PubMed] [Google Scholar]
- 18. Guo JJ, Pandey S, Doyle J, et al. A review of quantitative risk-benefit methodologies for assessing drug safety and efficacy-report of the ISPOR risk-benefit management working group. Value Health 2010;13:657–66. 10.1111/j.1524-4733.2010.00725.x [DOI] [PubMed] [Google Scholar]
- 19. Khosrow-Khavar F, Kim SC, Lee H, et al. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann Rheum Dis 2022;81:798–804. 10.1136/annrheumdis-2021-221915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med 2016;375:2519–29. 10.1056/NEJMoa1611593 [DOI] [PubMed] [Google Scholar]
- 21. Solomon DH, Husni ME, Wolski KE, et al. Differences in safety of nonsteroidal antiinflammatory drugs in patients with osteoarthritis and patients with rheumatoid arthritis: a randomized clinical trial. Arthritis Rheumatol 2018;70:537–46. 10.1002/art.40400 [DOI] [PubMed] [Google Scholar]
- 22. Mease P, Charles-Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis 2020;79:1400–13. 10.1136/annrheumdis-2019-216761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Taylor PC, Takeuchi T, Burmester GR, et al. Safety of baricitinib for the treatment of rheumatoid arthritis over a median of 4.6 and up to 9.3 years of treatment: final results from long-term extension study and integrated database. Ann Rheum Dis 2022;81:335–43. 10.1136/annrheumdis-2021-221276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sattin M, Towheed T. The effect of TNFα-inhibitors on cardiovascular events in patients with rheumatoid arthritis: an updated systematic review of the literature. Curr Rheumatol Rev 2016;12:208–22. 10.2174/1573397112666160404124655 [DOI] [PubMed] [Google Scholar]
- 25. Singh S, Fumery M, Singh AG, et al. Comparative risk of cardiovascular events with biologic and synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res 2020;72:561–76. 10.1002/acr.23875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Balanescu A-R, Citera G, Pascual-Ramos V, et al. Infections in patients with rheumatoid arthritis receiving tofacitinib versus tumour necrosis factor inhibitors: results from the open-label, randomised controlled ORAL Surveillance trial. Ann Rheum Dis 2022. 10.1136/ard-2022-222405. [Epub ahead of print: 03 Aug 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charles-Schoeman C, Buch M, Dougados M. Risk factors for major adverse cardiovascular events in patients aged ≥ 50 years with RA and ≥ 1 additional cardiovascular risk factor: results from a Phase 3b/4 randomized safety study of tofacitinib vs TNF inhibitors [abstract]. Arthritis Rheumatol 2021;73:0958. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2022-222259supp001.pdf (856.4KB, pdf)
ard-2022-222259supp002.pdf (294.9KB, pdf)
ard-2022-222259supp003.pdf (293.9KB, pdf)
ard-2022-222259supp004.pdf (294.9KB, pdf)
ard-2022-222259supp005.pdf (292.9KB, pdf)
ard-2022-222259supp006.pdf (294.2KB, pdf)
ard-2022-222259supp007.pdf (297.2KB, pdf)
ard-2022-222259supp008.pdf (293KB, pdf)
ard-2022-222259supp009.pdf (292.9KB, pdf)
ard-2022-222259supp010.pdf (292.9KB, pdf)
ard-2022-222259supp011.pdf (296.3KB, pdf)
ard-2022-222259supp012.pdf (293KB, pdf)
ard-2022-222259supp013.pdf (293.1KB, pdf)
ard-2022-222259supp014.pdf (293KB, pdf)
ard-2022-222259supp015.pdf (293.3KB, pdf)
ard-2022-222259supp016.pdf (293.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.