Abstract
Antibody responses to p66, a candidate integrin ligand of Borrelia burgdorferi, were studied in 79 patients with early or late manifestations of Lyme disease. The central portion of p66 was previously shown to contain all of the information required for specific recognition of β3-chain integrins, but work by others had suggested that the C-terminal portion of the protein contains a single surface-exposed, immunodominant loop. In examining antibody responses to full-length p66 and to three overlapping fragments of the protein, we found that the majority of Lyme disease patients had immunoglobulin M (IgM) and/or IgG responses to p66 and that, particularly early in the disease, epitopes throughout p66 were recognized. Among patients with later manifestations of the illness, antibody responses to the C-terminal portion of the protein were more prominent. These results demonstrate that Lyme disease patient sera recognize epitopes throughout p66.
Borrelia burgdorferi sensu lato, which includes B. burgdorferi sensu stricto, B. garinii, and B. afzelii, is the spirochetal agent of Lyme disease. B. burgdorferi is transmitted by the bite of certain Ixodes ticks, and infection results in a wide range of clinical manifestations that may affect the skin, joints, heart, and nervous system (22). Adhesion to host cell and tissue components is likely to participate in establishment of B. burgdorferi infection and in the apparent tropism of the spirochete for particular tissues. In in vitro experiments, B. burgdorferi has been shown to bind to glycosphingolipids (1), fibronectin (12, 15, 18), decorin (13), glycosaminoglycans (14, 16), and at least three integrins (7, 8).
A candidate ligand for β3-chain integrins was recently identified (9). This protein, termed p66, was cloned by two other groups (3, 19) on the basis of apparent surface localization and the previous observation (10) that a band of 66 kDa is commonly recognized by sera from Lyme disease patients in immunoblots of B. burgdorferi extracts. The central portion of p66, termed p66M, contains all the information required for integrin recognition, and this portion of the protein was contained in a filamentous phage clone that was selected from a B. burgdorferi library on the basis of integrin binding. Access to surface-exposed epitopes of p66 appears to be limited by the presence of Osp lipoproteins that are expressed by B. burgdorferi grown in laboratory culture (2). At the initiation of infection, however, expression of these proteins is down-regulated (20), and recent work has demonstrated that purified p66, which retains at least some of the native conformation of the protein, can serve as a protective antigen in mice (11).
It has also been proposed that p66 contains one surface-exposed, immunodominant loop near the C terminus (4). However, if p66 is an integrin ligand when expressed on the surface of B. burgdorferi, the central portion of the protein must, at least in part, also be surface exposed. This integrin-binding domain would therefore also potentially be targeted by the human antibody (B-cell) response. In support of this hypothesis, p66M was recognized by sera from a small number of Lyme arthritis patients in immunoblots (J. Coburn and W. Chege, unpublished data). The recognition of antibody epitopes throughout the length of p66, and the spectrum of reactivity to p66 among patients at different stages of disease, were therefore analyzed in this study. In addition, the availability of p66 in recombinant form allows, for the first time, the testing of a large number of human patient sera for immunoglobulin M (IgM) and IgG antibodies to this protein specifically, in the absence of other B. burgdorferi proteins that display similar electrophoretic mobility.
To determine whether p66 is recognized by sera from a diverse group of Lyme disease patients, 79 sera from North American patients representing different stages of disease were tested by enzyme-linked immunosorbent assay for reactivity to the recombinant protein. Twenty-five patients had early Lyme disease with localized erythema migrans (EM), 14 had acute (early) neuroborreliosis (acute neuro), 32 had Lyme arthritis (arthritis; a late manifestation of the disease), and 8 had late (chronic) neuroborreliosis (late neuro). All patients met the Centers for Disease Control and Prevention (CDC) criteria for the diagnosis of Lyme disease (5, 6). Sera from 72 patients with other illnesses were used as negative controls. All sera were coded to preclude biased interpretation of results.
The design and production of maltose-binding protein (MBP)-p66 fusion proteins used in this work were described elsewhere (9). Briefly, portions of the gene encoding p66 were cloned into pMalC2 (New England Biolabs, Beverly, Mass.), which results in the expression of the protein sequence of interest fused to the carboxyl terminus of MBP, a tag that facilitates purification of the recombinant protein by amylose affinity chromatography. Each preparation was at least 90% pure fusion protein; much of the remainder consisted of the native nonrecombinant MBP from the Escherichia coli expression host and degradation products of the fusion protein. Proteins tested included MBP fusions to the full-length mature p66 (p66FL; residues 19 to 618), the integrin-binding middle third (p66M; residues 142 to 384), and the portions of p66 amino terminal and carboxy terminal to the integrin binding domain, p66N (residues 19 to 178), and p66C (residues 396 to 618), respectively. MBP alone was also included as a control for p66-specific reactivity.
We began our studies by establishing conditions in which, on a molar basis, the microtiter wells were actually coated with equal amounts of protein. We had previously determined that even when equimolar concentrations of the different proteins were added to microtiter wells, the amounts that remained bound to the wells varied (possibly due to differential exposure of hydrophobic domains). Coating concentrations that resulted in equivalent amounts of each protein actually being bound to microtiter wells were determined using a polyclonal rabbit antiserum directed against MBP (New England Biolabs), which reacts efficiently against each of the MBP-p66 fusion proteins and against the MBP control. The concentrations of MBP and the p66 fusion proteins that generated approximately equivalent levels of anti-MBP reactivity were MBP, 1 μg/ml; MBP-p66N, 0.3 μg/ml; MBP-p66M, 0.03 μg/ml; MBP-p66C, 0.1 μg/ml; and MBP-p66FL, 0.1 μg/ml. Each protein was freshly diluted in cold phosphate-buffered saline (PBS), and 50 μl per well was incubated overnight at 4°C in Linbro 96-well plates (ICN Biomedical, Inc., Irvine, Calif.). PBS was used in place of the more standard bicarbonate buffer because buffered saline solutions had previously been determined to be preferable for integrin-binding assays (J. Coburn, unpublished data), and we wished to maintain any epitopes that might be present in the integrin-binding domain. PBS alone was included as a negative control. Wells were washed twice with 200 μl of PBS, with a 5-min incubation at room temperature (RT) for the second wash, and then were blocked for 1 h at RT with 200 μl of PBS supplemented with 5% milk plus 10% normal goat serum (blocking buffer; optimized empirically). All subsequent antibody dilutions were made in blocking buffer.
Quadruplicate wells were probed with 50 μl of each patient's serum diluted 1:100. A Lyme arthritis patient serum that had previously been shown to react with p66 by immunoblot was used as a positive control for human IgG at a 1:100 dilution. For each assay, a parallel set of wells was probed with anti-MBP antiserum (1:10,000; New England Biolabs). This anti-MBP antiserum was used as a control that allowed us to objectively measure the relative amounts of each of the MBP-p66 fusion proteins and MBP with which the wells were coated in each experiment. This level of control for protein-to-protein and for experiment-to-experiment variation was not possible with any patient serum. Using this system, we were able to account for variations in the coating efficiencies of the different proteins and in the signals obtained from patient sera on different assay dates.
Wells were incubated for 2 h at RT and washed twice with 200 μl of PBS and then twice with 200 μl of PBS–0.2% Tween 20 for 5 min. Fifty microliters of alkaline phosphatase (AP) -conjugated secondary antibody against human IgM (1:30,000; Biosource, Camarillo, Calif.), human IgG (1:20,000; Biosource), or rabbit IgG (1:10,000; Promega, Madison, Wis.) was added to the appropriate wells and incubated for 1 h at RT. Wells were washed as described above and then were washed once with 0.1 M Tris (pH 9.5)–0.1 M NaCl–5 mM MgCl2 for 5 min. Colorimetric development of AP was performed by incubation at 37°C with 50 μl of paranitrophenyl-phosphate (1 mg/ml) in 0.1 M Tris (pH 9.5)–0.1 M NaCl–5 mM MgCl2, and the optical density (OD) was read at 405 nm.
For data analysis, reactivity in wells coated with PBS was subtracted from all OD values. Signals obtained with patient sera were then divided by the values obtained with the rabbit anti-MBP serum. Responses to MBP (which were usually but not always low if present at all) were then subtracted to determine fusion protein-specific responses. The cutoff for a positive value was defined as 2 standard deviations above the mean of the control patients. Fisher's exact test was used to compare the numbers of patients with reactivity to different protein epitopes within and across clinical groups. Statistical analysis for the comparison of OD results within and across clinical groups was performed using the Kruskal-Wallis test, a nonparametric statistical method. P values of ≤0.05 were considered significant. Analyses were performed using BMDP New System (version 1; BMDP Statistical Software, Los Angeles, Calif.).
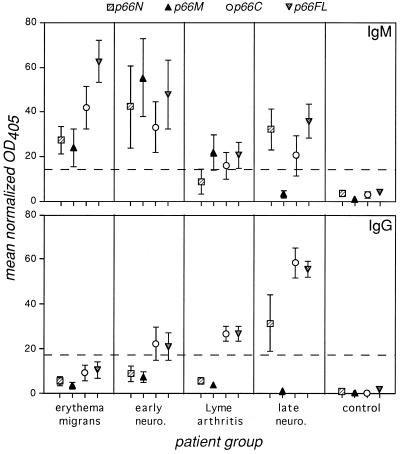
Antibody responses to full-length p66 (p66FL) were apparent in all patient groups (Fig. 1; Table 1), in agreement with previous results in which total B. burgdorferi sonicates were probed with patient sera in an immunoblot format (10). A total of 80% of the EM patients and 50% of the acute neuro patients showed an IgM response to p66FL, and the IgM response was maintained in many of the arthritis and late neuro patients. A total of 24% of the EM patients also showed an IgG response, and the percentage of patients with an IgG response to p66FL increased in later stages of disease (Table 1). Only a small percentage of the control patients showed IgM and/or IgG responses to p66FL above the cutoff value (Table 1). The EM and late neuro groups showed statistically higher IgM responses to p66FL than did the arthritis patients (Fig. 1). Patients in the late neuro group also had significantly higher IgG responses to p66FL than did those in any other group, and the arthritis patients had a higher IgG response than did the EM patients. No other comparisons between patient groups achieved statistical significance. A significantly higher IgG response to p66 in patients with late neuroborreliosis compared to those with Lyme arthritis is unusual among the known responses to B. burgdorferi proteins. The greatest response to spirochetal proteins is usually during the period(s) of arthritis, with somewhat less reactivity during late neuroborreliosis (10).
FIG. 1.
IgM and IgG responses to p66. Microtiter wells coated with MBP-p66 (full length) (p66FL; amino acids 21 to 618), p66N (p66 amino acids 21 to 178), p66M (amino acids 170 to 384), or p66C (amino acids 396 to 618) fusion proteins were probed with patient sera and then with anti-human IgM- or IgG-AP conjugates. Reactivity to control wells not coated with protein was subtracted from all values; signals from patient sera were divided by those from parallel wells probed with anti-MBP antiserum, and the resulting values were multiplied by 1,000. Any reactivity to MBP was then subtracted to determine the fusion protein-specific responses shown. Each point represents the mean ± the standard error of all serum samples from each patient group. The dotted lines indicate the values of the means + 2 standard deviations of the control patients' reactivity to p66FL (the cutoff for determining patient response to p66FL). Statistically significant differences for IgM reactivity to p66FL were as follows: EM > arthritis, P = 0.0004; late neuro > arthritis, P = 0.035. IgM responses to p66M were significantly higher among acute neuro patients than either late neuro or arthritis patients (P ≤ 0.018); the IgM response to p66M among acute neuro patients was also greater than that in EM patients, but the P value was 0.059. The IgG reactivities to p66FL in the late neuro patients were significantly higher than those in all other groups (P ≤ 0.0026 in all cases). The IgG responses to p66C were also higher in the late neuro group than in any other patient group (P ≤ 0.006 in all cases). The arthritis patients also had a significantly higher response to p66C than did the EM patients (P = 0.0008). The IgG response to p66M was significantly higher in the acute neuro patients than in the late neuro or arthritis patients (P ≤ 0.03). IgG reactivity to p66N was higher in the late neuro patients than in either the EM or arthritis patients (P ≤ 0.02).
TABLE 1.
Frequency of IgM and IgG responses to full-length p66 and p66 fragments
| Patient group | No. of patients | Antibody class | No. (%) positive patientsa
|
||||
|---|---|---|---|---|---|---|---|
| p66N | p66M | p66C | p66FL | Any portion of p66 | |||
| Erythema migrans | 25 | IgM | 12 (48) | 9 (36) | 16 (64) | 20 (80) | 23 (92) |
| IgG | 5 (20) | 7 (28) | 8 (32) | 6 (24) | 11 (44) | ||
| Early neuroborreliosis | 14 | IgM | 6 (42.9) | 10 (71.5) | 8 (57.5) | 7 (50) | 12 (85.8) |
| IgG | 6 (42.9) | 8 (57.2) | 7 (50) | 8 (57.2) | 12 (85.8) | ||
| Lyme arthritis | 32 | IgM | 14 (43.8) | 12 (37.5) | 12 (37.5) | 11 (34.5) | 20 (62.5) |
| IgG | 9 (28.2) | 10 (31.3) | 25 (78.2) | 24 (75) | 28 (87.5) | ||
| Late neuroborreliosis | 8 | IgM | 5 (62.5) | 2 (25) | 4 (50) | 5 (63) | 6 (75) |
| IgG | 4 (50) | 1 (12.5) | 8 (100) | 8 (100) | 8 (100) | ||
| Control | 72 | IgM | 1 (1.4) | 1 (1.4) | 3 (4.2) | 4 (5.6) | 5 (6.9) |
| IgG | 4 (5.6) | 0 | 5 (6.9) | 7 (9.1) | 9 (12.5) | ||
The cutoff value for patient positivity was defined as 2 standard deviations above the mean of the 72 control patients.
To determine which portions of p66 contain epitopes recognized by Lyme disease patients, the antibody responses to different portions of the protein were analyzed. IgM and IgG responses to all portions of p66 were demonstrated in at least some of the patients in all clinical groups (Fig. 1, Table 1). It should be noted that responses to any one of the p66 fragments did not always correspond to the response to p66FL, suggesting that the recombinant proteins may not be folded into precisely the same conformations and may not completely reflect the native conformation of the protein. The EM and late neuro patient groups both showed higher IgM responses to p66FL than to any of the fragments, supporting the suggestion that epitopes present in the full-length protein may not be represented in the fragments.
When antibody reactivity to each of the p66 fragments was compared within the EM, acute neuro, and arthritis groups, the IgM responses were similar, while the late neuro patients had a significantly higher IgM response to p66N than to p66M. When comparisons were made between different patient groups, reactivity to p66M was striking in the acute neuro group, with 71.5% of patients showing a positive IgM response, compared to less than 40% of patients in any of the other groups (Table 1). The level of both IgM and IgG responses to p66M was significantly higher in the acute neuro group than in the arthritis and late neuro groups (Fig. 1).
Analysis of the IgG responses within patient groups showed that no fragment of p66 was statistically more likely to yield a response than any other in either the EM or acute neuro patients (Fig. 1). However, the levels of IgG reactivity to p66C were considerably higher than to the other fragments among the arthritis and late neuroborreliosis patients, i.e., those with later stages of disease manifestations. Both of these patient groups also showed a higher response to p66N than to p66M, with the response to p66N being highest among the late neuro patients. Comparisons between patient groups revealed that the late neuro patients were significantly more likely to show an IgG response to p66C than were any other patients and showed a higher response to p66N than did EM or arthritis patients. Arthritis patients also had significantly higher IgG reactivity to p66C than did the EM patients.
The results presented here demonstrate that reactivity to multiple epitopes of p66, a candidate β3-chain integrin ligand, is widespread among Lyme disease patients. This conclusion is strengthened by the use of a large set of serum samples from well-characterized Lyme disease patients and by the use of MBP-p66 fusion proteins in conjunction with the anti-MBP serum that served as an important control throughout this work. The anti-MBP allowed us to demonstrate that, for every experiment, we had actually coated the wells with similar numbers of protein molecules for each of the proteins tested. This was not possible to determine using any patient serum, as reactivity to the fragments of p66 could not be objectively determined to be the same, and reactivity to MBP, if present at all, would not be comparable to reactivity to p66. The use of the anti-MBP serum also allowed a greater level of control for experiment-to-experiment variation, as it would have been extremely difficult to test 151 patient sera for IgM and IgG reactivity to all five proteins tested on the same day.
The nature of the epitopes recognized by Lyme disease patients, i.e., linear versus conformational, as well as the significance of recognition of particular epitopes by patients with particular manifestations of Lyme disease, remains to be determined. Patient sera recognize epitopes throughout the protein early in disease, but the antibody response against the C-terminal portion becomes more dominant with increasing duration of disease. The most likely explanation for these results is that, as the immune response matures, antibodies against the C-terminal portion of p66 are progressively selected. A second, far less likely but more intriguing possibility is that expression of p66 in the outer membrane of B. burgdorferi may change somewhat with either site or duration of infection. For example, p66 has been proposed to be a porin (21), and the different nutritional requirements of spirochetes in different environments (e.g., central nervous system versus joint) might affect the structure or expression level of the protein. The observation that reactivity to p66 is highest in the late neuroborreliosis patients supports the idea that p66 may be either expressed or processed differently in the nervous system, but this hypothesis would be strengthened by future testing of sera from larger numbers of patients. Further work will also be required to address this question with regard to the membrane topology of p66 and the regulation of its expression in multiple environments. At this point, however, the regulatory mechanisms governing p66 expression and the structure of this protein in the outer membrane of B. burgdorferi await resolution.
Acknowledgments
We thank John Leong and Nikhat Parveen for careful review of the manuscript.
This work was supported by a Biomedical Science Grant from the Arthritis Foundation, by National Institutes of Health (NIH) grant AI-40938 to J.C. and by the Center for Gastroenterology Research on Absorptive and Secretory Processes at New England Medical Center (Public Health Service grant 1 P30DK39428 awarded by the National Institute of Diabetes and Kidney Diseases). H.N. and H.R. received support from NIH training grant TAR-07570.
REFERENCES
- 1.Backenson P B, Coleman J L, Benach J L. Borrelia burgdorferi shows specificity of binding to glycosphingolipids. Infect Immun. 1995;63:2811–2817. doi: 10.1128/iai.63.8.2811-2817.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bunikis J, Barbour A G. Access of antibody or trypsin to an integral outer membrane protein (p66) of Borrelia burgdorferi is hindered by Osp lipoproteins. Infect Immun. 1999;67:2874–2883. doi: 10.1128/iai.67.6.2874-2883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunikis J, Noppa L, Bergstrom S. Molecular analysis of a 66-kDa protein associated with outer membrane of Lyme disease Borrelia. FEMS Microbiol Lett. 1995;131:139–145. doi: 10.1111/j.1574-6968.1995.tb07768.x. [DOI] [PubMed] [Google Scholar]
- 4.Bunikis J, Noppa L, Ostberg Y, Barbour A G, Bergstrom S. Surface exposure and species specificity of an immunoreactive domain of a 66-kilodalton outer membrane protein (p66) of the Borrelia spp. that cause Lyme disease. Infect Immun. 1996;64:5111–5116. doi: 10.1128/iai.64.12.5111-5116.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control. Case definitions for public health surveillance. Morb Mortal Wkly Rep. 1990;39:19–21. [Google Scholar]
- 6.Centers for Disease Control and Prevention. Recommendation for test performance and interpretation from the Second National Conference on the Serological Diagnostic of Lyme Disease. Morb Mortal Wkly Rep. 1995;44:590–591. [PubMed] [Google Scholar]
- 7.Coburn J, Leong J M, Erban J. Integrin αIIbβ3 mediates binding of the Lyme disease agent, Borrelia burgdorferi, to human platelets. Proc Natl Acad Sci USA. 1993;90:7058–7063. doi: 10.1073/pnas.90.15.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coburn J, Magoun L, Bodary S C, Leong J M. Integrins αvβ3 and α5β1 mediate attachment of Lyme disease spirochete to human cells. Infect Immun. 1998;66:1946–1952. doi: 10.1128/iai.66.5.1946-1952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coburn J, Chege W, Magoun L, Bodary S C, Leong J M. Characterization of a candidate Borrelia burgdorferi β3-chain integrin ligand identified using a phage display library. Mol Microbiol. 1999;34:926–940. doi: 10.1046/j.1365-2958.1999.01654.x. [DOI] [PubMed] [Google Scholar]
- 10.Dressler T, Whalen J A, Reinhardt B N, Steere A C. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis. 1993;167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 11.Exner M M, Wu X Y, Blanco D R, Miller J N, Lovett M A. Protection elicited by native outer membrane protein oms66 (p66) against host-adapted Borrelia burgdorferi: conformational nature of bactericidal epitopes. Infect Immun. 2000;68:2647–2654. doi: 10.1128/iai.68.5.2647-2654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grab D J, Givens C, Kennedy R. Fibronectin-binding activity in Borrelia burgdorferi. Biochim Biophys Acta. 1998;1407:135–145. doi: 10.1016/s0925-4439(98)00038-6. [DOI] [PubMed] [Google Scholar]
- 13.Guo B P, Norris S J, Rosenberg L C, Hook M. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect Immun. 1995;63:3467–3472. doi: 10.1128/iai.63.9.3467-3472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Issacs R. Borrelia burgdorferi bind to epithelial cell proteoglycan. J Clin Investig. 1994;93:809–819. doi: 10.1172/JCI117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopp P A, Schmitt M, Wellensiek H J, Blobel H. Isolation and characterization of fibronectin-binding sites of Borrelia garinii N34. Infect Immun. 1995;63:3804–3808. doi: 10.1128/iai.63.10.3804-3808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leong J M, Morrissey P E, Ortega-Barria E, Pereira M E, Coburn J. Hemagglutination and proteoglycan binding by the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:874–883. doi: 10.1128/iai.63.3.874-883.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parveen N, Leong J M. Identification of candidate glycosaminoglycan-binding adhesin of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:1220–1234. doi: 10.1046/j.1365-2958.2000.01792.x. [DOI] [PubMed] [Google Scholar]
- 18.Probert W S, Johnson B J. Identification of a 47 kDa fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 19.Probert W S, Allsup K M, Lefebvre R B. Identification and characterization of a surface-exposed, 66-kilodalton protein from Borrelia burgdorferi. Infect Immun. 1995;63:1933–1939. doi: 10.1128/iai.63.5.1933-1939.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skare J T, Mirzabekov T A, Shang E S, Blanco D R, Erdjument-Bromage H, Bunikis J, Bergstrom S, Tempst P, Kagan B L, Miller J N, Lovett M A. The oms 66 (p66) protein is a Borrelia burgdorferi porin. Infect Immun. 1997;65:3654–3661. doi: 10.1128/iai.65.9.3654-3661.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]