Key Teaching Points.
-
•
Ebstein’s anomaly (EA) is a rare congenital disorder that is chiefly characterized by apical displacement of the septal and posterior leaflets of the tricuspid valve (TV), leading to “atrialization” of the right ventricle and dilatation of the right atrium. This can lead to a spectrum of challenging clinical sequelae, including arrhythmia, conduction disease, and heart failure, which require careful consideration in order to optimize therapy.
-
•
Cardiac resynchronization therapy can be an effective treatment in patients with non–left bundle branch block (LBBB) electrocardiogram patterns with EA and associated left ventricular dysfunction. Clues to underlying left bundle branch disease (masked LBBB) include a prolonged PR interval, a slurred upstroke of the QRS in lead 1 and aVL, and left axis deviation.
-
•
Complications at device implant are more common in patients with EA owing to technical challenges from the severely enlarged right atrium, small right ventricle, frequent TV surgery, and frequent severe tricuspid regurgitation. Despite these aspects, transvenous defibrillation leads and coronary sinus pacing leads can be implanted successfully to treat patients with left ventricular dysfunction in this setting.
Introduction
Ebstein’s anomaly (EA) is a rare congenital heart disease accounting for less than 1% of all cardiac congenital anomalies. Numerous structural abnormalities may occur in EA; however, it is chiefly characterized by apical displacement of the septal and posterior leaflets of the tricuspid valve (TV). This leads to “atrialization” of the right ventricle and dilatation of the right atrium. Left ventricular (LV) noncompaction and ventricular pre-excitation are also recognized associations.1 EA can therefore manifest with challenging arrhythmias, conduction system disease, and biventricular failure, which, owing to anatomical considerations, can be difficult to manage. We describe a case of a patient with EA, LV noncompaction, and an atypical right bundle branch block who responded to cardiac resynchronization therapy (CRT). We also describe the challenges and possible indications for CRT implantation in EA.
Case report
A 54-year-old woman presented with progressive dyspnea and palpitations. The relevant background included a diagnosis of EA with an associated secundum atrial septal defect at the age of 15. The patient underwent successful surgical repair (GORE-TEX patch; W. L. Gore & Associates, Inc, Newark, DE) of her atrial septal defect and remained well until she required a TV repair (suture annuloplasty) 18 years later owing to increasing exertional dyspnea. At the time, transthoracic echocardiography confirmed EA of the TV with severe displacement of the septal leaflet with moderate TV regurgitation. Following TV repair, the patient experienced symptomatic paroxysmal atrial tachycardia. Electrophysiology study was performed and this identified a typical counterclockwise atrial flutter that was treated with cavotricuspid isthmus ablation. Flecainide was also used subsequently to manage other atrial tachyarrhythmias that could not be ablated.
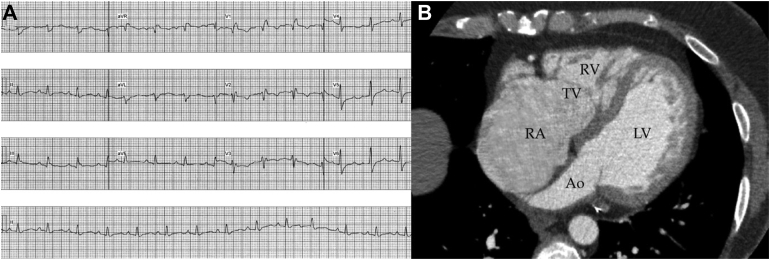
Ten years later, the patient presented with NYHA class II dyspnea and examination findings consistent with heart failure. A 12-lead electrocardiogram revealed sinus rhythm, first-degree atrioventricular block, and right bundle branch block with a QRS duration of 180 ms (Figure 1A). A transthoracic echocardiogram was performed which revealed new, severe left and right ventricular (RV) systolic impairment. Cardiac magnetic resonance and cardiac computed tomography imaging showed severely impaired LV (LV ejection fraction 30%) and RV (RV ejection fraction 25%) systolic function. There was circumferential noncompaction noted in the distal segments of the left ventricle (meeting Petersen’s criteria) and fibrosis in the basal-mid septum (Figure 1B). There was no evidence of mechanical dyssynchrony on cardiac imaging. The patient was commenced on heart failure therapy including furosemide, spironolactone, bisoprolol, and sacubitril/valsartan, which provided some improvement in symptoms. A repeat transthoracic echocardiogram was performed 3 months following optimal heart failure therapy and did not show any improvement in left or RV systolic function. After multidisciplinary discussion and given ongoing severe LV impairment, the patient went on to cardiac resynchronization therapy-defibrillator (CRT-D) implantation.
Figure 1.
A: The patient’s baseline electrocardiogram in sinus rhythm where the QRS shows a broad right bundle branch block with a slurred upstroke in leads 1 and aVL and a prolonged PR interval (suggesting concurrent conduction disease in the left bundle branch). The QRS duration was 180 ms. B: Cardiac computed tomography with severe right atrial dilatation, apical displacement of the septal and anterior leaflets of the tricuspid valve, and a very small right ventricle. Noncompacted myocardium is seen from the base to the apex on the free wall of the left ventricle. Ao = aorta; LV = left ventricle; RA = right atrium; TV = tricuspid valve.
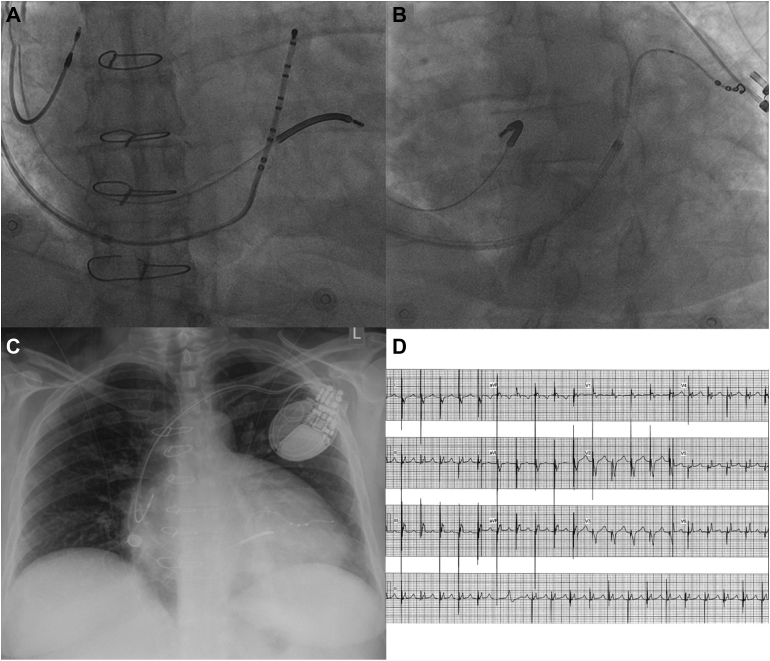
Insertion of the RV defibrillation lead was challenging owing to the severe right atrial dilatation and the need to position the coil within the small right ventricle to avoid any atrial oversensing. Satisfactory lead parameters were obtained by placing a wide curve on a medium-stiffness stylet and with counterclockwise rotation advancing the tip of a Boston Scientific Reliance 4-Front (Boston Scientific Corporation, Marlborough, MA) lead through the tricuspid annulus and into the high mid RV septum. Owing to severe right atrial dilatation (even with a Boston Scientific Wide outer sheath) the coronary sinus (CS) could not be reached. To confirm its position a Bard deflectable decapolar catheter could be advanced to the CS via a Cook Medical SL2 (Bard Dynamic XT™ Steerable Diagnostic CatheterCook Medical © Cook, Bloomington, IN) sheath from the groin and this position was recorded in right and left anterior oblique projections. This deflectable decapolar catheter was then advanced through the Boston Scientific Wide outer sheath from the left axillary access and after reaching the tricuspid annulus position in right anterior oblique the catheter could be flexed up gently and rotated counterclockwise into the CS and the outer sheath could be advanced over this (Figure 2A). Through this sheath a Boston Scientific X4 Spiral lead was advanced over a Sion (Asahi Intecc, Seto, Japan) angioplasty wire to a high lateral CS branch (Figure 2B). A Boston Scientific Ingevity lead was placed into the right atrial appendage and all leads were connected to the Boston Scientific Resonate X4 CRT-D, which was placed into a prepectoral pocket. The patient’s chest radiograph (Figure 2C) and electrocardiogram (Figure 2D) following implant are shown.
Figure 2.
A: Right anterior oblique view with the defibrillation lead in the right ventricular septum with the coil across the tricuspid annulus. The deflectable decapolar catheter is shown in the coronary sinus through the wide curve sheath traversing the severely dilated right atrium. B: Left anterior oblique view of the quadripolar left ventricular pacing lead positioned into a high lateral coronary sinus branch. C: Chest radiograph of the final lead positions in the heart. Severe right atrial enlargement can be appreciated with the significant prominence of the right heart border. D: Electrocardiogram following cardiac resynchronization therapy implant. Pacing is set up to fuse with the native QRS to obtain a width of 130 ms.
At 3 months follow-up post CRT-D implantation, the patient’s QRS duration had shortened from 180 ms to 130 ms (Figure 2D) and LV ejection fraction on echocardiography improved from 30% to 50%. Clinically, the patient described NYHA class I symptoms with resolution of her palpitations and exertional dyspnea.
Discussion
We report the first case of de novo CRT in a patient with EA and LV noncompaction resulting in normalization of LV function. This case demonstrates the possible utility of CRT in patients with adult congenital heart disease with a non–left bundle branch block (LBBB) QRS morphology and biventricular failure. It also demonstrates techniques that can be used for CRT in this challenging setting.
Previously, Numata and colleagues2 demonstrated the use of an upgrade to CRT in a patient with EA who developed pacing-induced LV failure; however, we were unable to find any de novo reports of CRT in EA patients. This may relate to the fact that permanent pacing is relatively rare in EA patients (3.7% in a study by Allen and colleagues3), so pacing-induced cardiomyopathy would be uncommon and may also represent a reluctance to perform de novo CRT in patients with non-LBBB QRS morphologies and concurrent RV dysfunction. The response to CRT in our patient may be due to a degree of masked LBBB, as suggested by the prolonged PR interval and the slurred upstroke of the QRS in leads 1 and aVL on the baseline electrocardiogram.4 These features suggest delayed conduction down both the right and left bundle branches. This masked LBBB may occur as a result of infra-Hisian conduction disturbance in EA patients or as part of the LV noncompaction cardiomyopathy itself and may lead to intraventricular dyssynchrony of the LV, similar to manifest LBBB.5 In a study of the MADIT-CRT population, the presence of a prolonged PR interval in patients with non-LBBB QRS morphologies predicted response to CRT (possibly owing to underlying masked LBBB).6 Although there are limited data, it is possible that in patients with non-LBBB restoration of atrioventricular synchrony itself can contribute to the clinical response to CRT, such as improving mechanical dyssynchrony or reducing diastolic mitral regurgitation.7 This suggests a need to more carefully scrutinize these patients with non-LBBB QRS morphologies (particularly since up to 18% of patients with EA may have some degree of LV impairment).1
In a retrospective review of implantable cardioverter-defibrillator (ICD) recipients by Gleva and colleagues,8 EA was associated with an increase in device implantation complication rates in adult congenital heart disease patients. This is likely related to the technical difficulties with lead implantation in EA patients who have an indication for ICD, as they are more likely to have a severely enlarged right atrium and a small right ventricle and may often have had prior TV surgery and or significant tricuspid regurgitation. Ventricular pacing leads can be placed into the atrialized right ventricle proximal to the TV or into the CS, but this is not possible for defibrillation leads.9 In the setting of EA it is often not possible to prolapse a lead across the TV and instead with the use of a wide curve on the medium-stiffness stylet the lead can be advanced through the TV in left anterior oblique and can be manipulated counterclockwise onto the septum while making sure the position is sufficiently apical in right anterior oblique. If there is integrated bipolar sensing, then it is particularly important that the whole ICD coil is across the valve. The intent was to position the coil completely in the right ventricle to create an optimal defibrillator vector, and atrial far-field oversensing was carefully checked for at implant. The use of a larger-curve outer sheath may provide additional support for RV lead placement in patients with EA and very severe right atrial dilatation; however, conventional outer sheaths (designed for CS leads) will not accommodate an ICD lead.
Severe right atrial enlargement is a known reason for failed CS lead implantation.10 In this case there may have been stenosis or distortion of the CS ostium following TV repair as well. Intraprocedural options for identifying a difficult CS include performing a coronary angiogram and capturing the venous phase or placing a CS electrophysiology catheter from a femoral vein approach. The later was performed in our case and requires careful attention to aseptic technique during femoral access. Once the CS was identified it could be targeted with a deflectable decapolar catheter inside a wide-curve outer sheath to enable the reach and support to both intubate the CS and advance the outer sheath over the electrophysiology catheter into a very posteriorly directed CS body. From this point implantation of an LV pacing lead for CRT was quite conventional.
Conclusion
This is the first case of de novo CRT therapy in a patient with EA and LV noncompaction resulting in normalization of LV function. Certain aspects of the QRS in non-LBBB patients may help to predict response to CRT in this setting, and with careful attention to technique and available tools challenging anatomy can be overcome for an excellent clinical result.
Footnotes
Funding Sources: None.
Disclosures: The authors have no conflicts to disclose.
References
- 1.Attenhofer Jost C.H., Connolly H.M., Dearani J.A., Edwards W.D., Danielson G.K. Ebstein's anomaly. Circulation. 2007;115:277–285. doi: 10.1161/CIRCULATIONAHA.106.619338. [DOI] [PubMed] [Google Scholar]
- 2.Numata G., Amiya E., Kojima T., et al. Cardiac resynchronization therapy in patients with Ebstein's anomaly. Int Heart J. 2017;58:816–819. doi: 10.1536/ihj.16-580. [DOI] [PubMed] [Google Scholar]
- 3.Allen M.R., Hayes D.L., Warnes C.A., Danielson G.K. Permanent pacing in Ebstein’s anomaly. Pacing Clin Electrophysiol. 1997;20:1243–1246. doi: 10.1111/j.1540-8159.1997.tb06776.x. [DOI] [PubMed] [Google Scholar]
- 4.Henin M., Ragy H., Mannion J., David S., Refila B., Boles U. Indications of cardiac resynchronization in non-left bundle branch block: clinical review of available evidence. Cardiol Res. 2020;11:1–8. doi: 10.14740/cr989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hebe J. Ebstein's anomaly in adults. Arrhythmias: diagnosis and therapeutic approach. Thorac Cardiovasc Surg. 2000;48:214–219. doi: 10.1055/s-2000-6897. [DOI] [PubMed] [Google Scholar]
- 6.Kutyifa V., Stockburger M., Daubert J.P., et al. Response to letter regarding, "PR interval identifies clinical response in patients with non-left bundle branch block: a multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy sub-study" by Kutyifa et al. Circ Arrhythm Electrophysiol. 2014;7:1280. doi: 10.1161/CIRCEP.114.002303. [DOI] [PubMed] [Google Scholar]
- 7.Solodky A., Zafrir N. Electrical and mechanical dyssynchrony in patients with right bundle branch block. J Nucl Cardiol. 2020;27:631–633. doi: 10.1007/s12350-018-1460-z. [DOI] [PubMed] [Google Scholar]
- 8.Gleva M.J., Wang Y., Curtis J.P., Berul C.I., Huddleston C.B., Poole J.E. Complications associated with implantable cardioverter defibrillators in adults with congenital heart disease or left ventricular noncompaction cardiomyopathy (from the NCDR® Implantable Cardioverter-Defibrillator Registry) Am J Cardiol. 2017;120:1891–1898. doi: 10.1016/j.amjcard.2017.07.103. [DOI] [PubMed] [Google Scholar]
- 9.Jayaprakash S., Mond H.G., Sparks P.B., et al. Transvenous ventricular pacing options in Ebstein's anomaly. Indian Heart J. 1998;50:565–568. [PubMed] [Google Scholar]
- 10.Bax J.J., Abraham T., Barold S.S., et al. Cardiac resynchronization therapy: Part 1--issues before device implantation. J Am Coll Cardiol. 2005;46:2153–2167. doi: 10.1016/j.jacc.2005.09.019. [DOI] [PubMed] [Google Scholar]