Abstract
Cross-inhibition by quorum-sensing pheromones between Staphylococcus aureus and Staphylococcus epidermidis was investigated using all known S. aureus agr pheromone subgroups. All S. aureus subgroups were sensitive towards the S. epidermidis pheromone, with the exception of the recently identified subgroup 4. The subgroup 4 pheromone was also the only S. aureus pheromone able to inhibit the S. epidermidis agr response. The close relation of subgroup 4 to subgroup 1 suggests that subgroup 4 might have evolved from subgroup 1 by mutation under the selective pressure of competition with S. epidermidis. The competition between S. aureus and S. epidermidis by means of quorum-sensing cross talk seems to be generally in favor of S. epidermidis, which might explain the predominance of S. epidermidis on the skin and in infections on indwelling medical devices.
Quorum-sensing systems, which sense and signal the state of cell density, are of high importance for the survival of bacteria, as they enable them to respond to changing environmental conditions (14). The agr system of staphylococci is a quorum-sensing system which controls the expression of exoproteins and surface proteins in a growth phase-dependent manner (10). The extracellular signal, which is used by the staphylococcal agr system, is a small peptide pheromone that harbors an unusual posttranslational modification (2). For Staphylococcus aureus and Staphylococcus epidermidis, it has been shown that this posttranslational modification is an intramolecular thioester, which links the thiol group of a central cysteine to the C-terminal carboxy group (5, 8). Interestingly, the primary sequence of the pheromones of different species and also of different pheromone subgroups within one species varies completely. Only the central cysteine and its distance to the C terminus are conserved (5, 8). For three S. aureus subgroups it has been demonstrated that the corresponding pheromones can inhibit the agr response of foreign subgroups (1). We have shown that this is also the case between different staphylococcal species, as the S. epidermidis pheromone has proven to be an efficient inhibitor of the agr response of S. aureus strain Newman (9). agr controls the expression of several important virulence factors in S. aureus, such as alpha-toxin, beta-toxin, delta-toxin, serine protease, DNase, fibrinolysin, enterotoxin B, and toxic shock syndrome toxin 1 (12). Suppression of the agr response in S. aureus by the above-mentioned pheromones also suppresses the expression of various virulence factors in in vitro studies (5, 9). Furthermore, the development of S. aureus-induced lesions in mice was efficiently suppressed when the infecting strain was injected subcutaneously together with the inhibiting pheromone of a foreign subgroup (5). Therefore, agr pheromones and their derivatives have been proposed as new antistaphylococcal drugs, especially in the treatment of infections by S. aureus and S. epidermidis, which rank among the most important pathogens in nosocomial infections (11).
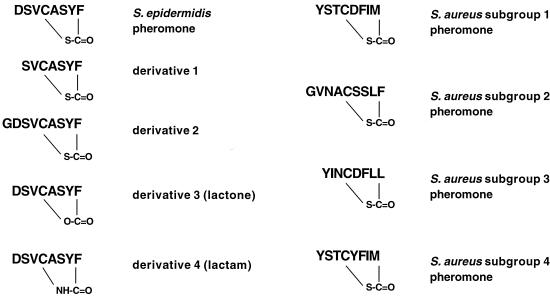
S. aureus causes many acute severe infections, such as impetigo, wound infections, or in toxic shock syndrome, whereas chronic infections tend to be caused in a higher relative proportion by S. epidermidis (13). The prevalence of S. epidermidis in many nosocomial infections, among them infections on indwelling medical devices, raises the question about the advantage that S. epidermidis possesses in these situations compared to S. aureus. Among the infections predominantly caused by S. epidermidis one can often find the involvement of biofilms (15), which constitute a high-density population. This led us to the assumption that the more frequent participation of S. epidermidis in these infections might be due to interspecies concurrence based on cell density control mechanisms. It has been proposed that the inhibiting properties of the staphylococcal pheromones serve as weapons in a struggle between different staphylococcal strains (1). To address the question of interspecies concurrence, we added synthetic natural S. epidermidis pheromone to S. aureus strains of subgroups 1 to 4 and synthetic pheromones of S. aureus subgroups to S. epidermidis. S. aureus subgroup 4 has only recently been discovered independently by us and by G. Lina (G. Lina, personal communication). In a previous study, we investigated 15 S. epidermidis strains by sequencing the DNA coding for the AgrD prepheromone and found only a single S. epidermidis pheromone sequence, DSVCASYF (8), suggesting that there is only one S. epidermidis agr pheromone group or that this one is by far the most frequent. All pheromones and pheromone derivatives were prepared by solid-phase synthesis as previously described (8) and are shown in Fig. 1. The staphylococcal strains used were S. epidermidis ATCC 14990, S. aureus strains A950227 (subgroup 1), A950085 (subgroup 2), A920226 (subgroup 3), A970377 (subgroup 4), A970392 (subgroup 4), A850484 (subgroup 4), and 1527/97 (subgroup 4). All S. aureus strains are clinical isolates. Strain 1527/97 was kindly provided by W. Witte, Robert-Koch Institut, Berlin, Germany, and classified as subgroup 4 strain in our laboratory by DNA sequencing; the other S. aureus strains were kindly provided by G. Lina, Centre Hospitalier et Universitaire de Lyon, Lyon, France, and were classified by G. Lina.
FIG. 1.
Synthetic agr pheromone and derivatives used in this study.
Inhibition of the agr system was monitored as we have previously described (7). Briefly, delta-toxin expression was determined by a high-performance liquid chromatography assay using a Pharmacia Resource PHE column and a water-acetonitrile gradient with 0.1% trifluoroacetic acid. Main cultures that were inoculated 1:100 from precultures were grown for 8 h, with pheromone addition at the time of inoculation. Afterwards, the samples were centrifuged and the supernatant was injected onto the column. Delta-toxin is encoded within the gene for the regulatory RNAIII, which is the intracellular effector of the agr system (6). Its expression is therefore a means to measure the activity of the agr system.
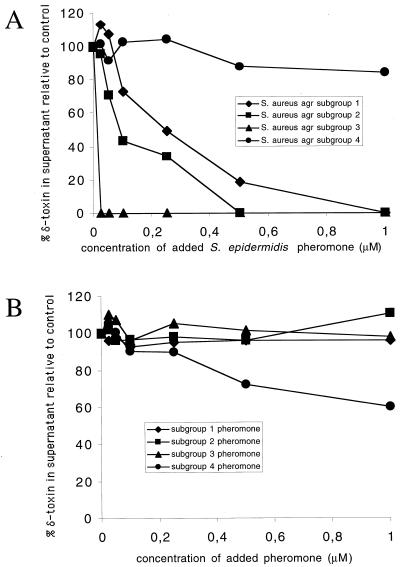
As shown in Fig. 2A, using pheromone concentrations ranging from 25 nM to 1 μM, the synthetic peptide corresponding to the natural S. epidermidis pheromone was very active against S. aureus subgroup 3. It had considerable activity against subgroups 1 and 2, but it was inactive against subgroup 4. On the other hand, S. epidermidis was insensitive to S. aureus pheromones from subgroups 1 to 3 and showed moderate sensitivity against S. aureus pheromone of subgroup 4 (Fig. 2B). This sensitivity was lower than that of the S. aureus subgroups 1 to 3 towards the S. epidermidis pheromone. At the very high pheromone concentration of 10 μM, the S. epidermidis pheromone completely inhibited delta-toxin expression in S. aureus subgroups 1, 2, and 3 but showed no effect on subgroup 4. At this concentration, the pheromone of S. aureus subgroup 4 was able to entirely suppress delta-toxin expression by S. epidermidis, whereas the pheromones of S. aureus subgroups 1 to 3 still did not show any inhibiting effect (data not shown).
FIG. 2.
Cross-inhibition of S. epidermidis and S. aureus by agr pheromones. The amount of delta-toxin in 500 μl of supernatant was determined by high-performance liquid chromatography after 8 h of growth and under the influence of different concentrations of added pheromone or pheromone derivatives. Pheromones were diluted in dimethyl sulfoxide; the control received only dimethyl sulfoxide. Cultures were grown in basic medium (1% tryptone [Difco], 0.5% yeast extract [Gibco BRL], 0.5% NaCl, 0.1% K2HPO4, 0.1% glucose) with shaking at 37°C. (A) Effect of the S. epidermidis pheromone on delta-toxin production of S. aureus agr subgroups. (B) Effect of the pheromones of different S. aureus agr subgroups on delta-toxin production of S. epidermidis.
These difference are most likely best explained by the more or less tight interaction of the pheromones with their receptor, the histidine kinase membrane enzyme AgrC. The third extracellular loop of this enzyme has been demonstrated to interact with the pheromone (4). It remains unclear if this interaction is a covalent one (by a trans-acylation reaction), as has been proposed (5). The charge of the pheromones does not correlate with the observed inhibiting properties, as the S. epidermidis pheromone and S. aureus pheromones of subgroups 1 and 3 have a net charge of zero, whereas the other tested pheromones harbor one positive charge. Therefore, other structural properties might be responsible for the differing interaction of the various pheromones with AgrC.
The most interesting result is the complete inactivity of the S. epidermidis pheromone against S. aureus subgroup 4 and the fact that only the pheromone of S. aureus subgroup 4 showed activity against S. epidermidis. We have tested three more subgroup 4 strains, which also proved to be completely insensitive to the S. epidermidis pheromone (data not shown).
Recently it has been observed that subgroup 4 strains are often involved in infections leading to scalded skin syndrome (G. Lina, personal communication). This means that these strains live on the skin, where they come into close contact and concurrence with S. epidermidis, which normally is the predominant strain on the skin (3). The subgroup 4 pheromone differs from that of subgroup 1 only by one amino acid (primary sequence YSTCYFIM instead of YSTCDFIM). It is therefore tempting to speculate that subgroup 4 might have evolved from subgroup 1 in order to be able to compete with S. epidermidis.
Activity of S. epidermidis pheromone derivatives against S. aureus agr subgroups.
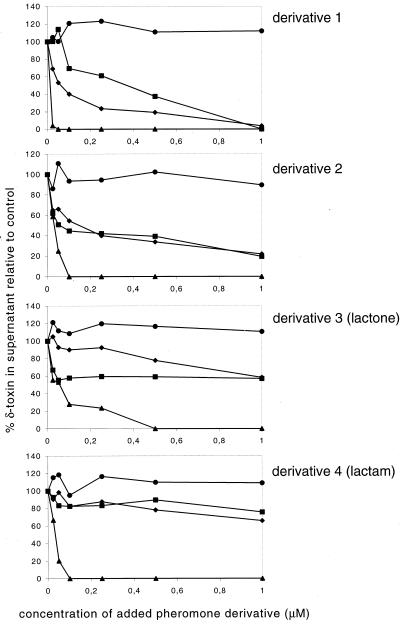
Among the tested S. epidermidis pheromone derivatives are lactone and lactam derivatives (derivatives 3 and 4) and derivatives with different lengths of the N-terminal tail, adjacent to the thiolactone-bearing ring structure (derivatives 1 and 2). Derivative 2 was slightly less active than the natural pheromone against subgroups 1, 2, and 3. This is in accordance with earlier data by which we could also show a reduced activity against S. aureus Newman (9), which belongs to subgroup 1. Derivative 1 exhibited an activity similar to that of the natural pheromone against subgroups 1 and 3 but was less active against subgroup 2. Both derivatives were inactive against subgroup 4. The derivatives in which the thiolactone structure was replaced by a lactone (derivative 3) or a lactam (derivative 4) showed a slightly further reduced activity against all subgroups, as already reported for subgroup 1 (9), but again no activity against subgroup 4 (Fig. 3).
FIG. 3.
Inhibition of the agr response in S. aureus agr subgroups by S. epidermidis pheromone derivatives. The amount of delta-toxin in the supernatants of S. aureus agr subgroup strains after addition of the S. epidermidis pheromone derivatives was determined as outlined in the legend for Fig. 2. Symbols used for S. aureus agr subgroups are the same as in Fig. 2A. Structures of the derivatives are shown in Fig. 1.
In summary, the S. epidermidis pheromone seems to be a more potent inhibitor of the S. aureus agr system, compared to the activity of S. aureus pheromones against S. epidermidis. The predominance of S. epidermidis on the skin and in chronic infections, for example, on indwelling medical devices, might be due to this advantage. As a normal resident of the skin's microflora, S. epidermidis might contribute to the body's barrier to colonization by the pathogenic S. aureus via quorum-sensing cross-inhibition. An interesting exception is S. aureus subgroup 4, which seems to have escaped from this unfortunate situation by mutation, probably because of close contact with S. epidermidis on the skin.
As far as agr pheromones are concerned in terms of potential therapeutics, our results show that an agr pheromone or a derivative may have strongly varying activity against different staphylococcal strains. It is therefore not easy to evaluate their therapeutic use in a patient who normally carries a lot of different staphylococcal strains. Furthermore, selection of resistant strains may quickly occur, as might have occurred already during the competition between staphylococcal strains during evolution, as our results with subgroup 4 suggest.
Acknowledgments
We thank Vera Augsburger for excellent technical assistance and Gérard Lina and Wolfgang Witte for providing S. aureus subgroup strains.
REFERENCES
- 1.Ji G, Beavis R, Novick R P. Bacterial interference caused by autoinducing peptide variants. Science. 1997;276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 2.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kloos W E, Schleifer K-H, Götz F. The genus Staphylococcus. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes, a handbook on the biology of bacteria: ecophysiology, isolation, identification, application. 2nd ed. II. New York, N.Y: Springer-Verlag; 1992. pp. 1369–1420. [Google Scholar]
- 4.Lina G, Jarraud S, Ji G, Greenland T, Pedraza A, Etienne J, Novick R P, Vandenesch F. Transmembrane topology and histidine protein kinase activity of AgrC, the agr signal receptor in Staphylococcus aureus. Mol Microbiol. 1998;28:655–662. doi: 10.1046/j.1365-2958.1998.00830.x. [DOI] [PubMed] [Google Scholar]
- 5.Mayville P, Ji G, Beavis R, Yang H, Goger M, Novick R P, Muir T W. Structure-activity analysis of synthetic autoinducing thiolactone peptides from Staphylococcus aureus responsible for virulence. Proc Natl Acad Sci USA. 1999;96:1218–1223. doi: 10.1073/pnas.96.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novick R P, Ross H F, Projan S J, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otto M, Götz F. Analysis of quorum sensing activity in staphylococci by RP-HPLC of staphylococcal delta-toxin. BioTechniques. 2000;28:1088–1090. doi: 10.2144/00286bm07. , 1092, 1096. [DOI] [PubMed] [Google Scholar]
- 8.Otto M, Süssmuth R, Jung G, Götz F. Structure of the pheromone peptide of the Staphylococcus epidermidis agr system. FEBS Lett. 1998;424:89–94. doi: 10.1016/s0014-5793(98)00145-8. [DOI] [PubMed] [Google Scholar]
- 9.Otto M, Süssmuth R, Vuong C, Jung G, Götz F. Inhibition of virulence factor expression in Staphylococcus aureus by the Staphylococcus epidermidis agr pheromone and derivatives. FEBS Lett. 1999;450:257–262. doi: 10.1016/s0014-5793(99)00514-1. [DOI] [PubMed] [Google Scholar]
- 10.Peng H L, Novick R P, Kreiswirth B, Kornblum J, Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988;170:4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raad I, Alrahwan A, Rolston K. Staphylococcus epidermidis: emerging resistance and need for alternative agents. Clin Infect Dis. 1998;26:1182–1187. doi: 10.1086/520285. [DOI] [PubMed] [Google Scholar]
- 12.Recsei P, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick R P. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 13.Rupp M E, Archer G D. Coagulase-negative staphylococci: pathogens associated with medical progress. Clin Infect Dis. 1994;19:231–245. doi: 10.1093/clinids/19.2.231. [DOI] [PubMed] [Google Scholar]
- 14.Swift S, Throup J P, Williams P, Salmond G P C, Stewart G S A B. Quorum sensing: a population-density component in the determination of bacterial phenotype. Trends Biochem Sci. 1996;21:214–219. [PubMed] [Google Scholar]
- 15.von Eiff C, Heilmann C, Herrmann M, Peters G. Basic aspects of the pathogenesis of staphylococcal polymer-associated infections. Infection. 1999;27(Suppl. 1):7–10. doi: 10.1007/BF02561610. [DOI] [PubMed] [Google Scholar]