Abstract
In hens, egg production depends on the development of germ cells in the ovary. Germ cells are established before birth, and their number gradually decreases during their lifespan. Therefore, it is essential to determine the time points of massive germ cell loss and the underlying mechanism. In this study, a gene-edited chicken with mCherry fluorescence specifically expressed in the germline was generated by the integration of the mCherry gene into the 3’-end of the DAZL locus, which facilitated the isolation of germ cells from the gonads of DAZL–mCherry embryos or chicks and quantification using flow cytometry based on the observation of red fluorescence. The results demonstrated the dynamics of germ cell development from embryos at 17 d of hatching (dh) to chickens at 7 d post-hatch (dph) and revealed a substantial loss of germ cells in the late embryonic stage (18 –19 dh) and post-hatch period (2 –3 dph). Additionally, the number of germ cells in DAZL × Guangxi Ma chicken was significantly higher than that in DAZL × Lohmann Pink chicken at 19 dh and 3 dph (P < 0.05). Furthermore, the numbers of germ cells positively correlated with the body weight in DAZL × Lohmann Pink chicken. In conclusion, our results showed the dynamics of germ cell development in chicken ovaries during peri-hatch periods and indicated the time point of substantial germ cell loss. The results provide evidence for further exploration of the underlying mechanism and serve as a reference for chicken breeding and management.
Key words: chicken, germ cell, dynamics, peri-hatch periods, gene-editing
INTRODUCTION
Primordial germ cells (PGCs) are the precursors of oocytes and sperms, which aid the transmission genetic information from one generation to the next (Mishra et al., 2019). The PGCs of chicken emerge as early as the Eyal-Giladi and Kochav (EGK) stage 3 of embryogenesis and then migrate to the germinal crescent at the Hamilton and Hamburger (HH) stage 4 (Hamburger and Hamilton, 1951). Subsequently, PGCs enter the circulation gradually as the blood vessels form at approximately HH stage 10 (Nakajima et al., 2014). These PGCs then circulate in the blood to colonize the developing gonads at approximately HH stage 24 (Yang et al., 2018).
In the embryonic ovaries of mice, PGCs proliferate through mitosis with incomplete cytokinesis, producing oocyte groups connected by cytoplasmic bridges to form germ cell cysts (Pepling and Spradling, 2001; Tingen et al., 2009). The germ cells of mice in the same germ cell cysts undergo mitosis synchronously, eventually enter meiosis, and pause at the diplotene stage in the prophase of the first meiotic division (Pepling and Spradling, 1998). Following this, germ cell cyst breakdown occurs along with the invasion of pre-granulosa cells before and after birth (Tingen et al., 2009). However, only a fraction of germ cells survive and eventually develop into oocytes after germ cell cysts break down, whereas up to 98% of germ cells serve as nurse cells to surviving oocytes (Pepling and Spradling, 2001; Nashchekin et al., 2021). In chickens, the total number of germ cells is estimated to range from 1.75 × 105 to 4.8 × 105 on the day of hatch, and germ cell cyst breakdown initiates at 3 to 4 d post-hatch. This leads to a significant decrease in the number of germ cells (to 75,000 at 7 dph) (Hughes, 1963; Gonzalez-Moran, 2011), with up to 84% of germ cells lost during germ cell cyst breakdown. The surviving germ cells, along with the emerging granular cells, form primordial follicles and constitute the follicle pools on the ovaries, which determine the reproductive performance of females during their lifespan (Zheng et al., 2014). However, the dynamics of germ cell development as well as the mechanism underlying critical events related to germ cell loss in chickens during the peri-hatch period (17 dh–7 dph) are largely unknown.
To quantify ovarian germ cells in an embryo or a chick, a serial section is commonly used and has been applied for counting germ cells in chicken (Hughes, 1963; Gonzalez-Moran, 2011) and turkey (Hall et al., 2020). An antibody-based counting technique was also used to quantify germ cells in chickens during embryonic development (6–20 dh) (Yang et al., 2018). However, the methods mentioned above are usually associated with discrepancies in statistical data owing to variations in factors such as the thickness of sections, antibody specificity, and cell morphology. Moreover, owing to the low efficiency of these methods, tracking the dynamics of germ cell development in embryonic gonads is challenging, and the accurate quantification of germ cells before and after germ cell cyst breakdown has not been reported in chickens. Therefore, the development of a precise method to track and quantify germ cells at the critical time point will be helpful for improving our understanding of chicken germ cell development.
DAZL (deleted in azoospermia like) is a gene specifically expressed in early germ cells (Bertocchini and Chuva, 2016; Kim and Han, 2018). Specific expression of the reporter gene driven by the DAZL promoter facilitates the tracking of germ cells. Here, we use a site-specific integration strategy and insert the mCherry gene into the 3’-end of the DAZL locus to achieve germline-specific fluorescence expression in PGCs and generate a DAZL–mCherry gene-edited chicken model. Using this chicken model, gonadal PGCs are visualized under a fluorescence microscope. Germ cells from embryonic gonads and post-hatched chicken could be isolated and quantified efficiently. The results of this study add to our knowledge of germ cell development in chickens.
MATERIALS AND METHODS
Animals and Ethics Statement
In this study, DAZL–mCherry chimeric chickens were first produced by gene editing technology, and then DAZL–mCherry gene-edited homozygous roosters were obtained by crossing of male and female F1. The embryos and chicks used in the experiment were produced by crossing of the homozygous DAZL–mCherry roosters with Lohmann Pink chicken or Guangxi Ma chickens. The eggs were incubated under 60% humidity at 37.8°C. The study protocol was approved by the Animal Experimental Committee of Guangxi University (GXU2018-003) and performed in compliance with the guidelines of the government of China. The animal experiments were conducted according to the guidelines for the Care and Use of Laboratory Animals.
PGC Isolation and Culture
PGCs were isolated and cultured as reported in our previous study (Xie et al., 2019). Briefly, embryo gonads were collected from 7-day-old fertilized egg (Guangdong Five-black chickens), trypsinized by treating with 0.05% trypsin-EDTA (Gibco, 25300062, USA) at 37°C for 10 min, neutralized in DMEM/F12 (BI, 01-170-1A, China) supplemented with 10% fetal bovine serum (Hyclone, SH30070.03, USA), and plated in 24-well plates. After incubation for 4 to 5 h at 37°C under 5% CO2, the suspended cells were collected and transferred to a new 24-well plate with transparent membrane inserts (1.0 mm; Millipore, PIRP 12L 04, USA), placed on mouse embryo fibroblast feeder layers, and cultured in mKO culture medium. The culture medium was refreshed every 3 d. The mKO culture medium was composed of knockout DMEM (osmolality: 250 mOsmol/kg, customized by Thermo Fisher Scientific, USA) supplemented with 0.2% chicken serum (Sigma, C5405, USA), 1% fetal bovine serum, 2 mM GlutaMax, 1 × non-essential amino acids, 0.1 mM β-mercaptoethanol, 4 ng/mL human recombinant FGF (R&D, 233-FB, USA), 1.2 mM sodium pyruvate (Sigma, P5280, USA), 1 × GS nucleoside (Millipore, GSS-1016_C, USA), 100 μg/mL sodium heparin (Sigma, H3149-100KU, USA), 25 ng/mL activin A (PeproTech, 120-14E, USA), and 1 × B27 supplement (Thermo, 17504044, USA). Unless otherwise specified, all reagents used in the study were acquired from Thermo Fisher Scientific.
Plasmid Construction and Transfection
The sgRNA oligos were annealed and constructed into the plasmid PX458 (Addgene#48138, USA). The homology arms of the donor, the DAZL-2a-mCherry-expressing cassette, were cloned from chicken genomic DNA (Gene ID: 374054) and assembled into the donor plasmid using the NEBuilder Gibson assembly master mix (NEB, E2611, USA). Cas9, sgRNAs plasmids, and donor plasmids were transfected into PGCs using Lipofectamine 3000 (Thermo Fisher, L3000015, USA) in accordance with the instructions provided by the manufacturer. Three days after the transfection, mCherry-positive PGCs were sorted using FACS and seeded in mKO medium for expanding the culture.
PGC Transplantation and Generation of DAZL–mCherry Chicken
For cell transplantation, 5,000 PGCs were injected into the cardiac vessel of chicken embryos at the HH15 stage. After PGC transplantation, the recipient embryos were incubated to hatch, and the derived chimeric chickens were raised to sexual maturation and crossed (F0 × F0) to generate homozygous F1 offspring.
Ovarian Cells Immunocytochemistry Analysis
The gonads of DAZL–mCherry embryos were isolated at 7 dh, washed 3 times with PBS, digested using 0.05% Trypsin (Gibco, 25300120, USA) at 37°C for 8 min and inactivation by culture in a serum-containing culture medium. All ovarian cells were fixed with 4% paraformaldehyde at room temperature (RT) for 15 min, and were blocked with a blocking buffer (PBS; 5% BSA; 0.1% Triton X-100) at RT for 45 min. Then, the ovarian cells were incubated at RT for 1 h with rabbit anti-cDAZL polyclonal antibodies (Homemade). After washing 3 times with PBS, ovarian cells were incubated with secondary antibodies (BOSTER, BA1127, China) at RT for 1 h. Finally, ovarian cells were mounted by ProLong Gold antifade reagent with DAPI (Invitrogen, P36935, USA) and visualized by a fluorescence microscope (OLYMPUS, IX73, Japan).
Ovarian Tissue Sectioning
The DAZL–mCherry female embryos and chicks were sacrificed, and the ovaries were dissected and fixed in Bouin's solution (25% formaldehyde, 70% saturated picric acid solution, and 5% glacial acetic acid) for 24 h at 4°C. After fixation, the ovaries were washed with PBS and stored in 70% ethanol at 4°C until further processing (Hall et al., 2020). The samples were dehydrated in gradient ethanol, embedded in paraffin, and sectioned with an automatic rotary microtome (RM2255, Leica, Wetzlar, Germany) at a thickness of 5 µm. The paraffinized sections were mounted on microscope slides. The paraffinized sections were deparaffinized, rehydrated, and stained with hematoxylin and eosin (HE) in accordance with protocol provided with the HE Staining Kit (G1120, Solarbio, Beijing, China). The results were examined under a light microscope (EX20, SDPTOP, Shanghai,China).
Quantification of Germ Cells
The left ovaries were separated from DAZL–mCherry female embryos at 19 dh and 3 dph and from chicken at 7 dph. Following this, the ovaries were washed 3 times with PBS, minced using sterile scissors, and digested using the following steps: 1) First digestion in 0.25% Trypsin (Gibco, 15050057, USA) at 37°C for 10 min and inactivation by culture in a serum-containing culture medium. The digested cells were resuspended by repetitive pipetting, followed by centrifugation at 500 rpm for 3 min, and the cell supernatant was collected; 2) The undigested tissue pellets at the bottom of the centrifuge tube were digested with Collagenase Type 4 (Sangon Biotech, A004186, China) for 30 min at 37°C, mixed every 5 min, and eventually inactivated by culturing in a serum-containing culture medium. The digested cells were resuspended by repetitive pipetting and centrifuged at 250 × g for 3 min, and the cell supernatant was collected.
All digested cells were pooled and centrifuged at 750 × g for 5 min. The supernatant was removed and the cell pellet was resuspended in the culture medium. The mCherry-positive cells were analyzed by Attune NxT flow cytometry (Thermo Fisher Scientific, Singapore).
Statistical Analysis
The results of flow cytometry were analyzed using FlowJo. Data were statistically analyzed with t tests using GraphPad Prism version 9. The results are expressed as arithmetic mean and SEM. Differences were considered significant at P < 0.05. The Pearson Product-Moment Correlation Coefficient given by R =, , and are the average values of samples in each group.
RESULTS
Generation of DAZL–mCherry Chicken
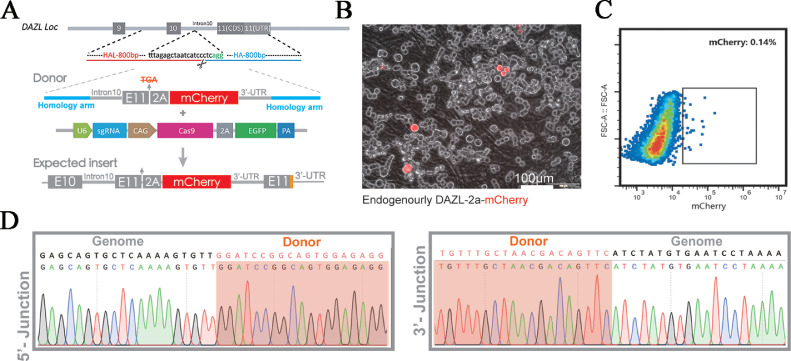
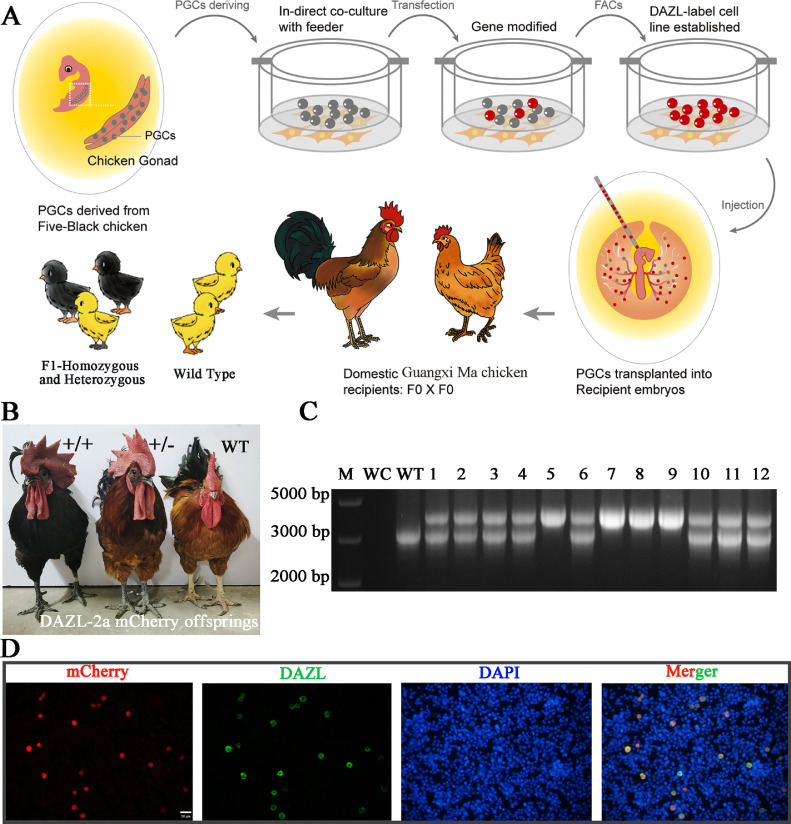
To induce the expression of the mCherry gene in germ cells using the DAZL endogenous promoter, a foreign donor cassette that contained 2A-mCherry flanked by DAZL 3’-UTR arms was introduced at the 3’-end of the DAZL using a CRISPR/Cas9-mediated site-specific gene integration strategy (Figure 1A). Three days after the co-transfection of CRISPR/Cas9, sgRNA, and donor plasmids, the expression of mCherry was observed in PGCs using fluorescence microscopy (Figures 1B and 1C). DNA sequencing of flow cytometry-sorted cells (mCherry+) revealed the successful integration of the mCherry gene in the DAZL locus of the chicken (Figure 1D). mCherry-positive PGCs were sorted using FACS, expanded, and transplanted into host embryos at stage HH14 (Figure 2A). After incubation to hatch, the male and female chimeras (F0) were crossed to obtain the F1 offspring (Figure 2B, Table S1). The donor PGCs were derived from F1 chicken, which were initially identified by black color in their feather or skin and were further validated by genotyping. Genotyping of the offspring revealed the integration of the mCherry gene into the 3’-end of the DAZL locus (Figure 2C). In addition, offspring from DAZL-2a-mCherry homozygotes presented as normal ones in terms of development and hatching rates (Table S2), indicating that the modification of the DAZL locus did not influence the development of chicken. Immunofluorescence for DAZL of ovarian cells showed that DAZL expression was detected only in cells expressing mCherry (Figure 2D), indicating that the mCherry can be used to indicate germ cells in this model of DAZL–mCherry chicken.
Figure 1.
Site-specific integration of mCherry into DAZL locus. (A) mCherry gene site-specific integration in chicken DAZL locus. (B) mCherry fluorescence was observed 48 h after co-transfection of Cas9 and donor plasmids. (C, D) mCherry positive PGCs were isolated by FACS and then genotyped by nest PCR and sanger sequencing.
Figure 2.
The generation of DAZL-2a-mCherry genetic modified chicken. (A) The strategy for generation of gene editing chicken. (B, C) F1 heterozygotes and homozygotes generated by F0 homozygotes were identified by feather and PCR genotyping. (D) Immunocytochemistry analysis of the ovarian cells in the DAZL–mCherry embryos at 7 dh.
Quantification of Germ Cells Using DAZL–mCherry Chicken
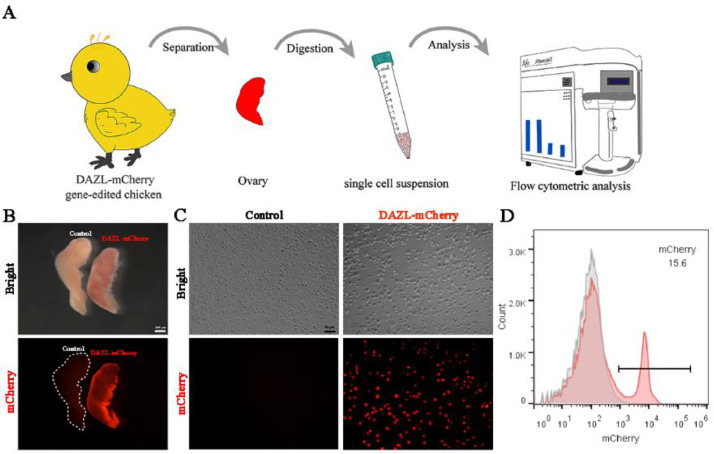
The DAZL–mCherry female embryos and chicks were derived after mating DAZL–mCherry roosters (F1) with wild-type Lohmann Pink hens to generate DAZL × Lohmann Pink chicken. To quantify germ cells in peri-hatched chickens, the ovaries of the embryos or chickens were isolated and digested into a single-cell suspension, and the number of mCherry-positive cells was counted using a flow cytometer (Figure 3A). Significant fluorescence was observed in the ovaries derived from DAZL–mCherry female embryos or chicken, but not in wild-type ovaries (Figure 3B), indicating the specific and strong expression of mCherry in germline cells. A significant number of cells showing red fluorescence was observed in cell suspensions obtained using the digested ovarian tissues (Figure 3C) and could be efficiently analyzed and counted using flow cytometry (Figure 3D), suggesting the efficacy of germ cell quantification.
Figure 3.
Establishment of the method for germ cell quantification using DAZL × Lohmann Pink chicken. (A) Workflow for germ cell quantification. (B) Fluorescent imaging of ovaries in wild-type and genetically modified Lohmann Pink chicken at 7 dph. (C) Single-cell suspension of ovarian tissue at 7 dph, as observed using fluorescence microscopy. (D) Flow cytometric analysis of germ cells in the ovarian tissue at 7 dph. Scale bar: 100 μm (B) and 50 μm (C).
Dynamics of Germ Cell Development in Peri-hatched Chickens
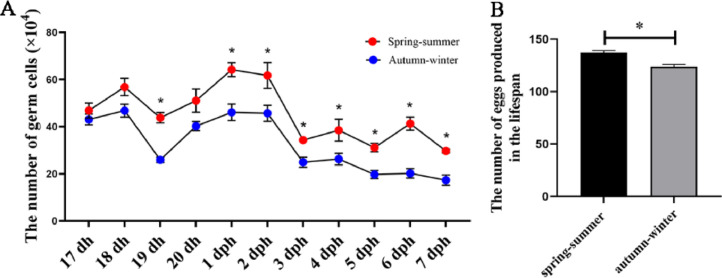
According to the established method of germ cell quantification above, the germ cells in the ovaries during the peri-hatch period (17 dh–7 dph) were quantified in the autumn to winter season. The number of germ cells in DAZL × Lohmann Pink chicken decreased significantly at 18 to 19 dh (P < 0.05) and 2 to 3 dph (P < 0.05) of ovarian development. The germ cells were quantified again in the spring to summer season, and a similar pattern of germ cell dynamics was observed. However, the number of germ cells detected in the spring–summer season was significantly higher than that detected in the autumn–winter season at 19 dh (P < 0.05) and 1 to 7 dph (P < 0.05) (Figure 4A, Table S3). Interestingly, we revisited the data record of a local farm of Guangxi Ma chicken and found that the number of eggs produced in the lifespan of chicks that hatched in the spring–summer season was significantly higher than that of chicks that hatched in the autumn–winter season (P < 0.05) (Figure 4B).
Figure 4.
Comparison of the number of germ cells in chicken and eggs production from hens hatched in different seasons. Comparison of the number of germ cells produced during the peri-hatch periods in the spring–summer and autumn–winter seasons (A). Comparison of the number of eggs produced in the lifespan of chicks hatched between the spring–summer and autumn–winter seasons (B). *P < 0.05.
Correlation of the Number of Germ Cells With the Body Weight
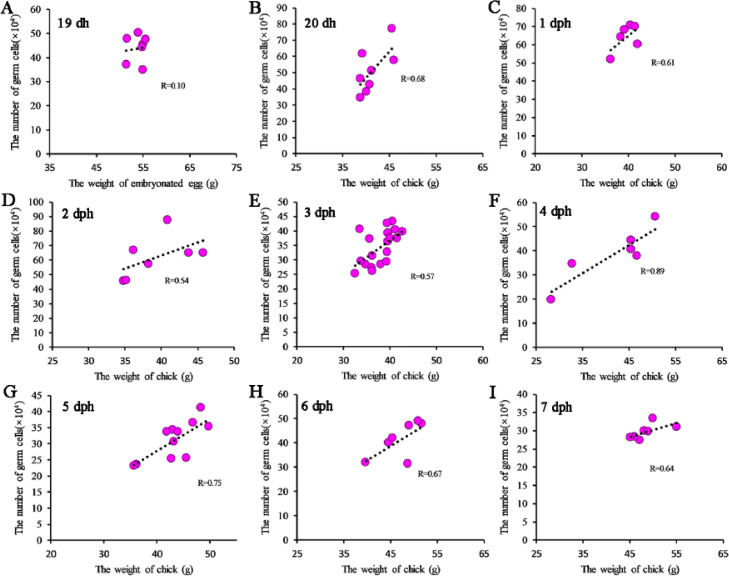
Corresponding body weights were recorded for the embryos and chicks in the autumn–winter season. A strong correlation was observed between the number of germ cells and the body weight at 1 dph (R = 0.63), 2 dph (R = 0.66), 3 dph (R = 0.55), 4 dph (R = 0.58), 5 dph (R = 0.87), 6 dph (R = 0.79), and 7 dph (R = 0.90), and a weak correlation was observed at 20 dph (R = 0.42), in the chicks. However, no correlation was observed between the number of germ cells and the egg weight at 19 dh (R = 0.10) (Figure 5). A similar correlation was observed in the spring–summer season (Figure S1).
Figure 5.
Correlation between the number of germ cells and body weight in Lohmann Pink chickens. Correlation between the number of germ cells and body weight in Lohmann Pink chicken at 19 dh (A), 20 dh (B), 1 dph (C), 2 dph (D), 3 dph (E), 4 dph (F), 5 dph (G), 6 dph (H), and 7 dph (I). R = , , and are the average values of samples in each group.
Difference Between the Number of Germ Cells in DAZL × Lohmann Pink and DAZL × Guangxi Ma Chickens
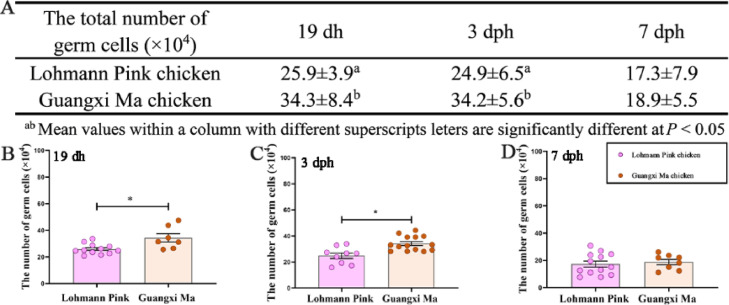
To compare the germ cell number in different genetic backgrounds, the mCherry–DAZL female chicks were derived by crossing mCherry–DAZL roosters (F1) with the wild-type Lohmann Pink hens and Guangxi Ma chicken (DAZL × Guangxi Ma chicken). The number of germ cells in the 2 genotypes was counted at 19 dh, 3 dph, and 7 dph. The results showed that the total number of germ cells in DAZL × Lohmann Pink and DAZL × Guangxi Ma chickens was approximately 25.9 × 104 and 34.3 × 104 at 19 dh, 24.9 × 104 and 34.2 × 104 at 3 dph, and 17.3 × 104 and 18.9 × 104 at 7 dph (Figure 6A). The number of germ cells in DAZL × Guangxi Ma chicken was significantly higher than that in DAZL × Lohmann Pink chicken at 19 dh and 3 dph (P < 0.05), whereas no significant difference was observed between the number of germ cells at 7 dph (Figures 6B–6D).
Figure 6.
Number of germ cells in chickens derived from cross of Guangxi Ma and Lohman Pink with DAZL-mCherry rooster. Average number of germ cells in Lohmann Pink chicken and Guangxi Ma chicken (A). Comparison of the number of germ cells between Lohmann Pink chicken and Guangxi Ma chicken (B–D). *P < 0.05.
Development of Female Germ Cells in DAZL–mCherry Chicken
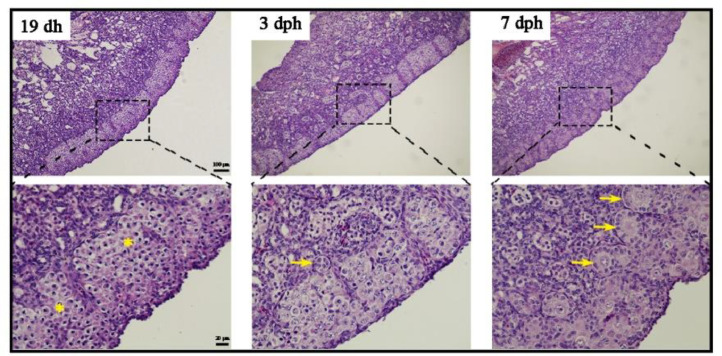
HE staining was performed to examine germ cell development in DAZL × Lohmann Pink chicken during peri-hatch periods (19 dh, 3 dph, and 7 dph). Intact germ cell cysts were observed at 19 dh. The primordial follicles were observed until 3 dph, and multiple follicles were observed at 7 dph (Figure 7). HE staining was also performed to examine the germ cell development in DAZL × Guangxi Ma chicken. The DAZL × Guangxi Ma chickens exhibited similar patterns of germ cell development at 19 dh, 3 dph, and 7 dph (Figure S2). These results indicated that the pattern of germ cell development in DAZL × Guangxi Ma chicken was consistent with that in DAZL × Lohmann Pink chicken.
Figure 7.
Histological analysis of germ cells in DAZL × Lohmann Pink chicken. Histological analysis of the ovarian tissues of DAZL × Lohmann Pink chicken (17 dh–7dph). The asterisk indicates the germ cell nest and the arrow indicates the follicle. Areas within the dashed boxes are magnified below. Scale bar: 100 μm and 20 μm.
DISCUSSION
Fluorescent labels facilitate cell tracing in live embryos or animals, and the use of tissue-specific promoters to drive the expression of reporter genes is a common strategy (Minematsu et al., 2008; Nicholas et al., 2009; Rengaraj et al., 2022). In the current study, a DAZL–mCherry gene-edited chicken was generated for the efficient tracking and accurate quantification of germ cells. It revealed the dynamics of germ cell development during 17 dh to 7 dph and the relationship between the number of germ cells and body weight. A difference in the number of germ cells was also observed in DAZL × Lohmann Pink and DAZL × Guangxi Ma chicken. The results of the study add to our knowledge on the efficient and accurate quantification of germ cells and help improve our understanding of germ cell development in peri-hatched chicken.
To decipher these critical events during germ cell development, accurate quantification of germ cells is necessary. Previously, serial sectioning was considered the preferred method for quantifying germ cells in whole ovaries (Hughes, 1963; Tilly, 2003; Hall et al., 2020). Unfortunately, using this method, the germ cell counts were affected by the morphological criteria for a nucleus (Brown et al., 2010), and the total number of germ cells was obtained in serial sections by sampling counting, which further reduced the accuracy of the method. Cell count determined by flow cytometry after immune-staining is more accurate for the quantification of cells than serial section sampling. Yang et al. explored germ cell dynamics during chicken embryonic development via flow cytometry using a germ cell-specific anti-cVASA antibody and propidium iodide staining (Yang et al., 2018). However, immune-staining is a time-consuming process and usually leads to a significant loss of cells. In our study, mCherry-positive cells can be easily tracked in the gonads of embryos and neonatal chickens. Additionally, these cells can be clearly distinguished from other ovarian cells using flow cytometry. The quantification of germ cells using DAZL–mCherry chicken only requires the isolation of gonadal cells followed by flow cytometry analysis, and the use of this method helps overcome the time-intensive and inaccurate counting procedure of the previously mentioned technique (Hughes, 1963; Yang et al., 2018).
Avian germ cells have been reported to proliferate as early as 9 dh (Hughes, 1963; Méndez et al., 2005), with meiosis initiated late at 15.5 dh (Smith et al., 2008), the diplotene stage of the first meiotic division occurring at 19 dh (Hughes, 1963), and germ cell cyst breakdown initiated at 3 to 4 dph (Gonzalez-Moran, 2011). An early study on chicken germ cell proliferation by serial sectioning showed that the germ cell numbers increased considerably from 9 dh to 17 dh, peaking at 6.8 × 105 and declining thereafter (Hughes, 1963). Another study showed that the number of germ cells increased continuously from 8 to 14 dh, from approximately 1.0 × 104 to 2.0 × 105 (Méndez et al., 2005). Yang et al. used the antibody-based technique to count the number of germ cells and found that the number increased to approximately 8.9 × 104 from 6 dh to hatch (Yang et al., 2018). The discrepancy in the results obtained from these studies might be attributable to the difference in the methods of germ cell counting or the genetics of the chicken. In our study, the dynamics of germ cell development from 17 dh to 7 dph was recorded. A decline in the number of germ cells was observed at 18 to 19 dh and 2 to 3 dph, suggesting massive germ cell loss during prophase of the first meiotic division and before germ cell breakdown. We observed the same pattern of germ cell dynamics in 2 different seasons (autumn–winter and spring–summer), which demonstrates the reliability of the quantitative approach. Additionally, results from the HE staining of gonads at 17 dh to 7 dph also confirmed massive germ cell loss and the relationship between biological events.
Apoptosis is a principal cause of the loss of germ cells, in addition to that caused by germ cell extrusion and autophagy (Wordinger et al., 1990; Pepling and Spradling, 2001; Lobascio et al., 2007; Rodrigues et al., 2009). Both apoptosis and autophagy were observed during the transformation of oogonia to oocytes (Sun et al., 2017), and germ cell extrusion was observed in the perinatal mouse ovary (Pepling and Spradling, 2001). Previous studies have shown that many germ cells were lost with germ cell cyst breakdown (Pepling and Spradling, 2001). We speculated that several germ cells underwent apoptosis, whereas some germ cells underwent autophagy and extrusion during these 2 periods, and a large number of germ cells successfully served as nurse cells and were lost through programmed cell death to accommodate granulosa cells that surrounded the oocytes before 3 dph.
The feed consumption, weight, and egg production of laying hens decreased at high temperatures (Kilic and Simsek, 2013; Kamanli et al., 2015). A higher relative humidity lowered the feed intake and induced a stress response (Kim et al., 2022). The number of germ cells in spring–summer was significantly higher than that in autumn–winter. Notably, the differences in germ cell numbers between seasons indicate that the season may be one of the key factors affecting germ cell numbers. Although the conditions of hatching and raising of chicks were standardized and consistent, the variation in temperature or humidity continue to be observed in large-scale laying houses in different seasons. Data collected during our revisit to the local farm revealed that the number of eggs produced in the lifespan of chicks hatched in spring–summer was significantly higher than that of those hatched in autumn–winter, suggesting that the seasonal effect influenced the reproductive performance of layers by affecting the growth of chicks.
Up to 98% of germ cells serve as nurse cells, supplying RNA, proteins, and organelles to the oocytes (Pepling and Spradling, 2001; Silva and Jemc, 2015). An animal's body weight is an indicator of its nutritional status (Ortiz et al., 2009). The number of germ cells in DAZL × Lohmann Pink chicks was positively correlated with the body weight after hatch, and we speculated that lighter chicks undergo the loss of a greater number of germ cells that act as nurse cells to feed oocytes. This finding suggests that to avoid the loss of germ cells caused by the weight of chicks, during the breeding of laying hens, the incubation conditions should be improved, and to reduce economic losses, underweight chicks should be removed in a timely manner.
Guangxi Ma chicken is a local characteristic chicken species in China with excellent meat quality and flavor (Yue-Hui et al., 2003; Tiemann et al., 2022). The average annual egg production of Guangxi Ma chicken is only 98 to 112; however, the average annual egg production of Lohmann Pink chicken is 300 to 320 eggs, and the laying period is longer than that of Guangxi Ma chicken (Huang et al., 2009). Further, by comparing the number of germ cells between Lohmann Pink chicken and Guangxi Ma chicken, the rate of germ cell loss in DAZL × Guangxi Ma chicken was found to be greater than that in DAZL × Lohmann Pink chicken during the peri-hatch periods. Therefore, we speculated that the varying numbers of germ cells were those lost from DAZL × Lohmann Pink and DAZL × Guangxi Ma chicken during germ cell cyst breakdown, which is one of the reasons for the difference between the egg-laying performances of the 2 breeds.
In summary, we produced a DAZL-mCherry chicken and established a method to accurately quantify the number of germ cells during peri-hatch periods for chickens. The results revealed the dynamic changes in chicken germ cells during 17 dh to 7 dph and determine the timepoint of massive germ cell loss pre- and post-hatch, providing useful data for exploring germ cell development in chickens.
Acknowledgments
This study was jointly supported by the National Key R&D Program of China (2021YFD1300100), National Natural Science Foundation of China (31960157), Guangxi Key R&D Program (AB21220005). The authors thank Zhiqiang Wang for his assistance flow cytometry analysis and appreciate the Core Facility Center of SKLCUSA (State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources) for technical support.
Disclosures
The authors declare that they have no competing interests.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.102377.
Appendix. Supplementary materials
REFERENCES
- Bertocchini F., Chuva D.S.L.S. Germline development in amniotes: a paradigm shift in primordial germ cell specification. Bioessays. 2016;38:791–800. doi: 10.1002/bies.201600025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C., Larocca J., Pietruska J., Ota M., Anderson L., Smith S.D., Weston P., Rasoulpour T., Hixon M.L. Subfertility caused by altered follicular development and oocyte growth in female mice lacking pkbalpha/akt1 1. Biol. Reprod. 2010;82:246–256. doi: 10.1095/biolreprod.109.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Moran M.G. Histological and stereological changes in growing and regressing chicken ovaries during development. Anat. Rec. (Hoboken) 2011;294:893–904. doi: 10.1002/ar.21364. [DOI] [PubMed] [Google Scholar]
- Hall G.B., Long J.A., Wood B.J., Bedecarrats G.Y. Germ cell dynamics during nest breakdown and formation of the primordial follicle pool in the domestic turkey (Meleagris gallopavo) Poult. Sci. 2020;99:2746–2756. doi: 10.1016/j.psj.2019.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V., Hamilton H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Huang Y.K., Liu J., Fan W.J., Feng D.W., Cui L.L., Han Z.B. Analysis of laying character of Lohmann Pink parent breeders in different time in each day. Guizhou. Nongye. Kexue. 2009;4:114–115. [Google Scholar]
- Hughes G.C. The population of germ cells in the developing female chick. J. Embryol. Exp. Morphol. 1963;11:513–536. [PubMed] [Google Scholar]
- Kamanli S., Durmuş I., Yalçın S., Yıldırım U., Meral Ö. Effect of prenatal temperature conditioning of laying hen embryos: hatching, live performance and response to heat and cold stress during laying period. J. Therm. Biol. 2015;51:96–104. doi: 10.1016/j.jtherbio.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Kilic I., Simsek E. The effects of heat stress on egg production and quality of laying hens. J. Anim. Vet. Adv. 2013;12:42–47. [Google Scholar]
- Kim D., Lee Y., Lee S., Lee K. Impact of relative humidity on the laying performance, egg quality, and physiological stress responses of laying hens exposed to high ambient temperature. J. Therm. Biol. 2022;103 doi: 10.1016/j.jtherbio.2021.103167. [DOI] [PubMed] [Google Scholar]
- Kim Y.M., Han J.Y. The early development of germ cells in chicken. Int. J. Dev. Biol. 2018;62:145. doi: 10.1387/ijdb.170283jh. [DOI] [PubMed] [Google Scholar]
- Lobascio A.M., Klinger F.G., Scaldaferri M.L., Farini D., De Felici M. Analysis of programmed cell death in mouse fetal oocytes. Reproduction. 2007;134:241–252. doi: 10.1530/REP-07-0141. [DOI] [PubMed] [Google Scholar]
- Méndez C., Carrasco E., Pedernera E. Adenohypophysis regulates cell proliferation in the gonads of the developing chick embryo. J. Exp. Zool A Comp. Exp. Biol. 2005;303A:179–185. doi: 10.1002/jez.a.141. [DOI] [PubMed] [Google Scholar]
- Minematsu T., Harumi T., Naito M. Germ cell-specific expression of GFP gene induced by chickenvasa homologue (Cvh) promoter in early chicken embryos. Mol. Reprod. Dev. 2008;75:1515–1522. doi: 10.1002/mrd.20894. [DOI] [PubMed] [Google Scholar]
- Mishra A.K., Campanale J.P., Mondo J.A., Montell D.J. Cell interactions in collective cell migration. Development. 2019;146 doi: 10.1242/dev.172056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Hattori T., Asano A., Ishikawa N., Tajima A. Migration and differentiation of gonadal germ cells under cross-sex germline chimeras condition in domestic chickens. J. Reprod. Dev. 2014;60:406–410. doi: 10.1262/jrd.2013-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashchekin D., Busby L., Jakobs M., Squires I., St. Johnston D. Symmetry breaking in the female germline cyst. Science. 2021;374:874–879. doi: 10.1126/science.abj3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas C.R., Xu E.Y., Banani S.F., Hammer R.E., Hamra F.K., Reijo Pera R.A. Characterization of aDazl-GFP germ cell-specific reporter. Genesis. 2009;47:74–84. doi: 10.1002/dvg.20460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz R., Cortés L., Cortés E., Medina H. Malnutrition alters the rates of apoptosis in splenocytes and thymocyte subpopulations of rats. Clin. Exp. Immunol. 2009;155:96–106. doi: 10.1111/j.1365-2249.2008.03796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepling M.E., Spradling A.C. Female mouse germ cells form synchronously dividing cysts. Development (Cambridge) 1998;125:3323–3328. doi: 10.1242/dev.125.17.3323. [DOI] [PubMed] [Google Scholar]
- Pepling M.E., Spradling A.C. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- Rengaraj D., Cha D.G., Lee H.J., Lee K.Y., Choi Y.H., Jung K.M., Kim Y.M., Choi H.J., Choi H.J., Yoo E., Woo S.J., Park J.S., Park K.J., Kim J.K., Han J.Y. Dissecting chicken germ cell dynamics by combining a germ cell tracing transgenic chicken model with single-cell RNA sequencing. Comput. Struct. 2022;20:1654–1669. doi: 10.1016/j.csbj.2022.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues P., Limback D., McGinnis L.K., Plancha C.E., Albertini D.F. Multiple mechanisms of germ cell loss in the perinatal mouse ovary. Reproduction. 2009;137:709–720. doi: 10.1530/REP-08-0203. [DOI] [PubMed] [Google Scholar]
- Silva D., Jemc J.C. Sorting out identities: an educational primer for use with “novel tools for genetic manipulation of follicle stem cells in the drosophila ovary reveal an integrin-dependent transition from quiescence to proliferation”. Genetics. 2015;201:13–22. doi: 10.1534/genetics.115.179911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.A., Roeszler K.N., Bowles J., Koopman P., Sinclair A.H. Onset of meiosis in the chicken embryo; evidence of a role for retinoic acid. BMC Dev. Biol. 2008;8:85. doi: 10.1186/1471-213X-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Sun X., Dyce P.W., Shen W., Chen H. The role of germ cell loss during primordial follicle assembly: a review of current advances. Int. J. Biol. Sci. 2017;13:449–457. doi: 10.7150/ijbs.18836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiemann I., Becker S., Büscher W., Meuser V. Exploring animal genetic resources of the domestic chicken and their behavior in the open field. J. Appl. Poult. Res. 2022;31 [Google Scholar]
- Tilly J.L. Ovarian follicle counts – not as simple as 1, 2, 3. Reprod. Biol. Endocrin. 2003;1:11. doi: 10.1186/1477-7827-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingen C., Kim A., Woodruff T.K. The primordial pool of follicles and nest breakdown in mammalian ovaries. Mol. Hum. Reprod. 2009;15:795–803. doi: 10.1093/molehr/gap073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordinger R., Sutton J., Brun-Zinkernagel A.M. Ultrastructure of oocyte migration through the mouse ovarian surface epithelium during neonatal development. Anat. Rec. 1990;227:187–198. doi: 10.1002/ar.1092270207. [DOI] [PubMed] [Google Scholar]
- Xie L., Lu Z., Chen D., Yang M., Liao Y., Mao W., Mo L., Sun J., Yang W., Xu H., Lu K., Lu Y. Derivation of chicken primordial germ cells using an indirect Co-culture system. Theriogenology. 2019;123:83–89. doi: 10.1016/j.theriogenology.2018.09.017. [DOI] [PubMed] [Google Scholar]
- Yang S.Y., Lee H.J., Lee H.C., Hwang Y.S., Park Y.H., Ono T., Han J.Y. The dynamic development of germ cells during chicken embryogenesis. Poult. Sci. 2018;97:650–657. doi: 10.3382/ps/pex316. [DOI] [PubMed] [Google Scholar]
- Yue-Hui M.A., Gui-Fang X.U., Wang D.Y., Liu H.L., Yang Y. Study on dynamic information of animal genetic resources in China. Agric. Ences China. 2003;2:80–84. [Google Scholar]
- Zheng W., Zhang H., Gorre N., Risal S., Shen Y., Liu K. Two classes of ovarian primordial follicles exhibit distinct developmental dynamics and physiological functions. Hum. Mol. Genet. 2014;23:920–928. doi: 10.1093/hmg/ddt486. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.