Abstract
Taurine (TAU), a sulfur-containing amino acid that synthesized from methionine and cystine, plays vital roles in maintenance of redox balance. The effect of substitution of TAU for methionine was evaluated in vivo and in vitro. The effects of replacing methionine with TAU and additional TAU supplementation on the performance and antioxidant capacity of laying hens were evaluated. The in vitro cultured chicken primary hepatocytes and intestinal epithelial cells were further employed. Two hubdred eighty-eight 40-wk-old Isa brown laying hens were divided into 4 groups and subjected one to the following treatments: fed with basal diet with 0.17% crystallized DL-Met (CON), the control diet and replace 25% (21% total Met, 21TAU) or 50% (42% total Met, 42TAU) of crystallized DL-Met with taurine, the control diet supplemented with 0.1% taurine (0.1% TAU). The laying rate, feed intake, egg weight, and feed efficiency were not influenced (P > 0.05) by TAU replacement or additional TAU supplementation. In the liver, 0.1% TAU decreased SOD but increased GSH-Px activity (P < 0.01). In duodenum, 42TAU decreased SOD activity (P < 0.05) while 0.1% TAU decreased GSH level and SOD activity (P < 0.05). In the hepatocytes, TAU treatment decreased (P < 0.05) the MDA and GSH contents, whereas increased SOD and GSH-Px activities (P < 0.05). Meanwhile, TAU treatment decreased (P < 0.05) the protein expression of Nrf2 while increase Keap1 expression. The mRNA expression of Nrf2, SOD1, SOD2, CAT, and GCLC were increased (P < 0.05) and GSR were decreased (P < 0.05) by 0.1% TAU. In the intestinal epithelial cells, TAU treatment decreased (P < 0.05) SOD activity, increased (P < 0.05) CAT activity, and decreased (P < 0.05) the mRNA and protein expression of Nrf2. In summary, partial substitution methionine for taurine (21–42%) has no influence on egg performance of hens. Taurine enhances the antioxidative capacity in hepatocyte but not in the enterocytes and if taurine could offer an improved effect on antioxidant capacity needs to be verified under oxidative stress-challenged conditions.
Key words: taurine, methionine, antioxidative capacity, laying hens
INTRODUCTION
Methionine (Met) is the initiating amino acid in the synthesis of almost all eukaryotic proteins (Brosnan and Brosnan, 2006). Met is the first limiting amino acids in laying hens fed corn-soybean meal-based diets (Bunchasak, 2009). Moreover, Met participate the synthesis of glutathione, which play an important role in the maintenance of redox balance (Seidel et al., 2019). Dietary supplementation of DL-Met stimulates the synthesis of the glutathione (Wang et al., 2019) and the redox status of broilers (Magnuson et al., 2020). DL-Met supplementation can increase glutathione peroxidase (GSH-Px) activity, glutathione (GSH) content to reduce the free radical injury caused in acute heat-stressed quails (Del Vesco et al., 2014).
Sulfur-containing amino acids include methionine (Met), cysteine (Cys), homocysteine, and taurine (TAU). TAU is a catabolite of Cys and can be synthesized by Met. In mammals, Met is catalyzed by methionine adenosyltransferase (MAT) to form S-adenosylmethionine (SAM), which, in turn, metabolizes to Cys via the catalysis of S-adenosylhomocysteine hydrolase (SAHH), cystathionine β-synthase (CβS), and cystathionine γ-lyase (CγL), and then Cys is metabolized into TAU, inorganic sulfate, or GSH (Brosnan and Brosnan, 2006). In chickens, excess SAA are metabolized to taurine by the transsulfuration pathway as well as mammals (Hosokawa et al., 1988; Ohta and Ishibashi, 1997). In chicken enterocytes, Cys and TAU content is higher when DL-2-hydroxy-(4-methylthio) butanoic acid is used as a source of DL-Met (Martín-Venegas et al., 2006). TAU, one of the most abundant free amino acids in many excitable tissues including the brain, skeletal and cardiac muscles, serves many important functions in mammals (El Idrissi, 2019). TAU is rich in the blood of layer-type chickens (Takawaki et al., 2013) and in the liver and thigh muscle of broilers (Huang et al., 2014). TAU level in blood is 6- to 11-fold higher in avian species than in humans (Szwergold and Miller, 2014). Moreover, TAU is an essential nutrient against oxidative damage in mammal (Brosnan and Brosnan, 2006) and in broilers (Xu et al., 2020). TAU repairs and regulates the balance of the redox state by acting on the endogenous antioxidant like sulfhydryl and GSH (Terrill et al., 2016; Han et al., 2020). In chicken, TAU is involved in the regulation of antioxidant enzymes like SOD, GSH-Px to resist cellular damage from free radical (Li et al., 2020). In methionine cycle, homocysteine is converted to cysteine, which feeds into the generation of glutathione (GSH) and taurine to maintain the redox balance in the cell via the transsulfuration pathway (Lauinger and Kaiser, 2021). Hence, we hypothesized that taurine could spare methionine for the maintenance of redox balance.
In poultry, a portion of the dietary requirement for sulfate by the growing chick is utilized in the synthesis of TAU, which is augmented by DL-Met and repressed by high concentrations of Cys (Martin, 1972). In broilers fed with diet containing 2% Met, appropriate levels of TAU in replacement of Met can improve growth performance, immune system, T-AOC, and lipid metabolism (Lv et al., 2017). Dietary taurine supplementation (0.25 or 0.50%), however, has the potential to decrease egg weight without affecting egg production, feed conversion, or body weight (Yamazaki and Takemasa, 1998). Therefore, the effect of TAU in laying hens remains to be elucidated further.
The aim of this study was to explore the effect of substitution of TAU for methionine on laying performance egg quality, organ index, blood metabolites, and antioxidative system. The laying performance, egg quality, organ index, blood metabolites, and antioxidative system were determined in vivo. Moreover, the antioxidative effect of TAU was evaluated in the cultured chicken primary hepatocytes and enterocytes in vitro.
MATERIALS AND METHODS
All procedures in the study were approved by the Animal Care Committee of Shandong Agricultural University and were performed in accordance with the guidelines for experimental animals of the Ministry of Science and Technology (Beijing, China).
Birds and Management
A total of two hundred eighty-eight 40-wk-old Isa brown laying hens with similar body weight (1.836 ± 0.171 kg) and laying rate were used. The hens were randomly divided into 4 groups and subjected to one of following treatments: fed with basal diet supplemented with 0.17% crystallized DL-Met (Control, CON), the basal diet replace 21.03% (21Treplaced 0.0425% crystallized DL-Met with taurine (99%), approximately 21% total dietary methionine) or 42.07% methionine with taurine (42Treplaced 0.085% crystallized DL-Met with taurine), the basal diet supplemented with 0.17% DL-Met and 0.1% taurine (0.1% TAU). The doses of TAU were selected according to Surai et al. (2020). The basal diet was formulated according to the recommendations of the National Research Council standard (NRC, 1994, Table 1). In each treatment, there were 6 replicates and each replicate had 12 hens. The experimental hens were reared in battery cage (60-cm length × 45-cm width × 50-cm height) and each hen had approximately 900 cm2 of floor space. Each cage was equipped with one nipple drinker and a feeder. All the hens had free access to feed and water during the experimental period. Housing temperature and relative humidity were maintained at 23 ± 2°C and 65 ± 5%, respectively. The photoperiod was 16 h light and 8 h dark. The number of egg and egg weight was recorded daily, feed intake was recorded weekly, and feed conversion ratio (FCR) was calculated. Taurine (99%) was purchased from Shijiazhuang Richland Biochemical Co. (Shijiazhuang, Hebei, China).
Table 1.
Composition and nutrient of experimental diets of laying hens (%).
| Ingredients, % | Control diet |
|---|---|
| Corn, 8.5% | 54.055 |
| Wheat bran | 7.99 |
| Soybean oil | 1.79 |
| Soybean meal, 43% | 24.29 |
| Salt | 0.35 |
| Limestone powder | 9.60 |
| Calcium hydrogen phosphate | 1.44 |
| Choline chloride, 50% | 0.09 |
| Lysine, 99% | 0.10 |
| Methionine, 99% | 0.17 |
| Taurine | – |
| Trace element feed1 | 0.10 |
| Vitamin premix feed2 | 0.025 |
| Total | 100.00 |
| Nutrient level, %3 | |
| Crude protein | 16.95 |
| Metabolic energy, MJ/Kg | 11.29 |
| Calcium | 4.45 |
| Total Phosphorus | 0.41 |
| Lysine | 0.84 |
| Methionine | 0.20 |
| Aspartic acid | 1.37 |
| Threonine | 0.59 |
| Serine | 0.78 |
| Glutamate | 2.43 |
| Glycine | 0.60 |
| Alanine | 0.70 |
| Cysteine | 0.12 |
| Valine | 0.51 |
| Isoleucine | 0.48 |
| Leucine | 1.26 |
| Tyrosine | 0.39 |
| Phenylalanine | 0.74 |
| Histidine | 0.43 |
| Arginine | 0.93 |
| Proline | 0.74 |
Note:
Premix provided the following per kg of diet: vitamin A, 3.96 mg; vitamin D3, 0.06 mg; vitamin E, 20 mg; vitamin K, 3 mg; thiamin mononitrate, 1.70 mg; riboflavin, 5.50 mg; pantothenic acid, 9.40 mg; niacin, 28 mg; pyridoxine, 6.60 mg; vitamin B12, 3.30 mg; biotin, 0.10 mg; folic acid, 0.60 mg; choline chloride, 470 mg.
Premix provided the following per kg of diet: Fe, 55 mg; Zn, 88 mg; Mn, 88 mg; Cu, 5.50 mg; I, 1.70 mg; Se, 0.30 mg.
The amino acid composition was measured values and the other nutrients were calculated values.
Sample Collection
After 8-wk treatment, 2 hens were randomly selected from each replicate and 12 hens in total for each treatment. Blood sample was obtained from a wing vein of hen after overnight feed withdrawal. The blood samples were centrifuged at 3,000 g for 15 min to obtain the serum, which was stored at −20°C for further analysis. Thereafter, the hens were sacrificed and the liver, duodenum, jejunum, and ileum tissue sample were obtained. The samples were snap-frozen in liquid nitrogen and stored at −80°C for further analysis. The weight of chickens, liver, abdominal fat, duodenum, jejunum, ileum was recorded to calculate the organ index.
Determination of Feed Nutrients Contents
The experimental diets were analyzed for dry matter (method 930.15), crude protein (CP; method 990.03), calcium (Ca; method 984.01), and phosphorus (AP; method 965.17) of control diet as described by AOAC International (1996). Dietary AAs were determined by ion-exchange chromatography using a Hitachi L-8900 AA Analyzer (Tokyo, Japan) after acid hydrolysis with 6 N HCl and reflux for 24 h.
For taurine measurement, 100 mg feed sample was mixed with 10 mL 0.1% formic acid in a 15 mL tube, vortexed for 5 min, and ultrasonic extracted for 30 min. The mixture was centrifuged at 4°C and 12,000 rpm for 5 min. The supernatant was purified on Sep-Pak C18 microcolumn (Waters, Milford, MA), filtered through 0.22 μm PTFE filter (Forneeds, Shanghai), and then used for measurement of taurine. Taurine was measured by LC-MS/MS (Sciex 4500) according to the method by Ricciutelli et al. (2014). Taurine was not detected in control diet and were 0.39 ± 0.02, 0.77 ± 0.06, and 1.07 ± 0.05 mg/kg, respectively.
Egg Quality
For the last 2 d of the 8-wk experimental period, 10 eggs around mean egg weight of the replicate were collected and 60 eggs in each treatment were collected for determination of egg quality. Eggshell thickness was measured by averaging the 3 locations on the egg (air cell, equator, and sharp end) using an eggshell thickness tester (ETG-1061, Tokyo, Japan). Eggshell strength was measured using an egg force reader (EFG-0503, Tokyo, Japan). Haugh unit, albumen height, and yolk color were determined with Multifunctional Egg Detector (EMT-5200, ROBOTMATION, Japan). Yolk and eggshell percentages were expressed as the ratio of yolk weight and shell weight to egg weight.
Serum Variables
Serum glucose (GLU), creatine kinase (CK), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total protein (TP), triglyceride (TG), total cholesterol (TCHO), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C) were measured spectrophotometrically (UV 2450, Shimadzu Corp., Nakagyo-ku, Kyoto, Japan) with commercial diagnostic kits (Sichuan Maccura Biotechnology Co., Sichuan, China).
Malondialdehyde (MDA), Glutathione (GSH), and Antioxidant Enzyme Activity Assays
MDA (A003-1-2), GSH (A006-2-1), GSH-Px (A005-1-2), CAT (A007-1-1), and SOD (A001-3-2) were measured with commercial kits (Institute of Nanjing Jiancheng Biological Engineering, Nangjing, China).
Primary Culture of Chicken Hepatocytes
Hepatocytes cultured from specific pathogen-free (SPF) chicken embryo of 17 d. In a nutshell, Livers with removed gallbladder, sarcolemma and connective tissue were collected and washed in HBSS. Livers were cut into small pieces then washed in HBSS, and collagenase IV Sigma-Aldrich Trading Co.Ltd. (Shanghai, China) was used to digested at 37°C. After filtration, the cells were collected and washed in HBSS with centrifugation of 1,000 rpm. To detached the hepatocytes the method of Density gradient centrifugation in a layer with 60% Percoll was used (Sigma). The cell suspension was pushed on the Percoll layer with Pasteur pipette and centrifuged for 15 min at 3,000 rpm. The cells were collected and washed in HBSS with centrifugation of 1,000 rpm then seeded in the in 6 well plates with William's E Medium (GIBCO,Grand Island, NY) supplemented with 10% FBS (Crystalgen, United States). When the cells bespread over 80%, the taurine treatment (0, 1, 5, 10 mM) on hepatocytes for 24 h after rinsed with HBSS then collected the cells prepare for mRNA and protein expression measurement.
Primary Culture of Chicken Intestinal Epithelial Cells
Intestinal epithelial cells (IECs) cultured from SPF chicken embryo of 19 d. In brief, the duodenal mucosa was extruded and in Hank's Balanced Salt Solution (HBSS) (Solarbio, China). After remove the blood cells and intestinal content, the material was digested with collagenase I (MP Biomedicals, United States) at 37°C then was filtered following centrifuged at 800 rpm for 10 min. The cellular pellet was saved then washed twice to thrice with HBSS at 800 rpm for 10 min and resuspended in DMEM-F12 (GIBCO) supplemented with 10% FBS (Crystalgen, United States). The ICEs were treated when cultured with over 80% cell at 37°C and 5% CO2 in 6 well plates (Zhang et al., 2019). The taurine treatment (0, 1, 5, 10 mM) on IECs for 24 h after rinsed with HBSS then collected the cells prepare for mRNA and protein measurement.
Quantitative Reverse Transcription PCR Analysis
The mRNA expression levels of Nrf2 and the antioxidant enzyme-related genes SOD1, SOD2, CAT, GSS, and GSR in tissues or cells were quantified using quantitative real-time PCR. In short, total RNA was extracted with Trizol (15,596-026, Invitrogen, San Diego, CA) and checked the clarity and quality with Aspectrophotometer (Eppendorf, Germany). Reverse transcription was performed using Commercial kits (0489,703,0001, Roche, Switzerland). The cDNA was amplified with SYBR Green (0491,391,4001, Roche, Switzerland) and the real-time PCR reaction on a Q5 Real-Time PCR System (Applied Biosystems, Thermo, Waltham, MA). Primers were designed and synthesized by Shanghai Sangon Biotechnology Company (Shanghai, China). The GAPDH as a reference gene. Using the comparative CT method (2−ΔΔCT) to regularize the differences (Wang et al., 2013). The expression levels of genes encoding superoxide dismutase 1 (SOD1), superoxide dismutase 2 (SOD2), catalase (CAT), glutathione synthetase (GSS), glutathione reductase (GSR), nuclear factor erythroid 2-related factor 2 (Nrf2), glutamate-cysteine ligase catalytic subunit (GCLC), glutamate-cysteine ligase modifier subunit (GCLM), and heme oxygenase 1 (HO-1) were determined. The primer sequences are listed in Table 2.
Table 2.
Primers for the targeted and reference transcripts.
| Gene | Primer sequence | Accession no. | Product size (bp) |
|---|---|---|---|
| GAPDH | F:ACATGGCATCCAAGGAGTGAG R:GGGGAGACAGAAGGGAACAGA |
NM_204305.1 | 244 |
| SOD1 | F:TTGTCTGATGGAGATCATGGCTTC R:TGCTTGCCTTCAGGATTAAAGTGAG |
NM_205064.1 | 98 |
| SOD2 | F:CAGATAGCAGCCTGTGCAAATCA R:GCATGTTCCCATACATCGATTCC |
NM_204211.1 | 86 |
| CAT | F:GTTGGCGGTAGGAGTCTGGTCT R:GTGGTCAAGGCATCTGGCTTCTG |
NM_001031215.1 | 182 |
| GSS | F:GCTCAGTGCCAGTTCCAGTT R:GGTCCCACAGTAAAGCCAAG |
XM_425692.4 | 115 |
| GSR | F:CCAGAACACCACCAGAAAGG R:TTACCAAAGAGCCGAAGTGC |
XM_001235016.3 | 114 |
| Nrf2 | F:TGACCCAGTCTTCATTTCTGC R:GGGCTCGTGATTGTGCTTAC |
NM_205117.1 | 186 |
| GCLC | F:CAACCACCCAACACTCTGG R:CTCTTGCCTCCTCTTCCTCA |
XM_419910.3 | 130 |
| GCLM | F:CCTGAAGAAAGGGATGAACTG R:CCTGAAGAAAGGGATGAACTG |
NM_001031215.1 | 114 |
| HO-1 | F:CTTCGCACAAGGAGTGTTAAC R:CATCCTGCTTGTCCTCTCAC |
NM_205344.1 | 78 |
Abbreviations: CAT, catalase; GAPDH, glyceraldehydes-3-phosphate dehydrogenase; GCLC, glutamate-cysteine ligase catalytic subunit; GCLM, glutamate-cysteine ligase modifier subunit; GSR, glutathione reductase; GSS, glutathione synthetase; HO-1, heme oxygenase 1; Nrf2, nuclear factor erythroid 2-related factor 2; SOD1, superoxide dismutase 1; SOD2, superoxide dismutase 2.
Western Blot Analysis
The expression of Nrf2 protein were measured by western blotting. Briefly, the nuclear protein was collected using nuclear protein extraction kit (Beyotime Biotech Co., Jiangsu, China). The supernatant quantified by BCA protein assay kit (Beyotime Biotech Co., Jiangsu, China). The 7.5% SDS-polyacrylamide gel electrophoresis was run and blocked for 1 h (Beyotime Biotech Co., Jiangsu, China). The membrane was incubated with primary and secondary antibody (Nrf2, rabbit and polyclonal antibody from Abcam; Lamin B1, mouse monoclonal antibody from Abcam; horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG from Beyotime). Used the LumiGlo Reserve Substrate (KPL Inc., Gaithersburg, MD) and VisionWorks LS Biospectrum 500 Imaging System (UVP LLC, Upland, CA) to quantified the protein expression. The Lamin B1 as an internal reference.
Statistical Analysis
All data were analyzed using one-way ANOVA with Statistical Analysis Systems statistical software package (Version 9.4, SAS Institute, Cary, NC), and the results were presented as means ± SEM. Tukey's test was used for mean comparisons. P < 0.05 was considered significantly different.
RESULTS
The ADFI, egg weight, laying rate, and FCR were not changed by dietary treatment (P > 0.05, Table 3). Compared with the control, the organ index of jejunum was decreased (P < 0.05) by 42TAU while was not significantly changed (P > 0.05) by 21TAU and 0.1% TAU treatments (Table 4). The duodenal (P = 0.051) and abdominal fat (P = 0.090) showed a tendency to be changed by dietary treatment. In contrast, liver and ileum indexes were not altered by treatment (P > 0.05, Table 4).
Table 3.
Effect of replacement of methionine with taurine and additional taurine supplementation on laying performance of hens (n = 6).
| Control | 21TAU | 42TAU | 0.1% TAU | Pooled SEM | P-value | |
|---|---|---|---|---|---|---|
| ADFI (g/d) | 116.08 | 115.31 | 115.17 | 115.91 | 2.11 | 0.909 |
| Laying rate (%) | 82.05 | 83.86 | 85.96 | 84.45 | 4.65 | 0.697 |
| Egg weight (g) | 61.35 | 60.84 | 61.21 | 61.32 | 0.79 | 0.778 |
| FCR (g/g) | 1.98 | 1.99 | 1.96 | 1.98 | 0.03 | 0.510 |
ADFI, average daily feed intake; FCR, feed conversion ratio; 21AU, replacement of 21% methionine with taurine; 42TAU, replacement of 42% methionine with taurine; 0.1% TAU, fed the basal diet supplemented with 0.1% taurine.
Table 4.
Effect of replacement of methionine with taurine and additional taurine supplementation on relative organ weight of laying hens (n = 12).
| % | Control | 21TAU | 42TAU | 0.1% TAU | Pooled SEM | P-value |
|---|---|---|---|---|---|---|
| Liver | 1.70 | 1.63 | 1.64 | 1.66 | 0.10 | 0.762 |
| Duodenum | 0.78 | 0.62 | 0.68 | 0.68 | 0.08 | 0.051 |
| Jejunum | 1.10a | 0.96ab | 0.89b | 0.91b | 0.11 | 0.041 |
| Ileum | 0.71 | 0.58 | 0.65 | 0.60 | 0.08 | 0.119 |
| Abdominal Fat | 2.46 | 3.33 | 2.71 | 2.17 | 0.64 | 0.090 |
21TAU, replacement of 21% methionine with taurine; 42TAU, replacement of 42% methionine with taurine; 0.1% TAU, fed the basal diet supplemented with 0.1% taurine.
P < 0.05.
Compared with control, serum TP content was not changed by dietary treatments, whereas the 21TAU and 42TAU groups had lower TP level than that of 0.1% TAU groups (P < 0.05, Table 5). The ALT activity (P = 0.065) and HDL-C content (P = 0.054) tended to be changed by dietary treatment. Serum AST and CK activities, GLU, TG, TCHO, and LDL-C levels were not significantly influenced (P > 0.05, Table 5).
Table 5.
Effect of replacement of methionine with taurine and additional taurine supplementation on blood parameters of laying hens (n = 12).
| Item | CON | 21TAU | 42TAU | 0.1% TAU | Pooled SEM | P-value |
|---|---|---|---|---|---|---|
| AST, U/L | 2.42 | 1.40 | 1.66 | 2.50 | 1.02 | 0.426 |
| ALT, U/L | 165.9 | 153.8 | 139.5 | 156.0 | 13.7 | 0.065 |
| CK, U/L | 1028 | 956 | 983 | 1060 | 274 | 0.953 |
| TP, g/L | 47.78a | 42.54ab | 40.51b | 52.06a | 4.28 | 0.005 |
| GLU, mmol/L | 11.58 | 11.81 | 11.33 | 11.57 | 0.56 | 0.716 |
| TG, mmol/L | 8.81 | 6.57 | 7.74 | 11.31 | 3.25 | 0.278 |
| TCHO, mmol/L | 1.98 | 1.44 | 1.56 | 2.16 | 0.46 | 0.145 |
| HDL-C, mmol/L | 1.81 | 1.39 | 1.45 | 2.15 | 0.39 | 0.054 |
| LDL-C, mmol/L | 0.59 | 0.41 | 0.46 | 0.67 | 0.19 | 0.271 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; CK, creatine kinase; GLU, glucose; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; TCHO, total cholesterol; TP, total protein; LDL-C, low-density lipoprotein cholesterol; 21TAU, replacement of 21% methionine with taurine; 42TAU, replacement of 42% methionine with taurine; 0.1% TAU, fed the basal diet supplemented with 0.1% taurine.
P < 0.05.
Eggshell breaking strength and eggshell percentage were decreased (P < 0.05) by dietary treatment and 21TAU group had the lowest ones compared with other groups (Table 6). Eggshell thickness showed a tendency (P = 0.058) to be decreased in 21TAU-hens, compared to other treatment. Egg shape index, albumen height, Haugh unit, yolk color, and yolk percentage were not changed by dietary treatment (P > 0.05, Table 6).
Table 6.
Effect of replacement of methionine with taurine and additional taurine supplementation on egg quality of laying hens (n = 60).
| Item | Control | 21TAU | 42TAU | 0.1% TAU | Pooled SEM | P-value |
|---|---|---|---|---|---|---|
| Shape index | 1.28 | 1.29 | 1.30 | 1.29 | 0.02 | 0.668 |
| Shell thickness, mm | 3.18 | 3.06 | 3.18 | 3.14 | 0.71 | 0.058 |
| Breaking strength, kg·f | 4.08a | 3.53b | 4.06ab | 4.03ab | 2.98 | 0.028 |
| Haugh unit | 77.1 | 80.7 | 81.2 | 78.3 | 3.81 | 0.342 |
| Albumen height, mm | 6.24 | 8.22 | 6.94 | 6.73 | 0.48 | 0.174 |
| Yolk percentage, % | 25.90 | 26.33 | 25.95 | 26.29 | 0.01 | 0.505 |
| Eggshell percentage, % | 9.35ab | 9.24b | 9.71ab | 9.75a | 0.00 | 0.012 |
21TAU, replacement of 21% methionine with taurine; 42TAU, replacement of 42% methionine with taurine; 0.1% TAU, fed the basal diet supplemented with 0.1% taurine.
P < 0.05.
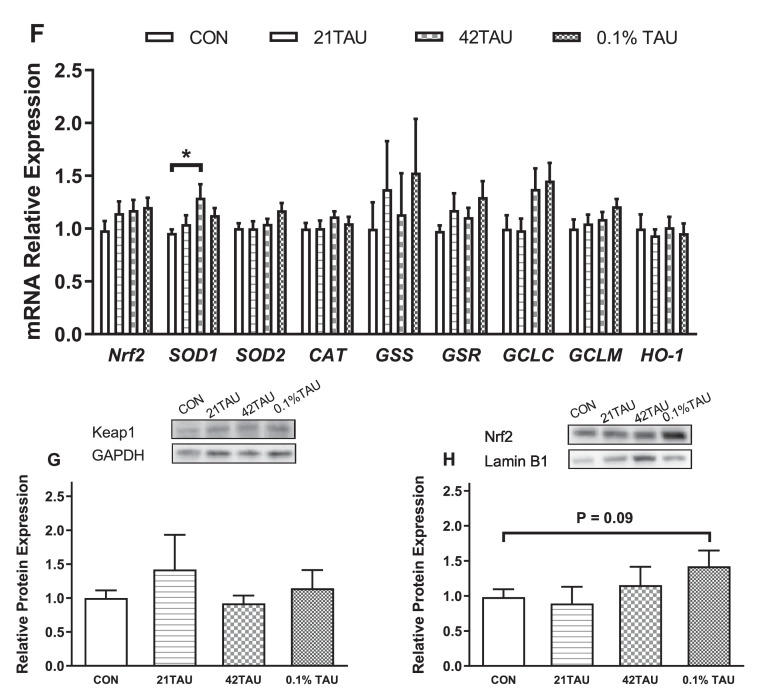
In the liver, compared with control, MDA content tended to be decreased by 42TAU group (P = 0.08) but not altered (P > 0.05) by 21TAU and 42TAU dietary treatment (Figure 1A, B). Compared with control, SOD activity was decreased (P < 0.01) whereas GSH-Px activity was increased (P < 0.01) by 0.1% TAU group, but was not influence (P > 0.05) by 21TAU and 42TAU treatments (Figure 1D, E). In contrast, GSH level and CAT activity were not changed by dietary treatment (P > 0.05, Figure 1B, C). Compared with control, the mRNA level of SOD1 was increased (P < 0.05) by 42TAU treatment but not by other treatments. By contrary, the expressions of genes SOD2, CAT, GSS, GSR, GCLC, GCLM, HO-1, and Nrf2 were not significantly altered (P > 0.05) by dietary treatments (Figure 1F). Similarly, dietary treatment has no significant influence (P > 0.05) on the protein expression level of Keap1, while Nrf2 showed a tendency (P = 0.09) to be increased by 0.1% TAU treatment (Figure 1G, H).
Figure 1.
Effect of replacement of methionine with taurine on contents of MDA (A) and GSH (B), the activities of CAT (C), SOD (D), and GSH-Px (E), mRNA expression levels of genes encoding antioxidant enzymes (F), and protein levels of Nrf2 (G) and keap1 (H) in the liver of laying hens. Data were presented as mean ± SE (n = 12). 21TAU: replacement of 21% methionine with taurine; 42TAU: replacement of 42% methionine with taurine. *P < 0.05; ⁎⁎P < 0.01.
In duodenum, compared with control, 0.1% TAU group showed a tendency to decrease (P = 0.07) MDA level and decreased GSH (P < 0.05; Figure 2A, B). In contrast, 21TAU and 42TAU has not detectable influence on MDA and GSH levels (P > 0.05). SOD activity was decreased significantly by 42TAU and 0.1%TAU treatments (P < 0.05) but not by 21TAU (P > 0.05) (Figure 2D). Dietary treatment, however, had no influence on CAT and GSH-Px activities (P > 0.05, Figure 2C, E). Compared with control, the level of Nrf2 mRNA was increased by 21TAU, 42TAU, and 0.1% TAU treatments (P < 0.05). The expression of SOD2 and GCLC mRNA was increased by 0.1% Tau treatment (P < 0.05, Figure 2F). Compare to control, the expression of CAT (P = 0.09) and GSS (P = 0.09) showed a tendency to be increased by 0.1% TAU and 21TAU, respectively. The expressions of genes SOD1, GSR, GCLM, and HO-1 were not changed (P > 0.05) by dietary TAU treatment.
Figure 2.
Effect of replacement of methionine with taurine on contents of MDA (A) and GSH (B), the activities of CAT (C), SOD (D), and GSH-Px (E), and mRNA expression levels of genes encoding antioxidant enzymes (F) in duodenum of laying hens. Data were presented as mean ± SE (n = 12). 21AU: replacement of 21% methionine with taurine; 42TAU: replacement of 42% methionine with taurine. *P < 0.05.
In jejunum and ileum, the MDA and GSH concentrations and activities of CAT, SOD, and GSH-Px were not altered (P > 0.05) by dietary treatment (Figures 3, 4). No significant change was detected (P > 0.05) for the transcriptional levels of genes Nrf2, SOD1, SOD2, CAT, GSS, GSR, GCLC, GCLM, and HO-1 (Figures 3, 4).
Figure 3.
Effect of replacement of methionine with taurine on contents of MDA (A) and GSH (B), and the activities of CAT (C), SOD (D), and GSH-Px (E), and mRNA expression levels of genes encoding antioxidant enzymes (F) in jejunum of laying hens. Data were presented as mean ± SE (n = 12).
Figure 4.
Effect of replacement of methionine with taurine on contents of MDA (A) and GSH (B), and the activities of CAT (C), SOD (D), and GSH-Px (E), and mRNA expression levels of genes encoding antioxidant enzymes (F) in ileum of laying hens. Data were presented as mean ± SE (n = 12).
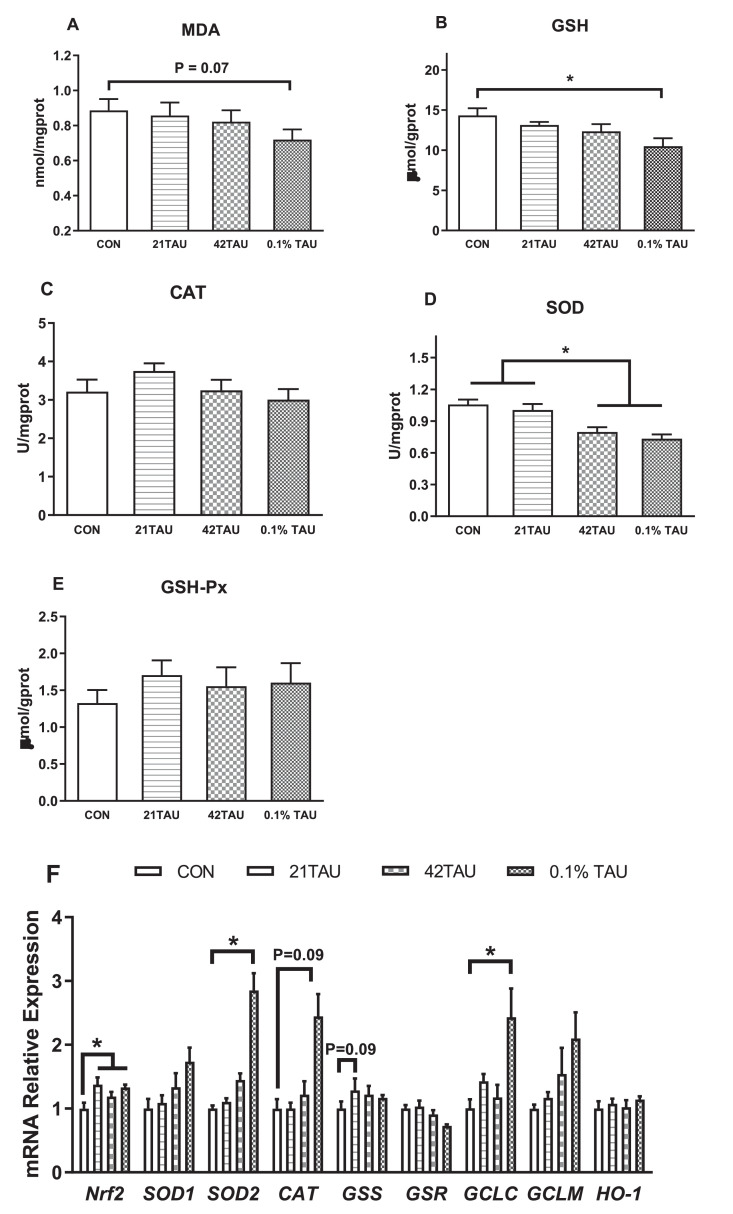
In the in vitro cultured hepatocytes, MDA and GSH contents were decreased (P < 0.05) by TAU at a dose of 5 mM or 10 mM but not altered by 21TAU, compared to control (Figure 5A, B). Compared with control, the activities of SOD and GSH-Px were increased only by 10 mM TAU treatment (Figure 5D, E), whereas CAT was not changed by TAU in the ranged of 1 to 10 mM (Figure 5C). The mRNA expressions of Nrf2, SOD1, SOD2, CAT, and GCLC were increased (P < 0.05) while GSR was decreased (P < 0.05) by 10 mM TAU but not changed by 1- and 5-mM TAU treatments (Figure 5F). In contrast, the expression of GSS, GCLM, and SOD2 was not changed by TAU treatment (P > 0.05). The protein level of Nrf2 was decreased by TAU from 1 to 10 mM whereas Keap1 was increased by 1 and 5 mM TAU treatments (P < 0.05, Figure 5G, H).
Figure 5.
Effect of taurine (TAU: 0, 1, 5, 10 mM) on MDA (A), GSH (B), and antioxidant enzyme activity CAT (C), SOD (D), and GSH-Px (E), mRNA expression levels of genes encoding antioxidant enzymes (F), and protein levels of keap1 (G) and Nrf2 (H) in primary cultured hepatocytes. Data were presented as mean ± SE (n = 6). *P < 0.05.
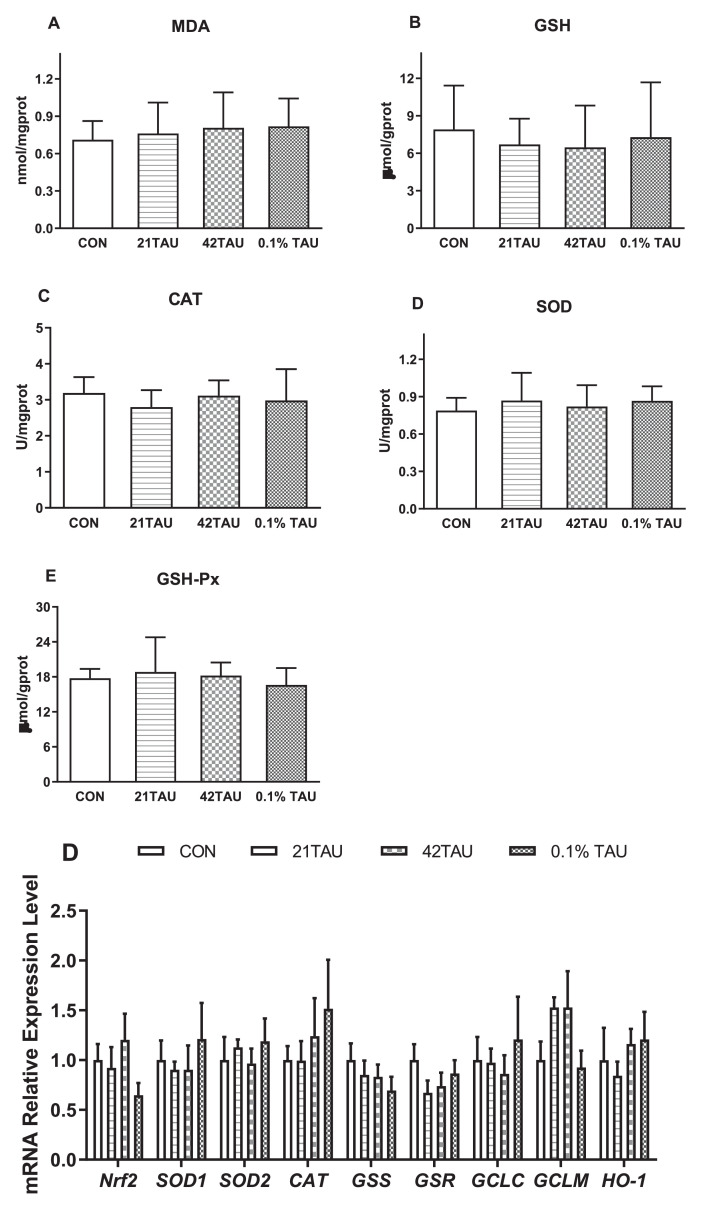
In ICEs, both MDA and GSH concentrations were not influenced (P > 0.05) by TAU treatment. The activities of SOD and CAT were elevated (P < 0.05) in 10 mM TAU treatment, whereas GSH-Px was not changed by TAU treatment (P > 0.05) at all the tested dosages (Figure 6C, D, E). All the expression level of tested genes was not changed by TAU, except Nrf2 mRNA was decreased by 10 mM TAU treatment (P < 0.05, Figure 6F). TAU administration decreased the protein level of Nrf2 at a dose of 10 mM (P < 0.05) and the expression of Keap1 was not altered by TAU (P > 0.05, Figure 6G, H).
Figure 6.
Effect of taurine (TAU: 0, 1, 5, 10 mM) on MDA (A), GSH (B), and antioxidant enzyme activity CAT (C), SOD (D), and GSH-Px (E), mRNA expression levels of genes encoding antioxidant enzymes (F), and protein levels of Nrf2 (G) and keap1 (H) in primary cultured enterocytes. The results are expressed as the mean ± SE (n = 6). *P < 0.05.
DISCUSSION
Methionine, as the first limiting amino acid in corn-soybean diet, is necessary for the maintenance of laying performance of hens (Conde-Aguilera et al., 2016; Reda et al., 2020). Methionine is the precursor for cellular methylation and the synthesis of cysteine and the methionine-sparing effect of cystine/cysteine has been proved by previous works (Finkelstein and Mudd, 1967; Finkelstein et al., 1988). The cysteine, in turn, can be used in the synthesis of taurine (Stipanuk, 2020). Hence, it is speculated the taurine may have a methionine-sparing effect. Taurine is one of the most abundant amino acids that exist in organism in free state. For example, taurine concentrations in breast and thigh muscle tissues of broilers are 10.4 and 165.5 mg/100 g tissue, respectively (Huang et al., 2014), which is comparable to that in skeletal muscle of lamb (59.9–75.5 mg/100 g, Radzik-Rant et al., 2020) and rats (17–33 μmol/g wet tissue, Iwata et al., 1986). TAU can be synthesized from Met in chicken (Martin, 1972). Dietary TAU addition slightly improves the performance of chicks fed a purified diet deficient in sulfur AA, whereas the addition of a mixture of methionine and cystine achieves the best growth performance (Anderson et al., 1975). Dietary supplementation with encapsulated DL-Met + Lys changes plasma sulfur AA profile with elevated TAU and decreased Cys in laying hens and broilers (Sun et al., 2020a,b). In this study, the substitution of TAU for Met was further evaluated. The unobvious changed laying performance by 21TAU and 42TAU and 0.1%TAU treatment indicated that dietary supplementation of TAU or partial replacement of Met with TAU has no unfavorable influence on the laying performance of hens. This result was in line with the previous work by Hajjarmanesh et al. (2022), who reported that dietary TAU supplementation (1.96 g/kg diet) had no significant effect on egg production and egg weight of laying hens fed a basal diet with 0.42% digestible Met during 71 to 76 wk of age. Similarly, laying hens fed with diet supplemented with TAU (0.25 or 0.50%) showed a potential to decrease egg weight without affecting egg production, feed conversion, or body weight (Yamazaki and Takemasa, 1998). Tau is normally considered to be non-essential for poultry, whereas it could become semi-essential amino acid under stress states (Surai et al., 2020; Uyanga et al., 2022). Met replacement by taurine resulted in decreased TP and a tendency of decreased HDL-C, which was not detected in 0.1% TAU treatment. If the observation was a result of reduced Met supplementation needs to be investigated further. Collectively, the results suggested that dietary supplementation of taurine within a range from 0.04% to 0.08% have a methionine-sparing effect.
In this study, decreased egg breaking strength and the tendency of decreased eggshell thickness was observed in 21TAU treatment but not in 42TAU and 0.1% TAU groups. As the negative effect was not detected in 42TAU group, hence, it seems that the reduced dietary Met level play a minor role in the deteriorated eggshell quality of 21TAU hen. This speculation was supported by the work of Safaa et al. (2008), who reported that reducing dietary Met level (0.36 vs. 0.31%) had no influence on eggshell quality. Moreover, this result was contrary to the previous result that adding taurine (1.96 g/kg diet) increased the egg shell breaking strength in hens after peak production by increasing the integrity of calcium columns and decreased the size of egg shell pores (Hajjarmanesh et al., 2022). The effect of TAU and/or Met on calcium absorption and deposition during eggshell formation needs to be investigated further.
It is interesting to note that the small intestinal tract weight was reduced by TAU treatment. This result was in line with the result in broilers by Huang et al. (2014), who reported that dietary supplementation of taurine retards small intestinal growth especially the jejunum. The impaired subepithelial morphology partly by increasing the concentration of conjugated bile acids was speculated to be responsible for the reduced intestinal tract weight. In mice, dietary taurine supplementation (165 mg/kg) could regulate the gut micro-ecology, which might be of benefit to health by inhibiting the growth of harmful bacteria (Yu et al., 2016). In human, taurine is the most abundant free amino acid found in duodenal mucosa (Ahlman et al., 1993). It is presumed that such a high intracellular level in the gut may imply taurine play an important role in intestinal mucosa metabolism and function. Hence, the effect of taurine on intestinal tract function needs to be investigated further.
Methionine has been proved to be associated with antioxidant capacity of organisms. Met restriction can intervene in the natural antioxidant capacity by leading to the production of endogenous enzymes that reduce oxidative stress and, in turn, DNA damage, cancer, cardiovascular disease, neuropsychiatric disorders, and neurodegenerative diseases (Martínez et al., 2017). Dietary Met restriction decreases mitochondrial ROS production and oxidative stress in brain and kidney of rat (Caro et al., 2009), which, however, cannot be explained by the changes in activities of antioxidant enzymes (Maddineni et al., 2013). In broiler, additional dietary supplementation of DL-Met mitigates oxidative stress in digestive tract induced by high stocking density conditions (Miao et al., 2021) or Eimeria spp. challenge (Khatlab et al., 2019). Moreover, TAU was a classic free radical scavenger and displayed notable scavenging potential like peroxyl radical, nitric oxide, and superoxide donors (Oliveira et al., 2010). TAU has been proved to play an important role in the maintenance of the redox balance (Brosnan and Brosnan, 2006; Terrill et al., 2016; Han et al., 2020). Hence, the effect of substitution of TAU for Met on antioxidant system of liver and intestine was further determined. In the hens fed diet with TAU in replacement of Met, the MDA and GSH contents and CAT and GSH-Px activities in the liver, duodenum, jejunum, and ileum were not significantly changed, except SOD activity was decreased by 42TAU in the liver and duodenum, indicating TAU in replacement of partial Met has no unfavorable influence on the antioxidant system of hens. In contrast, 0.1% TAU decreased SOD but increased GSH-Px in the liver, decreased SOD activity and GSH level in duodenum, and had no influence on all the parameters in jejunum and ileum. The different responses of the antioxidative enzymes may relate to their biochemical characteristics. Superoxide dismutase (SOD) is the first detoxification enzyme and most powerful antioxidant in the cell (Ighodaroab and Akinloye, 2018), while CAT has a relative higher Km for hydrogen peroxide whereas GSH-Px has a lower one (Little et al. 1970; Ausin et al., 1988). Collectively, the result indicates that partially substitution of TAU for Met or 0.1% additional supplementation of TAU takes little effect on the antioxidative system of liver and small intestine. Numerous studies showed that TAU was beneficial for the redox balance in tissues, and reduction of intracellular TAU may cause severe cell dysfunction (Das et al. al., 2011; Zhang et al., 2021). Dietary supplementation of TAU enhances cardiomyocyte antioxidant ability of broilers (Li et al., 2020). Therefore, the antioxidative effect of TAU was further investigated in the primary cultured hepatocytes and ICEs from chicken embryos.
In the hepatocytes, the reduced MDA was in accordance with the elevated SOD, CAT, and GSH-Px activities in TAU treatments, suggesting TAU enhances the antioxidative capacity by increasing the antioxidative enzyme system. Moreover, in the hepatocytes, GSH level was suppressed by TAU treatment. This result seems to be related to the increased sulfur amino acids in the cultured medium in TAU treatment. Bagley and Stipanuk (1995) reported that glutathione production was markedly lower in hepatocytes from rats fed excess sulfur amino acids. In this study, the in vitro result was different from the in vivo observation, which may be a result of activated Keap1/Nrf2 pathway. The Keap1/Nrf2 pathway is the principal protective response to oxidative stress. Under homeostatic conditions, Keap1 represses Nrf2 activity under quiescent conditions, whereas Nrf2 liberates from Keap-1-mediated repression on exposure to stress, translocates to the nucleus, and activates the antioxidant transcription program (Yamamoto et al., 2018). In the present study, the protein levels of Keap1 and Nrf2 in the liver were not significant changed by dietary TAU treatments. In the in vitro cultured hepatocytes, however, the increased Keap1 and decreased Nrf2 was observed after 24-h TAU treatment. The result may imply the possibility of activated Keap1/Nrf2 pathway and augmented Nrf2 degradation. This speculation was supported by the increased expression of genes SOD1, SOD2, and CAT by TAU treatment.
Different from the hepatocytes, SOD was decreased while CAT was increased by 10 mM TAU in the in vitro cultured enterocytes, suggesting that the antioxidative effect of TAU depends on the types of cells. Indeed, taurine is primarily synthesized in the liver and play an antioxidative protect role on hepatocytes (Baliou et al., 2021). TAU is the most abundant free amino acid found in human duodenal mucosa and its transport in the intestine have gained more attention (O'Flaherty et al., 1997). The role of taurine plays in the gut, however, has not been well studied. In intestinal Caco-2 cells, the increased membrane lipid peroxidation by docosahexaenoic acid was partially retarded by the addition of taurine (Roig-Pérez et al., 2009). In this study, the protein levels of Keap1 and Nrf2 were not significantly changed except of decreased Nrf2 by 10 mM TAU, in line with the unchanged transcriptional levels of genes encoding antioxidant enzymes, suggesting that taurine supplementation at the dose from 1 to 10 mM have not activated the Keap1/Nrf2 pathway. In this study, the effect of taurine was not evaluated with an oxidative-stress challenged model. Hence, the regulating effect of TAU on antioxidative enzyme system of enterocytes remains to be elucidated further.
In summary, replacement of 25% and 50% of crystallized DL-Met with taurine and supplement with 0.1% Tau had no unfavorable effect on the laying performance of hens fed a diet with 0.2% Met. Taurine supplementation facilitates the maintenance of redox balance in the liver of laying hens through multiple mechanisms, in which, Keap1/Nrf2 pathway is suggests to be involved. The result suggests that dietary supplementation of taurine have a methionine-sparing effect for laying hens. The beneficial effect of taurine on antioxidant capacity needs to be verified under oxidative stress-challenged conditions. Further study is needed to understand the metabolic relationship among methionine, cystine, and taurine action in laying hens.
Acknowledgments
The authors would thank Mei Zhao, Hui Wang, Kelin Li, MingHui Wang, Liqin Zhao, Sheng Li, Zengmin Liu and Mongze Song for their assistance in the collection of animal samples and experiment of molecular.
Disclosures
The authors have declared no conflicts of interests.
References
- Ahlman B., Leijonmarck C.E., Wernerman J. The content of free amino acids in the human duodenal mucosa. Clin. Nutr. 1993;12:266–271. doi: 10.1016/0261-5614(93)90044-5. [DOI] [PubMed] [Google Scholar]
- Anderson J.O., Warnick R.E., Dalai R.K. Replacing dietary methionine and cystine in chick diets with sulfate or other sulfur compounds. Poult. Sci. 1975;54:1122–1128. doi: 10.3382/ps.0541122. [DOI] [PubMed] [Google Scholar]
- AOAC International . 17th ed. AOAC Int.; Gaithersburg, MD: 1996. Official Methods of Analysis. [Google Scholar]
- Austin L., Arthur H., de Niese M., Gurusinghe A., Baker M.S. Micromethods in single muscle fibers. 1. Determination of catalase and superoxide dismutase. Anal. Biochem. 1988;174:568–574. doi: 10.1016/0003-2697(88)90057-7. [DOI] [PubMed] [Google Scholar]
- Bagley P.J., Stipanuk M.H. Rats fed a low protein diet supplemented with sulfur amino acids have increased cysteine dioxygenase activity and increased taurine production in hepatocytes. J. Nutr. 1995;125:933–940. doi: 10.1093/jn/125.4.933. [DOI] [PubMed] [Google Scholar]
- Baliou S., Adamaki M., Ioannou P., Pappa A., Panayiotidis M.I., Spandidos D.A., Christodoulou I., Kyriakopoulos A.M., Zoumpourlis V. Protective role of taurine against oxidative stress (review) Mol. Med. Rep. 2021;24:605. doi: 10.3892/mmr.2021.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan J.T., Brosnan M.E. The sulfur-containing amino acids: an overview. J. Nutr. 2006;136:1636S–1640S. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- Bunchasak C. Role of dietary methionine in poultry production. J. Poult. Sci. 2009;46:169–179. [Google Scholar]
- Caro P., Gomez J., Sanchez I., Naudi A., Ayala V., Lopez-Torres M., Pamplona R., Barja G. Forty percent methionine restriction decreases mitochondrial oxygen radical production and leak at complex I during forward electron flow and lowers oxidative damage to proteins and mitochondrial DNA in rat kidney and brain mitochondria. Rejuvenat. Res. 2009;12:421–434. doi: 10.1089/rej.2009.0902. [DOI] [PubMed] [Google Scholar]
- Conde-Aguilera J.A., Cholet J.C., Lessire M., Mercier Y., Tesseraud S., Van Milgen J. The level and source of free-methionine affect body composition and breast muscle traits in growing broilers. Poult. Sci. 2016;95:2322–2331. doi: 10.3382/ps/pew105. [DOI] [PubMed] [Google Scholar]
- Das J., Ghosh J., Manna P., Sil P.C. Taurine suppresses doxorubicin-triggered oxidative stress and cardiac apoptosis in rat via up-regulation of PI3-K/Akt and inhibition of p53, p38-JNK. Biochem. Pharmacol. 2011;81:891–909. doi: 10.1016/j.bcp.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Del Vesco A.P., Gasparino E., Grieser D.O., Zancanela V., Gasparin F.R., Constantin J., Oliveira Neto A.R. Effects of methionine supplementation on the redox state of acute heat stress-exposed quails. J. Anim. Sci. 2014;92:806–815. doi: 10.2527/jas.2013-6829. [DOI] [PubMed] [Google Scholar]
- El Idrissi A. Taurine regulation of neuroendocrine function. Adv. Exp. Med. Biol. 2019;1155:977–985. doi: 10.1007/978-981-13-8023-5_81. [DOI] [PubMed] [Google Scholar]
- Finkelstein J.D., Martin J.J., Harris B.J. Methionine metabolism in mammals. The methionine-sparing effect of cystine. J. Biol. Chem. 1988;263:11750–11754. [PubMed] [Google Scholar]
- Finkelstein J.D., Mudd S.H. Trans-sulfuration in mammals-the methionine-sparing effect of cystine. J. Biol. Chem. 1967;242:873–880. [PubMed] [Google Scholar]
- Hajjarmanesh M., Zaghari M., Hajati H., Ahmad A.H. Effects of zinc, manganese, and taurine on egg shell microstructure in commercial laying hens after peak production. Biol. Trace Elem. Res. 2022 doi: 10.1007/s12011-022-03388-z. [DOI] [PubMed] [Google Scholar]
- Han H., Zhang J., Chen Y., Shen M., Yan E., Wei C., Yu C., Zhang L., Wang T. Dietary taurine supplementation attenuates lipopolysaccharide-induced inflammatory responses and oxidative stress of broiler chickens at an early age. J. Anim. Sci. 2020;98:skaa311. doi: 10.1093/jas/skaa311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa Y., Niizeki S., Tojo H., Sato I., Yamaguchi K. Hepatic cysteine dioxygenase activity and sulfur amino acid metabolism in rats: possible indicators in the evaluation of protein quality. J. Nutr. 1988;118:456–461. doi: 10.1093/jn/118.4.456. [DOI] [PubMed] [Google Scholar]
- Huang C.X., Wang B., Min Z., Yuan J. Dietary inclusion level and time effects of taurine on broiler performance, meat quality, oxidative status and muscle taurine content. Br. Poult. Sci. 2014;55:598–604. doi: 10.1080/00071668.2014.943692. [DOI] [PubMed] [Google Scholar]
- Ighodaroab O.M., Akinloye O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018;54:287–293. [Google Scholar]
- Iwata H., Obara T., Kim B.K., Baba A. Regulation of taurine transport in rat skeletal muscle. J. Neurochem. 1986;47:158–163. doi: 10.1111/j.1471-4159.1986.tb02844.x. [DOI] [PubMed] [Google Scholar]
- Khatlab A.S., Del Vesco A.P., de Oliveira Neto A.R., Fernandes R.P.M., Gasparino E. Dietary supplementation with free methionine or methionine dipeptide mitigates intestinal oxidative stress induced by Eimeria spp. challenge in broiler chickens. J. Anim. Sci. Biotechnol. 2019;10:58. doi: 10.1186/s40104-019-0353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauinger L., Kaiser P. Sensing and signaling of methionine metabolism. Metabolites. 2021;11:83. doi: 10.3390/metabo11020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Yang J., Lyu Q., Wu G., Lin S., Yang Q., Hu J. Taurine prevents cardiomyocyte apoptosis by inhibiting the calpain-1/cytochrome c pathway during RVH in broilers. Amino Acids. 2020;52:453–463. doi: 10.1007/s00726-020-02824-5. [DOI] [PubMed] [Google Scholar]
- Little C., Olinescu R., Reid K.G., O'Brien P.J. Properties and regulation of glutathione peroxidase. J. Biol. Chem. 1970;245:3632–3636. [PubMed] [Google Scholar]
- Lv Q., Sun L., Cui Y., Yang J., Yang Q., Yu X., Liu M., Ning Z., Hu J. Effects of replacement of methionine in diets with taurine on growth performance and blood index in broilers. Adv. Exp. Med. Biol. 2017;2:989–1000. doi: 10.1007/978-94-024-1079-2_78. [DOI] [PubMed] [Google Scholar]
- Maddineni S., Nichenametla S., Sinha R., Wilson R.P., Richie J.P. Methionine restriction affects oxidative stress and glutathione-related redox pathways in the rat. Exp. Biol. Med (Maywood). 2013;238:392–399. doi: 10.1177/1535370213477988. [DOI] [PubMed] [Google Scholar]
- Magnuson A.D., Liu G., Sun T., Tolba S.A., Xi L., Whelan R., Lei X.G. Supplemental methionine and stocking density affect antioxidant status, fatty acid profiles, and growth performance of broiler chickens. J. Anim. Sci. 2020;98:skaa092. doi: 10.1093/jas/skaa092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W.G. Sulfate metabolism and taurine synthesis in the chick. Poult. Sci. 1972;51:608–612. doi: 10.3382/ps.0510608. [DOI] [PubMed] [Google Scholar]
- Martinez Y., Li X., Liu G., Bin P., Yan W., Mas D., Valdivie M., Hu C.A., Ren W., Yin Y. The role of methionine on metabolism, oxidative stress, and diseases. Amino Acids. 2017;49:2091–2098. doi: 10.1007/s00726-017-2494-2. [DOI] [PubMed] [Google Scholar]
- Martin-Venegas R., Geraert P.A., Ferrer R. Conversion of the methionine hydroxy analogue DL-2-hydroxy-(4-methylthio) butanoic acid to sulfur-containing amino acids in the chicken small intestine. Poult. Sci. 2006;85:1932–1938. doi: 10.1093/ps/85.11.1932. [DOI] [PubMed] [Google Scholar]
- Miao Z., Dong Y., Qin X., Yuan J., Han M., Zhang K., Shi S., Song X., Zhang J., Li J. Dietary supplementation of methionine mitigates oxidative stress in broilers under high stocking density. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . 9th, Rev. Ed. National Academy Press; Washington, DC: 1994. Nutrient Requirements of Poultry. [Google Scholar]
- O'Flaherty L., Stapleton P.P., Redmond H.P., Bouchier-Hayes D.J. Intestinal taurine transport: a review. Eur. J. Clin. Invest. 1997;27:873–880. doi: 10.1046/j.1365-2362.1997.2000747.x. [DOI] [PubMed] [Google Scholar]
- Ohta Y., Ishibashi T. Effects of days after changing diets, body weight and feed consumption on excretion of taurine in broilers. Jpn. Poult. Sci. 1997;34:282–291. [Google Scholar]
- Oliveira M.W., Minotto J.B., de Oliveira M.R., Zanotto-Filho A., Behr G.A., Rocha R.F., Moreira J.C., Klamt F. Scavenging and antioxidant potential of physiological taurine concentrations against different reactive oxygen/nitrogen species. Pharmacol. Rep. 2010;62:185–193. doi: 10.1016/s1734-1140(10)70256-5. [DOI] [PubMed] [Google Scholar]
- Radzik-Rant A., Rant W., Sosnowiec G., Świątek M., Niżnikowski R., Szymańska Ż. The effect of genotype and muscle type on the physico-chemical characteristics and taurine, carnosine and L-carnitine concentration in lamb meat. Arch. Anim. Breed. 2020;63:423–430. doi: 10.5194/aab-63-423-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda F.M., Swelum A.A., Hussein E.O.S., Elnesr S.S., Alhimaidi A.R., Alagawany M. Effects of varying dietary DL-methionine levels on productive and reproductive performance, egg quality, and blood biochemical parameters of quail breeders. Animals (Basel) 2020;10:1839. doi: 10.3390/ani10101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciutelli M., Caprioli G., Cortese M., Lombardozzi A., Strano M., Vittori S., Sagratini G. Simultaneous determination of taurine, glucuronolactone and glucuronic acid in energy drinks by ultra high performance liquid chromatography-tandem mass spectrometry (triple quadrupole) J. Chromatogr. A. 2014;1364:303–307. doi: 10.1016/j.chroma.2014.08.083. [DOI] [PubMed] [Google Scholar]
- Roig-Pérez S., Ferrer C., Rafecas M., Moretó M., Ferrer R. Correlation of taurine transport with membrane lipid composition and peroxidation in DHA-enriched Caco-2 cells. J. Membr. Biol. 2009;228:141–150. doi: 10.1007/s00232-009-9166-4. [DOI] [PubMed] [Google Scholar]
- Safaa H.M., Serrano M.P., Valencia D.G., Arbe X., Jiménez-Moreno E., Lázaro R., Mateos G.G. Effects of the levels of methionine, linoleic Acid, and added fat in the diet on productive performance and egg quality of brown laying hens in the late phase of production. Poult Sci. 2008;87:1595–1602. doi: 10.3382/ps.2008-00005. [DOI] [PubMed] [Google Scholar]
- Seidel U., Huebbe P., Rimbach G. Taurine: a regulator of cellular redox homeostasis and skeletal muscle function. Mol. Nutr. Food. Res. 2019;63 doi: 10.1002/mnfr.201800569. [DOI] [PubMed] [Google Scholar]
- Stipanuk M.H. Metabolism of sulfur-containing amino acids: how the body copes with excess methionine, cysteine, and sulfide. J. Nutr. 2020;150:2494S–2505S. doi: 10.1093/jn/nxaa094. [DOI] [PubMed] [Google Scholar]
- Sun M., Jiao H., Wang X., Uyanga U.A., Zhao J., Lin H. Encapsulated crystalline lysine and DL-methionine have higher efficiency than the crystalline form in broilers. Poult. Sci. 2020;99:6914–6924. doi: 10.1016/j.psj.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Zhao J., Wang X., Jiao H., Lin H. Use of encapsulated L-lysine-HCl and DL-methionine improves postprandial amino acid balance in laying hens. J. Anim. Sci. 2020;98:skaa315. doi: 10.1093/jas/skaa315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surai P.F., Kochish I.I., Kidd M.T. Taurine in poultry nutrition. Anim. Feed Sci. Technol. 2020;260 [Google Scholar]
- Szwergold B.S., Miller C.B. Potential of birds to serve as pathology-free models of type 2 diabetes, part 2: do high levels of carbonyl-scavenging amino acids (e.g., taurine) and low concentrations of methylglyoxal limit the production of advanced glycation end-products? Rejuvenation Res. 2014;17:347–358. doi: 10.1089/rej.2014.1561. [DOI] [PubMed] [Google Scholar]
- Takawaki M., Tanizawa H., Nakasai E., Shiraishi J.I., Kawakami S.I., Oka T., Tsudzuki M., Bungo T. Comparison of plasma amino acid levels of two breeds of Japanese native chicken and commercial layer line. Int. J. Poultry Sci. 2013;12:90–93. [Google Scholar]
- Terrill J.R., Pinniger G.J., Graves J.A., Grounds M.D., Arthur P.G. Increasing taurine intake and taurine synthesis improves skeletal muscle function in the mdx mouse model for Duchenne muscular dystrophy. J. Physiol. 2016;594:3095–3110. doi: 10.1113/JP271418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyanga V.A., Oke E.O., Amevor F.K., Zhao J., Wang X., Jiao H., Onagbesan O.M., Lin H. Functional roles of taurine, L-theanine, L-citrulline, and betaine during heat stress in poultry. J Anim Sci Biotechnol. 2022;13:23. doi: 10.1186/s40104-022-00675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.J., Li Y., Song Q.Q., Guo Y.Y., Jiao H.C., Song Z.G., Lin H. Corticosterone regulation of ovarian follicular development is dependent on the energy status of laying hens. J Lipid Res. 2013;54(7):1860–1876. doi: 10.1194/jlr.M036301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yin X., Yin D., Lei Z., Mahmood T., Yuan J. Antioxidant response and bioavailability of methionine hydroxy analog relative to DL-methionine in broiler chickens. Anim. Nutr. 2019;5:241–247. doi: 10.1016/j.aninu.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S.W., Lu Z., Ma B.B., Xing T., Li J.L., Zhang L., Jiang Y., Gao F. Dietary taurine supplementation enhances antioxidative capacity and improves breast meat quality of broiler chickens. Br. Poult. Sci. 2020;61:140–145. doi: 10.1080/00071668.2019.1691147. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M., Takemasa M. Effects of dietary taurine on egg weight. Poult. Sci. 1998;77:1024–1026. doi: 10.1093/ps/77.7.1024. [DOI] [PubMed] [Google Scholar]
- Yu H., Guo Z., Shen S., Shan W. Effects of taurine on gut microbiota and metabolism in mice. Amino Acids. 2016;48:1601–1617. doi: 10.1007/s00726-016-2219-y. [DOI] [PubMed] [Google Scholar]
- Zhang J., Sun Y., Zhao L., Chen T., Fan M., Jiao H., Zhao J., Wang X., Li F., Li H., Lin H. SCFAs-induced GLP-1 secretion links the regulation of gut microbiome on hepatic lipogenesis in chickens. Front. Microbiol. 2019;10:2176. doi: 10.3389/fmicb.2019.02176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wei Z., Yang M., Liu D., Pan M., Wu C., Zhang W., Mai K. Dietary taurine modulates hepatic oxidative status, ER stress and inflammation in juvenile turbot (Scophthalmus maximus L.) fed high carbohydrate diets. Fish Shellfish Immunol. 2021;109:1–11. doi: 10.1016/j.fsi.2020.11.029. [DOI] [PubMed] [Google Scholar]