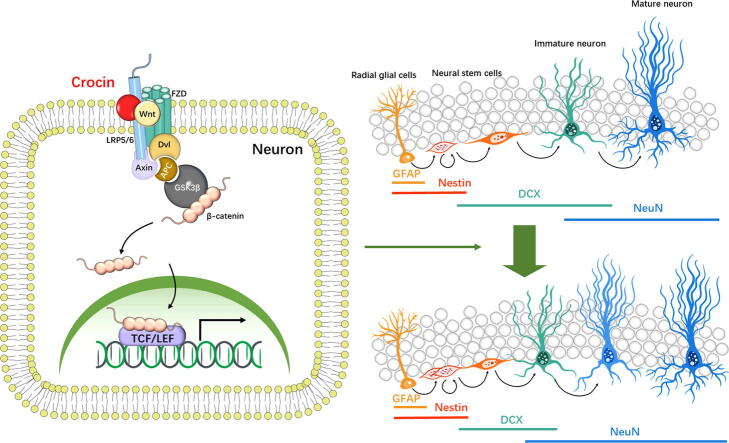
Graphical abstract
Keywords: Depression, Adult hippocampal neurogenesis, Wnt/β-catenin, Crocin
Highlights
-
•
Crocin exhibits antidepressant property without disturbing male sexual function.
-
•
Crocin promotes hippocampal adult neurogenesis by activating Wnt/β-catenin signaling.
-
•
Adult neurogenesis inhibition compromises crocin-induced antidepressant action.
-
•
Blockage of Wnt signaling suppresses crocin-induced antidepressant response.
-
•
Crocin rescues depressive symptoms induced by chronic unpredictable mild stress.
Abstract
Introduction
Adult hippocampal neurogenesis (AHN) is acknowledged to play a critical role in depression. Emerging evidence suggests that the Wnt/β-catenin pathway can modulate hippocampal neurogenesis. Crocin, a natural carotenoid, possesses antidepressant property. Yet, how it affects neurogenesis and exerts antidepressant response remains unknown.
Objective
To explore the role of AHN and Wnt/β-catenin in the antidepressant action of crocin.
Methods
Depressive-related behaviors, including sucrose preference test (SPT), tail suspension test (TST), forced swimming test (FST), and sexual behaviors were performed following crocin treatment. Neurogenesis was characterized via immunohistochemistry, immunofluorescence, Golgi staining and electrophysiology approach. Wnt/β-catenin signaling was examined with western blot analysis. The role of AHN Wnt/β-catenin cascade in crocin’s antidepressant response was assessed by conditional removal of glial fibrillary acidic protein (GFAP)-expressing newborn neural cells, temozolomide administration, microinfusion of Dkk1 or viral-mediated shRNA of Wnt3a.
Results
Crocin decreased the immobility duration in TST and FST without impairing the performance in sexual behaviors. Crocin boosted the proliferation and differentiation of progenitors, and promoted dendritic maturation and functional integration of hippocampal newborn neurons. Conditional removal of GFAP-expressing neural cells or temozolomide administration impaired the antidepressant response of crocin. Additionally, Wnt/β-catenin signaling was promoted following crocin treatment. In chronic unpredictable mild stress (CUMS) murine model, crocin treatment displayed antidepressant response in SPT, FST and TST, and restored the neurogenesis levels and Wnt/β-catenin signaling impaired by CUMS. Infusion of Dickkopf-1 (DKK1) or knockdown of Wnt3a in the hippocampus impaired the antidepressant response of crocin.
Conclusion
Crocin exerted antidepressant response, which was dependent on enhancement of AHN and activation of the Wnt/β-catenin pathway.
Introduction
Major Depressive Disorder (MDD) is a highly prevalent disease which causes huge society and economic burden around the world [1]. MDD impacts an individual’s quality of life and even induces suicide [2]. Although monoamine-based antidepressants are widely used, slow-onset, low response rates and side effects limit their clinical use [3]. Other options, fast-onset antidepressant therapies including sleep deprivation (SD) and low-dose ketamine also have several disadvantages, e.g., short duration of efficacy and side effects such as headache and hallucination [4], [5]. Thus, it is necessary to develop more effective and safer antidepressant treatments.
Adult hippocampal neurogenesis (AHN) acts as a critical role in hippocampal plasticity and is critically involved in mood regulation [6]. Neurogenesis persists throughout life of mammals, mainly occurring in the subgranular zone (SGZ) and subventricular zone (SVZ) of the lateral ventricle in hippocampus [7]. These areas continuously proliferate neural stem cells (NSCs) and neural progenitor cells (NPCs), leading to the generation of new neurons [8]. The adult neurogenesis in SGZ area is the highly multiple and complicated process which drives the proliferation, differentiation, and maturation into functional neurons [9]. In the past decade, the role of hippocampal neurogenesis in depressive disorders development and antidepressant response has been highlighted [10], [11]. Multiple antidepressants are shown to attenuate depression via the increase of hippocampal neurogenesis in dentate gyrus (DG) area of adult rodent models [11]. In addition, the selective serotonin reuptake inhibitors exert antidepressant effects via the hippocampal neurogenesis enhancement [12].
The Wnt/β-catenin signal is a critical regulator of adult neural hippocampus. It has been illustrated that the blockade of Wnt event almost eliminates adult hippocampal neurogenesis in vivo, while overexpression of Wnt3a increases neurogenesis in vivo and in vitro [13], [14]. The deletion of secreted frizzled-related protein 3 (sFRP3), a selective Wnt inhibitor, promotes maturation, dendritic growth, and dendritic spine formation of newborn neurons in the adult mouse hippocampus [15]. Enhancement of Wnt/β-catenin signaling improves dendritic architecture in the adult hippocampus [16]. The development of morphologically highly stereotypic dendritic arbor of new neurons are critical for their functionally integration into the existing neural circuitry [17].
Crocin, a carotenoid, is mainly produced in Crocus sativus stigmas and fruits of Gardenia jasminoides Ellis [18]. The antidepressant property of crocin has been verified not only in various rodent depression models [19], [20], [21], but also clinical trials [22]. A clinical study showed that crocin significantly reduced the Baker Depression Scale score as compared to the placebo group [22]. Furthermore, crocin is confirmed capable of entering the blood–brain barrier [23]. However, to our knowledge, the effect of the adult hippocampal neurogenesis in the antidepressant activity of crocin remains largely unknown.
The present work was aimed to explore the potential mechanism of adult hippocampal neurogenesis in crocin-mediated antidepressant effects. We found that crocin mitigated the depressive symptoms of mice without disturbing male sexual function. Mechanism studies revealed that crocin activated the Wnt/β-catenin signaling and increased adult neurogenesis in the hippocampus. However, genetic or pharmacological inhibition of neurogenesis abolished crocin-induced improvement in depressive behaviors, and the suppression of Wnt/β-catenin signaling cascade reversed the effects of crocin on both depression-like symptoms and neurogenesis enhancement. These results demonstrated that crocin exhibited antidepressant effects by improving adult hippocampal neurogenesis via Wnt/β-catenin. This study provided the foundation for further application of crocin for depression.
Materials and methods
Animals
Male C57BL/6 mice with (18–22 g) were supplied from Sipper-BK Co. Ltd (Shanghai, China) 1 week prior to the experiment. Wild-type mice (WT) and transgenic mice (aged 7 weeks) expressing herpes simplex virus thymidine kinase (TK) under the human glial fibrillary acidic protein (GFAP) promoter (GFAP-TK mice) were purchased from The Jackson Laboratory for estimating neurogenesis role 1 week before experiment and individually housed. The mice were housed in a 22 ± 1 °C and 60% humidity environment with a 12 h/12 h dark/light cycle. All the animals had free access to food and water.
Ethics statement
All experiments involving animals were conducted according to the ethical policies and procedures approved by the Institutional Animal Care and Use Committee at Nanjing University of Chinese medicine, Nanjing University of Chinese medicine, China (Approval no. 202105A004).
Drugs
Crocin (purity ≥ 98 %, CAS No. 42553–65-1, Cat. No. B21336) and fluoxetine (CAS No. 56296–78-7, Cat. No. PHR1394) were provided by Yuanye Technology Co. Ltd (Tianjin, China) and Sigma-Aldrich (St. Louis, USA) respectively. Valganciclovir (VGCV) (V129457) was purchased from Shanghai Aladdin (Shanghai, China). Dickkopf-1 (DKK1; 57248-M08H) was purchased from Sino Biological Inc (Beijing, China). Temozolomide (TMZ; T2577) and bromodeoxyuridine (BrdU; 19–160) were supplied from Sigma-Aldrich (St. Louis, USA). Crocin (12.5 or 25 mg/kg) was dissolved in normal saline and administrated intragastrically (i.g). Fluoxetine (20 mg/kg, i.p.), valganciclovir (35 mg/kg, i.p.) and BrdU (50 mg/kg, i.p.) were dissolved in normal saline and administrated intraperitoneally (i.p.). TMZ (25 mg/kg, i.p.) was dissolved in 5% dimethyl sulfoxide (DMSO). Dkk1 was dissolved in PBS and stereotactically injected to DG region.
Behavior tests
Sucrose preference test (SPT)
The SPT was conducted according to former research with slight alteration [24]. In brief, animals were habituated to 1% sucrose solution (w/v) and water for 24 h, followed by 12 h of water and food deprivation. Two bottles with 150 ml 1% sucrose solution (w/v) or water were offered to mice. The bottles were weighted prior to or following the test. After calculating the consumption of water and sucrose solution, the formula was applied for determining the sucrose preference:
sucrose preference = [(sucrose consumption)/(sucrose consumption + water consumption)] × 100%.
Tail suspension test (TST)
TST is an acknowledged murine model to evaluate antidepressant property [24]. In a visually and acoustically isolated environment, animals were suspended above the floor 50 cm using adhesive tape placed 1 cm from the tail tip for 6 min. Immobile behaviors were recorded when the animals were suspended passively and motionless. The immobility time was assessed within the final 4 min using ANY-MAZE software.
Force swimming test (FST)
FST was carried out based on the previous method with slight modification [24]. Animals were allowed to swim in a clear glass cylinder with 30 cm height and 15 cm diameter filled with 12 cm 24 ± 2 °C water for 6 min. The immobility duration, defined as no movement of the limb or body except those necessary for the mice to keep their heads above water, were analyzed by the ANY-MAZE software.
Open field test (OFT)
The OFT was examined by previous OFT method with minor changes [12]. A black metallic apparatus (40 × 40 × 15 cm3) virtually divided into 16 equal squares was applied for OFT. Each animal was individually placed in the center of arena facing the wall and video-recorded for 5 min. The time spent in center and total distance travelled were automatically analyzed. The box was cleaned with 75% alcohol during the interval of OFT test.
Sexual behavior test
The sexual behavior test was conducted as before with minor modifications [25]. Adult female mice and male mice were taken into a cage (female: male = 3:1) and observed in a dark room for 30 min. The latency to mate, mating times (number of bouts) and mating duration of male mice were recorded within 30 min.
Stereotactic surgeries
The stereotactic surgeries were performed as previously described with minor changes [26]. Briefly, surgical anesthesia was induced with 1.5% isoflurane mice were secured in a stereotaxic instrument. A skin incision was made, and bilateral guide cannula implantation was performed to target DG region using skull drill at medial–lateral (ML) ± 2.0 mm, and anterior-posterior (AP) −2.8 mm, relative to midline and bregma line, respectively. The 2.2 mm guide cannula connected to infusion cannula was projected to dorsal–ventral (DV) coordinate. The dental cement was applied for fixing the microscrews. Infusion cannulas were replaced by dummy cannulas to connect the guide cannula for the prevention of clogging. 1-week recovery was allowed after the surgical procedure.
Multipotent neural stem/progenitor cells isolation
Primary NSCs were dissociated from the brains of male 1 day old mice as described previously [27]. Briefly, the hippocampus of C57BL/6 mice was separated from surrounding tissues and transferred into the NSC culture media consisting of 2% B-27 supplement, 20 ng/mL of epidermal growth factor, 20 ng/mL of basic fibroblast growth factor in Dulbecco's modified Eagle's medium/nutrient mixture F-12 (DMEM/F-12) media. The primary NSCs were then cultured in an incubator with a humidified atmosphere in a standard condition with 5% CO2 at 37 °C to form neurospheres. Half of the medium was replaced with fresh medium every 5 days. Three days later, half of the medium was refreshed. The passage of neurospheres was performed every 6 days to 5 × 105/mL at the final density in poly-L-ornithine coated coverslips in a 48-well plate. The 3rd passage was used for further analyses.
Immunofluorescence analysis
The immunofluorescence analysis for brain tissues was performed in accordance with the previous report [12]. The mice were sacrificed when the behavior tests ended. Tissues were perfused with 4% paraformaldehyde, immersed in 20%-40% sucrose, and maintained at 4 °C for frozen section. The samples were sliced through the whole hippocampus by cryostat (Leica CM1950). Samples were blocked with 3% bovine serum albumin (BSA) at 25 °C followed by incubating with anti-BrdU, anti-NeuN and anti-doublecortin (anti-DCX) antibodies overnight at 4 °C. Thereafter, the slides were treated with fluorescent secondary antibody. The proportions of BrdU+, NeuN+, DCX+, DCX- cells and dividing cells in sections were visualized under a microscope (n = 6). The images were analyzed with Image J, and statistical significance was investigated by Kruskal-Wallis test. The number of BrdU+, NeuN+, DCX+, DCX- cells were calculated through the rostral/caudal extent of the DG region (bregma −0.82 mm to −4.16 mm) with 6 interval serial sections (180-μm interval). The sections were fixed with 4% paraformaldehyde, rehydrated, and the treated with 0.2% TritonX-100 for 5 min. Then, the sections were blocked with 3% BSA. After washing, the slices were treated with anti-NeuN, anti-DCX, anti-Ki67, anti-Nestin primary antibodies at 4 °C overnight. Afterwards, the cells were exposed to Alexa Fluor 594 conjugated secondary antibodies at 25 °C, and further counterstained for approximate 15 min with 4′-6-Diamidino-2-phenylindole (DAPI) solution. Immunofluorescent images were analyzed with a confocal laser-scanning microscope (Olympus).
Sholl analysis
The dendritic arbors were evaluated using 3D Sholl analysis by several indices including dendritic length and intersection number. The immunostaining for DCX was observed under confocal microscopy (Olympus) with 63 × Z-stack thickness at 512 × 512 resolution using stereo-investigator software (Olympus) and Image J. Each Z-stacks was harvested with 3 frames. And the surrounding manual process was removed and the dendritic length was determined using Fiji. The intersection number within radii 1–14 μm around the cell body was counted by cell counter plugin.
Immunohistochemistry staining
The immunohistochemistry staining was conducted as reported elsewhere with minor modifications [28]. The brain tissues of experimental animals were immediately removed and fixed in 4% paraformaldehyde. The brain tissues encompassing entire DG region were sliced into 30 μm section using a cryostat (Leica, Model M1950). The paraffin sections were deparaffinized by xylene and rehydrated gradually in different graded ethanol solution. Then the slides were microwaved in sodium citrate and treated with 3% fresh hydrogen peroxide. After the blockage with 3% BSA at room temperature, the sections were incubated with anti-BrdU, anti-Ki67 and anti-GFAP antibodies at 4 °C overnight. Thereafter, the slides were treated with secondary antibody and third antibody. DAB and haematoxylin solution were treated until the expected staining intensity was achieved. Consequently, the slides were dehydrated in various ethanol solution and paraffinized by xylene. The proportions of BrdU+, Ki67+ were calculated using Stereo Investigator 7 (MBF bioscience, Williston, VT) with 6 interval serial sections (180-μm interval) through the rostral/caudal extent of the hippocampus (bregma −0.82 mm to −4.16 mm). [29] The cells were observed under Olympus bx53 microscope at 40 × magnification (n = 6). The counting frame size and sampling frame size were 78 μm × 78 μm, and 80 μm × 80 μm, respectively. The design error coefficient of the sampling scheme less than 10% was regarded as accurate.
Golgi staining
Golgi-Cox OptimStain Kit (Hitobiotec, USA) was used for Golgi staining based on the manufacturer’s instructions. Briefly, brain tissues were placed into the premixed Golgi-Cox impregnation solution (containing Solutions 1 and 2) for 14 d in the dark. Next, brain samples were transferred into Solution 3 and at 4 °C for 48 h in the dark. Afterward, the brains were cut in 150-μm-thick coronal slices using a cryostat (Leica CM1950). For staining, the sample was placed on gelatin-coated slides and then exposed to solutions 4 and 5, followed by dehydration in 70% ethanol (30 s), 80% ethanol (30 s), 95% ethanol (60 s), 100% ethanol Ⅰ (60 s), 100% ethanol Ⅱ (60 s). The analysis of spine density was performed using microscope and Neurolucida 360 software.
Electrophysiological recording
The electrophysiology was conducted as previously reported with slight modifications [30]. After the behavioral tests, the animals were anesthetized with halothane and then sacrificed. The brain tissues were immediately collected and the transverse hippocampal slices (400 μm) were placed at 32 °C in an interface chamber. The slides were perfused with artificial cerebrospinal fluid (CSF) with following ingredients (in mM, pH7.4): 26.2 NaHCO3, 119 NaCl, 1 NaH2PO4, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2 and 11 glucose. The slices were recovered for 2 h prior to the electrodes stimulation and recording. Stimulation isolation unit (Sarasota, FL) and a bipolar tungsten stimulating electrode was inserted in dentate gyrus by medial performant path (MPP). The evoked potentials were assayed at the layer above upper blade of DG region by capillary microelectrode containing artificial CSF. The paired-pulse depression (PPD) at 50 ms was determined to verify the isolation of MPP. After recording a stable baseline for 10 min, the input–output curves were obtained. The test stimuli were delivered with a 30 s interval. The reactions were monitored in each 20 s within 60 min after long-term potentiation (LTP) induction caused by a tetanic theta-burst stimulation pulses at 100 Hz for 2 s. Field excitatory postsynaptic potentials (fEPSPs) was recorded continuously for at least 45 min followed by the high-frequency stimulation by Digidata 1322 A and Multiclamp 700B amplifier (Axon Instruments, Foster City, USA). The Values of fEPSP amplitude were presented as mean ± standard error of the mean (SEM) percentage alteration relative to the mean baseline slope.
Western blot
The western blot was performed according to a former protocol with minor modifications [31]. The mice were sacrificed and brain tissues were quickly harvested. The concentrations of total proteins were measured by commercial Bicinchoninic Acid (BCA) kit. The sample was subjected to 7.5% polyacrylamide gels for electrophoresis. Afterwards, the proteins were then transferred onto a Polyvinylidene fluoride (PVDF) membrane and blocked with 2% BSA for 1 h. The membranes were incubated with appropriated primary antibodies overnight at 4 °C. The samples were incubated with immunoglobulin (IgG) horseradish peroxidase (HRP) anti-rabbit secondary antibody for 1 h at room temperature after washing 3 times with Tris-buffered saline containing 0.1% (v:v) Tween 20 (TBST). Consequently, the proteins were visualized with enhanced chemiluminescence kit. The Bands were quantified using Image J software and normalized to Tubulin.
Statistical analysis
The results were presented as mean ± SEM and analyzed by GraphPad Prism 7 software. Two-sample comparisons were conducted using unpaired student’s t-test. The multiple comparisons were carried out using one-way analysis of variance (ANOVA) supplemented with a Bonferroni post hoc analysis or two-way ANOVA with a Tukey post hoc analysis. Unless specifically indicated, p < 0.05 was identified as statistically significant.
Results
Crocin exhibited comparable antidepressant response to fluoxetine without disturbing male sexual function
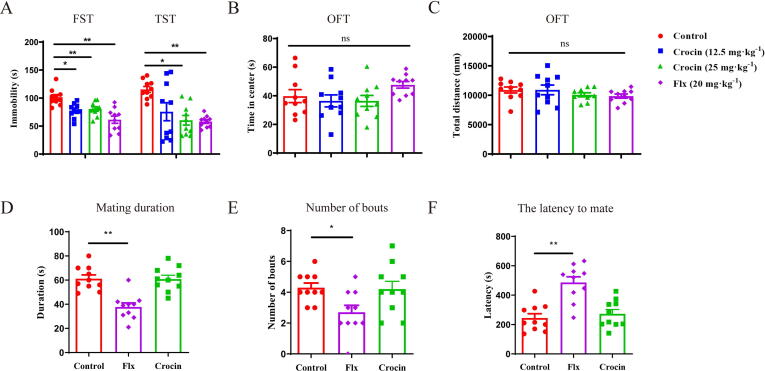
To clarify the antidepressant effect of crocin, we gave the male mice crocin (12.5 or 25 mg/kg, i.g.) for 2 weeks and used fluoxetine (20 mg/kg, i.p.) as the positive control [32]. Fourteen days later, crocin treatment caused a significant reduction in the immobile durations of FST and TST (Fig. 1A; FST: F (3, 36) = 11.67, p < 0.01; TST: F (3, 36) = 7.630, p < 0.05), while it did not obviously influence the time in center (Fig. 1B; F (3, 36) = 1.920, p = 0.1438) and total distance (Fig. 1C; F (3, 36) = 1.040, p = 0.3864) of OFT, indicating that the antidepressant effect of crocin was not confounded by its impact on locomotor activity. It seems that crocin exerted similar antidepressant response to fluoxetine. Furthermore, the mating duration, number of bouts, and mate latency of male mice were detected to evaluate the sexual function in fluoxetine- or crocin-treated mice. Results demonstrated that the sexual function of fluoxetine-treated mice was significantly decreased (Fig. 1D-F; Duration: F (2, 27) = 17.59, p < 0.01; Number of bouts: F (2, 27) = 4.335, p < 0.05; Latency: F (2, 27) = 16.20, p < 0.01), whereas crocin treatment did not influence the estrus of mice, indicating that crocin exhibited less adverse effect than fluoxetine.
Fig. 1.
Crocin attenuated depressive-like behaviors without inducing adverse effect on sexual behavior. (A) Effects of crocin on the immobility time in FST and TST. (B-C) Effects of crocin on the spontaneous locomotor activity in OFT. (D-F) The mating duration, number of bouts and latency to mate in the sexual behavior test. Results are presented as mean ± SEM. One-way ANOVA followed by Bonferroni post hoc test. *p < 0.05; **p < 0.01; ns, no significance.
Crocin promoted different stages of hippocampal adult neurogenesis
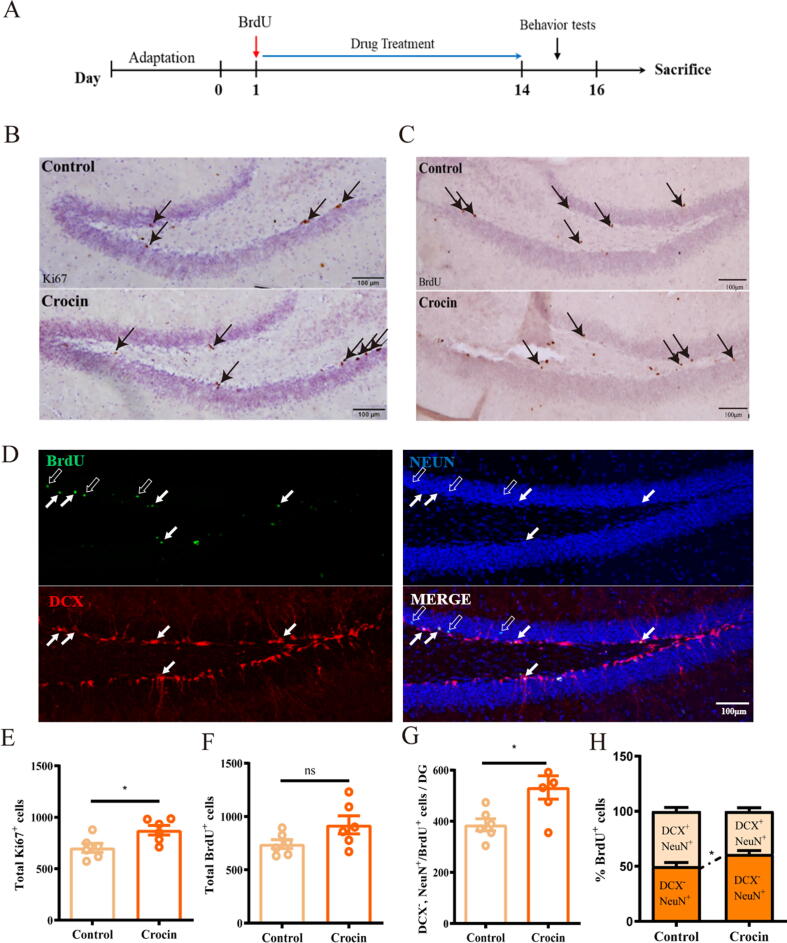
For labeling of proliferating cells, animals were treated with BrdU (50 mg/kg, i.p.) twice daily at first day of starting drug treatment for 3 consecutive days. Afterwards, 25 mg/kg of crocin was given for 2 weeks (Fig. 2A, n = 6). The effect of crocin on hippocampal neurogenesis was then assessed. As shown in Fig. 2B, treatment with crocin markedly increased the Ki67+ positive cell population in DG region (Fig. 2B and 2E; t = 2.708, p < 0.05), whereas it did not influence the survival proportion of newborns neurons detected by BrdU staining (Fig. 2C and 2F; t = 1.907, p = 0.086). Radial glia-like neural stem/progenitor cell (Nestin+) developed to DCX+ progenitor cell within 2 weeks, and further developed to mature neuron (NeuN+/DCX-) within the third week [33], [34]. The expression of DCX in BrdU+NeuN+ cell was commonly considered to identify whether radial glia-like neural stem/progenitor cell progressed into mature neuron [33], [34]. The results illustrated that BrdU prematurely labeled the mature neuron (NeuN+/DCX-) population. The total number of NeuN+/DCX- cells, and the proportion of NeuN+/DCX- cell were all pronouncedly increased (Fig. 2D, 2G, 2H and Fig. S1A; t = 2.835, p < 0.05), suggesting that 2 weeks of crocin administration promoted the differentiation of BrdU+ cells into mature neurons. No significant change can be detected in number of SOX2+ cells between crocin-treated group and control group (Fig. S1B-S1C, t = 0.861, p = 0.414).
Fig. 2.
Crocin improved adult neurogenesis in the hippocampus. (A) Time line of BrdU injection and drug treatment. (B) Dividing cells (arrows) were identified using Ki67 immunohistochemistry in crocin-treated or control group. Scale bar: 100 μm. (C) BrdU immunohistochemistry (gray-brown) was used for detecting 2-week survival cells (arrows), Scale bar: 100 μm. (D) Representative images of DG area showed four types of cells: BrdU+ cells (green) which co-express strong DCX (red) or NeuN (blue); BrdU unlabeled DCX+/NeuN+ immature neurons (denoted by solid arrows); BrdU unlabeled DCX- /NeuN+ mature neurons (denoted by hollow arrow). (E-G) Quantification of Ki67+, total BrdU+ and NeuN+/DCX-/NeuN+ cells. (H) The percentage of DCX- /NeuN+ mature neurons in crocin-treated group or control group. Results are presented as mean ± SEM. Unpaired student’s t-test. *p < 0.05; ns, no significance.
The effect of crocin on the dendritic morphology of immature neuron was investigated by Sholl analysis on DCX+/BrdU+ cells with tertiary dendrites (Fig. 3A-B). The total number of DCX+/BrdU+ cells, intersection number and dendritic length of these cells were calculated in each group. As presented in Fig. 3C-E, with crocin treatment, the total number of DCX+/BrdU+ cells (t = 2.239, p < 0.05), the intersection number (t = 2.705, p < 0.05) and dendritic length (t = 2.481, p < 0.05) of these cells were markedly increased. We also examined whether crocin has effect on neural plasticity which may functionally integrated to local circuit. As indicated in Fig. 3F-H and S1D, crocin treatment greatly increased spine density versus control group (t = 2.573, p < 0.05), and the fEPSP slope (t = 2.701, p < 0.05) in crocin group was evidently larger than that in control group during the last 10 min. To conclude, these results indicated that crocin was able to facilitate the maturation of young neurons, and result in the re-adjustment of local circuits, leading to increased long-term potentiation (LTP) induction and enhanced synaptic transmission.
Fig. 3.
Crocin enhanced adult neurogenesis and synaptic plasticity in hippocampus. (A) Images of DCX+/BrdU+ immunohistochemistry after crocin treatment. Scale bar: 100 μm. (B) Sholl analysis of DCX+/BrdU+ neurons on crocin-treated or control group. (C-E) Chronic crocin administration significantly increased DCX+/BrdU+ cells, intersection number and dendritic length. (F-G) Dendritic spine density in DG region of hippocampus. Scale bar: 10 μm. (H) Crocin improved the ACSF–LTP recording of the last 10 min. Results are presented as mean ± SEM. Unpaired student’s t-test. *p < 0.05, **p < 0.01; ns, no significance.
The effect of crocin on the proliferation and differentiation of NSCs in vitro
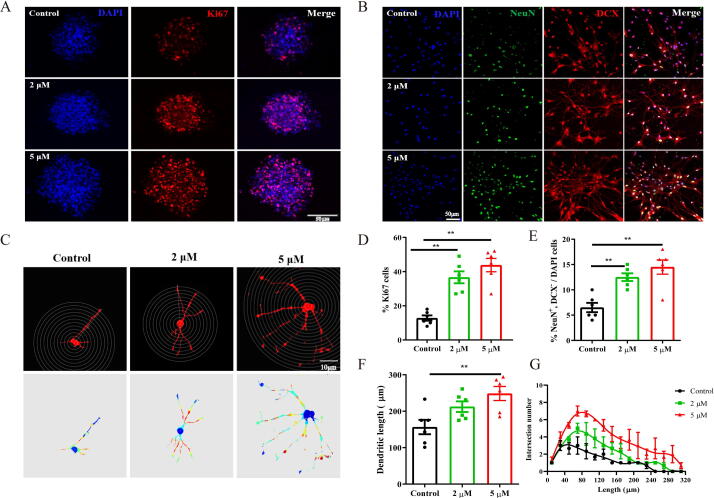
The effect of crocin on the differentiation and proliferation of NSCs was also investigated in vitro. Primary mouse NSCs were obtained from 1-day-old male C57BL/6 mice. Seven days later, cells were treated with 2 µM or 5 µM crocin for a week. The purity of neutrosphere was identified using Nestin (Fig. S2). The immunofluorescence staining of Ki67 and DCX was used to determine the effect of crocin on the differentiation and proliferation of neural stem cell in vitro. As presented in Fig. 4A and 4D, the percentage of Ki67+ cells were significantly increased after crocin treatment (F (2, 15) = 26.61, p < 0.01). In parallel, the percentage of NeuN+/DCX- was clearly up-regulated under crocin treatment (Fig. 4B and 4E, F (2, 15) = 15.22, p < 0.01). The sholl analysis was carried out to measure the morphology of dendritic spines (Fig. 4C). Results indicated that treatment with crocin was able to increase the dendritic length and intersection number after differentiation. (Fig. 4 F-G; Dendritic length: F (2, 15) = 6.64, p < 0.01; Intersection number: F (15, 30) = 15.09, p < 0.01). Collectively, crocin enhanced the proliferation and differentiation of the primary NSCs.
Fig. 4.
Effect of crocin on the differentiation and proliferation of stem neural cell in vitro. (A) Immunofluorescence staining of primary NSC cells with Ki67 antibodies. Cells were treated with 2 μM or 5 μM crocin. Scale bar: 50 μm. (B) Immunofluorescence staining of primary NSC cells with NeuN, DCX, DAPI antibodies. Cells were treated with 2 μM or 5 μM crocin. Scale bar: 50 μm. (C) The representative pictures of primary NSCs in concentric sholl radii. ells were treated with 2 μM or 5 μM crocin. Scale bar: 10 μm. (D-E) Quantification of Ki67+ and NeuN+/DCX-/DAPI cells. (F) The dendritic length after differentiation in 2 μM crocin-treated, 5 μM crocin-treated or control group. (G) The intersection number after differentiation in 2 μM crocin-treated, 5 μM crocin-treated or control group. Results are presented as mean ± SEM. Statistical analysis: one-way ANOVA followed by Bonferroni post hoc test. **p < 0.01.
Genetic or pharmacological blockade of neurogenesis blunted the antidepressant effect of crocin
We assumed that the antidepressant property of crocin was depended on the adult neurogenesis enhancement. To test this hypothesis, genetic- and pharmacological- blockade approaches were used to ablate neurogenesis in the DG.
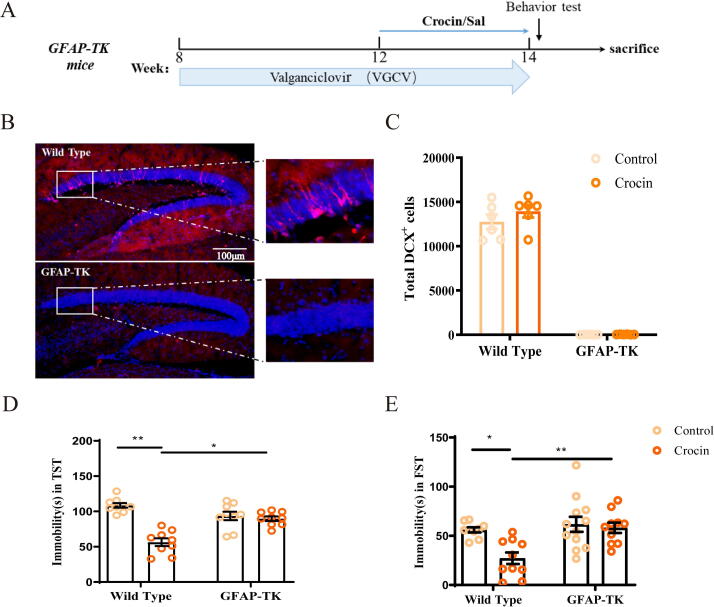
Functional granule neuron is produced in the hippocampus during the entire lifetime through a complex process that starts with GFAP-expressing radial cell precursors [6]. The GFAP-TK mouse is universally regarded as a murine transgenic model in which virus TK is expressed from the promoter of the GFAP gene [35]. Upon the stimulation of TK, mitotic cells, but not post-mitotic cells, are sensitive to VGCV [6]. Eight weeks old GFAP-TK mice (Fig. S3A) were treated with VGCV (35 mg/kg, i.p.) for continuous 6 weeks and crocin (25 mg/kg, i.g.) was treated at the last two weeks (Fig. 5A). Compared with wild-type mice, the expression of DCX was completely eliminated in GFAP-TK mice (Fig. 5B-C; two-way ANOVA: DCX+: F (1, 20) = 1.19, p = 0.284), which was similar with the results of previous literature [6]. The number of SOX2+ and S100β+ cells remained no change in both groups, indicating that quiescent radial glial and mature glial cells were not affected in GFAP-TK mice (Fig. S3B-S3D, S100β+ /GFAP+: t = 1.515, p = 0.161; SOX2+/GFAP+: t = 16.46, p < 0.01).
Fig. 5.
Crocin-related effect on depressive-like behaviors and neurogenesis were blocked in GFAP-TK mice with VGCV treatment. (A) Timeline of GFAP-TK mice experiment. GFAP-TK mice were treated with VGCV for 6 weeks. Crocin treatment started from 12th week. (B) Young granule neurons (red) cells expressing DCX in DG of wild type mice. No DCX+ cells can be detected in DG of GFAP-TK mice. Scale bar: 100 μm. (C) Quantification of total number of DCX+ cells in DG under crocin treatment. (D-E) The effect of crocin on immobility time of GFAP-TK mice or wild type mice in TST and FST. Results are presented as mean ± SEM. Two-way ANOVA followed by Tukey post hoc test. *p < 0.05; **p < 0.01; ns, no significance.
VGCV treated GFAP-TK mice no longer displayed antidepressant activity in TST and FST after crocin administration (Fig. 5D-E; two-way ANOVA: TST: F (1, 32) = 26.53, p < 0.05; FST: F (1, 32) = 7.69, p < 0.05).
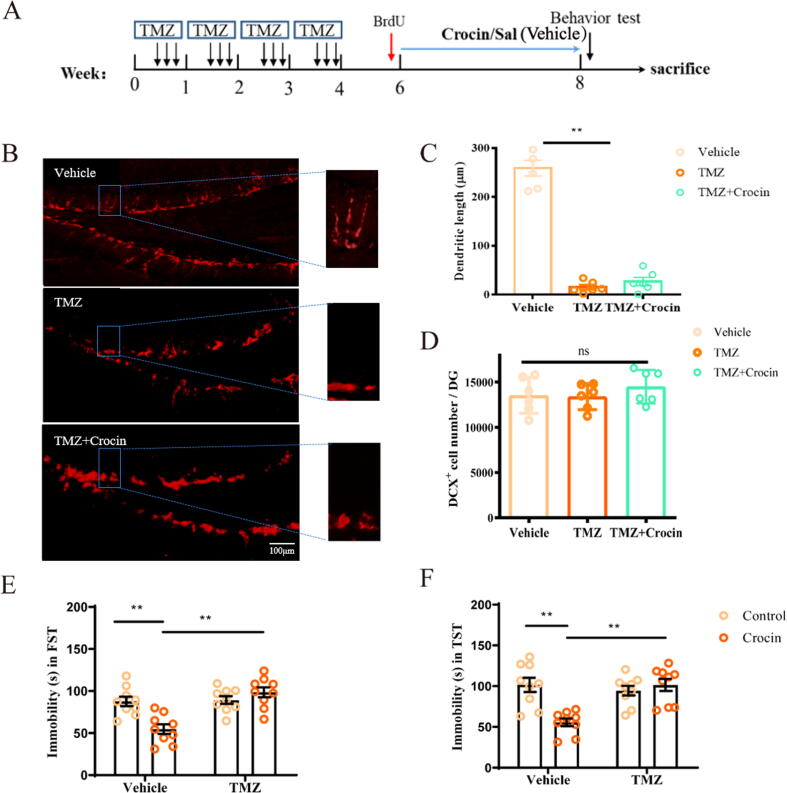
TMZ, a drug which displays antimitotic effect, is widely used for the treatment of glioblastoma multiforme [36]. It also allows us to chemically inhibit AHN by targeting late-phase DCX+ progenitors, while the total number of DCX+ remains unaffected [33]. Mice received 4 rounds of TMZ (25 mg/kg, i.p.) treatment at 1-week intervals, with each round consisting of 3 daily injections of TMZ or vehicle followed by 4 days of no injections (Fig. 6A). Ten days after the four rounds of TMZ treatment, the thymidine analog BrdU was intraperitoneal injected to mice for 3 days to mark the proliferating cells. Then mice were administrated with crocin (25 mg/kg, i.g.) once daily for 2 weeks. TMZ-treated DCX+ cells showed the characteristics of early-phase DCX+ progenitors including decreased branch and dendrite length compared with DCX+ cells in the control group, indicating suppression of late-phase DCX+ progenitor cells by TMZ, treatment with crocin could not reverse this effect (Fig. 6B-C; F (2, 15) = 162.8, p < 0.01). However, the number of the DCX+ progenitor cells in TMZ-treated mice, or in crocin-treated mice was similar to that of control mice (Fig. 6D; F (2, 15) = 0.702, p = 0.511). As illustrated in Fig. 6E-F, the antidepressant activity of crocin was blocked by TMZ stimulation (two-way ANOVA: FST: F (1, 32) = 14.70, p < 0.01; TST: F (1, 32) = 14.95, p < 0.05).
Fig. 6.
Crocin-related effect on depressive-like behaviors and neurogenesis were blocked in mice with TMZ treatment. (A) Experimental timeline for the impact of TMZ blockade of late-phase DCX+ progenitors on crocin’s antidepressant activity. (B) Sections of TMZ, Vehicle or crocin treated mice were immunolabled with DCX (red). Amplified pictures of the dash-line rectangle region were shown to observe DCX+ cell morphology alteration following TMZ treatment. Scale bar: 100 μm. (C-D) Quantification of dendritic length and total DCX+ cell number in DG (unpaired student’s t-test). (E-F) The effect of crocin on immobility time of TMZ-treated mice or vehicle control mice in TST and FST. (two-way ANOVA followed by Tukey post hoc). Results are presented as mean ± SEM. **p < 0.01; ns, no significance.
Crocin exerted antidepressant effect through Wnt/β-catenin signaling
To study the interaction of crocin and Wnt3a, the molecular docking analysis was conducted using the Autodock program. The docking results demonstrated that crocin binds to Wnt3a by forming stable hydrogen bonds at SER-239, ER-237, ASP-270, GLU-248, ARG-185, ARG-250 and GLU-174 (Fig. S4).
As Wnt/β-catenin signaling was pivotal for AHN, we then measured the Wnt/β-catenin signaling in hippocampi of control and crocin-treated mice. The Wnt3a and p-GSK3β (ser9) expressions were notably upregulated, whereas p-β-catenin expression was downregulated compared with those of control group (Fig. S5; Wnt3a: t = 4.385, p < 0.01; p-GSK3β: t = 3.420, p < 0.01; p-β-catenin: t = 3.064, p < 0.05). It was indicated that crocin activated the Wnt/β-catenin signaling in the hippocampus.
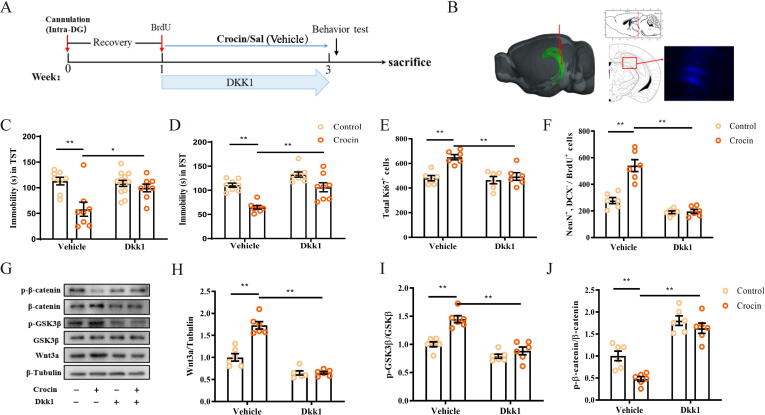
We further blocked Wnt3a signaling to examine whether the Wnt/β-catenin cascade was necessary for crocin-induced antidepressant effects. Dkk1 competed LRP6 with Wnt, preventing the complex formation of LRP6, Wnt and Fz to block the downstream signaling. Herein, Dkk1 was used to specifically inhibit the Wnt/β-catenin cascade. One week recovery after the implantation of a cannula, BrdU was injected twice daily for 3 days. Then 5 μL of Dkk1 (dissolved in 0.5 µL PBS) was stereotactically injected into the DG region once daily for 2 weeks (Fig. 7A-B). In addition, crocin (25 mg/kg, i.g.) was given to mice 1 h post Dkk1 injection. The results showed that crocin-mediated antidepressant effects of in TST and FST were hampered in Dkk1-injected mice (Fig. 7C-D; two-way ANOVA: TST: F (1, 28) = 6.12, p < 0.05; FST: F (1, 28) = 2.65, p < 0.05). The accelerated activities on progenitor cell proliferation and immature neuron differentiation caused by crocin were inhibited (Fig. 7E-F and Fig. S6; two-way ANOVA: Ki67: F (1, 20) = 7.19, p < 0.05; NeuN+/DCX-/BrdU+: F (1, 20) = 23.11, p < 0.01)
Fig. 7.
Crocin-related effect on depressive symptoms and neurogenesis were reversed by Dkk1 treatment. (A) Timeline of Dkk1 experiment. (B) Cannula position and Hoechst33342 dye infusions into the DG of mice. (C-D) Mice pretreated with Dkk1 followed by crocin treatment did not exhibit reduced immobility time in TST and FST. (E-F) Crocin significantly increased the number of Ki67 positive cells and number of mature neurons in Vehicle group, but not in Dkk1 group. (G-J) Effects of crocin treatment on Wnt3a, p-β-catenin and p-GSK3β protein levels in the hippocampus of Dkk1 mice. Results are presented as mean ± SEM. Statistical analysis: two-way ANOVA followed by Tukey post hoc test. *p < 0.05; **p < 0.01.
The effects of Dkk1 on key proteins of Wnt signaling including p-β-catenin, β-catenin, p-GSK3β, GSK3β and Wnt3a were determined by Western blot (Fig. 7G). Following the pretreatment of Dkk1 antagonist, the levels of Wnt3a, p-GSK3β/GSK3β were decreased, whereas p-β-catenin/β-catenin was up-regulated, indicating that Wnt signaling activated by crocin can be successfully blocked by Dkk1. (Fig. 7H-J; two-way ANOVA: Wnt3a: F (1, 20) = 30.43, p < 0.01; p-GSK3β: F (1, 20) = 10.58, p < 0.01; p-β-catenin: F (1, 20) = 3.06, p = 0.09).
We also utilized shRNA lentiviral particles to knockdown the Wnt3a expression (Fig. S7A). The lentivirus Wnt3ashRNA was stereotactically injected into the DG region followed by a week of recovery. Crocin (25 mg/kg, i.g.) was then given to mice for 14 days. As indicated in Fig. S7B, Wnt3a shRNA transfection significantly reversed crocin-mediated antidepressant like efficacy in TST and FST (two-way ANOVA: TST: F (1, 28) = 8.03, p < 0.01; FST: F (1, 28) = 5.41, p < 0.05). The data demonstrated the essential role of Wnt/β-catenin pathway in crocin-modulated neurogenesis enhancement and antidepressant effects.
The effect of crocin on CUMS-exposed mice
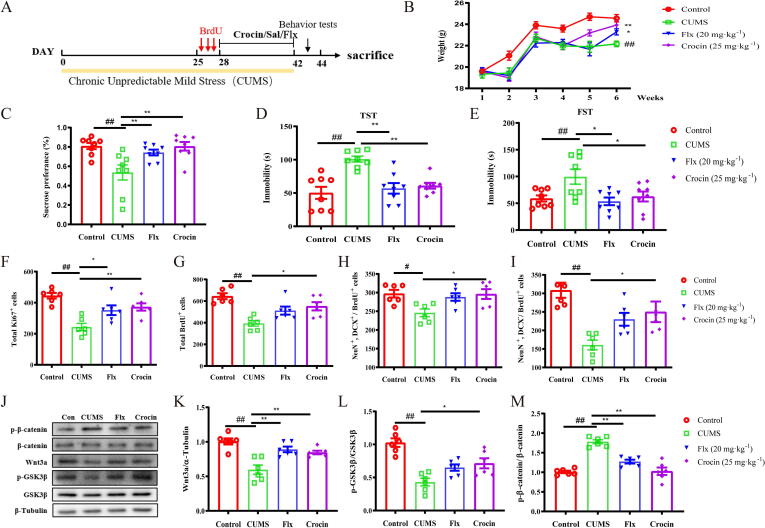
The chronic unpredictable mild stress (CUMS) protocol is a robust model which mimics the stress-induced depression state [37]. The antidepressant effect of crocin and related signaling were then tested in the CUMS model (Fig. 8A) [38]. SPT was used to confirm the successful establishment of depressive model after 4 weeks of CUMS exposure (Fig. S8A; t = 4.490, p < 0.01). Starting the 5th week of CUMS exposure, crocin or fluoxetine was treated for 2 weeks. As presented in Fig. 8B-E, crocin administration increased the body weight, relieved the anhedonia-like response in SPT and decreased immobile duration in both FST and TST in CUMS treated mice (weight: F (15, 210) = 3.925, p < 0.01; SPT: F (3, 28) = 6.70, p < 0.01; FST: F (3, 28) = 4.81, p < 0.01; TST: F (3, 28) = 11.44, p < 0.01). For neurogenesis, crocin or fluoxetine treatment elevated Ki67+, BrdU+ cells, NeuN+/DCX+/BrdU+ as well as the number of NeuN+/DCX-/BrdU+ cells (Fig. 8F-I; Fig. S8B-C; Fig. S9; Ki67+: F (3, 20) = 11.46, p < 0.01; BrdU: F (3, 20) = 10.38, p < 0.01; NeuN+/DCX+/BrdU+: F (3, 20) = 4.92, p < 0.05; NeuN+/DCX-/BrdU+: F (3, 20) = 8.94, p < 0.01) in CUMS-exposed mice. The results of western blot showed that the up-regulated p-β-catenin, down-regulated p-GSK3β and Wnt3a in CUMS-challenged model can be reversed by crocin treatment. In parallel, fluoxetine treatment decreased the level of p-β-catenin, increased the level of Wnt3a, while it did not affect the level of p-GSK3β. (Fig. 8J-M; Wnt3a: F (3, 20) = 13.45, p < 0.01; GSK3β: F (3, 20) = 15.48, p < 0.01; p-β-catenin: F (3, 20) = 34.74, p < 0.01), indicating the activation of Wnt/β-catenin pathway in CUMS mice following crocin treatment.
Fig. 8.
Effects of crocin on depressive-like behaviors and adult hippocampal neurogenesis in CUMS-induced mice. (A) Timeline of CUMS experiment. (B) The body weight of mice in control, CUMS, CUMS treated with Flx (20 mg/kg) or CUMS treated with crocin (25 mg/kg). Body weight was measured for 6 weeks. (C) Sucrose preference rate of mice in each group. (D-E) The immobility time (TST and FST) of mice in four groups were quantified. (F-I) Crocin reversed CUMS-induced decline of Ki67+ cells, BrdU+ cells, NeuN+/DCX+/BrdU+ cells and mature neurons (NeuN+/DCX-/BrdU+) in DG area. (J-M) Effects of crocin treatment on Wnt3a, p-β-catenin and p-GSK3β protein levels in the hippocampus of CUMS mice. Results are presented as mean ± SEM. Results are presented as mean ± SEM. One-way ANOVA followed by Bonferroni post hoc test. CUMS vs Control, #p < 0.05; ##p < 0.01; Crocin vs CUMS, *p < 0.05; **p < 0.01.
Discussion
The discovery of crocin’s therapeutic effect on depression has raised a new question about the mechanism of how it exerts antidepressant action. Moreover, its regulatory properties of Wnt pathway may provide the possibility of facilitation in hippocampal neurogenesis. In the present study, we made use of neurogenesis blockage and Wnt inhibition in depressive animal model to monitor the treatment effect of crocin upon neurogenesis and Wnt/β-catenin signaling cascade. Our major finding was that crocin treatment attenuated the depressive symptoms of mice via activating Wnt/β-catenin pathway and promoting neurogenesis in the hippocampus.
Crocin, a natural bioactive product extracted from Gardenia jasminoides Ellis, is a diester consisted of the dicarboxylic acid crocetin and disaccharide gentiobiose with a clearly examined structure [39]. Despite its well-known anti-inflammatory and antioxidant properties [40],crocin was also reported to have antidepressant-like activity in various experimental depressive models, e.g., lipopolysaccharide (LPS) [21], corticosterone (CORT) [20], perinatal anxiety (PNA) [41], chronic obstructive pulmonary disease (COPD) [19] and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [41]. For clinical usage, several well-designed randomized clinical trials confirmed the efficacy of crocin as adjunctive treatment in mild, moderate, and even major depression [22], [42]. In line with these results, our study found that crocin attenuated the depression like symptoms of mice without disturbing male sexual function. As its mechanism of antidepressant-like effect, previous studies demonstrated that crocin enhanced the branch points and complexity of dendrites in depressed rats [43]. In addition, hippocampal synaptic plasticity-related proteins could be increased via the modification of GHSR-PI3K signaling under crocin treatment [44]. Despite these findings, the panoramic view of how crocin exerts its antidepressant action remains largely unclear.
Hippocampal neurogenesis occurs mainly in DG region, with a 1.75% annual turnover rate of renewing neurons in adult brain [45]. Several clinical studies highlighted the correlation between adult neurogenesis and depression, for patients with depression showed a decreased hippocampal volume [46]. Moreover, multiple antidepressants were shown to attenuate depression via the increase of adult neurogenesis in DG area of hippocampus [47]. The hypothesis of neurogenesis emphasizes that stress/glucocorticoids-induced decreased number of newborn neurons and neural precursor cells result in re-adjustment of local circuits and synaptic transmission, leading to the structural and morphological changes in the DG of hippocampus [48]. In our study, crocin administration not only increased the number of different stages of hippocampal cells, e.g., DCX+ neuronal precursor cells, BrdU+ NeuN+ late immature neurons, but also promoted their function, e.g., increased the dendritic length and intersection number. Similar phenomena were also detected in fluoxetine-treated mice, showing that fluoxetine treatment has positive impact on the maturation and functional integration of newborn neurons [49]. On this basis, results of in vitro experiments further demonstrated that crocin treatment stimulated NSCs to enter the cell cycle, enhanced NPC proliferation, and promoted immature DCX+ neurons to transform into mature neurons. These findings suggested that crocin administration accelerated the proliferation as well as the maturation of newborn neurons and promoted the functional maturation of the hippocampal neural network. Furthermore, specific elimination of DCX+ neurons in GFAP-TK transgenic mice [6] and TMZ model significantly inhibited the effect of crocin on depression-like behaviors of mice, indicating that the antidepressant efficacy of crocin required the participation of neonatal hippocampal neurogenesis in mice. However, it seems that crocin have no significant effect on the total number of NSCs in DG region of mice. There are two possible explanations: 1) The number of experimental animals was insufficient to give a solid result (n = 5 for each group). We need to extent the number of candidate animals in the further study. 2) The microenvironment in animal’s brain is totally different from that of in vitro single environment which only have one type of cell. Mutiple factors from the complicated microenvironment may compromise the effect of crocin on NSCs in vivo.
In the nervous system, Wnt activity in the hippocampus was associated with the proliferation, differentiation and maturation of neural progenitor cells [13]. Emerging evidence showed that Wnt3a was necessary for brain development. The loss of hippocampus was found in the absence of Wnt3a [50]. Correspondingly, overexpression of Wnt3 was sufficient to increase neurogenesis from adult hippocampal stem/progenitor cells. Previous studies reported crocin exerted anti-tumor activity through Wnt/PI3K pathway [51]. Moreover, crocin was found to alleviate pain hyperalgesia by suppressing the spinal Wnt5a/β-Catenin signaling pathway [52].
It is noteworthy that although crocin has been demonstrated to activate the Wnt/β-catenin cascade, which resulted in enhanced neurogenesis and profound antidepressant effects, however, how crocin binds to Wnt, and the downstream mechanisms are need to be further clarified. In this work, we assessed the effect of crocin on the Wnt signaling pathway in hippocampus of mice. Our results indicated that crocin could activate the Wnt/β-catenin signaling pathway in hippocampus. Our data was in accordance with the role of crocin in adjuvant-induced arthritis model, in which crocin also has impact on Wnt/β-catenin pathway [52]. The Wnt inhibition experiments further validated this finding, as the inhibition of Wnt/β-catenin cascade compromised the effect of crocin on immature neurons and further abolished the effect of crocin on depressive behaviors in mice. We also carried out the molecular docking study to evaluate the binding ability of crocin and Wnt, which revealed that crocin and Wnt can directly bind, indicating that there might be a potential direct interaction between crocin and the Wnt/β-catenin signaling pathway.
Taking advantage of CUMS as a useful model to study desperate behavior [53], we also found that crocin reversed the desperate behavior, activated Wnt/β-catenin signaling and promoted the proliferation and differentiation of hippocampal neurons. Interestingly, crocin increased the survival rate of newborn neurons in CUMS mice (Fig. 8G), but not in normal mice (Fig. 2F), which indicated that crocin exhibited no effect on the normal apoptosis of neurons in the state of low tissue damage but could reduce the pathological damage to hippocampal neurons caused by external stimuli, meanwhile, with lower side effects. However, there is still a limited number of well-controlled, multiple center clinical trials to provide the evidence of antidepressant action of crocin. Moreover, it is better to clarify the effect of crocin on Wnt/ β-catenin signaling activation in a more simplified experimental system or in a certain type of cells. Thus, further research works are desperately required.
In summary, the current research displayed that crocin exerted antidepressant effects with lower side effects than fluoxetine. We showed that the antidepressant response of crocin was accompanied by hippocampal neurogenesis in mice and activation of the Wnt/β-catenin cascade. These results elucidated a neuronal basis for the antidepressant effect of crocin, demonstrating the important role of neurogenesis. Our studies provided the foundation for further establishment of crocin application for improving the treatment outcome of depression.
Compliance with Ethics Requirements
All Institutional and National Guidelines for the care and use of animals were followed.
CRediT authorship contribution statement
Weiwei Tao: Conceptualization, Investigation, Methodology, Validation, Writing – original draft, Project administration. Jie Ruan: Methodology, Resources, Formal analysis. Ruyan Wu: Methodology, Resources, Formal analysis. Min Zhao: Data curation, Methodology. Tong Zhao: Methodology, Software. Mingming Qi: Methodology, Software. Sonata S.Y. Yau: Formal analysis, Methodology. Guangda Yao: Formal analysis, Methodology. Hongru Zhang: Conceptualization, Visualization, Investigation, Writing – review & editing. Yue Hu: Conceptualization, Visualization, Investigation, Writing – review & editing. Gang Chen: Project administration, Funding acquisition, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by National Natural Science Foundation of China (81873096, 82174002, 81874374), Key research and development projects of east-west cooperation (2021BEG02040), Open Projects of the Discipline of Chinese Medicine of Nanjing University of Chinese Medicine Supported by the Subject of Academic priority discipline of Jiangsu Higher Education Institutions (NO. ZYX03KF73), and Blue Project of Middle-aged in Jiangsu province (2020).
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.02.015.
Contributor Information
Hongru Zhang, Email: 170947@njucm.edu.cn.
Yue Hu, Email: yuehu@njucm.edu.cn.
Gang Chen, Email: hdn_2001@126.com.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Arioz B.I., Tastan B., Tarakcioglu E., Tufekci K.U., Olcum M., Ersoy N., et al. Melatonin Attenuates LPS-Induced Acute Depressive-Like Behaviors and Microglial NLRP3 Inflammasome Activation Through the SIRT1/Nrf2 Pathway. Front Immunol. 2019;10:1511. doi: 10.3389/fimmu.2019.01511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullins N., Bigdeli T.B., Børglum A.D., Coleman J.R.I., Demontis D., Mehta D., et al. GWAS of Suicide Attempt in Psychiatric Disorders and Association With Major Depression Polygenic Risk Scores. Am J Psychiatry. 2019;176(8):651–660. doi: 10.1176/appi.ajp.2019.18080957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machado-Vieira R., Henter I.D., Zarate C.A., Jr. New targets for rapid antidepressant action. Prog Neurobiol. 2017;152:21–37. doi: 10.1016/j.pneurobio.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemmeter U.-M., Hemmeter-Spernal J., Krieg J.-C. Sleep deprivation in depression. Expert Rev Neurother. 2010;10(7):1101–1115. doi: 10.1586/ern.10.83. [DOI] [PubMed] [Google Scholar]
- 5.Murrough J.W., Iosifescu D.V., Chang L.C., Al Jurdi R.K., Green C.E., Perez A.M., et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snyder J.S., Soumier A., Brewer M., Pickel J., Cameron H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ming G.-l., Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70(4):687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmann O., Spalding K.L., Frisén J. Adult Neurogenesis in Humans. Cold Spring Harb Perspect Biol. 2015;7(7):a018994. doi: 10.1101/cshperspect.a018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulose S.M., Miller M.G., Scott T., Shukitt-Hale B. Nutritional Factors Affecting Adult Neurogenesis and Cognitive Function. Adv Nutr. 2017;8(6):804–811. doi: 10.3945/an.117.016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramírez-Rodríguez G.B., Palacios-Cabriales D.M., Ortiz-López L., Estrada-Camarena E.M., Vega-Rivera N.M. Melatonin Modulates Dendrite Maturation and Complexity in the Dorsal- and Ventral- Dentate Gyrus Concomitantly with Its Antidepressant-Like Effect in Male Balb/C Mice. IJMS. 2020;21(5):1724. doi: 10.3390/ijms21051724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brymer K.J., Johnston J., Botterill J.J., Romay-Tallon R., Mitchell M.A., Allen J., et al. Fast-acting antidepressant-like effects of Reelin evaluated in the repeated-corticosterone chronic stress paradigm. Neuropsychopharmacology. 2020;45(10):1707–1716. doi: 10.1038/s41386-020-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tunc-Ozcan E., Peng C.Y., Zhu Y., Dunlop S.R., Contractor A., Kessler J.A. Activating newborn neurons suppresses depression and anxiety-like behaviors. Nat Commun. 2019;10(1):3768. doi: 10.1038/s41467-019-11641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inestrosa N.C., Varela-Nallar L. Wnt signalling in neuronal differentiation and development. Cell Tissue Res. 2015;359(1):215–223. doi: 10.1007/s00441-014-1996-4. [DOI] [PubMed] [Google Scholar]
- 14.Harada H., Farhani N., Wang X.-F., Sugita S., Charish J., Attisano L., et al. Extracellular phosphorylation drives the formation of neuronal circuitry. Nat Chem Biol. 2019;15(11):1035–1042. doi: 10.1038/s41589-019-0345-z. [DOI] [PubMed] [Google Scholar]
- 15.Lie D.-C., Colamarino S.A., Song H.-J., Désiré L., Mira H., Consiglio A., et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 16.Singh S., Mishra A., Srivastava N., Shukla S. MK-801 (Dizocilpine) Regulates Multiple Steps of Adult Hippocampal Neurogenesis and Alters Psychological Symptoms via Wnt/beta-Catenin Signaling in Parkinsonian Rats. ACS Chem Neurosci. 2017;8(3):592–605. doi: 10.1021/acschemneuro.6b00354. [DOI] [PubMed] [Google Scholar]
- 17.Heppt J., Wittmann M.T., Schaffner I., et al. beta-catenin signaling modulates the tempo of dendritic growth of adult-born hippocampal neurons. EMBO J. 2020;39(21) doi: 10.15252/embj.2020104472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu T., Yu S., Xu Z., Tan J., Wang B., Liu Y.-G., et al. Prospects and progress on crocin biosynthetic pathway and metabolic engineering. Comput Struct Biotechnol J. 2020;18:3278–3286. doi: 10.1016/j.csbj.2020.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie Y., He Q., Chen H., Lin Z., Xu Y.i., Yang C. Crocin ameliorates chronic obstructive pulmonary disease-induced depression via PI3K/Akt mediated suppression of inflammation. Eur J Pharmacol. 2019;862:172640. doi: 10.1016/j.ejphar.2019.172640. [DOI] [PubMed] [Google Scholar]
- 20.Xiao Q., Xiong Z.e., Yu C., Zhou J., Shen Q., Wang L., et al. Antidepressant activity of crocin-I is associated with amelioration of neuroinflammation and attenuates oxidative damage induced by corticosterone in mice. Physiol Behav. 2019;212:112699. doi: 10.1016/j.physbeh.2019.112699. [DOI] [PubMed] [Google Scholar]
- 21.Zhang L., Previn R., Lu L., Liao R.-F., Jin Y.i., Wang R.-K. Crocin, a natural product attenuates lipopolysaccharide-induced anxiety and depressive-like behaviors through suppressing NF-kB and NLRP3 signaling pathway. Brain Res Bull. 2018;142:352–359. doi: 10.1016/j.brainresbull.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Ghaderi A., Rasouli‐Azad M., Vahed N., Banafshe H.R., Soleimani A., Omidi A., et al. Clinical and metabolic responses to crocin in patients under methadone maintenance treatment: A randomized clinical trial. Phytother Res. 2019;33(10):2714–2725. doi: 10.1002/ptr.6445. [DOI] [PubMed] [Google Scholar]
- 23.Lautenschläger M., Sendker J., Hüwel S., Galla H.J., Brandt S., Düfer M., et al. Intestinal formation of trans-crocetin from saffron extract (Crocus sativus L.) and in vitro permeation through intestinal and blood brain barrier. Phytomedicine. 2015;22(1):36–44. doi: 10.1016/j.phymed.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y., Huang R., Cheng M., Wang L., Chao J., Li J., et al. Gut microbiota from NLRP3-deficient mice ameliorates depressive-like behaviors by regulating astrocyte dysfunction via circHIPK2. Microbiome. 2019;7(1) doi: 10.1186/s40168-019-0733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nomura M., Durbak L., Chan J., et al. Genotype/age interactions on aggressive behavior in gonadally intact estrogen receptor beta knockout (betaERKO) male mice. Horm Behav. 2002;41(3):288–296. doi: 10.1006/hbeh.2002.1773. [DOI] [PubMed] [Google Scholar]
- 26.Lan T., Li Y.e., Fan C., Wang L., Wang W., Chen S., et al. MicroRNA-204-5p reduction in rat hippocampus contributes to stress-induced pathology via targeting RGS12 signaling pathway. J Neuroinflammat. 2021;18(1) doi: 10.1186/s12974-021-02299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qin T., Yuan Z., Yu J., Fu X., Deng X., Fu Q., et al. Saikosaponin-d impedes hippocampal neurogenesis and causes cognitive deficits by inhibiting the survival of neural stem/progenitor cells via neurotrophin receptor signaling in mice. Clin Transl Med. 2020;10(8) doi: 10.1002/ctm2.v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan H., Tang H.-B., Shan L.-Q., Liu S.-C., Huang D.-G., Chen X., et al. Quercetin prevents necroptosis of oligodendrocytes by inhibiting macrophages/microglia polarization to M1 phenotype after spinal cord injury in rats. J Neuroinflammat. 2019;16(1) doi: 10.1186/s12974-019-1613-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockenstein E., Mante M., Adame A., Crews L., Moessler H., Masliah E. Effects of Cerebrolysin on neurogenesis in an APP transgenic model of Alzheimer's disease. Acta Neuropathol. 2007;113(3):265–275. doi: 10.1007/s00401-006-0166-5. [DOI] [PubMed] [Google Scholar]
- 30.Leng L., Zhuang K., Liu Z., Huang C., Gao Y., Chen G., et al. Menin Deficiency Leads to Depressive-like Behaviors in Mice by Modulating Astrocyte-Mediated Neuroinflammation. Neuron. 2018;100(3):551–563.e7. doi: 10.1016/j.neuron.2018.08.031. [DOI] [PubMed] [Google Scholar]
- 31.Ma X.L., Shen M.N., Hu B., et al. CD73 promotes hepatocellular carcinoma progression and metastasis via activating PI3K/AKT signaling by inducing Rap1-mediated membrane localization of P110beta and predicts poor prognosis. J Hematol Oncol. 2019;12(1):37. doi: 10.1186/s13045-019-0724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostadhadi S., Imran Khan M., Norouzi-Javidan A., Dehpour A.-R. Antidepressant effect of pramipexole in mice forced swimming test: A cross talk between dopamine receptor and NMDA/nitric oxide/cGMP pathway. Biomed Pharmacother. 2016;81:295–304. doi: 10.1016/j.biopha.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 33.Ma Z., Zang T., Birnbaum S.G., Wang Z., Johnson J.E., Zhang C.-L., et al. TrkB dependent adult hippocampal progenitor differentiation mediates sustained ketamine antidepressant response. Nat Commun. 2017;8(1) doi: 10.1038/s41467-017-01709-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Encinas J.M., Vaahtokari A., Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. PNAS. 2006;103(21):8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Jimenez F.J., Clemente E., Moreno-Manzano V., Erceg S. Organized Neurogenic-Niche-Like Pinwheel Structures Discovered in Spinal Cord Tissue-Derived Neurospheres. Front Cell Dev Biol. 2019;7:334. doi: 10.3389/fcell.2019.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallego O. Nonsurgical treatment of recurrent glioblastoma. Curr Oncol. 2015;22(4):e273–e281. doi: 10.3747/co.22.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie W., Meng X., Zhai Y., Ye T., Zhou P., Nan F., et al. Antidepressant-like effects of the Guanxin Danshen formula via mediation of the CaMK II-CREB-BDNF signalling pathway in chronic unpredictable mild stress-induced depressive rats. Ann Transl Med. 2019;7(20):564. doi: 10.21037/atm.2019.09.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramaker M.J., Dulawa S.C. Identifying fast-onset antidepressants using rodent models. Mol Psychiatry. 2017;22(5):656–665. doi: 10.1038/mp.2017.36. [DOI] [PubMed] [Google Scholar]
- 39.Alavizadeh S.H., Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol: Int J Published Brit Ind Biol Res Assoc. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Hashemzaei M., Mamoulakis C., Tsarouhas K., Georgiadis G., Lazopoulos G., Tsatsakis A., et al. Crocin: A fighter against inflammation and pain. Food Chem Toxicol: Int J Published Brit Ind Biol Res Assoc. 2020;143:111521. doi: 10.1016/j.fct.2020.111521. [DOI] [PubMed] [Google Scholar]
- 41.Tang J., Lu L., Wang Q., Liu H., Xue W., Zhou T., et al. Crocin Reverses Depression-Like Behavior in Parkinson Disease Mice via VTA-mPFC Pathway. Mol Neurobiol. 2020;57(7):3158–3170. doi: 10.1007/s12035-020-01941-2. [DOI] [PubMed] [Google Scholar]
- 42.Talaei A., Hassanpour Moghadam M., Sajadi Tabassi S.A., Mohajeri S.A. Crocin, the main active saffron constituent, as an adjunctive treatment in major depressive disorder: a randomized, double-blind, placebo-controlled, pilot clinical trial. J Affect Disord. 2015;174:51–56. doi: 10.1016/j.jad.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 43.Ghalandari-Shamami M., Nourizade S., Yousefi B., Vafaei A.A., Pakdel R., Rashidy-Pour A. Beneficial Effects of Physical Activity and Crocin Against Adolescent Stress Induced Anxiety or Depressive-Like Symptoms and Dendritic Morphology Remodeling in Prefrontal Cortex in Adult Male Rats. Neurochem Res. 2019;44(4):917–929. doi: 10.1007/s11064-019-02727-2. [DOI] [PubMed] [Google Scholar]
- 44.Wu R., Xiao D., Shan X., Dong Y.u., Tao W.-W. Rapid and Prolonged Antidepressant-like Effect of Crocin Is Associated with GHSR-Mediated Hippocampal Plasticity-related Proteins in Mice Exposed to Prenatal Stress. ACS Chem Neurosci. 2020;11(8):1159–1170. doi: 10.1021/acschemneuro.0c00022. [DOI] [PubMed] [Google Scholar]
- 45.Fuchs E., Gould E. Mini-review: in vivo neurogenesis in the adult brain: regulation and functional implications. Europ J Neurosci. 2000;12(7):2211–2214. doi: 10.1046/j.1460-9568.2000.00130.x. [DOI] [PubMed] [Google Scholar]
- 46.Videbech P., Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 47.Hill A.S., Sahay A., Hen R. Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology. 2015;40(10):2368–2378. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boku S., Nakagawa S., Toda H., Hishimoto A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3–12. doi: 10.1111/pcn.12604. [DOI] [PubMed] [Google Scholar]
- 49.Wang J.-W., David D.J., Monckton J.E., Battaglia F., Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28(6):1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee S.M., Tole S., Grove E., McMahon A.P. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127(3):457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]
- 51.Amerizadeh F., Rezaei N., Rahmani F., Hassanian S.M., Moradi‐Marjaneh R., Fiuji H., et al. Crocin synergistically enhances the antiproliferative activity of 5-flurouracil through Wnt/PI3K pathway in a mouse model of colitis-associated colorectal cancer. J Cell Biochem. 2018;119(12):10250–10261. doi: 10.1002/jcb.27367. [DOI] [PubMed] [Google Scholar]
- 52.Wang J.-F., Xu H.-J., He Z.-L., Yin Q., Cheng W. Crocin Alleviates Pain Hyperalgesia in AIA Rats by Inhibiting the Spinal Wnt5a/β-Catenin Signaling Pathway and Glial Activation. Neural plasticity. 2020;2020:1–10. doi: 10.1155/2020/4297483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Antoniuk S., Bijata M., Ponimaskin E., Wlodarczyk J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci Biobehav Rev. 2019;99:101–116. doi: 10.1016/j.neubiorev.2018.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.