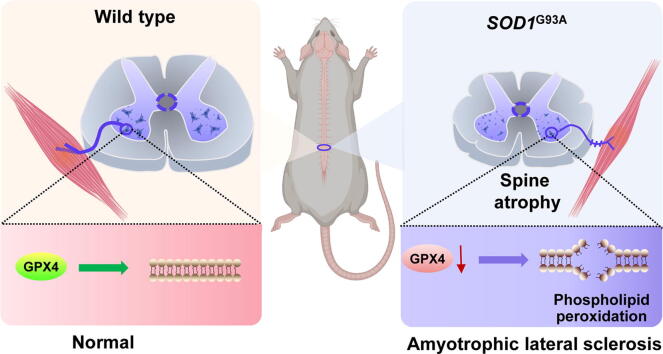
Graphical abstract
Keywords: ALS, Phospholipid peroxidation, GPX4, SOD1G93A transgenic mice, Spinal cord
Highlights
-
•
The level of phospholipid peroxidation in spinal cord is increased in ALS mouse model.
-
•
Anti-phospholipid peroxidation treatment reduces spinal motor neuron loss in ALS mice.
-
•
The expressions of GPX4 are decreased in lumbar spinal cords of ALS mice and patients.
-
•
Knockdown of Gpx4 results in loss of spinal motor neurons and abnormal neuromuscular connections.
-
•
Intrathecal injection of GPX4-AAV to overexpress GPX4 alleviates the pathological phenomenon of ALS.
Abstract
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease characterized by oxidative stress that triggers motor neurons loss in the brain and spinal cord. However, the mechanisms underlying the exact role of oxidative stress in ALS-associated neural degeneration are not definitively established. Oxidative stress-generated phospholipid peroxides are known to have extensive physiological and pathological consequences to tissues. Here, we discovered that the deficiency of glutathione peroxidase 4 (GPX4), an essential antioxidant peroxidase, led to the accumulation of phospholipid peroxides and resulted in a loss of motor neurons in spinal cords of ALS mice. Mutant human SOD1G93A transgenic mice were intrathecally injected with neuron-targeted adeno-associated virus (AAV) expressing GPX4 (GPX4-AAV) or phospholipid peroxidation inhibitor, ferrostatin-1. The results showed that impaired motor performance and neural loss induced by SOD1G93A toxicity in the lumbar spine were substantially alleviated by ferrostatin-1 treatment and AAV-mediated GPX4 delivery. In addition, the denervation of neuron-muscle junction and spinal atrophy in ALS mice were rescued by neural GPX4 overexpression, suggesting that GPX4 is essential for the motor neural maintenance and function. In comparison, conditional knockdown of Gpx4 in the spinal cords of Gpx4fl/fl mice triggered an obvious increase of phospholipid peroxides and the occurrence of ALS-like motor phenotype. Altogether, our findings underscore the importance of GPX4 in maintaining phospholipid redox homeostasis in the spinal cord and presents GPX4 as an attractive therapeutic target for ALS treatment.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by progressive degeneration of motor neuron in the brain and spinal cord [1], [2], [3]. Patients with ALS usually show muscle atrophy and gradually develop paralysis, eventually dying of respiratory muscle failure 3 to 5 years after being diagnosed [4]. Although ALS is a rare disease, the number of new cases continues to rise thereby imposing great socioeconomic burden on patients and their families [5], [6], [7]. Oxidative stress plays a central role in the onset and progression of ALS through the induction of neuronal excitotoxicity and astrocyte activation [8], [9]. To date, due to undefined mechanism and the lack of specific therapeutic targets, there are still no effective disease-modifying therapies for ALS and current FDA-approved medications, such as edaravone and riluzole, only show modest effects on disease progression [10], [11], [12]. Hence, there is an urgent need to identify potential targets for ALS therapy.
In recent years, there is emerging evidence to support a correlation between accumulation of phospholipid peroxides and a variety of diseases including neurodegenerative diseases, cancer, etc [13], [14], [15], [16]. Neuronal membrane phospholipids are rich in polyunsaturated fatty acid (PUFA) that are susceptible to peroxidative damage, ultimately leading to membrane structure modification and neuron death [17], [18]. Hence, the peroxidation of phospholipids with PUFA has been regarded as an important parameter that reflects the degree of oxidative damage in neurons [19]. Given the importance of oxidative stress in ALS pathogenesis, further studies exploring the role of phospholipid peroxidation in ALS are warranted. Altered phospholipid metabolism and elevated level of 4-hydroxynonenal (4HNE), an end product of phospholipid peroxidation, have been revealed in the spinal cord of ALS mice and patients [20], [21]. Accordingly, we speculate that phospholipid peroxidation-induced spinal cord damage might contribute to ALS disease progression.
A phospholipid peroxidation-associated cell death type, known as ferroptosis, is initiated by doubly- and triply-oxygenated arachidonic acid-containing phosphatidylethanolamines (PEs) [22]. Importantly, these highly active oxidized PEs can be reduced to less toxic hydroxy-PEs by an antioxidant enzymes glutathione peroxidase 4 (GPX4) [23]. This phospholipid-specific hydroperoxidase is essential for maintaining phospholipid redox balance, due to its unique ability to directly remove phospholipid peroxides in the presence of glutathione (GSH) [24], [25]. Several studies have underscored the protective role of GPX4 in brain diseases, such as Alzheimer’s disease, Parkinson's disease, hemorrhagic brain damage, etc [26], [27], [28]. Furthermore, the ablation of GPX4 in neurons has been demonstrated to cause motor neuron degeneration and paralysis [29]. However, the relevance of GPX4 and phospholipid peroxidation-induced neuronal damage to ALS is still unclear. In this study, we report the phenotypic significance of GPX4 deficiency and the accumulation of phospholipid peroxides in the spinal cord of SODG93A mice. Besides, intrathecal injection of neuron-targeted GPX4 and the treatment of phospholipid peroxidation inhibitor, ferrostatin 1 (Fer-1), significantly attenuated motor dysfunction in ALS mice. Our findings provide novel insights on a heretofore unknown role for GPX4 deficiency in phospholipid peroxidation during ALS pathogenesis, which may provide new direction for developing novel treatments.
Materials and methods
Chemicals and reagents
Malondialdehyde (MDA) detection kit was purchased from Beyotime (Shanghai, China). NADPH assay kit was obtained from Comin Biotechnology (Suzhou, China). Fer-1 was acquired from Selleck Chemicals (Houston, TX, USA). Primary antibodies against 4HNE (#Ab46545), SOD1 (#Ab16831), NeuN (#Ab104224) and GPX4 (#Ab125066) were purchased from Abcam (Cambridge, UK). Primary antibodies against ChAT (#Ab144P) and NeuN (#ABN78) were obtained from Millipore (Billerica, MA, USA). Primary antibody against GFAP (#3670P) was purchased from Cell Signaling Technology (Beverly, MA, USA). Primary antibodies for GAPDH (#FD0063) and β-Actin (#FD0060) were obtained from Fdbio science (Hangzhou, China). Secondary antibodies including goat anti-rabbit IgG-HRP (#FDR007), goat anti-mouse IgG-HRP (#FDM007) and rabbit anti-goat IgG-HRP (#FD8000) were obtained from Fdbio science. Secondary antibodies including Alex Fluor 594 anti-rabbit (#A-21207), Alex Fluor 488 anti-rabbit (#A-21206) and Alex Fluor 555 anti-mouse (#A-31570) were obtained from Life Technology (Gaithersburg, MD, USA).
Animals
Transgenic male mice with high copy number (20–25 copies, #004435), low copy number (7–10 copies, #002299) of mutant human SOD1G93A, and Gpx4fl/fl mice (#027964) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). SOD1G93A mice were bred with C57BL/6J background female mice obtained from Guangdong Medical Laboratory Animal Center (Guangzhou, China). The mice were genotyped by PCR using DNA extracted from tail biopsies. Littermates were used as controls and the mice used in the experiment were equally male and female. The mice were housed in a room at a mean constant temperature (24 ± 2 °C), a relative humidity of 50–60%, and provided free access to standard pellet chow and water under a 12-h light-dark cycle.
Ethics statement
All animal experiments were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised in 1996) and were approved by the Animal Ethics Committee of Jinan University (Approval Number: 20130904001).
Intrathecal injection
Intrathecal injection was done under sterile conditions and performed as previously described [30], [31] with minor modifications. Fer-1 (5 mg/mL, 10 μL), a total of 3 × 1010 particles of adeno-associated virus (AAV) serotype 2/9 vector carrying genetically encoded homo sapiens glutathione peroxidase 4 (GPX4, ref. seq. NM_002085.5) or Cre-EGFP under the hSyn promoter (BrainVTA, Wuhan, China) were intrathecally injected in a 10 μL volume using a Hamilton syringe. Briefly, mice were anesthetized with 1.25% avertin. The surgical area was shaved and disinfected with iodine tincture. After placing mice on a prone position and de-iodinating with 75% alcohol, the skin was cut to expose the lumbar spine. A Hamilton syringe was perpendicularly inserted into the gap between the L5 and L6 in the lumbar vertebrae. Once contact was made with the bone of the spinal column, the needle angle was reduced to approximately 30° and inserted into the subarachnoidal space. The solution containing AAV or Fer-1 were slowly released into the cerebrospinal fluid when there is a sudden lateral movement or ‘S’ shape of the tail. The syringe was removed 1 min later to minimize cerebrospinal fluid and vector leakage. After the surgery, animals were housed in a cage with free access to food and water till the end point. Age-matched transgenic and WT littermates were injected with AAV vector or solvent (2% DMSO + 50% PEG300 + 5% Tween 80 + ddH2O) as controls. Intrathecal injection of Fer-1 into SOD1G93A was carried in mice that were about 252–260 days-old. Intrathecal injection of GPX4-AAV into SOD1G93A mice and Cre-EGFP-AAV into Gpx4fl/fl were performed in mice that 28.5 weeks and 6.5 weeks-old, respectively. EGFP fluorescent optical images were taken of the stripped spinal cord using an in vivo fluorescence imaging system (PerkinElmer, USA).
Behavioral analysis
Motor function was assessed once a week using the rotarod test, wire hanging test and neurological scoring. All animals were pre-trained for three trials before the formal tests of motor function.
For the rotarod test [32], mice were placed onto an accelerating rotating rod (Ugo Basile, Comerio, Italy) with an increasing speed from 5 to 30 RPM within 5 min. The ability of the mouse to stay on the rod without falling was automatically timed by the apparatus. Each mouse was given three trials and the average value was recorded.
The wire hanging test was applied to measure the muscular strength of mice [33], [34]. The wire mesh used for this test was 50 × 40 cm consisting of 1.2 mm diameter wires with an interval of 0.8 mm between adjacent in the mesh. The mouse was placed on the center of the wire mesh and then carefully turned over, keeping it 60 cm off the ground. The time taken for the mouse to fall off was recorded. The test was terminated at 180 s for each trial. Each mouse was given three trials and the average value was recorded.
Neurological scoring was measured with reference to a system developed by the ALS Therapy Development Institute [35]. Briefly, the mouse's movement and performance of its hind limbs were recorded. Each mouse is given a maximum score of 4 points when the hindlimb presents a normal splay. A score of 0 is given if the mouse cannot straighten itself within 30 s.
Western blot analysis
Dissected spinal cords from mice were homogenized at 4 °C by sonication in cold lysis buffer (Beyotime, Shanghai, China) containing a protease inhibitor cocktail (Targetmol, Shanghai, China). The supernatant was collected after centrifugation of the homogenate at 12,000 RPM for 10 min at 4 °C, and the protein concentration was measured using BCA protein assay kit (Pierce, Rockford, IL, USA). Protein extracts were separated on 12% SDS polyacrylamide gel and transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). After blocking the membrane with 5% nonfat milk in Tris buffered saline-Tween 20 for 1 h at room temperature, target proteins on the membrane were incubated overnight at 4 °C with the primary antibody. On the second day, the membrane was rinsed with Tris buffered saline-Tween 20 and following incubated with the corresponding secondary antibodies for 2 h at room temperature. Bound antibody was visualized using an ECL system (Tanon 5200, Shanghai, China). Band intensity was quantified using Quantity One software and target protein bands normalized to the housekeeping protein band GAPDH. Results were plotted using GraphPad prism software 8.0.
Measurements of MDA, GSH and NADPH
MDA levels were assessed using the thiobarbituric acid reactive substances assay kit (Beyotime, Shanghai, China) according to the manufacturer’s instructions.
GSH content analysis was performed as described in our previous study [15] using a C18 column (4.6 × 150 mm, 5 μm, Cosmosil, Japan) and a four-channel ESA 5600A CoulArry Detector to detect the GSH signal.
NADPH content was assessed using a commercial chemical assay kit (Keming, Suzhou, China) according to the manufacturer’s instruction. Briefly, the method was based on the spectrophotometric measurement of the color produced when NADPH reduced oxidized thiazole blue to formazan (570 nm).
Quantitative reverse transcription polymerase chain reaction
Dissected spinal cords from mice were extracted using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and RNA concentrations determined using a spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, Waltham, MA, USA). RNA was transcribed into cDNA (cDNA synthesis kit, TransGen Biotech, Beijing, China). Relative expression of RNA was measured using SYBR green (TransGene Biotech) on a CFXConnectTM reverse transcription (RT) machine (Bio-Rad, Hercules, CA, USA) and calculated using the 2−ΔCt method. The primer sequences are shown in Supplementary Table S1 and S2.
Tissue processing, Nissl staining, and immunofluorescence
Anesthetized mice were sacrificed and transcardially perfused with saline followed by 4% paraformaldehyde in PBS. The lumbar region (L1–L5) of the spinal cord was isolated and post-fixed in 4% paraformaldehyde overnight at 4 °C, cryoprotected for 48 h in PBS containing 30% sucrose, embedded in O.C.T (TisssueTek, Sakura) and cut at a thickness of 20 μm using a Leica vibratome (Leica Biosystem, Nussloch, Germany).
For Nissl staining, frozen slides were air-dried for 2 h. Sections were immersed in 0.1% (w/v) cresyl violet acetate (MilliporeSigma, Burlington, MA, USA) using a standard protocol, dehydrated and imaged (PreciPointGmbh M8 microscope, Freising, Germany). Nissl-stained motor neurons cells having a diameter at least 25 μm in the ventral horn (VH) were counted.
For immunofluorescent staining, frozen sections were air-dried for 2 h and then blocked with 5% normal donkey serum/0.5% TritonX-100/PBS for 2 h at room temperature. After spinning off the liquid, slides were incubated overnight at 4 °C with the indicated primary rabbit anti-NeuN (Millipore, Bedford, USA) or mouse anti-GFAP (Millipore, Bedford, USA) antibodies diluted in 5% normal donkey serum/0.1% Triton X-100 in PBS. Sections were then washed with PBS and incubated for 2 h at room temperature with an appropriate fluorescent secondary antibody. Cover slips were mounted over one drop of anti-fade mounting medium (Beyotime, Shanghai, China) and examined under a fluorescent microscope (Zeiss Axio Observer Z1, Carl Zeiss, Oberkochen, Germany). Photographs of GPX4, NeuN and GFAP were analyzed using Image-pro plus 6.0 software.
LC-MS analysis of phospholipids
Lipids in the spinal cord were extracted by the Folch procedure [36] and were further analyzed by LC-MS. Phospholipids were separated on a normal-phase column (Luna Silica (2), 3 μm, 150 × 2.0 mm (Phenomenex)) at a flow rate of 0.2 mL/min on a DionexUltiMate 3000 HPLC system (Thermo Fisher Scientific). The mobile phase consisted of 10 mM ammonium formate in propanol/hexane/water (285:215:5, v/v/v, solvent A) and 10 mM ammonium formate in propanol/hexane/water (285:215:40, v/v/v, solvent B). The elution gradient program was set as follow: 0 min, 10% B; 20 min, 32% B; 30 min, 70% B; 32 min, 100% B; 58 min, 100% B; 60 min, 10% B; 75 min, 10% B. The column temperature was maintained at 35 °C. The injection volume was 2 μL.
MS analysis of phospholipids was performed on a Q-Exactive Hybrid Quadrupole-Orbitrap mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Analysis was performed in full MS negative mode at resolution setting of 70,000 and data-dependent-MS/MS mode at resolution setting of 17,500. Ion source conditions were set as follows: spray voltage, 3.0 kV; vaporizer temperature, 320 °C; transfer tube temperature, 350 °C; S-lens Rf level, 60; sheath gas, 35 (arbitrary units); auxiliary gas, 15 (arbitrary units); sweep gas, 1 (arbitrary units). For MS, the scan range was m/z 400–1800 and the maximum ion injection time was 128 ms using 1 microscan per MS scan. For MS/MS, high energy collision induced dissociation analysis was performed with the collision energy set to 24 eV and the maximum ion injection time of 500 ms. An isolation window of 1.5 Da was set for MS/MS scans.
ST analysis
ST is a transcriptomics technology based on in situ capture, and the operation steps are described previously [37]. GPX4 gene expression analysis was performed on the large-scale and high-resolution ALS transcriptomics data in the public interactive data browsing website, https://als-st.nygenome.org. The ALS database collected about 1200 mouse lumbar spinal cord tissue samples from4 periods (pre-symptomatic, onset, symptomatic and end-stage) and a total of ∼1200 spinal cord sections of 67 mice. It also collected 80 postmortem sections of lumbar and cervical spinal cord tissues from 7 sporadic ALS patients with lumbar or bulbar-onset.
Resting EMG recording
Resting EMG recording analysis was performed as previous study [38]. Mice were anesthetized with 1.25% avertin and the left hind limbs were shaved. To record EMG potentials, two 30-G platinum transcutaneous needle electrodes were inserted into the gastrocnemius, keeping two recording electrodes ∼2 mm apart. Electrodes were connected to an active head stage and the signal amplified (X100) and digitized using the PowerLab 16/32 data-acquisition system (ADInstruments). The signal within 5 mins was recorded at a sampling rate of 20 kHz and stored in a PC, and then analyzed using LabChart 8.15 (ADInstruments).
MRI and DTI
MRI and DTI analysis were performed as previous research [39]. Paraformaldehyde (4%)-fixed spinal cords from mice were placed in 5 mL Eppendorf tubes and immersed in perfluorocarbon oil media (Fluorinert, FC-40, 3 M). MRI scanning was performed using a Bruker 9.4 T Biospec 9430 USR (Ettlingen, Germany) with a TX Volume Array and RX Surface Array. The diffusion images were acquired by using a spin-echo diffusion weighted sequence with the following acquisition parameters: TR = 3 s, TE = 20 ms, NEX = 8, FOV = 18 mm × 18 mm, matrix size = 108 × 96, and b = 650 s/mm2). Total imaging time was 14 min. Slice thickness was 0.7 mm. A DTI diffusion scheme was used, 17 sequential imaging sections centered in the lumbar spinal cord and a total of 30 diffusion sampling directions were acquired. The values of fractional anisotropy (FA) and mean diffusivity (MD) were calculated by ParaVision 360 V1.1. Volume analyses were performed by manually segmenting the boundary between the gray matter (GM) and the white matter (WM), slice by slice, using Carimas 2.9 RayVisualization v.2.9.4.0 (Turku PET Centre). The total volumes of GM and WM around lumbar 3–5 enlargement (3.5 mm spinal cord length) were counted.
Statistics
Data were analyzed using Graphpad 8.0 software and expressed as the mean ± SEM. Differences among groups were determined by either Student’s t test or One-way ANOVA or Two-way ANOVA. P < 0.05 was considered statistically significant.
Study approval
All animal experiments were performed in accordance with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (NIH publication No. 80-23, revised in 1996) and were approved by the Animal Ethics Committee of Jinan University (Approval Number: 20130904001).
Results
Accumulation of phospholipid peroxides is an essential feature in ALS mice
The G93A mutation of SOD1 is an inherited ALS causative factor, which produces toxic SOD1 protein aggregates and contributes to the pathogenesis of ALS [40]. In order to explore the relationship between phospholipid peroxidation and ALS disease progression, low copy (∼8 copies) and high copy (∼25 copies) mutated SOD1G93A (hereinafter referred to as G93A) mouse strains with different speeds of the occurrence and development of ALS [41] were utilized in this study. Firstly, the impact of high- and low-copy G93A on motor function in mice were evaluated by behavioral tests for 35 weeks. The rotarod test (Fig. 1a), wire hanging test (Supplementary Fig. S1a), and neurological scores (Supplementary Fig. S1b) showed that the average age of disease onset of high copy and low copy mice was ∼12 weeks and ∼28 weeks, respectively, and the average age of disease endpoint was ∼21 weeks and ∼35 weeks, respectively. These observations suggest that increased copy number of G93A mutation led to an earlier onset of motor dysfunction. Since the loss of motor neurons in the lumbar spine is one of the key pathological features of ALS, the motor neurons in ventral horn (VH) of lumbar spine were marked using NeuN and Nissl staining. The results show that when compared with WT group, the motor neurons in G93A mice were significantly shrunken (Fig. 1b and 1c). Also, the number count decreased as the copy number increased (Fig. 1d and 1e).
Fig. 1.
The number of motor neuron decreased and the phospholipid peroxides increased with the accumulation of SOD1G93A transgene copy number. a Persistence time of mice on the rotating rod. NWT, G93A-low = 6. NG93A-high = 8.b, c Representative images and integrated fluorescence density of NeuN-stained motor neurons in the lumbar ventral horn (VH) of the spinal cord at the terminal age of G93A mice. Scale = 100 μm. d, e Representative Nissl staining images and corresponding quantification of large motor neurons in the lumbar VH of the spinal cord at the terminal age of G93A mice. Scale = 100 μm. N = 6. f MDA concentration in the spinal cord of mice at 120 day. N = 5. g, h Western blotting and quantifications of 4HNE and SOD1 in the spinal cord of mice at 120 day measured by Western blotting. N = 3. i Multivariate statistical analysis of oxidative phospholipids using orthogonal partial least squares–discriminate analysis. j Values of variable importance for the projection of oxidative phospholipids. k Volcano plot of oxidative phospholipids represented by log2 (fold change) of G93A 120 day/WT 120 day groups plotted against the -log10 (p-value). Statistical significance was evaluated by t test (p < 0.05). l Amount of PE(38:4) + 2O in G93A 120 day and WT 120 day mice. NWT = 7. NG93A = 6. Data are represented as mean ± SEM, **p < 0.01, ***p < 0.001 vs. WT group by t test.
To delineate the relationship between phospholipid peroxidation and G93A mutation, we generally assessed the levels of phospholipid peroxides in ALS, concentrations of two important phospholipid peroxidation end-products, malondialdehyde (MDA) and 4HNE in the spinal cords of 120-day-old high copy and low copy G93A mice were measured. As shown in Fig. 1f-h, MDA and 4HNE levels increased with a higher transgene copy number. Also, the mutant SOD1 was significantly up-regulated in 120-day-old high copy G93A mice corresponding to the mid-stage state of ALS disease (Fig. 1f-h).
Further, LC-MS/MS-based redox phospholipidomics analysis was performed to determine the molecular species of oxidized phospholipids, focusing on the five major categories of phospholipids in WT and G93A low copy mice, including PE, phosphatidylcholine (PC), phosphatidylinositol (PI), phosphatidylserine (PS), and phosphatidylglycerol (PG). As shown in Fig. 1i, the composition of oxidized phospholipids in G93A mice was significantly different from that in WT mice. Among the five phospholipids discussed above, oxidized PEs were found to be primary contributors to this difference (Fig. 1j). Interestingly, oxidized arachidonic acid-PE (PE(38:4) + 2O), a ferroptosis-specific cell death signal [22], showed the most significant elevation (Fig. 1k and 1 l). These data appear to reveal the accumulation of PE peroxides as a major characteristic feature in the pathogenesis of ALS.
Inhibition of phospholipid peroxidation alleviates motor dysfunction and neuron loss of ALS mice
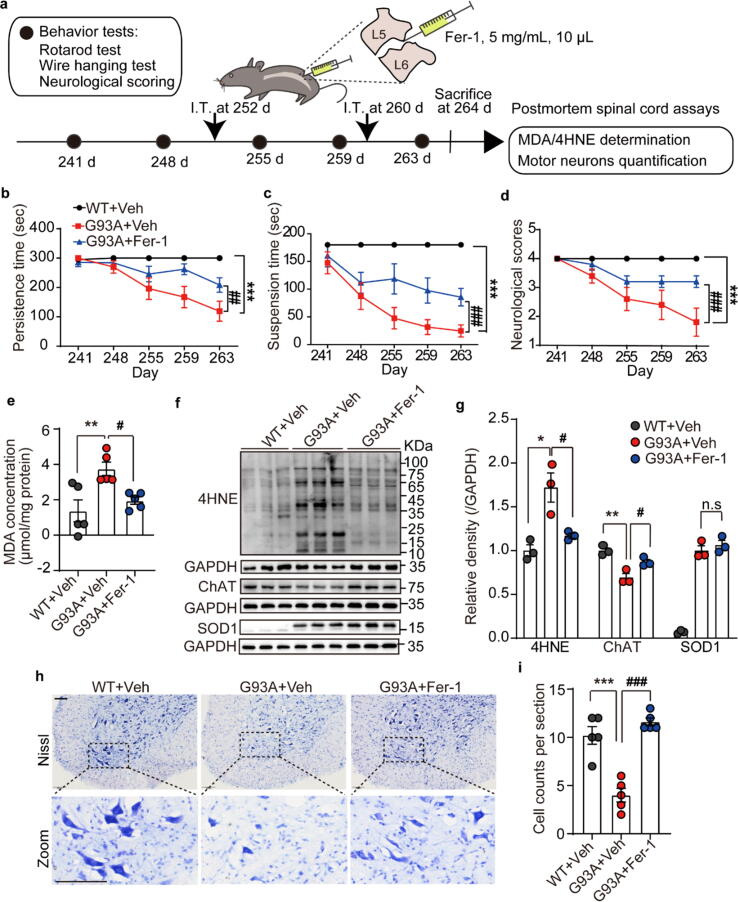
In order to evaluate the potential role of phospholipid peroxidation-induced injury in ALS, Fer-1, a specific inhibitor of lipid peroxidation was intrathecally injected (5 mg/mL, 10 μL) into the L5-L6 lumbar spine of symptomatic low copy G93A mice on day 252 and day 260, respectively (Fig. 2a). As shown by the rotarod test, wire hanging test and neurological scores (Fig. 2b-d), Fer-1 administration significantly mitigated behavior deficits in G93A mice. Meanwhile, the contents of MDA and 4HNE in the spinal cords were also significantly reduced in Fer-1 treated animals, comparing with G93A group (Fig. 2e-g). These data indicate that the restriction of phospholipid peroxidation in the spinal cord can improve the motor performance of ALS mice. Furthermore, the damage of motor neuron caused by G93A mutation was examined. The protein expression of a motor neuron marker ChAT was increased in Fer-1 treated ALS mice (Fig. 2f and 2 g). Consistent with this, the results of Nissl staining also showed that Fer-1 could restore the decrease of large motor neurons (>25 μm) in the VH of the lumbar spinal cord of G93A mice (Fig. 2h and 2i). This is a very interesting observation since it suggests that inhibition of lipid peroxidation is upstream to motor neuron loss in ALS. This raises the inspiring possibility of therapeutic options directed at preventing phospholipid peroxidation in the spinal cord in an effort to delay the progression of ALS.
Fig. 2.
Lipid peroxidation inhibitor Fer-1 alleviated motor dysfunction and neuron loss of G93A mice. a Schematic diagram of Fer-1 administration, behavior test and ‘postmortem’ assays. b-d Persistence time on the rotating rod, the hanging time on the wire mesh, and statistics of neurological scores of G93A mice intrathecally injected with Fer-1. Data are represented as mean ± SEM. N = 5 mice per group, ***p < 0.001 G93A + Veh vs. WT + Veh group; ##p < 0.01, ###p < 0.001 G93A + Fer-1 vs. G93A + Veh group by Two-way ANOVA. e MDA concentration in the spinal cord of mice. N = 5. f, g Western blotting and band quantifications of 4HNE, ChAT and SOD1 in the spinal cord of mice. N = 3. h, i Representative Nissl staining images and quantification of the large motor neurons in the lumbar VH of the spinal cord of mice. Scale = 100 μm. N = 5. Data are represented as mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001 G93A + Veh vs. WT + Veh group; #p < 0.05, ##p < 0.01, ###p < 0.001 G93A + Fer-1 vs. G93A + Veh group by One-way ANOVA. I.T.: intrathecal injection; Veh: vehicle; Fer-1: ferrostatin 1.
GPX4 expression is impaired in ALS mice and patients
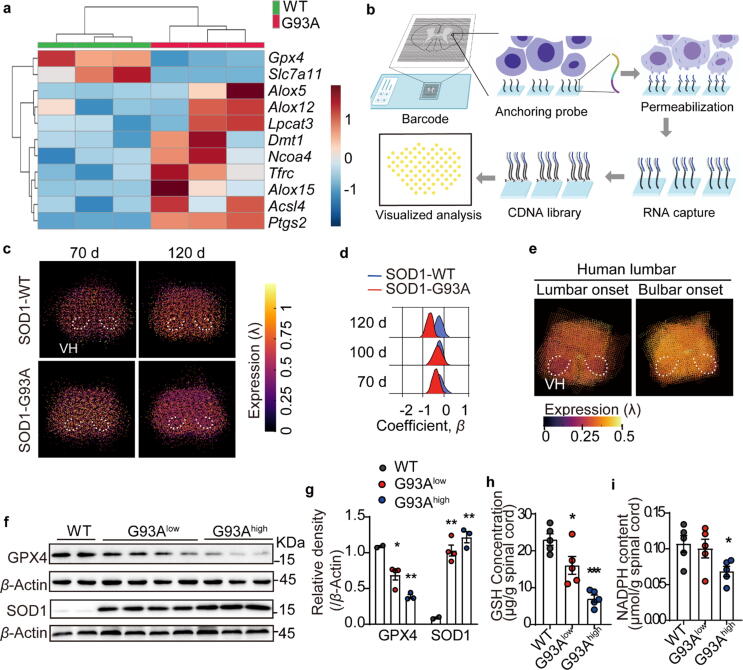
The reasons for increased phospholipid peroxidation in ALS animals could be either, an accumulation of membrane phospholipid peroxides or a reduction in antioxidant capacities such as GPX4 activity [42]. To analyze the contribution of phospholipid peroxidation-related genes to ALS, comparative qPCR analysis was performed using spinal cord extracts from WT and G93A transgenic animals. As shown in Fig. 3a, the expression levels of genes that promote phospholipid peroxidation (Lpcat3, Ncoa4, Tfrc, Alox15, Acsl4, Alox5, Alox12, Dmt1, Ptgs2) increased, while genes that can reduce phospholipid peroxidation (Gpx4, Slc7a11) decreased in the spinal cord of ALS mice. Among them, GPX4, a key antioxidant enzyme that is known to contribute to maintenance of redox balance in phospholipid [43], was significantly under-expressed in the spinal cords from ALS mice (Fig. 3a).
Fig. 3.
GPX4 was down-regulated in the spinal cord of both G93A mice and ALS patients. a Lipid peroxidation-related genes expression in the spinal cord of mice at 120 day detected by qPCR, and expressed using a heatmap. N = 3 mice each group. b Schematic diagram of spine spatial transcriptomics. c, d Spatial transcriptomics representative images of Gpx4 gene expression in the lumbar spine and statistics of the coefficient parameter β of Gpx4 gene in the lumbar VH of the spinal cord at 70,100 and 120 days age-matched G93A mice and WT mice. e Spatial transcriptional expression of GPX4 in the lumbar VH of the spinal cord of ALS patients with lumbar onset and bulbar onset. f, g Protein expression and quantifications of GPX4 and SOD1 in the spinal cord of G93A high copy, low copy mice and WT littermates at 120 days age. h, i GSH concentration and NADPH content in the spinal cord of mice at 120 day. Data are expressed as mean ± SEM. N = 5 mice each group, *p < 0.05, ***p < 0.001 vs. WT group by t test. VH: ventral horn.
Next, we utilized an interactive data exploration portal for the spatiotemporal transcriptome atlas of the ALS spinal cord (https://als-st.nygenome.org) to perform precise spatial gene expression analysis of Gpx4 in ALS spinal cord samples derived from both human and mice. Spatial transcriptomics (ST) is a transcriptomics technology based on in situ capture, which accurately correlates RNA-seq data with spatial information through specific sequence barcodes, so as to quantify and visualize the transcriptomics of tissue sections in space. The principle of ALS ST is showed in the Fig. 3b. As shown in Fig. 3c, Gpx4 gene expression in G93A mice at the age of 120 days was lower compared to WT control mice. Spatially, the decrease of Gpx4 gene expression occurred in the ventral horn (VH) region (Fig. 3d) as well as other areas of the lumbar spine (Supplementary Fig. S2).
ALS patients can be categorized into lumbar-onset and bulbar-onset. By comparing the GPX4 gene expression in the lumbar spinal cord of ALS patients with lumbar onset and bulbar onset, one can determine the difference between the onset and non-onset sites. Analysis of lumbar spine sections from ALS patients using the ST database revealed that the level of GPX4 gene in the VH of onset site was lower than that in the non-onset site (Fig. 3e), thereby providing proof of clinical relevance linking the reduction in GPX4 with ALS. Similarly, western blot analysis from spinal cords extracts showed a significant reduction in GPX4 protein expression in ALS mice, likely due to the presence of the mutant SOD1 protein (Fig. 3f and 3 g).
The lipid peroxides-clearing function of GPX4 depends on the small antioxidant molecular GSH. GPX4 converts reduced GSH to oxidized glutathione (GSSG) while reducing lipid hydroperoxides. GSSG is then recycled back to GSH by the actions of glutathione reductase and nicotinamide adenine dinucleotide phosphoric acid (NADPH) [44]. Hence, GSH and NADPH play critical roles in maintaining the function of GPX4 to prevent phospholipid peroxidation. Interestingly, there is a significant decreased in the levels of both GSH and NADPH in the spinal cords of high copy ALS mice (Fig. 3h and 3i), implying a deficiency of GPX4 function. Taken together, the above data reveal impaired GPX4 expression in ALS that likely leads to accumulation of peroxidized phospholipids in the spinal cord.
Intrathecal GPX4 deficiency drives pathological features of ALS in mice
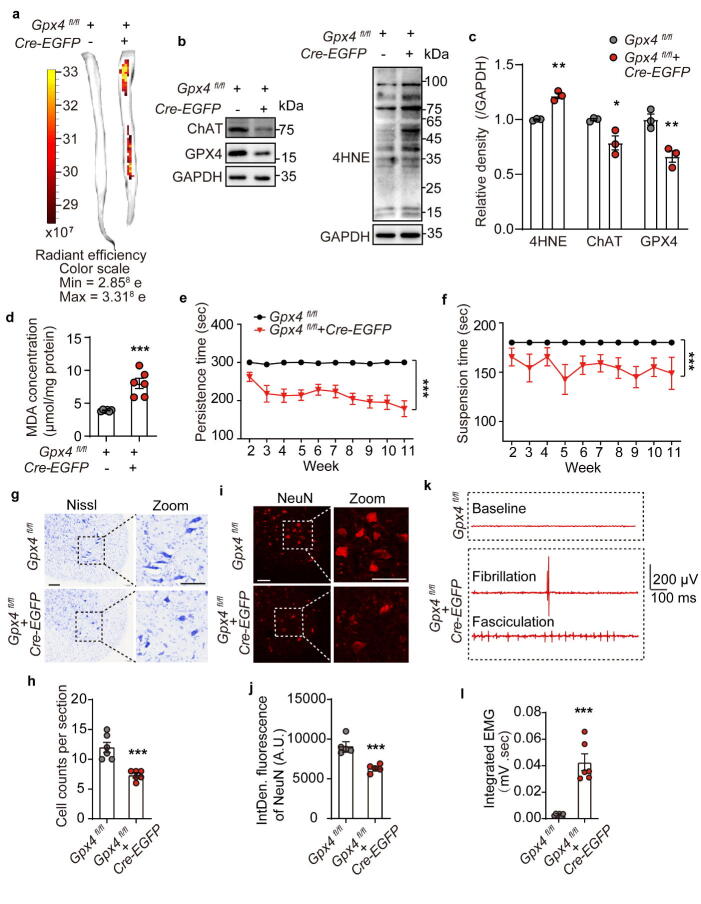
To address the role of GPX4 in ALS, a conditional knockdown mouse model for the Gpx4 gene was established (Gpx4 CKD) by intrathecally injecting neuronal-targeted Cre-EGFP-AAV under the hSyn promoter (3 × 1010 particles, 10 μL) into the spinal cord of 6-weeks-old Gpx4fl/fl mice. Fluorescence imaging of the isolated spinal cord showed that the Cre-EGFP-AAV successfully infected the mouse spinal cord and expressed fluorescent EGFP protein 12 weeks after the intrathecal injection (Fig. 4a). Western blot analysis of these spinal cord extracts showed that protein expression of GPX4 and ChAT was reduced post Cre-EGFP-AAV infection (Fig. 4b and 4c). Additionally, 4HNE and MDA levels were significantly elevated in the post infection spinal cords (Fig. 4b-d), indicating that the loss of GPX4 led to lipid peroxidation.
Fig. 4.
Intrathecal Gpx4 knockdown induced sever motor performance deficits in mice. a Fluorescence imaging of the isolated spinal cord of Gpx4fl/fl mice intrathecally injected with Cre-EGFP-AAV under the hSyn promoter. The red/yellow gradient scale indicates the intensity of EGFP signal. b, c Expression levels and quantifications of GPX4, ChAT and 4HNE in the spinal cord of mice. N = 3 mice each group. d MDA concentration in the spinal cord. N = 6 mice each group. e, f Persistence time on the rotating rod and suspension time on the wire mesh of mice. Data are represented as mean ± SEM. N = 6 mice per group, ***p < 0.001 Gpx4fl/fl + Cre-EGFP vs. Gpx4fl/fl by Two-way ANOVA. g, h Representative Nissl staining images and quantification of the large motor neurons in the lumbar VH. N = 6. i, j Representative images and integrated fluorescence density of NeuN-stained motor neurons in the lumbar VH. N = 5. k EMG activity and the presence of fibrillation and fasciculation in the GCM of Gpx4fl/fl mice treated with Cre-EGFP-AAV or AAV before sacrifice. l Quantitative analysis of the integrated EMG signals. N = 6 mice each group. Data are expressed as mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001 Gpx4fl/fl + Cre-EGFP vs. Gpx4fl/fl by t test. EMG: electromyography; GCM: gastrocnemius muscle. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To test whether Gpx4 CKD in spinal cord neurons led to motor dysfunction, the rotarod test and wire hanging test were performed every week after intrathecal injection of Cre-EGFP-AAV. In line with the results obtained from G93A mice, Gpx4 CKD led to an apparent deficit in persistence time on the rotating bar (Fig. 4e) and wire mesh (Fig. 4f). To demonstrate the effect of GPX4 on the survival of motor neurons, we employed Nissl staining and NeuN immunofluorescence and results showed that Gpx4 deficiency resulted in a loss of large motor neurons in the lumbar VH of these spinal cords (Fig. 4g-j). To further assess the motor neuron damage caused by loss of GPX4 in the spinal cord, electromyography (EMG), an electro-diagnostic medicine technique was employed to evaluate muscle function in these animals. The resting EMG images of the gastrocnemius muscles (GCM) showed acute fibrillation and fasciculation in Gpx4 CKD mice when compared to controls, which remained at the baseline (Fig. 4k). Moreover, the integrated EMG signals in the GCM of Gpx4 CKD mice were remarkably higher than that of control mice (Fig. 4l), suggesting that the loss of GPX4 function in neurons led to the denervation of the muscle through neuron death (Fig. 4g-j). In sum, the deficiency of Gpx4 in mouse spinal cord neurons leads to neuronal death through lipid peroxides accumulation, resulting in pathological features similar to ALS.
Neuron-targeted GPX4-AAV delivery improves the pathological characteristics of ALS mice
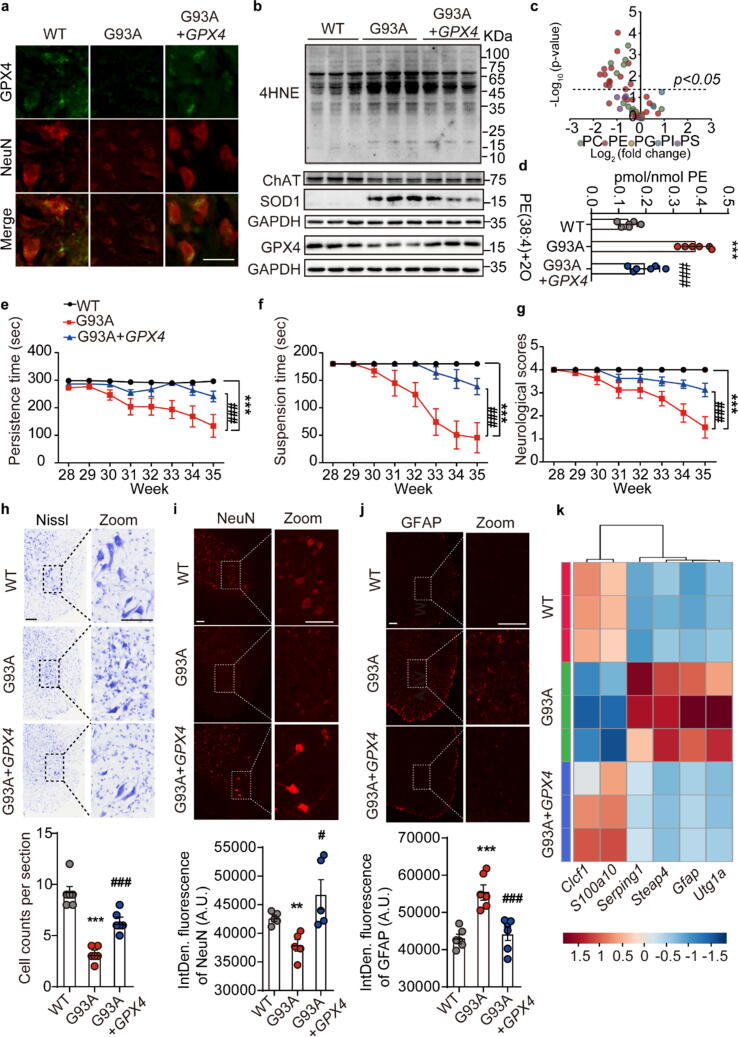
To explore whether restoring GPX4 could alleviate ALS, neuron-targeted GPX4-AAV under the hSyn promoter (3 × 1010 particles, 10 μL) was intrathecally injected into the spinal cords of low copy G93A mice at the early stage of disease onset (28.5 w). Firstly, GPX4 was successfully expressed in the spinal cord neurons of G93A mice as confirmed by immunofluorescence co-localization of GPX4 and NeuN (Fig. 5a) and western blotting (Fig. 5b). Secondly, GPX4 expression significantly inhibited the accumulation of phospholipid peroxidation end product 4HNE and prevented the loss of the neuronal marker ChAT compared to G93A mice (Fig. 5b). Further, oxidized phospholipidomics results demonstrated that the accumulation of phospholipid peroxides represented by PE(38:4) + 2O in G93A mice could be reversed by administering GPX4-AAV (Fig. 5c and 5d, Supplementary Fig. S3). We also determined that GPX4 expression in G93A mice significantly improved the motor performances compared to G93A mice (Fig. 5e-g). Additionally, loss of Nissl-reactive and NeuN-positive large motor neurons in the lumbar VH of G93A mice was reversed by intrathecally GPX4-AAV injection (Fig. 5h and 5i).
Fig. 5.
Intrathecal overexpressing GPX4 rescued motor performance disorder in G93A mice. a Representative fluorescence co-localization images of GPX4 and NeuN in the lumbar ventral horn. Scale = 50 μm. N = 3. b Expression of GPX4, ChAT, and 4HNE in the spinal cord of mice. N = 3. c Volcano plot of oxidative phospholipids represented by log2 (fold change) of G93A + GPX4-AAV/G93A + AAV groups plotted against the -log10 (p-value). Statistical significance was evaluated by t test (p < 0.05). d Amount of PE(38:4) + 2O in the spinal cord of mice. N = 6. e-g Persistence time on the rotating rod, suspension time on the wire mesh, and statistics of neurological scores of mice intrathecally injected with GPX4-AAV. Data are represented as mean ± SEM. N = 6 mice each group, ***p < 0.001 G93A + AAV vs. WT + AAV group; ###p < 0.05 G93A + GPX4-AAV vs. G93A + AAV group by Two-way ANOVA. h Representative Nissl staining images and quantification of motor neurons in the lumbar VH. N = 6. i Representative images and the integrated fluorescence density of NeuN-stained motor neurons in the lumbar VH at the terminal age. N = 5. j Representative images and the integrated fluorescence density of GFAP-stained astrocytes in lumbar VH at the terminal age. Scale = 100 μm. N = 6 mice each group. k Pan (Gfap, Steap4), A1 (Serping1, Utg1a) and A2 (Clcf1, S100a10) astrocyte genes expression in the spinal cord of mice detected by qPCR, and expressed using a heatmap. N = 3. Data are expressed as mean ± SEM, **p < 0.01, ***p < 0.001 G93A + AAV vs. WT + AAV group; #p < 0.05, ###p < 0.001 G93A + GPX4-AAV vs. G93A + AAV group by One-way ANOVA.
In addition to monitor the changes of motor neurons, the status of astrocytes is sensitive to redox states and its activation has been deemed as one of the main pathological characteristics in the progression of ALS [45], [46]. To clarify the relationship between GPX4 and astrocytes, we firstly performed immunofluorescence analysis of astrocyte marker GFAP in the spinal cord sections. The images showed that GPX4 overexpression by AAV delivery decreased the number of GFAP-positive cells in the lumbar spine of G93A mice (Fig. 5j). On the other hand, gene profile of pan, type A1 and A2 astrocyte markers was also determined to assess the activation of astrocytes in GPX4-AAV treated mice. Results showed that GPX4 overexpression remarkably reduced the expressions of pan (Gfap, Steap4) and protective A1 (Serping1, Utg1a) astrocyte signature genes, whereas upregulated the mRNA level of neuro-protective A2 astrocyte marker genes, including Clcf1 and S100a10 in G93A mice (Fig. 5k). These findings provide primary evidences linking the lack of GPX4 and the activation of astrocytes.
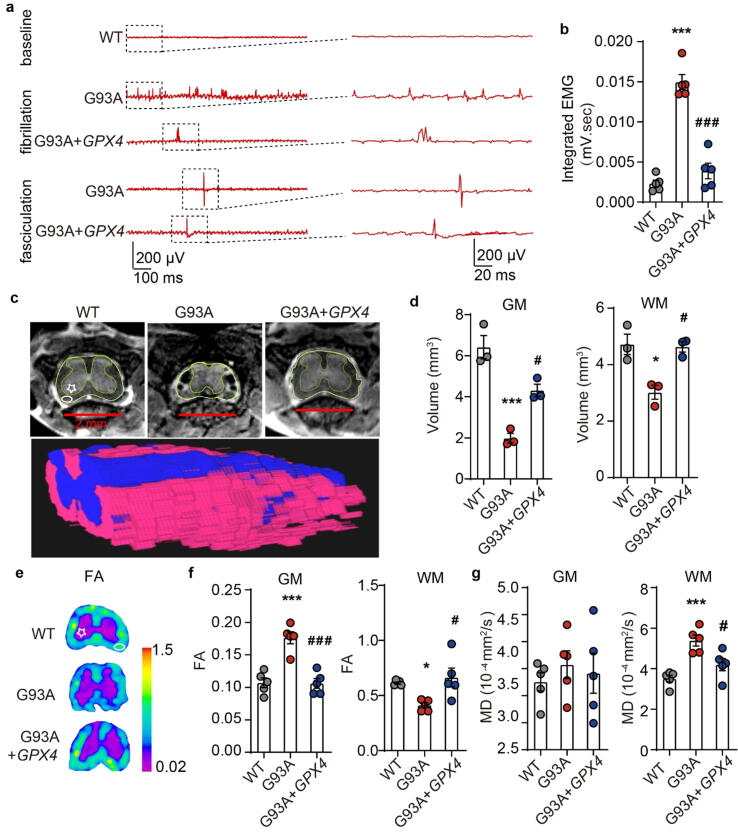
Furthermore, the therapeutic effect of intrathecally GPX4-AAV delivery was confirmed by EMG, MRI and diffusion tensor imaging (DTI) detection. EMG examination suggested that GPX4-AAV decreased the frequency of fibrillation and fasciculation in the GCM of ALS mice (Fig. 6a). Correspondingly, total EMG signals were significantly attenuated (Fig. 6b), demonstrating an overall improvement in neuromuscular connections following GPX4-AAV administration to G93A ALS mice. MRI and DTI are important clinical imaging techniques used in diagnosis of ALS. They were performed to unveil the changes in the volume of the lumbar spine and the pathological changes of lumbar white matter (WM) and gray mater (GM) in ALS mice. Structure and volume analysis based on MRI showed that both the GM and the WM volumes of lumbar spine of sham-operated G93A mice were significantly reduced compared with sham-operated WT mice, and were rescued by GPX4-AAV administration (Fig. 6c and 6d), suggesting the positive effect of GPX4-AAV delivery on spinal atrophy in ALS mice.
Fig. 6.
Intrathecally overexpressing GPX4 in SOD1G93A mice improved the GCM fibrillation and fasciculation, and blocked spine atrophy. a Fibrillation and fasciculation recordings in the GCM of G93A mice administrated with AAV and GPX4-AAV. b Quantitative analysis of the integrated EMG signals. N = 5 mice each group. c, d Representative MRI images and volume analysis of the lumbar GM region and the lumbar WM region. N = 3 mice each group. e Representative FA maps of lumbar spinal cord. Colors represent FA values, where on pink & purple shows lower FA values and on blue & cyan displays higher FA values. f, g Quantitative values of FA and MD in the GM and WM of lumbar VH. N = 5 mice each group. Data are represented as mean ± SEM. *p < 0.05, ***p < 0.001 G93A + EGFP-AAV vs. WT + EGFP-AAV group; #p < 0.05, ###p < 0.001 G93A + GPX4-AAV vs. G93A + EGFP-AAV group by One-way ANOVA. FA: fractional anisotropy, MD: mean diffusivity. WM: white matter, indicated by ellipse. GM: gray matter, indicated by pentagram. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
DTI provides quantitative parameters of fractional anisotropy (FA) and mean diffusion (MD), which can evaluate the anisotropy and diffusion of water molecules in the WM and GM of mice spine to reveal the integrity of neuronal pathways. An increase of FA in the GM region and a reduction of FA in the WM region of lumbar VH indicated a loss of neuronal pathway integrity in G93A mice, which was significantly reversed by GPX4-AAV administration (Fig. 6e and 6f). Moreover, MD in the WM region increased in the VH of sham-operated G93A mice, also indicating a loss of neuronal pathway integrity, which was attenuated by administration of GPX4-AAV, while MD in the GM region remained unchanged (Fig. 6g).
Discussion
ALS is a fatal neurodegenerative disease that results in the progressive loss of motor neurons in the spinal cord and brain. Due to the complexity of the disease pathogenesis, there is still a lack of effective treatments [47], [48]. Hence, it is urgent to obtain a better understanding of the molecular mechanism underlying disease progression in ALS, with a focused goal of identifying new therapeutic strategies. Here, we report that the accumulation of phospholipid peroxidation products, represented by doubly-oxygenated arachidonic acid-containing PEs(38:4), due to G93A mutation of SOD1. Since PUFA-enriched PEs are essential phospholipids on the double layer of the cell membrane with a rapid turnover rate [49], [50], the oxidation of PEs(38:4) is likely to affect the cell membrane function. In fact, an elevation in lipid peroxides and the rupture of the motor cortex plasma membrane have been previously observed in ALS patients and mice [51], [52]. As expected, intrathecal injection of lipid peroxidation inhibitor Fer-1 significantly reduced motor neuron loss and improved the motor dysfunction of ALS mice, proposing that anti-phospholipid peroxidation agents might be potential ALS treatment. Our data identified phospholipid peroxidation as an essential feature of the onset and progression of ALS.
One possible explanation for the accumulation of phospholipid peroxides is the impaired antioxidant pathways. GPX4 is a well-known antioxidant enzyme that can directly reduce phospholipid peroxides [42], [53]. Our results showed that GPX4 expression in the spinal cord of ALS mice was significantly declined, which has been confirmed by the spatial gene expression in patients’ spinal cord samples from clinical ST database of ALS. Consistently, the conditional knockdown of Gpx4 was demonstrated to enhance the level of phospholipid peroxidation in the spinal cord and impair mice motor capacity. This result was supported by the study of Chen et al., which has reported that the absence of neuronal GPX4 led to the degeneration of motor neurons and onset of paralysis [29].
During ALS, the activation of astrocytes, the immune cells in the CNS, contributes to the disease progression by promoting inflammation and motor neuron death [54], [55]. Activated astrocytes are divided into pro-inflammatory AI and protective A2 types [56]. Thus, we attempted to clarify the relationship between GPX4 and astrocyte activation. Our results demonstrated that the inhibition of GPX4 expression induced by SOD1G93A led to astrocyte activation in G93A ALS mice. Interestingly, we found that the activation of neurotoxic type A1 astrocytes in the ALS spinal cord was attenuated by neuron-targeted overexpression of GPX4 while aggravated by knockdown of GPX4. In fact, apart from the activation, the amount of astrocytes was also affected by GPX4 level, reflected by GFAP immunostaining the spinal cord sections (Fig. 5j, Supplementary Fig. S4). These primary observations gave us a hint that GPX4 might play a key role in maintaining brain immune homeostasis. Since we had previously reported that PE(38:4) + 2O expressed on the surface of ferroptotic cells facilitated their elimination by phagocytosis [57], we speculate that PE peroxides resulted from GPX4 deficiency might also act as signals to activate astrocytes, possibly through the transfer of lipid peroxides from neurons to astrocytes [58]. The deep and exact mechanism is worthy of further investigation.
In this study, we also provide strong evidence for the applicability of GPX4-related treatment as a promising therapy for ALS, as neuron-targeted GPX4-AAV delivery prohibited phospholipid peroxidation and improved the viability of motor neurons. In addition to AAV delivery system, tetrahedral framework nucleic acids (tFNAs) are also another optimistic choice to deliver exogenous GPX4. tFNAs are novel nucleic acid nanoparticles with neuroprotective and anti-inflammatory advantages. Since the tFNAs are beneficial for cell proliferation and can freely pass across cell membranes, they are showing promising applications in the field of ALS therapy [59], [60]. However, in the road of developing new drug transport carriers, many obstacles are emerging, such as low resistance to in vivo degradation. At all events, the best therapeutic route against ALS by targeting GPX4 is in need of further exploration.
Although the present study has revealed the critical role of GPX4 in the pathological changes of ALS and defined its potential as therapeutic target, the specific molecular mechanism by which G93A inhibits GPX4 expression warrants further investigation. It has been reported that the expression and activity of GPX4 are under the control of selenium or GSH [61], [62]. At the transcriptional level, the GPX4 mRNA level can be regulated by selenium via the transcription factor AP-2 gamma and the specific protein 1 [63], [64]. At the post-transcriptional modification level, alternative splicing of the GPX4 mRNA joint linker based on different signal stimuli or developmental stages is a factor that regulates GPX4 [24]. In addition, GPX4 expression at gene level is also regulated by Sec-tRNA, which is one of the key regulatory elements during the maturation of GPX4 by incorporating selenium as a selenocysteine. At the translational level, the guanine-rich sequence-binding factor 1 has been reported to promote the translation of mitochondrial GPX4 during embryonic brain development [65]. Meanwhile, GPX4 protein level can also be regulated by posttranslational modification, as it has been uncovered to degrade via HSP90-mediated autophagy [66]. Ubiquitination and other degradation ways of GPX4 protein remains to be explored. In the case of GPX4 loss in ALS, the precise mechanism requires deep examination from transcriptional, translational and posttranslational modification perspectives.
Conclusion
This study characterizes the loss of GPX4, a key phospholipid peroxidation regulator, in the lumbar spines of ALS patients and mice. Moreover, PE(38:4) + 2O is identified as the main type of oxidized phospholipids in the spinal cord of ALS mice. Importantly, neuron-targeted GPX4 gene therapy for scavenging phospholipids peroxidation in spinal cord is proposed as an essential therapeutic strategy to alleviate motor neuron loss, behavioral dysfunction, abnormal neuromuscular connection, and lumbar spine atrophy. Therefore, this study provides an important research basis for the application of GPX4 or other anti-peroxidation agents in anti-ALS treatments, albeit further studies on the molecular mechanisms of GPX4 in neuron degeneration and astrocyte activation are warranted.
Compliance with Ethics Requirements
All Institutional and National Guidelines for the care and use of animals (fisheries) were followed.
CRediT authorship contribution statement
Long-Fang Tu: Investigation, Data curation, Writing – original draft. Tian-Ze Zhang: Investigation. Yang-Fan Zhou: Validation. Qing-Qing Zhou: Validation. Hai-Biao Gong: Methodology. Lei Liang: Project administration, Funding acquisition. Lin-Na Hai: Validation. Nan-Xin You: Validation. Yang Su: Methodology. Yong-Jun Chen: Supervision. Xu-Kai Mo: Methodology. Chang-Zheng Shi: Supervision. Liang-Ping Luo: Supervision. Wan-Yang Sun: Formal analysis. Wen-Jun Duan: Visualization, Writing – review & editing, Funding acquisition. Hiroshi Kurihara: Supervision, Project administration. Yi-Fang Li: Writing – review & editing, Funding acquisition. Rong-Rong He: Conceptualization, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported, in part, by National Key Research and Development Program of China (2017YFC1700404), Natural Science Foundation of China (82125038, 81873209, U1801284, 81903821 and 81973718), the Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2017BT01Y036), GDUPS (2019), Natural Science Foundation of Guangdong (2019A1515010909, 2021A1515011297), Science and Technology Program of Guangzhou (201903010062, 907158833068), and the Innovation Team Project of Guangdong Provincial Department of Education (2020KCXTD003). The authors (R.R. He and Y.F. Li) also gratefully acknowledge the support of K. C. Wong Education Foundation.
Footnotes
Peer review under responsibility of Cairo University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2022.02.016.
Contributor Information
Lei Liang, Email: leiliang@jnu.edu.cn.
Wen-Jun Duan, Email: duanwj@jnu.edu.cn.
Yi-Fang Li, Email: liyifang706@jnu.edu.cn.
Rong-Rong He, Email: rongronghe@jnu.edu.cn.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Longo D.L., Brown R.H., Al-Chalabi A. Amyotrophic Lateral Sclerosis. N Engl J Med. 2017;377(2):162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 2.Kiernan M.C., Vucic S., Cheah B.C., Turner M.R., Eisen A., Hardiman O., et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–955. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 3.van Es M.A., Hardiman O., Chio A., Al-Chalabi A., Pasterkamp R.J., Veldink J.H., et al. Amyotrophic lateral sclerosis. Lancet. 2017;390(10107):2084–2098. doi: 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- 4.Chiò A., Logroscino G., Hardiman O., Swingler R., Mitchell D., Beghi E., et al. Prognostic factors in ALS: A critical review. Amyotroph lateral Scler Off Publ World Fed Neurol Res Gr Mot Neuron Dis. 2009;10(5-6):310–323. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowicka N., Juranek J., Juranek J.K., Wojtkiewicz J. Risk Factors and Emerging Therapies in Amyotrophic Lateral Sclerosis. Int J Mol Sci. 2019 May;20(11):2616. doi: 10.3390/ijms20112616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu L.u., Chen L.u., Wang S., Feng J., Liu L., Liu G., et al. Incidence and prevalence of amyotrophic lateral sclerosis in urban China: a national population-based study. J Neurol Neurosurg Psychiatry. 2020;91(5):520–525. doi: 10.1136/jnnp-2019-322317. [DOI] [PubMed] [Google Scholar]
- 7.Arthur K.C., Calvo A., Price T.R., Geiger J.T., Chiò A., Traynor B.J. Projected increase in amyotrophic lateral sclerosis from 2015 to 2040. Nat Commun. 2016 Aug;7:12408. doi: 10.1038/ncomms12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le Gall L., Anakor E., Connolly O., Vijayakumar U., Duddy W., Duguez S. Molecular and Cellular Mechanisms Affected in ALS. J Pers Med. 2020 Aug;10(3):101. doi: 10.3390/jpm10030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiong L., McCoy M., Komuro H., West X.Z., Yakubenko V., Gao D., et al. Inflammation-dependent oxidative stress metabolites as a hallmark of amyotrophic lateral sclerosis. Free Radic Biol Med. 2022;178:125–133. doi: 10.1016/j.freeradbiomed.2021.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson C., Heiman-Patterson T., Kittrell P., Baranovsky T., McAnanama G., Bower L., et al. Radicava (edaravone) for amyotrophic lateral sclerosis: US experience at 1 year after launch. Amyotroph Lateral Scler Frontotemporal Degener. 2019;20(7-8):605–610. doi: 10.1080/21678421.2019.1645858. [DOI] [PubMed] [Google Scholar]
- 11.Yoshino H. Edaravone for the treatment of amyotrophic lateral sclerosis. Expert Rev Neurother. 2019;19(3):185–193. doi: 10.1080/14737175.2019.1581610. [DOI] [PubMed] [Google Scholar]
- 12.Chiò A., Mazzini L., Mora G. Disease-modifying therapies in amyotrophic lateral sclerosis. Neuropharmacology. 2020;167:107986. doi: 10.1016/j.neuropharm.2020.107986. [DOI] [PubMed] [Google Scholar]
- 13.Sultana R., Perluigi M., Butterfield D.A. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med. 2013;62:157–169. doi: 10.1016/j.freeradbiomed.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W., SriRamaratnam R., Welsch M., Shimada K., Skouta R., Viswanathan V., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1-2):317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M., Liu C.-Y., Wang T., Yu H.-M., Ouyang S.-H., Wu Y.-P., et al. (+)-Clausenamide protects against drug-induced liver injury by inhibiting hepatocyte ferroptosis. Cell Death Dis. 2020;11(9):781. doi: 10.1038/s41419-020-02961-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedmann Angeli J.P., Schneider M., Proneth B., Tyurina Y.Y., Tyurin V.A., Hammond V.J., et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–1191. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X., Michaelis E.K. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson E.P., Henry Y.K., Henkel J.S., Smith R.G., Appel S.H. Increased lipid peroxidation in sera of ALS patients: a potential biomarker of disease burden. Neurology. 2004;62(10):1758–1765. doi: 10.1212/wnl.62.10.1758. [DOI] [PubMed] [Google Scholar]
- 19.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 20.Shibata N., Nagai R., Uchida K., Horiuchi S., Yamada S., Hirano A., et al. Morphological evidence for lipid peroxidation and protein glycoxidation in spinal cords from sporadic amyotrophic lateral sclerosis patients. Brain Res. 2001;917(1):97–104. doi: 10.1016/s0006-8993(01)02926-2. [DOI] [PubMed] [Google Scholar]
- 21.Smith R.G., Henry Y.K., Mattson M.P., Appel S.H. Presence of 4-hydroxynonenal in cerebrospinal fluid of patients with sporadic amyotrophic lateral sclerosis. Ann Neurol. 1998;44(4):696–699. doi: 10.1002/ana.410440419. [DOI] [PubMed] [Google Scholar]
- 22.Kagan V.E., Mao G., Qu F., Angeli J.P.F., Doll S., Croix C.S., et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13(1):81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang S., Chapa-Dubocq X.R., Tyurina Y.Y., St Croix C.M., Kapralov A.A., Tyurin V.A., et al. Elucidating the contribution of mitochondrial glutathione to ferroptosis in cardiomyocytes. Redox Biol. 2021;45:102021. doi: 10.1016/j.redox.2021.102021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savaskan N.E., Ufer C., Kühn H., Borchert A. Molecular biology of glutathione peroxidase 4: from genomic structure to developmental expression and neural function. Biol Chem. 2007 Oct;388(10):1007–1017. doi: 10.1515/BC.2007.126. [DOI] [PubMed] [Google Scholar]
- 25.Maiorino M., Conrad M., Ursini F. GPx4, Lipid Peroxidation, and Cell Death: Discoveries, Rediscoveries, and Open Issues. Antioxid Redox Signal. 2018;29(1):61–74. doi: 10.1089/ars.2017.7115. [DOI] [PubMed] [Google Scholar]
- 26.Yoo M.H., Gu X., Xu X.M., Kim J.Y., Carlson B.A., Patterson A.D., et al. Delineating the role of glutathione peroxidase 4 in protecting cells against lipid hydroperoxide damage and in Alzheimer’s disease. Antioxid Redox Signal. 2010;12(7):819–827. doi: 10.1089/ars.2009.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellinger F.P., Bellinger M.T., Seale L.A., Takemoto A.S., Raman A.V., Miki T., et al. Glutathione Peroxidase 4 is associated with Neuromelanin in Substantia Nigra and Dystrophic Axons in Putamen of Parkinson’s brain. Mol Neurodegener. 2011;6(1):8. doi: 10.1186/1750-1326-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Q., Han X., Lan X.i., Gao Y., Wan J., Durham F., et al. Inhibition of neuronal ferroptosis protects hemorrhagic brain. JCI insight. 2017;2(7):e90777. doi: 10.1172/jci.insight.90777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen L., Hambright W.S., Na R., Ran Q. Ablation of the Ferroptosis Inhibitor Glutathione Peroxidase 4 in Neurons Results in Rapid Motor Neuron Degeneration and Paralysis. J Biol Chem. 2015;290(47):28097–28106. doi: 10.1074/jbc.M115.680090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuster D.J., Dykstra J.A., Riedl M.S., Kitto K.F., Belur L.R., McIvor R.S., et al. Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat. 2014;8:42. doi: 10.3389/fnana.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu H., Lin H., Duan W., Cui C., Li Z., Liu Y., et al. Intrathecal Injection of scAAV9-hIGF1 Prolongs the Survival of ALS Model Mice by Inhibiting the NF-kB Pathway. Neuroscience. 2018;381:1–10. doi: 10.1016/j.neuroscience.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L., Haraguchi S., Koda T., Hashimoto K., Nakagawara A. Muscle atrophy and motor neuron degeneration in human NEDL1 transgenic mice. J Biomed Biotechnol. 2011;2011:1–7. doi: 10.1155/2011/831092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deacon R.M.J. Measuring the strength of mice. J Vis Exp. 2013 Jun;76 doi: 10.3791/2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aartsma-Rus A., van Putten M. Assessing functional performance in the mdx mouse model. J Vis Exp. 2014;85 doi: 10.3791/51303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatzipetros T., Kidd J.D., Moreno A.J., Thompson K., Gill A., Vieira F.G. A Quick Phenotypic Neurological Scoring System for Evaluating Disease Progression in the SOD1-G93A Mouse Model of ALS. J Vis Exp. 2015 Oct;104 doi: 10.3791/53257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Folch J., Lees M., Stanley G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 37.Maniatis S., Äijö T., Vickovic S., Braine C., Kang K., Mollbrink A., et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science. 2019;364(6435):89–93. doi: 10.1126/science.aav9776. [DOI] [PubMed] [Google Scholar]
- 38.Golabchi F.N., Sapienza S., Severini G., Reaston P., Tomecek F., Demarchi D., et al. Assessing aberrant muscle activity patterns via the analysis of surface EMG data collected during a functional evaluation. BMC Musculoskelet Disord. 2019;20(1):13. doi: 10.1186/s12891-018-2350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gatto R.G., Li W., Magin R.L. Diffusion tensor imaging identifies presymptomatic axonal degeneration in the spinal cord of ALS mice. Brain Res. 2018;1679:45–52. doi: 10.1016/j.brainres.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Shaw B., Valentine J. How do ALS-associated mutations in superoxide dismutase 1 promote aggregation of the protein? Trends Biochem Sci. 2007;32(2):78–85. doi: 10.1016/j.tibs.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 41.Gurney M.E. The use of transgenic mouse models of amyotrophic lateral sclerosis in preclinical drug studies. J Neurol Sci. 1997 Oct;152(Suppl 1):S67–S73. doi: 10.1016/s0022-510x(97)00247-5. [DOI] [PubMed] [Google Scholar]
- 42.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brigelius-Flohé R., Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830(5):3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 44.Ribas V., García-Ruiz C., Fernández-Checa J.C. Glutathione and mitochondria. Front Pharmacol. 2014;5:151. doi: 10.3389/fphar.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirrlinger J., Dringen R. The cytosolic redox state of astrocytes: Maintenance, regulation and functional implications for metabolite trafficking. Brain Res Rev. 2010;63(1-2):177–188. doi: 10.1016/j.brainresrev.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Díaz-Amarilla P., Olivera-Bravo S., Trias E., Cragnolini A., Martínez-Palma L., Cassina P., et al. Phenotypically aberrant astrocytes that promote motoneuron damage in a model of inherited amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2011;108(44):18126–18131. doi: 10.1073/pnas.1110689108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turner M.R., Hardiman O., Benatar M., Brooks B.R., Chio A., de Carvalho M., et al. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 2013;12(3):310–322. doi: 10.1016/S1474-4422(13)70036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oskarsson B., Gendron T.F., Staff N.P. Amyotrophic Lateral Sclerosis: An Update for 2018. Mayo Clin Proc. 2018;93(11):1617–1628. doi: 10.1016/j.mayocp.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 49.Patel D., Witt S.N. Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. Oxid Med Cell Longev. 2017;2017:1–18. doi: 10.1155/2017/4829180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calzada E., Onguka O., Claypool S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int Rev Cell Mol Biol. 2016;321:29–88. doi: 10.1016/bs.ircmb.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gautam M., Jara J.H., Kocak N., Rylaarsdam L.E., Kim K.D., Bigio E.H., et al. Mitochondria, ER, and nuclear membrane defects reveal early mechanisms for upper motor neuron vulnerability with respect to TDP-43 pathology. Acta Neuropathol. 2019;137(1):47–69. doi: 10.1007/s00401-018-1934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang W.S., Stockwell B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016;26(3):165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang W.S., Kim K.J., Gaschler M.M., Patel M., Shchepinov M.S., Stockwell B.R. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci USA. 2016;113(34):E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meyer K., Ferraiuolo L., Miranda C.J., Likhite S., McElroy S., Renusch S., et al. Direct conversion of patient fibroblasts demonstrates non-cell autonomous toxicity of astrocytes to motor neurons in familial and sporadic ALS. Proc Natl Acad Sci USA. 2014;111(2):829–832. doi: 10.1073/pnas.1314085111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puentes F., Malaspina A., van Noort J.M., Amor S. Non-neuronal Cells in ALS: Role of Glial, Immune cells and Blood-CNS Barriers. Brain Pathol. 2016;26(2):248–257. doi: 10.1111/bpa.12352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo X., Gong H.-B., Gao H.-Y., Wu Y.-P., Sun W.-Y., Li Z.-Q., et al. Oxygenated phosphatidylethanolamine navigates phagocytosis of ferroptotic cells by interacting with TLR2. Cell Death Differ. 2021;28(6):1971–1989. doi: 10.1038/s41418-020-00719-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ioannou M.S., Jackson J., Sheu S.-H., Chang C.-L., Weigel A.V., Liu H., et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell. 2019;177(6):1522–1535.e14. doi: 10.1016/j.cell.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 59.Ma W., Zhan Y., Zhang Y., Xie X., Mao C., Lin Y. Enhanced Neural Regeneration with a Concomitant Treatment of Framework Nucleic Acid and Stem Cells in Spinal Cord Injury. ACS Appl Mater Interfaces. 2020;12(2):2095–2106. doi: 10.1021/acsami.9b19079. [DOI] [PubMed] [Google Scholar]
- 60.Zhang T., Tian T., Lin Y. Functionalizing Framework Nucleic Acid-Based Nanostructures for Biomedical Application. Adv Mater. 2021 Nov;17 doi: 10.1002/adma.202107820. [DOI] [PubMed] [Google Scholar]
- 61.Sneddon A.A., Wu H.-C., Farquharson A., Grant I., Arthur J.R., Rotondo D., et al. Regulation of selenoprotein GPx4 expression and activity in human endothelial cells by fatty acids, cytokines and antioxidants. Atherosclerosis. 2003;171(1):57–65. doi: 10.1016/j.atherosclerosis.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 62.Ursini F., Maiorino M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic Biol Med. 2020;152:175–185. doi: 10.1016/j.freeradbiomed.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 63.Alim I., Caulfield J.T., Chen Y., Swarup V., Geschwind D.H., Ivanova E., et al. Selenium Drives a Transcriptional Adaptive Program to Block Ferroptosis and Treat Stroke. Cell. 2019;177(5):1262–1279.e25. doi: 10.1016/j.cell.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 64.Tang D., Chen X., Kang R., Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021;31(2):107–125. doi: 10.1038/s41422-020-00441-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ufer C., Wang C.C., Fähling M., Schiebel H., Thiele B.J., Billett E.E., et al. Translational regulation of glutathione peroxidase 4 expression through guanine-rich sequence-binding factor 1 is essential for embryonic brain development. Genes Dev. 2008;22(13):1838–1850. doi: 10.1101/gad.466308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Z., Geng Y., Lu X., Shi Y., Wu G., Zhang M., et al. Chaperone-mediated autophagy is involved in the execution of ferroptosis. Proc Natl Acad Sci USA. 2019;116(8):2996–3005. doi: 10.1073/pnas.1819728116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.