Graphical abstract
Keywords: Self-assembled, Peptides, Nanostructures, Optical bioapplication, Agriculture food analysis
Highlights
-
•
Supramolecular interactions of peptide self-assembly.
-
•
Common morphologies and photoactive properties of self-assembled peptide nanostructures.
-
•
Inherent photoluminescence of peptide self-assembly.
-
•
Promising fields of peptide self-assembly are explored, including optical bioapplication in agriculture food analysis.
-
•
The current challenges and future perspectives of optosensors based on short peptide self-assemblies.
Abstract
Background
Food processing plays an important role in the modern industry because food quality and security directly affect human health, life safety, and social and economic development. Accurate, efficient, and sensitive detection technology is the basis for ensuring food quality and security. Optosensor-based technology with the advantage of fast and visual real-time detection can be used to detect pesticides, metal ions, antibiotics, and nutrients in food. As excellent optical centres, self-assembled peptide-based nanostructures possess attractive advantages, such as simple preparation methods, controllable morphology, tunable functionality, and inherent biocompatibility.
Aim of review
Self-assembled peptide nanostructures with good fabrication yield, stability, dispersity in a complex sample matrix, biocompatibility, and environmental friendliness are ideal development goals in the future. Owing to its flexible and unique optical properties, some short peptide self-assemblies can possibly be used to achieve the purpose of rapid and sensitive detection of composition in food, agriculture, and the environment, expanding the understanding and application of peptide-based optics in analytical chemistry.
Key scientific concept of review
The self-assembly process of peptides is driven by noncovalent interactions, including hydrogen bonding, electrostatic interactions, hydrophobic interactions, and π-π stacking, which are the key factors for obtaining stable self-assembled peptide nanostructures with peptides serving as assembly units. Controllable morphology of self-assembled peptide nanostructures can be achieved through adjustment in the type, concentration, and pH of organic solvents and peptides. The highly ordered nanostructures formed by the self-assembly of peptides have been proven to be novel biological structures and can be used for the construction of optosensing platforms in biological or other systems. Optosensing platforms make use of signal changes, including optical signals and electrical signals caused by specific reactions between analytes and active substances, to determine the content or concentration of an analyte.
Introduction
Food analysis and inspection are based on some basic theories and technologies of physics, chemistry, and biochemistry and analyse compounds, including pesticides, metal ions, antibiotics, and nutrients, in food to ensure that food quality meets international standards. Many ingredients in food exist in only a tiny amount, but they can still have a far-reaching impact on health. Therefore, a visual, fast, sensitive, and on-site real-time analysis of optical sensors is necessary to ensure food quality and security. In recent years, food analysis has been achieved with optosensing arrays exhibiting optical properties of fluorescence, phosphorescence, or electrochemiluminescence based on organic and inorganic molecules or nanoprobes that could efficiently and accurately be used in qualitative and quantitative analysis.
Self-assembled peptide nanostructures have attracted increasing attention because of their simple preparation methods, modulated structures, and tunable characteristics, having promising potential in optical applications. Self-assembly of peptides can be defined as the spontaneous formation of ordered structures using short-chain amino acids as unit blocks. It is a typical bottom-up route for the preparation of organic nanostructures with complex and hierarchical architectures [1], [2], [3], [4], [5]. The key benefits of utilizing peptide self-assembly for the fabrication of nanostructures include the creation of a variety of remarkable structures and their inherent biocompatibility [6], [7], [8], [9], [10]. Peptide assemblies obtained from different peptides may possess many electrochemical properties, thermal stability, and environmental responsiveness. In addition to these captivating properties, the assemblies may also exhibit multiple optical and electrical properties, such as intrinsic fluorescence, allowing them to be integrated into real-time detection and quantitative evaluation systems [11].
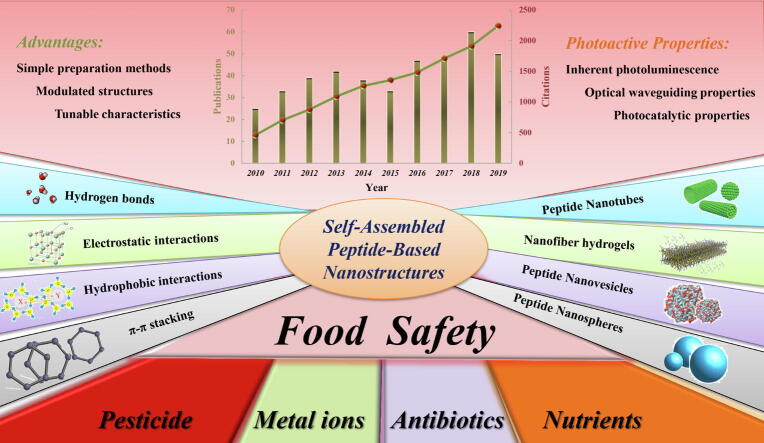
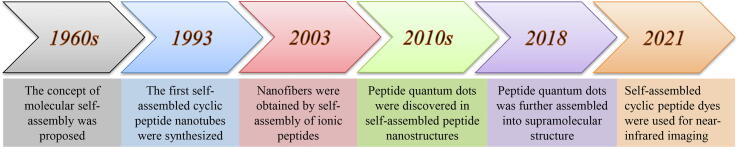
Publication and citation counts of peptide self-assembly from the Web of Science have increased steadily since its discovery, indicating that the scientific community is showing a growing interest in self-assembled peptide-based nanomaterials (Fig. 1). The key events in the development of self-assembled peptide-based nanomaterials can be outlined. The concept of molecular self-assembly began in the 1960s [12]. It referred to the process by which molecules of high-entropy systems can spontaneously form ordered micro/nanostructures under the driving forces of noncovalent interactions [13]. To date, the term 'molecular self-assembly' covers a broader area that also includes assemblies formed via covalent interactions [14]. Aside from biocompatibility, peptides have inherent biological activity and biodegradability, making them ideal components as biological materials. In terms of environmental responsiveness, peptides can induce self-assembly via external stimuli such as pH or temperature. Based on the structure and type of self-assembling peptides, they can be classified into α-helical, β-sheet, di-, cyclic peptides, and amphipathic peptides [15], [16], [8]. Many photosensitivity phenomena, such as photoluminescence and optical waveguide properties, that have been observed in inorganic nanocrystals and quantum dots (QDs) are attributed to peptide supramolecular assembly, showing great potential in nano-optical applications [17], [18], [19] (Fig. 2).
Fig. 1.
Schematic representation of the assembly driving force, morphology, photoactive properties, and optical bioapplication in food analysis of self-assembled peptide nanostructures. The annual publication and citation numbers in Web of Science are also presented.
Fig. 2.
The evolution of self-assembled peptide nanostructures since their discovery.
With the rapid development of self-assembled peptides as nanomaterials for the construction of semiconductors, this review discusses the driving forces of self-assembled peptide nanostructures, i.e., hydrogen bonding, electrostatic interactions, hydrophobic interactions, and π-π stacking. We also summarize the common morphologies of self-assembled peptide nanostructures, i.e., nanotubes, nanofibre hydrogels, nanovesicles, and nanospheres. The superior photoactive properties of self-assembled peptides and their potential in optical applications are also discussed. In particular, self-assembled peptide-based optical materials possess physicochemical properties superior to those of organic dyes and inorganic semiconductors, advancing towards new applications of optical sensing applications in food analysis. Ultimately, we discuss current challenges and future perspectives in the fabrication of self-assembled peptides to enhance their photoelectric properties to unleash more interdisciplinary technologies and applications.
Driving forces and morphology of self-assembled peptide nanostructures
Based on the structure and type of self-assembling peptides, common peptides as basic building blocks include α-helical peptides, β-sheet peptides, dipeptides, cyclic peptides and amphipathic peptides. With noncovalent interactions as key driving forces, controllable morphology of self-assembled peptide nanostructures can be achieved through adjustment in the type, concentration, and pH of organic solvents and peptides [20], [21], [22], [23], [24]. Common morphologies of self-assembled peptide nanostructures include nanotubes, nanofibre hydrogels, nanovesicles, and nanospheres.
Driving forces for self-assembly
The self-assembly process of peptides is driven by noncovalent interactions, including hydrogen bonding, electrostatic interactions, hydrophobic interactions, and π-π stacking, which are the key factors for obtaining stable self-assembled peptide nanostructures with peptides serving as assembly units [25], [26], [27]. Polar amino acids usually interact with each other via hydrogen bonding and electrostatic interactions, while nonpolar amino acids employ hydrophobic interactions and π-π stacking to cause aggregation [28].
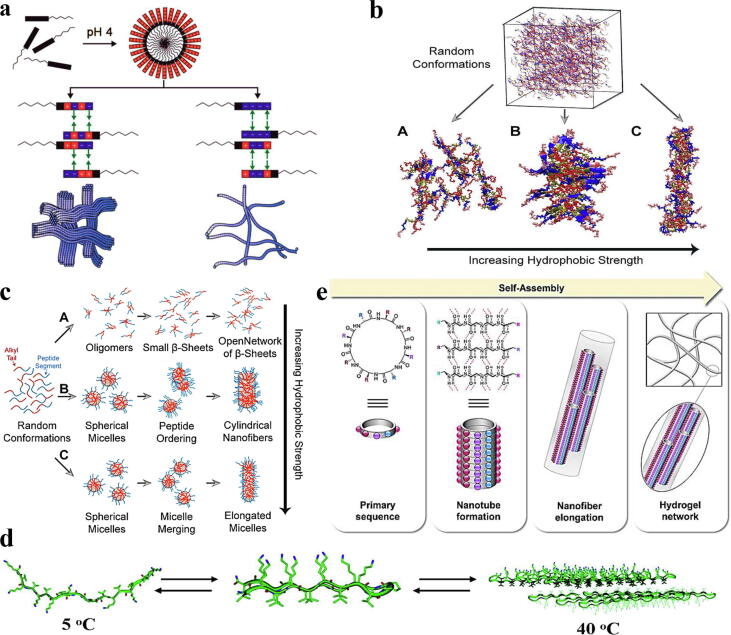
Natural peptides usually have clear secondary structures, i.e., an α-helix structure is formed via hydrogen bonding between main chain amides, where hydrogen amides form the right-handed helix while amino acids protrude from the helix. Side chains of the helix could interact with other helices in the presence of related amino acids, promoting their accessibility in solvents [7], [29]. Among the natural motifs of self-assembled structures, β-sheets are the driving force for self-assembled structures with the highest universality. In the early 1950s, Pauling et al. discovered that β-sheet structures in proteins are mainly composed of alternating hydrophobic and hydrophilic residues. The presence of these residues facilitates hydrogen bonding between backbone amides and carbonyl groups, stabilizing the arrangement of multiple peptide backbones [30], [31], [32], [33]. In fact, hydrogen bonding has the most important impact on the formation and stability of the secondary structure of peptides, acting as an important driving force for the self-assembly of peptides. Natural hydrogen has been found in α-helices, β-sheets, and coiled coils. Bonding phenomena have been involved in designing and developing peptide sequences for self-assembly into functional nanostructures [34]. Hydrogen bonds foster the self-assembly of peptides into one-dimensional or multidimensional nanostructure systems due to their high selectivity and directionality [35]. As the polarity or pH of a self-assembly system changes, the formation of hydrogen bonds is affected, resulting in the formation of positively and negatively charged amino acid peptides. The resulting electrostatic interaction drives these peptides to undergo charge-dipolar interactions or ionic bonding with water molecules, which positively impacts the stability of self-assembled peptide nanostructures. Gribbon et al. used a monopeptide with Glu and Lys at the end of the sequence to obtain a dimer α-helix coil structure, which allows the formation of peptide nanofibres with the orderly arrangement, excellent stability, and great expandability through ionic interactions [36]. In the presence of water, the polar regions in the peptide sequence further interact with water molecules, while the nonpolar regions are forced to gather close to each other to avoid contact with water molecules, generating hydrophobic forces; the effect is not directional [37]. In addition to hydrogen bonding, hydrophobic interactions also have an important impact on the self-assembly of peptide molecules. Derivative peptides conjugated with hydrophobic anticancer drugs self-assemble into nanofibres through hydrophobic interactions and intermolecular hydrogen bonding [38]. Peptides containing aromatic rings, i.e., phenylalanine or tryptophan, mainly employ π-π stacking as the main driving force for self-assembly. π-π stacking is an important force driving directional growth in the construction of self-assembled peptide-based nanostructures. As peptide molecules containing aromatic groups have limited solubility in water, π-π stacking interactions serve as the main driving force for peptide self-assembly under aqueous conditions [39].
Morphology of self-assembled peptide nanostructures
Peptide nanotubes
At the end of the 1980s, nanoscience and nanotechnology gradually advanced as new interdisciplinary fields of science and technology. Among nanomaterials that have been developed, nanotubes stand out due to their unique structures and properties, having broad application prospects in the fields of electronics, catalysis, and optics. In recent years, researchers have become interested in establishing efficient and controllable methods for obtaining peptide nanotubes in recent years.
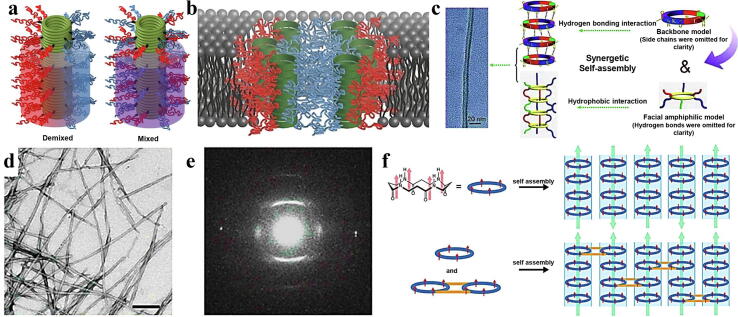
Among self-assembled peptide nanobodies, peptide nanotubes are a relatively classical configuration. Some representative self-assembly methods for acquiring peptide nanotubes include rod-shaped molecules forming cylindrical structures, folded molecules forming hollow spiral structures, sector-shaped or convergent molecules forming disc-like structures, and cyclic molecules forming continuous tubular structures [40], [41]. The use of multiple peptide molecules as self-assembly units to construct functionalized peptide nanotubes has become a popular research interest for researchers. Several amphiphilic peptides were used to obtain helical peptide nanotubes with diameters of 30–50 nm [42]. As the basic core sequence of amyloid-β peptide fragments, diphenylalanine (FF) has the ability to diversify and stabilize self-assembled structures. It can form various of micro/nanostructures by virtue of π-π stacking of benzene rings and hydrogen bonding in the peptide backbones. It was found that FF can undergo unidirectional stacking to form nanotubes [43]. In addition, FF nanotubes were reported to have good thermal stability [44]. FF nanotubes obtained via vapour deposition have advantages such as high yield, controllable density, and length [45]. Linear peptides offer flexibility that can be cyclized to obtain cyclic peptides with long half-lives and strong receptor selectivity for constructing the controllable self-assembled peptide nanostructures [46], [47]. Perrier et al. introduced different polymers on both sides of D- and L-cyclic octapeptides and employed side -chain modification to effectively embed phospholipid bilayers to obtain an ion channel with a Janus structure (Fig. 3a, b) [48]. Zhang et al. created a nanotube system with the combined advantages of chirality and facial amphiphilicity by stacking cyclic peptide backbones of alternating units of D- and L-amphiphilic α-cyclic hexapeptides. The system was formed on the basis of intermolecular hydrogen bonding between the chiral cyclic peptide backbones and synergistic hydrophobic interactions provided by facial amphiphilicity, further stabilizing the intermolecular hydrogen bonding. These factors ultimately extend the tubular assemblies longitudinally and afford nanotubes with a high length-diameter ratio (Fig. 3c) [49]. Peptide nanotubes can be obtained using the stacking effect of cyclic β-peptides by directing amide bonds to orient in one direction. The one-direction orientation can generate a large dipole moment and enhance the piezoelectric coefficient. Anti-parallel positioning of bundled peptide nanotubes would unfavourably affect the dipole moment [50]. To avoid nullification of the dipole moment, Tabata et al. synthesized three cyclic tetra-β-peptides (CP4s), namely, cyclo (L-β-homolysine (Z)-β-alanine-β-alanine-β-alanine) (CP4K), cyclo (L-β-homoglutamic acid (OBzl)-L-β-homolysine (Z)-β-alanine-β-alanine) (CP4KE) and bis-CP4 to avoid nullification of the dipole moment. When the mixture of CP4KE and bis-CP4 was exposed to methanol vapour, it was easy to obtain peptide nanotube bundles with the appearance of needle-like crystals (Fig. 3d, e). The addition of bis-CP4 directed peptide nanotubes to arrange in a parallel orientation to form dipole moments with enhanced direct and reverse piezoelectric coefficients (Fig. 3f). When evaluating the linear relationship between the polarization of peptide nanotubes and the surface potential according to Eq. (1), it was found that the addition of bis-CP4 would promote polarization of peptide nanotube bundles, thereby increasing the dipole moment and fostering the parallelism of the peptide nanotubes.
| (1) |
where V is the surface potential, P is the polarization corrected by the depolarization effect, L is the thickness, ε0 is the dielectric constant under vacuum, and εS is the relative dielectric constant. It was found that the addition of bis-CP4 would promote the polarization of the peptide nanotube bundles, thereby increasing the dipole and promoting the parallelism orientation of the peptide nanotubes, which is in line with the research results [51]. The design of this new peptide molecular material broadens the application of self-assembled peptide nanotubes in various fields.
Fig. 3.
a) Cyclic peptide–polymer nanotubes with 'demixed' corona configurations (left) and cyclic peptide–polymer nanotubes with 'mixed' corona configurations (right) [48]. b) Schematic diagram of pore formation using the Janus conjugate showing sequestration of polystyrene (in blue) forming the central macropore cavity, and poly-n-butyl acrylate (in red) interacts with the phospholipid bilayer [48]. c) Nonbonded intermolecular hydrogen bonding and surface hydrophobic interactions act as driving forces to form nanotubes [49]. d) TEM image of CP4KE/bis-CP4 peptide nanotube bundles [51]. e) Electron diffraction pattern of CP4KE/bis-CP4 peptide nanotube bundles [51]. f) Schematic diagram of the peptide nanotube bundles of CP4KE and CP4KE/bis CP4 (the orientation parallelism of the two peptide nanotubes is induced by Bis-CP4, embedded and merged into the two peptide nanotubes to form nanotube bundles to produce large dipoles) [51].
Peptide nanofibre hydrogel
Compared with other self-assembled peptide-based nanostructures, peptide nanofibres have excellent biodegradability, as they have superior biological properties, such as cell recognition, cell adhesion, and differentiation [25], [52], [53], [54]. Generally, peptide molecules that form self-assembled peptide nanofibres are mostly modified amphiphilic molecules composed of hydrophilic heads with active peptide molecules, hydrophobic tails with alkyl chains, and a few amino acids in between to provide sufficient space for the prevention of spontaneous aggregation of molecules after the introduction of negative charges [55].
Using the principle of molecular self-assembly, molecular structures of self-assembled peptides can be rationally designed, and the ability to respond to external stimuli such as pH, temperature, ionic strength, or solvent media can also be endowed to the assemblies. Peptide molecules can be assembled into nanofibres spontaneously or triggered by multiple noncovalent bonds. Chen et al. controlled the self-assembly of a series of peptide amphiphiles (PAs) with different lengths composed of alternative units of arginine and aspartic acid by altering the pH of buffer solutions. At pH 11, peptides aggregated into a bundle-like network structure via electrostatic attraction between positively and negatively charged sequences; at pH 13, peptides dispersed into nanofibres due to electrostatic repulsion between positively and negatively charged sequences. The close proximity of nanofibres will cause the PA molecules that cross each other to interact on the surface. The charged residues are arranged in a complementary-attractive manner, and the bound nanofibres are obtained; in contrast, the complementary-repulsive arrangement will lead to the appearance of fully dispersed nanofibres (Fig. 4a) [56]. Aside from pH, changes in temperature and ionic strength also affected the formation of nanofibres with β-sheet networks. Tantakitti et al. found that the β-sheet structure grew increasingly in cylindrical peptide nanofibres prepared from PAs by increasing the temperature when the surrounding ionic strength was higher than the critical ionic strength. The energy provided during annealing is expected to drive fibers' nucleation process and rapid growth of fibres with β-sheet secondary structures. At times when the surrounding ionic strength is lower than the critical ionic strength, hydrophobic attraction and the repulsive electrostatic effect compete with each other, resulting in a reduction in the percentage of β-sheet structure. This causes the infinite fibres to break apart and form almost monodisperse fibres [57]. Changes in solvent polarity can cause hydrophobic and hydrophilic ends of PA to be arranged in reverse order in the assembled structure due to their amphiphilic nature, thereby altering the hydrophobic interaction strength between peptides and inducing the formation of peptide nanobodies. Using R to represent the hydrophobic interaction strength, the larger the R value is, the stronger the hydrophobic interaction. The R value changes depending on the polarity of the solvent in a peptide-polymer model. When the R value is 1/6, the hydrophobic interaction is weak, forming an open β-sheet structure with no specific arrangement order; when the R value is 1/3, the hydrophobic effect is enhanced, allowing the formation of a closed β-sheet structure in which cylindrical nanofibres can be obtained; and when the R value is 1.3, peptide molecules are assumed to have a random coiled conformation and eventually form elongated cylindrical micelles (Fig. 4b, c) [58]. Considering that current construction methodologies of peptide nanofibres are mainly based on self-assembly of β-sheet peptides, Li et al. used a polyanion with a rigid backbone, namely, polyoxometalate (POM), to self-assemble with short peptides via electrostatic interaction to obtain a biologically stable multivalent nanofibre with positive charges on the surface that allows separation and trapping of bacterial cells [59]. Other biomaterials with similar properties to POM are also expected to be able to build a high-value hierarchical combination with peptide nanofibres, advancing the gap of biology, biochemistry, and materials science.
Fig. 4.
a) Schematic diagram of lateral self-assembly of nanofibres based on the surface interaction of PA molecules in adjacent nanofibers [56]. b) A schematic diagram of unique nanostructures, where a represents the open network of β-sheets, b represents cylindrical nanofibres, and c represents elongated micelles [58]. c) Three kinetic mechanisms obtained from the simulation results: a represents the formation of a β-sheet network when the hydrophobic interaction strength is weak, b represents the formation of cylindrical nanofibres when the hydrophobic interaction strength is medium, and c represents the formation of elongated micelles when the hydrophobic interaction is strong [58]. d) Schematic diagram of MAX3 self-assembly with temperature changes [63]. e) Hierarchical self-assembly process involved in hydrogel formation from D- and L-cyclic peptides [66].
Hydrogels are polymer network systems with water as the dispersion medium. It is soft in nature and stable in shape, and can absorb and consolidate a large volume of water molecules. Owing to its pore structure and three-dimensional network, hydrogels provide good permeability and mechanical support. At present, most hydrogels are prepared from chemically cross-linked synthetic polymer chains. They can also be obtained via the self-assembly of small molecules. Peptide molecules with the ability to self-assemble into physically cross-linked fibre-like structures are ideal alternatives [60], [61]. Peptide self-assembled hydrogels have outstanding advantages, such that they are biocompatible, biodegradable, and nontoxic [62]. At present, several studies have reported on the driving of supramolecular assembly and the formation of peptide hydrogels, stating that the first folding of peptide molecules into a conformation that is conducive to self-assembly is an important prerequisite for hydrogelation. In particular, for the purpose of tissue regeneration, β-sheet peptide hydrogels have received constant attention. Ozbas et al. developed a small peptide, MAX3, which assembled to form hydrogels as the temperature increased. As the temperature increases, nonpolar residues of the MAX3 peptide in the unfolded state are dehydrated and folded through hydrophobic collapse, affording an amphiphilic β-hairpin conformation. In contrast, a decrease in temperature causes the β-hairpin conformation to unfold and the subsequent dissolution of the hydrogel (Fig. 4d) [63]. In addition to temperature, ultraviolet irradiation can also trigger hydrogel formation through peptide folding via intermolecular and intramolecular hydrogen bonding to produce the amphiphilic β-hairpin conformation [64]. Amanda et al. constructed a type of multidomain peptide (MDP) that is assembled in β-sheet motifs. The structure of MDP is stable in the hydrogen bonding network along the long axis of the fibre and the hydrophobic domain in the fibre core. As MDP is amphiphilic, the composition of the aqueous solution can trigger its self-assembly to form nanofibres, each of which is composed of double-layer peptides. The hydrophilic surface is exposed to the aqueous solution, while the hydrophobic surface hydrophobically collapses in the MDP core, ultimately affording repeating units of MDP nanofibres. The length of nanofibres is significantly extended through the adjustment of electrostatic interactions, leading to fibrous entanglement and hydrogel formation. Since the self-assembly and cross-linking of MDP can take place under physiological conditions, their hydrogels have shown great potential as bioengineering scaffolds [65]. For many years, peptide self-assembled peptide hydrogels have primarily relied on linear peptides. Recent studies have proven that cyclic peptides composed of 11 or 12 amino acids have different self-assembly properties from their linear equivalents, stimulating the exploration of using cyclic peptides as building blocks for hydrogelation. Shaikh et al. used a cyclic peptide composed of alternating D- and L-amino acids for hydrogel formation. The cyclic peptide can self-assemble to form nanotubes in solution and aggregate to form a porous network of entangled nanofibres (Fig. 4e). Rheological measurements revealed that the hydrogels made of the cyclic peptide were soft hydrogels [66].
Peptide nanovesicles
In a different solvent environment, amphiphilic molecules can form ordered assemblies with a closed double-layer structure named vesicles. Vesicles usually have a hollow structure, which is conducive to loading small molecules. Polymer vesicles obtained from various self-assembled molecules generally have excellent tunability, specificity, and stability [67].
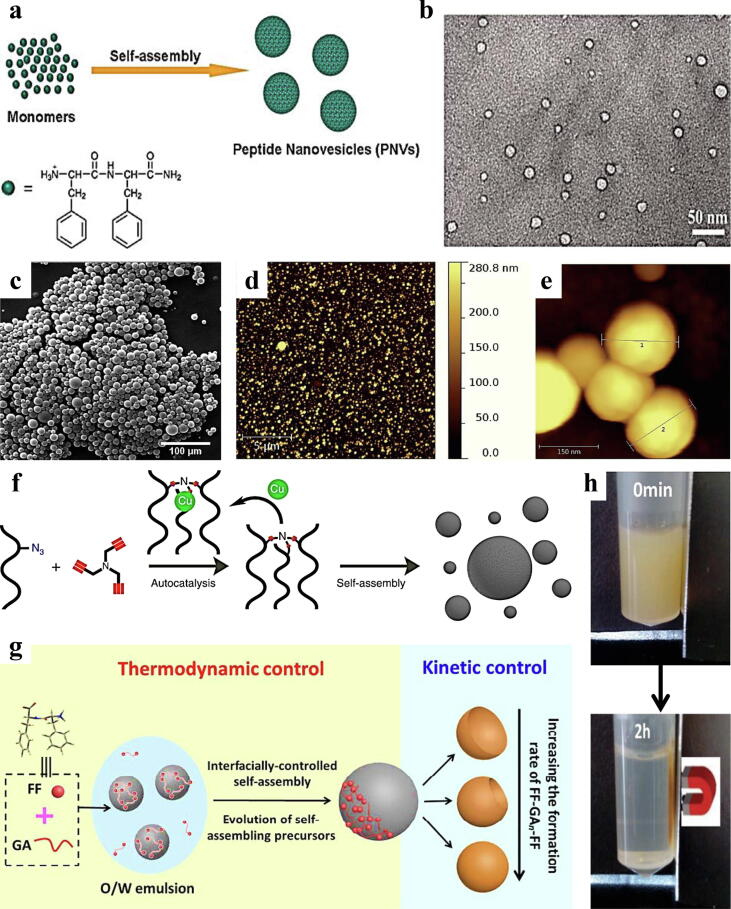
At present, vesicles formed by the assembly of amphiphilic block polymers have been widely studied. Most of the time, peptides are combined with synthetic hydrophobic fragments to foster the assembly process for the construction of vesicles. When block copolymer monomers are entirely composed of peptide molecules, the resulting nanovesicles are easier to biodegrade with better biocompatibility than synthetic polymer vesicles [68]. Cationic antennae were attached to the hydrophobic segments of a group of peptide units composed of natural amino acids for the formation of nanovesicles. Since the molecular structure of this assembly is similar to that of phospholipids, it can induce supramolecular self-assembly above a critical concentration and form a more structurally stable β-like complex that can encapsulate solute within the solution during the assembly process. Hydrophobic segments of peptides drive The formation of such a high-resolution double-layer spherical water injection structure is driven by hydrophobic segments of peptides. The TEM image of the nanovesicles after concentration or dilution shows that the lipid vesicles can complete the membrane fusion process well, verifying the feasibility of the vesicles to form a water injection structure. Correspondingly, coarse-grained simulation of the self-assembly process showed that these peptides have similar lateral sizes to common phospholipids and further confirmed the feasibility of a novel branched PA as the assembly unit of peptide nanovesicles to replace liposome delivery systems [69]. Similarly, Dimitrios et al. designed lipid-like peptide nanovesicles with both hydrophobic domains and hydrophilic heads to regulate drug release and loading capacity by altering amino acid sequences and charge distribution [70]. The quantum confinement (QC) phenomenon recently discovered in various peptide nanostructures highlights the optoelectronic properties of these self-assembled peptide nanostructures with quality control properties. For the first time in the research of peptide nanovesicles, Huang et al. reported the electrogenerated chemiluminescence (ECL) characteristics of peptide nanovesicles formed via self-assembly of cationic dipeptides derived from FF (Fig. 5a, b), opening a new route for the design and preparation of ECL devices using biologically inspired nanomaterials [71]. Current research has transitioned from the control of the morphology and size of peptide nanovesicles to characteristics and application feasibility, optimizing the specific properties of self-assembled peptide nanovesicles and diversifying their applications other than biomaterials.
Fig. 5.
a) Mechanism diagram of nanocaphyllin based on a cationic dipeptide [71]. b) TEM image of nanocaphyllin based on a cationic dipeptide [71]. c) SEM images of spherical nanostructures [73]. d) AFM image analysis of spherical nanostructures [73]. e) Magnification of some nanospheres labelled with numbers 1 and 2 [73]. f) Copper-dependent oligazole-based catalytic formation causes spontaneous assembly to form peptide nanospheres after continuous triphosphate production [76]. g) Schematic diagram of preparation based on dipeptide-concave nanospheres from crescent-like to solid-interior structures [79]. h) Magnetic response of nanospheres under a magnetic field [79].
Peptide nanospheres
Nanospheres have inherent advantages, such as a large specific surface area, tunable size, and easy surface functionalization. Current methods for preparing nanospheres include polymer cross-linking, self-assembly of copolymers, and self-assembly of amphiphilic molecules [72]. Peptides are excellent small bioorganic molecules that serve as the basic building units for the self-assembly of peptide nanospheres. Scelsi et al. developed peptides with a conformational triblock structure based on the influence of secondary structure tendency on the self-assembly performance of peptides. The peptides formed nanospheres with diameters of 100–400 nm (Fig. 5c, d and e), indicating that they have conformational flexibility in peptide self-assembly [73]. Matsuura et al. developed a novel β-cyclic peptide of the Sesbania mosaic virus. The peptide with a β-ring motif has an FKFE sequence at the C-terminus to enhance its solubility in water. Dynamic light scattering and transmission electronic microscopy showed that the peptides self-assembled into nanospheres with a diameter of approximately 30 nm in water at pH 3.8; at pH 2.2, the β-cyclic peptide with the FKFE sequence did not assemble; and at pH 6.4–11.6, larger aggregates were observed [74]. In brief, the formation of peptide nanospheres was dependent on pH. In view of the important role of self-replication in human life, the development of simpler autocatalytic systems has attracted much attention. The reaction products in the system can serve as catalysts for the formation of end products [75]. The extreme selectivity of the self-replication ability of peptide molecules has promoted the research and development of template-dependent peptides. There have been many reports on the use of molecular self-catalysts or self-catalytic ligands. Roberto et al. synthesized functional oligotriazole peptides capable of self-assembly in a simple method. Under the drive of the Cu-catalysed azide-alkyne cycloaddition reaction (CuAAC), the oligotriazole Cu1+ complex catalysed the formation of triazole. It is possible to realize autocatalytic production of triskelion-based peptides, which self-assemble to form peptide nanospheres with bioinspired structures (Fig. 5f). Continuous transfer experiments showed that self-assembly and autocatalysis of oligotriazole peptides lead to continuous self-assembly of three-dimensional peptide nanospheres in the presence of appropriate precursors, providing a promising strategy for the development of self-assembled synthetic biomaterials [76]. Kinetic and thermodynamic controls simultaneously drive the self-assembly process of biomolecules, providing unique opportunities for constructing functional and nonstatic peptide nanostructures such as concave-shaped particles. Concave-shaped particles are constructed using peptide molecules with good biocompatibility and structural programmability with intrinsic anisotropic structures, which are more functional than regular spherical particles. They have shown excellent application prospects in both protein-–protein interaction modelling and drug delivery [77], [78]. A concave nanosphere based on boron dipeptide was developed. The self-assembly pathway is driven by coordinated control of kinetics and thermodynamics. Based on the covalent Schiff reaction between the amino groups of FF and glutaraldehyde (GA), the thermodynamics of interface assembly are controlled by the gradual formation of FF-GAn-FF. In contrast, the kinetic growth process is controlled by the formation rate of FF-GAn-FF hyaluronic acid dipeptide and the interface nucleation rate of the concave nanospheres (Fig. 5g). In addition, further functionalization of concave nanospheres can be achieved by encapsulation of inorganic nanoparticles (such as magnetic nanoparticles) in an oil phase (Fig. 5h). This is an advancement compared to the traditional manufacturing technology of concave nanospheres [79].
Photoactive properties
In the past few decades, many ordered peptides have self-assembled into various nanostructures with excellent physical and chemical properties, such as chemical stability, thermal stability, and optical properties, showing their potential in technical applications and material processing. The excellent photoluminescence properties, optical waveguide properties, and photocatalytic properties of self-assembled peptide nanostructures are reported here.
Inherent photoluminescence of peptide QDs
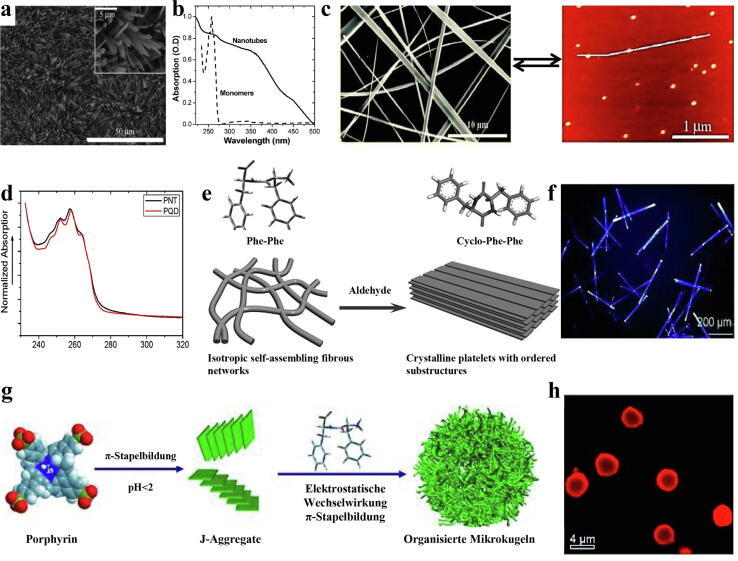
As a type of traditional fluorescent semiconductor nanoparticle, the optical properties of QDs are mainly attributed to the QC effect of their nanometre-scale size. Some bioinspiring short peptide self-assemblies can also form nanoscale QC structures due to the strong Coulomb interaction generated by the highly constrained “electron -hole,” resulting in structure-dependent optical properties [23], [80], [81], [82]. These short peptide self-assemblies may have attractive semiconductor properties, as their band gaps are comparable to those of conventional materials such as FF, which is derived from β-amyloid self-assembling peptides [82]. The directional aromatic interaction and hydrogen bonding network promote the formation of QC domains in the FF nanostructure, which is the molecular origin of its inherent semiconducting properties and the main force for the self-assembly of FF into a supramolecular semiconductor [83]. This phenomenon has also been proven by Amdursky et al., who prepared highly ordered FF nanotubes by vapour deposition (Fig. 6a). The nanotubes showed absorption spectra at 245–264 nm and 300–370 nm (Fig. 6b), as opposed to the narrow absorption peak of the FF monomer at 257 nm. This typical phenomenon of stepped light absorption indicates that a two-dimensional quantum well crystal structure can be obtained in FF nanotubes [84]. Two-dimensional quantum wells, one of the most common QC structures, have also been observed in Fmoc-FF nanohydrogels [85]. This QC structure corresponds to the semiconducting properties of peptide self-assembled nanostructures [86]. One-dimensional peptide QDs have also been observed to exist in the basic assembly of FF nanostructures in the form of dimers, e.g., tert-butoxy-modified FF (Boc-FF), which self-assembles to form rigid and stable peptide nanospheres. The absorption spectrum showed that there were peptide QDs with a radius of 1.3 nm in the assembled structure [17]. The discrete self-assembled peptide nanotubes formed by FF exhibited photoluminescence and optical characteristics. When the assembly medium was changed from water to methanol solution, it was decomposed into individual dimers, peptide QDs. Recovery of medium to the aqueous solution will lead the peptide QDs with an average diameter of 1.65 nm to self-assemble to form peptide nanotubes again, demonstrating the reversibility of the process, which also indicated that peptide QDs exist as a single entity in elemental FF nanotubes (Fig. 6c, d) [87]. Similarly, Tao et al. studied the self-assembly of aromatic cyclic dipeptide tryptophan by dynamic light scattering. The UV–Vis absorption spectrum showed the presence of peptide QDs in the assemblies, and the diameter was approximately 2.24 nm, which was twice the size of the monomer. Mass spectrometry proved the involvement of dimer QDs in the assembly process of cyclic peptides. In detail, cyclic dipeptides cause a spatial conjugation effect of the electron clouds of indole rings through π-π interactions between their aromatic side chains (indole rings) and form quantum-confined areas that restrict molecular motion. After dimerization occurs, peptide QDs acted as the building block for the self-assembly of the supramolecular structure [18]. Simple peptide molecules with the ability to self-assemble into ordered peptide nanostructures are promising candidates for the preparation of bioorganic peptide QDs. Peptide QDs with different diameters can be prepared by simply adjusting the morphology of peptides, which may be the key feature technology to bridge the research-application gap and advance the development of multifunctional photoluminescent devices.
Fig. 6.
a) SEM image of the conventional peptide nanotube (inset shows higher surface magnification) [84]. b) Optical absorption map of FF-based peptide nanotubes and FF [84]. c) SEM image of FF base peptide nanotubes (left), AFM image of the corresponding peptide quantum dots (right) [87]. d) Optical absorption spectrum of the FF peptide nanotubes (black line) and the FF peptide QDs (red curve) [87]. e) Schematic illustration of oriented crystallization over a long-range in self-assembling fibrous peptide networks [91]. f) PL image of platelets excited at 330–380 nm [91]. g) Schematic diagram of the preparation of biomolecule-based microspheres [95]. h) Confocal image of microspheres showing autofluorescence [95].
Optical waveguiding properties
As quantum-confined organic nanomaterials, self-assembled peptide nanostructures have easy-tuning optical properties and superior self-assembly tendency. They can realize the propagation and manipulation of light in the subwavelength range. They can become effective building blocks for next-generation organic optical components to manufacture future micro-optic devices [88]. Hexagonal peptide microtubes with micrometer diameters and millimetre lengths composed of layered and oriented single-level nanotubes were acquired under controlled solvothermal annealing [89]. Incorporation of Guest dyes, i.e., Nile red, was incorporated within the peptide microtubes to demonstrate its optical waveguiding property. Light excitation of one end of the peptide microtubule could cause another end to emit light, while the microtubule body has almost no photoluminescence phenomenon, showing optical waveguide characteristics. To enrich the field patterns of peptide-based components with waveguide characteristics, FF microtubes and cationic dipeptide (CDP) microrods that are suitable for use as optical waveguide materials were prepared using the reprecipitation method. Taking CDP microrods as an example, the assembly system of CDP incorporated the dye molecule fluorescein isothiocyanate (FITC) to obtain single-crystal microrods with optical properties. The incorporation of FITC did not significantly affect the original molecular arrangement and crystal structure of CDP microrods. When the middle of the CDP microrod was excited by light, strong light spots were detected at both ends; when the upper end of the CDP microrod was excited by light, a strong light spot was detected at the lower end. This phenomenon has indicated that FITC dye-doped microrods have good optical waveguide properties, expanding the potential applications of organic nanomaterials as optical devices [90]. Introduction of GA into a self-assembled fibrogel system based on FF can trigger the cyclization of linear FF, and cyclic FF can be obtained as a result of the Schiff reaction that transforms a specific structure to a three-dimensional sequential organization of crystalline platelets (Fig. 6e). Such FF platelets exhibited blue optical waveguide properties in the excitation wavelength range of 330–380 nm (Fig. 6f). This is due to the Schiff base bonds formed between the amino groups of linear FF and the aldehyde groups of glutaraldehyde, resulting in FF exhibiting an inherent photoluminescence n–p* transition [91]. The typical optical waveguide phenomenon is caused by light reflection inside the self-assembled peptide nanofibres. The introduction of formaldehyde can also realize the tunable thickness of FF-based self-assembled peptide nanoribbons for platelets with excellent optical waveguide properties.
Photocatalytic properties
In natural photosynthetic systems, peptides and self-assembled protein complexes play essential roles as regulatory molecules in the arrangement of pigments [92], [93]. Compared with proteins, peptide molecules have simple structures, good stability, and easy preparation. Therefore, peptide self-assembly has received extensive attention for the construction of biomimetic light-harvesting systems. In the design of peptides to regulate the self-assembly of pigment molecules, peptides are mainly used to regulate the aggregation behaviour of pigment molecules to allow more efficient light capture and energy transfer [21], [94]. In a system where phenylalanine dipeptide regulates tetrasulfonic phenylporphyrin (TPPS), TPPS itself first forms the J aggregation structure and generates electrostatic and π-π stacking interactions with phenylalanine dipeptide to create a multiscale ordered microsphere structure (Fig. 6g). The FT-IR spectrum of the obtained peptide-porphyrin microspheres has an absorption band at 1735 cm−1, corresponding to the free –COOH group of phenylalanine dipeptide. In the microspheres, the phyllin molecules are in an orderly aggregation state, have strong light stability, and can be used for in situ photocatalysis to generate platinum and other nanoparticles (Fig. 6h), which in turn can be utilized in more complex photocatalytic reactions [95]. In addition to porphyrin, graphite carbonitride (g-C3N4) possesses tunable electronic properties and superior thermal stability. It was coassembled with the Fmoc-FF hydrogel for high-efficiency photosensitive chromophores. Light-induced electron transfer occurs with exfoliation of g-C3N4 nanosheets, resulting in a doubled photocurrent density compared to that of pristine g-C3N4, demonstrating the outstanding photocatalytic activity of the complex [96]. Covalent coupling of a photoconductive device and peptides can further optimize the molecular structure and improve optical performance. Nanofibre-based porous microspheres were obtained by conjugating the N-terminus of FF with 5-mono (4-carboxyphenyl)-10,15,20-triphenyl porphine (MCpP). Extensive π-π interactions in the assembly region cause the microspheres to undergo photoelectron transfer, and the excitation wavelength is redshifted. Microspheres with spectral photosensitivity and weakened fluorescence attenuation ability are particularly stable in water. In addition, the excellent nicotinamide adenine dinucleotide (NADH) conversion frequency of the system can help in the biocatalytic production of L-Glu. Excitingly, the system offers advantages in recycling [97]. The improved photocatalytic properties and electronic properties of these peptide nanostructures have allowed them to gain promising potential in solar cell technology and biomedical applications.
Optical bioapplication of self-assembled peptide-based nanostructures in food analysis
In the past ten years, new detection tools for optosensing have developed rapidly. Optosensing platforms make use of signal changes, including optical signals and electrical signals caused by specific reactions between analytes and active substances, to determine the content or concentration of an analyte. The highly ordered nanostructures formed by the self-assembly of peptides have been proven to be novel biological structures and can be used for the construction of optosensing platforms in biological or other systems. Food safety issues are receiving increasing attention, and it is urgent to develop more effective and convenient optical sensing detection methods for the detection of ingredients in food matrices. Therefore, we summarize the existing optical detection methods for some components in food, summarize the corresponding peptide-based fluorescence sensing systems that have been studied, and prospect the optical bioapplications of self-assembled peptide-based nanostructures in food analysis (Table 1).
Table 1.
Optical sensor based on nanomaterials for agriculture food analysis.
| Optical nanomaterials | Target analyte | Linear range | R2 | LOD | Food ingredients | References |
|---|---|---|---|---|---|---|
| BSA-AuNCs | OPs | 0.33–6.67 ng /mL | 0.9982 | 0.14 ng /mL | Pesticides | [100] |
| MoS2 QDs | MP | 0.1 μg /mL-30 μg /mL | 0.99 | 0.085 μg /mL | Pesticides | [101] |
| CMC aerogel / EuMOFs / CDs | OPs | 5–40 μM | 0.99529 | 89 nM | Pesticides | [102] |
| GSH-capped CdTe QDs | Paraoxon | 10 ng /L-500 ng /L | 0.9935 | 1.62 × 10−15 M | Pesticides | [103] |
| Aptamer /Peptide /AuNPs | OPs | 0.01–0.75 nM | 0.9972 | 1.94 pM | Pesticides | [104] |
| TPE-Peptide | Ethyl paraoxon | 1–100 μM | 0.9923 | 0.6 μM | Pesticides | [105] |
| CDs | Cr3+ /Pb2+ | 0.1–6.0 μM /0.1–5.0 μM | 0.998 /0.99 | 27 nM /34 nM | Metal ions | [106] |
| GSSH-2TPE | Pb2+ | 0.1–5 μM | 0.9925 | 1.5 nM | Metal ions | [108] |
| TPE-Peptide | Hg2+ | 1–100 nM | 0.99 | 5.3 nM | Metal ions | [109] |
| Dipeptide /Pyrene fluorophore | Al(III) | 0–500 nM | 0.980 | 138.1 nM | Metal ions | [110] |
| Metal binding peptide | Zn2+ /Cu2+ | 0–300 nM | 0.9991 /0.9814 | 5.2 nM /5.5 nM | Metal ions | [111] |
| D-P5 | Hg2+ /Cu2+ | 0–50 μM | – | 25.0 nM /85.0 nM | Metal ions | [112] |
| QDG@MIPs | SS /KS | 3.00–118 μM / 3.00–105 μM | 0.9855 / 0.9800 | 0.22 μM /0.24 μM | Antibiotics | [113] |
| MNP–SiO2–QD | Enrofloxacin | 0.0156 mg /mL-2 mg /mL | 0.995 | – | Antibiotics | [114] |
| COFs | Tetracycline /Ofloxacin | 0.005–0.0625 mM/0.025–0.25 mM | 0.9905 /0.9937 | 0.002 mM /0.0065 mM | Antibiotics | [115] |
| CDP-NI | Doxorubicin / Rifampicin | 1.0 μM-25 nM /13 μM-300 nM | 0.96 /0.96 | 18 nM /202 nM | Antibiotics | [116] |
| Aptamer / FAM /AuNPs | Kanamycin | 0.1 pM-0.1 μM | 0.99 | 0.1 pM | Antibiotics | [117] |
| Aptamer /AuNPs | Kanamycin | 5–1000 pM | 0.9778 | 1.23 pM | Antibiotics | [118] |
| AIEgen /Peptide / AuNCs-apt | Vancomycin | 0.01–100 μg /mL | 0.9917 / 0.9834 | 2.79 ng /mL | Antibiotics | [119] |
| CPNsPBOC-COOH | Ascorbic acid | 0.57–17.10 μM | 0.9903 | 10 nM | Nutrients | [120] |
| CNDs | Vitamin B2 | 0.35–35.9 μM | 0.99693 | 37.2 nM | Nutrients | [121] |
| Self-assembled peptide hydrogel /QDs /Enzymes | Glucose | 1–10 mM | 0.989 | 3.12 mM | Nutrients | [125] |
Self-assembled peptide-based nanostructures in pesticide analysis
According to United Nations projections, by 2050, global demand for agricultural products will be 60% higher than current demand. The growing demand for high food productivity in agriculture has prompted countries worldwide to use pesticides extensively to prevent large-scale pests and diseases from causing devastating losses. In a survey, the Indian Agricultural Research Council found that compared with 2009–2010, the use of pesticides use in India increased by 50% in 2014–2015 [98]. Therefore, the problem of pesticide residues in food and water is inevitable. Excessive pesticides will bring risks to human health, such as cancer, birth defects, and interruption of hormone function [56], [99]. With increasing attention given to food safety, many countries in the world have formulated various laws and regulations that strictly restrict the environment, especially pesticide residues in food. Therefore, the requirements for the limit of detection (LOD) of the corresponding method are becoming increasingly stringent. One of the advantages of optical detection is its high sensitivity, which makes optical-related detection technology show broad prospects in the field of pesticide residue detection. With the rapid development of nanoscience, as a new type of nanostructure that is different from conventional optical materials, self-assembled peptide nanostructures with unique spectral characteristics and excellent biocompatibility can be used in the fields of food and agriculture, such as pesticide detection.
Short peptide self-assemblies have photoluminescence properties and are expected to be used as optical signal sources and chemiluminescence enhancers in pesticide residue detection, similar to other nanoparticles. According to the current research, the signal source of the optical detection technology of pesticide residues in food has a wide range. For example, bovine serum albumin-protected gold nanoclusters (BSA-AuNCs) were used to prepare highly sensitive fluorescent probes to determine organophosphorus pesticides (OPs). During the detection process, acetylthiocholine iodide (ATCI) is catalysed by acetylcholinesterase (AChE) to hydrolyse to form thiocholine (TCh). The bond reaction between TCh and BSA-AuNCs weakens the fluorescence of BSA-AuNCs.. In the presence of organophosphorus pesticides, the activity of AChE is inhibited, the production of TCh is reduced, and the fluorescence of BSA-AuNCs is restored, thereby realizing sensitive and rapid fluorescence detection of OPs in water and food samples, with an LOD of 0.14 ng/mL (S/N = 3) [100]. Compared with traditional organic fluorophores, quantum dots (QDs) have unique optical properties, such as a broad absorption spectrum and asymmetrical emission spectrum, making QDs suitable for high sensitivity single-particle optical detection with high sensitivity. For example, MoS2 QDs have been used to determine methyl parathion (MP) in rice. The functional mechanism of the nanoprobe relies on the photoluminescence quenching of MoS2 QDs induced by p-nitrophenol, in which p-nitrophenol (p-NP) is formed by rapid hydrolysis of MP under alkaline conditions. Under optimal conditions, this detection technology has rapidly detected MP with LODs as low as 0.085 μg/mL in rice and tap water [101]. Technologies based on optical sensors always need to break through the existing foundation of rapid and visual real-time detection. Therefore, Xu et al. developed a sensor based on wearable gloves for noninvasive OP monitoring through fluorescence detection technology. This new type of “lab glove” device integrates a flexible host material (CMC aerogel) and two fluorescent centres (EuMOF for red emission and nanosized carbon dots for blue emission). Based on the porous structure of the CMC aerogel and MOF, this real-time detection system achieved a fast response (30 s), naked-eye detection (red to blue emission transition corresponds to an increase in OP concentration), and high sensitivity (LOD = 89 nM). In addition, compared with other optical sensing technologies used in pesticide residue detection, this sensing system has a considerable cost advantage [102].
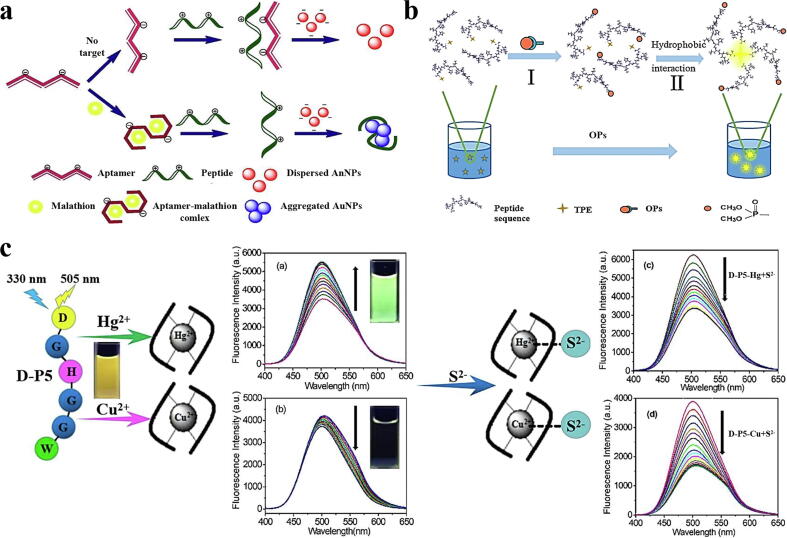
Several studies have proven that using biological elements such as enzymes, proteins, DNA, and whole-cell organisms as biorecognition elements for the detection of pesticides can further provide additional advantages of water solubility, affinity, specificity, and changes in ligand binding spectra. When self-assembled peptide nanostructures have not been fully used in single-particle optical detection systems, some researchers have explored the auxiliary functions of peptide units in optical sensing systems. For example, Korram and colleagues developed a fluorescence detection technology based on enzyme-immobilized glutathione (GSH)-terminated CdTe QDs to monitor OPs in food. GSH-terminated CdTe QDs show higher sensitivity to H2O2. The production of H2O2 is attributed to the active enzymatic reaction of acetylcholinesterase (AChE) and choline oxidase (CHOx). This leads to a decrease in the fluorescence intensity of GSH-terminated CdTe QDs. After exposure to OPs, the fluorescence of CdTe QDs at 520 nm resumes. The corresponding fluorescence change of this biosensing system reasonably corresponds to the amount of pesticide residues, which provides promising benefits for the detection of OP pesticides in food, water, and environmental samples [103]. Bala et al. used cationic peptides, aptamers, and unmodified gold nanoparticles to construct an optical sensing strategy, in which the role of the peptide unit was carefully evaluated. When connected to the aptamer, the peptide frees the gold nanoparticles, which now appear red. On the other hand, when the aptamer binds to the pesticide molecule, the peptide causes the aggregation of gold nanoparticles to make the suspension appear blue (Fig. 7a). The sensor system uses the optical change of gold nanoparticles to achieve the colorimetric detection of malathion, with a LOD of 1.94 pM, which is significantly lower than other available reports [104]. Additionally, Wang and colleagues synthesized a peptide linked to a tetraphenylethylene (TPE) molecule to form an aggregation-induced emission fluorescent probe (TPE-peptide) for the detection of OPs. The detection principle of the sensor system involves the covalent bond reaction between OPs and the active site serine in the peptide sequence. Through this reaction, an adduct is formed between OPs and the peptide to accelerate the aggregation of the peptide, thereby inducing strong emission of the TPE-peptide probe (Fig. 7b). This probe has been proven to show a highly sensitive fluorescence response to OPs, with a LOD of 0.6 μM, and shows selectivity [105].
Fig. 7.
a) Schematic diagram of colorimetric analysis of malathion based on aptamers, cationic peptides and gold nanoparticles [104]. b) Schematic diagram of the working principle of the TPE-peptide probe [105]. c) Proposed fluorescence detection mode of D-P5 for Cu2+, Hg2+, and S2−[112].
The goal of pesticide residue detection methods is rapid, sensitive, qualitative, quantitative, and multiresidue detection. Through existing research, organizing inorganic luminescent nanoparticles and peptides into hybrid self-assembly systems has provided a diversified toolbox for manufacturing optical sensors. It has been explained that some self-assembled nanostructures based on short peptides all show unique optical properties, which indicates that short peptide building blocks can be assembled into a QD-like structure at the nanoscale, with obvious exciton effects, which makes these new forms of self-assembled peptide nanostructures organic QDs suitable for use. In the contemporary environmentally friendly environment, self-assembled peptide nanostructures deserve to be further explored as single-particle luminescence centres to achieve high sensitivity and high precision pesticide residual detection.
Self-assembled peptide-based nanostructures in metal ion analysis
Heavy metal ions are nondegradable and biologically incompatible and will accumulate in the food chain, causing severe harm to humans. For example, excessive intake of Ag can cause stomach pain, nervous system damage, and even death. Excessive Pd content in the body can cause severe physical disorders. In addition, the world has always paid attention to mercury poisoning in organisms because Hg2+ easily penetrates biological membranes and causes toxicity. Therefore, the effective detection of metal ions in food has become a top priority. To date, a large number of precise and sensitive methods for the quantitative detection of metal ions have been established, including X-ray fluorescence spectroscopy (XRF), cold vapour atomic fluorescence spectroscopy (CVAFS), atomic absorption/emission spectroscopy (AAS), and inductively coupled plasma/atomic emission spectroscopy (ICP-MS). However, these methods are not well suited for in situ applications, so optical sensors, as a fast, reliable, and straightforward technology, have been widely used to detect metal ions in food inspection.
Nano-optical signal sources used in metal ion detection are constantly being updated. As one of the most common optical technologies, fluorescence sensing technology has been widely used in the detection of metal ions. For example, Lu and colleagues built an ion-imprinted fluorescent polymer based on blue and red dual-channel dual emission carbon dots (DDCDs), which can simultaneously realize dual-channel detection of Cr3+ and Pb2+. The LODs of Cr3+ and Pb2+ can reach 27 nM and 34 nM, respectively. The detection of Cr3+ and Pb2+ in actual water samples also obtained satisfactory recovery rates [106]. To reduce the fluorescent background, Wang's team designed and synthesized a 3-mercaptopropionic acid functionalized copper nanocluster, which is a nanohybrid with aggregation-induced luminescence properties, named Cu2+@MPA-Cu NCs. When the target molecule is present, Cu2+ will strongly bind to it, so the aggregated structure of Cu2+@MPA-Cu NCs is destroyed, and the fluorescence is quenched. Based on this principle, a fluorescent probe for detecting related ions in food additives was constructed, with a LOD as low as 26.3 nM [107].
Because peptides are highly soluble in aqueous solutions and have a strong binding affinity to heavy metal ions, metal-peptide interactions provide abundant resources and design principles for developing smart sensors. Recently, people have studied peptide-based fluorescent chemical sensors for detecting metal ions, which laid the foundation for detecting heavy metal ions in foods by self-assembled peptide nanostructures in the future. For example, Gui's team developed a biomimetic peptide-based fluorescent sensor based on the structure of the naturally occurring peptide glutathione. The well-known Lewis acid-base theory is used to guide molecular design and selective adjustment of targeting properties. Due to the integration of the aggregation-induced emission effect and peptide recognition, this sensing system can exhibit ideal sensing characteristics for Pb2+, including specific on-response, fast binding, signal output, and high sensitivity with a LOD of 1.5 nM [108]. The molecular configuration of the peptide-based sensor and the balance between the chelating groups contribute to the high selectivity of the complexation of specific metal ions.
Similarly, there are studies on metal ion-based peptide receptors using tetraphenylethylene fluorophores to construct a fluorescent peptide-based chemical sensor to detect Hg2+. This fluorescence sensing system showed the only selective turn-on response to Hg2+ among the 16 metal ions contained in the NaCl buffered aqueous solution. The peptide-based chemical sensor shows a highly sensitive response to Hg2+, and the LOD is 5.3 nM [109]. In addition, Lee and colleagues designed and developed a dipeptide receptor-based fluorescent probe with a pyrene fluorophore for ratiometric detection of Al(III) ions. The complete ratio response of the probe requires the concentration of Al(III). the change in the emission intensity ratio of the two fluorescence emission peaks shows a linear response to Al(III) with a concentration range of 0–500 nM, and the LOD is 138.1 nM. Importantly, the probe showed a highly selective response to Al(III) in the presence of other metal ions [110]. The sensitivity and affinity of the peptide-based fluorescence sensor in the detection of metal ions have been studied. Therefore, Notomista et al. designed a peptide with a dansylamide moiety connected to the N-terminus and a tryptophan residue connected to the C-terminus. This is mainly because they can be used as fluorescence resonance energy transfer (FRET) pairs, where dansyl fluorophores often exhibit strong chelation-enhanced fluorescence (CHEF). The designed and developed new metal ion-sensitive fluorescent peptide-based probe showed a selective fluorescence turn-on response to Zn2+ in aqueous solution when excited at 295 nm and 340 nm, proving that FRET and CHEF are active in metal/peptide complexes. Better elimination of the fluorescent background paves the way for the later development of highly selective peptide-based optical sensors for metal ion detection in food and the environment [111]. It is worth noting that metal ions usually exist in the actual sample in a coexisting state. Hence, the simultaneous detection of two or more metal ions is also urgently needed. Based on this, Li and colleagues used Fmoc solid-phase peptide synthesis (SPPS) to construct a new multifunctional fluorescent peptide sensor based on pentapeptide dansyl-Gly-His-Gly-Gly-Trp-COOH (D-P5). This peptide-based fluorescence sensor can show selective and sensitive responses to Hg2+ and Cu2+ in the coexistence of multiple ions. The probe distinguishes Hg2+ and Cu2+ ions mainly through the “turn on” response to Hg2+ and the “turn-off” response to Cu2+. The addition of Hg2+ or Cu2+ ions can cause the sensor to show a significant colour change under ultraviolet light (Fig. 7c). The LOD of Hg2+ was 25.0 nM, and the LOD of Cu2+ was 85.0 nM. This research provides new possibilities for using short peptide-based fluorescence sensors for multifunctional detection [112].
In general, peptides have the advantages of optional structural units, rich coordination chemistry, and high stability, making them attractive candidates for customized selective metal-binding ligands. Many works have focused on the construction of peptide-based fluorescence sensors. With the help of basic research on self-assembled peptide nanostructures, self-assembled peptide nanostructure-based optical sensors for detecting metal ions in food with high selectivity, sensitivity, and accuracy will undoubtedly show a wide range of application prospects.
Self-assembled peptide-based nanostructures in antibiotic analysis
For the treatment and prevention in the livestock and poultry industry, antibiotics are an essential low-molecular-weight chemical. However, the abuse of related antibiotics can cause excessive antibiotic residues in food, threatening human health. Therefore, antibiotic residues are undoubtedly an important issue in the field of food and animal feed safety. Many agencies, such as the European Union (EU) and the US Food and Drug Administration (FDA), have established maximum antibiotic residue limits for certain foods to protect human health and ensure food safety. A variety of analytical methods for the detection of antibiotics have been reported, such as high-performance liquid chromatography (HPLC), gas chromatography-mass spectrometry (GC–MS), capillary electrophoresis (CE), etc. Disadvantages such as expensive equipment and time-consuming procedures restrict the implementation of the abovementioned methods in field testing. For the supervision and monitoring of food safety, rapid multiple detection methods for antibiotic residues are necessary and urgent.
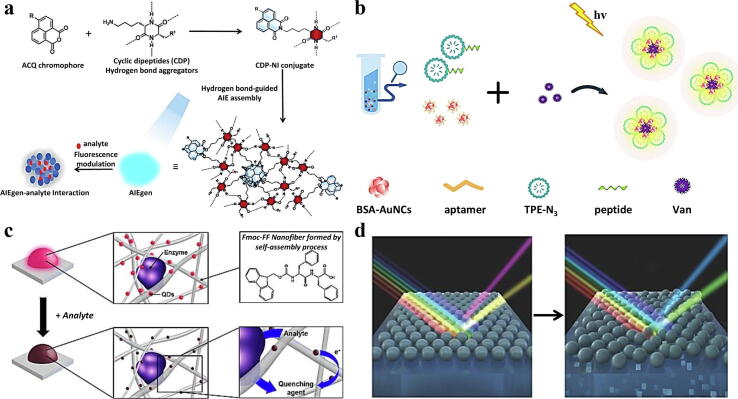
The fluorescence detection method is an excellent method to realize antibiotic residue detection. At present, a variety of fluorescent materials have been used to detect antibiotic residues by fluorescent luminescence. For example, quantum dots have attracted attention because of their excellent fluorescence properties. Peng and colleagues synthesized a molecularly imprinted double-emitting QD composite material that can be used to simultaneously detect streptomycin sulfate (SS) and kanamycin sulfate (KS). The realization of this detection goal is attributed to the preparation of two CdTe QDs with green (QDG) and red emission (QDR) colours [113]. Their recovery characteristics usually limit the use of fluorescence detection methods. Therefore, to solve this problem, a study designed and synthesized a magnetic QD material (MNP-SiO2-QD) with reusability and high fluorescence retention based on the characteristics of typical CdTe QDs and magnetic nanoparticles (MNPs). This type of material can realize the qualitative and quantitative detection of antibiotics within 5 min. Based on the excellent magnetic responsiveness of this material, after 5 cycles of use, the fluorescence intensity of MNP-SiO2-QD can still maintain its initial level of 50%. The sensor system provides a template for developing new antibiotic detection methods and further ensures food safety [114]. The synthesis and application of other fluorescent materials in food inspection are also being continuously studied. For example, Wang's team used a green method to construct a crystalline polyimide covalent organic framework (COF) without using organic solvents. The synthesized COF with a π-conjugated skeleton has bright fluorescence, which is mainly attributed to the n-π* transition in N,N-dimethylformamide and alkaline aqueous solutions. It is worth noting that this COF will quench the fluorescence in the presence of two antibiotics. The quenching mechanism may be an aggregation effect and π-π reaction. The sensing system can be used in actual samples to obtain good results with a wide linear range and low LOD [115]. Similar to the detection of other hazardous substances in food, there is also work exploring a new aggregation-induced emission fluorescence system for the detection of antibiotics. Cyclic dipeptide (CDP) is used as a multifunctional molecular assistant to form strong intermolecular hydrogen bonds, which can be linked to naphthalene anhydride through the quenching property caused by aggregation. The introduction of CDP promotes the molecular assembly of the resulting naphthalimide product, thereby forming aggregation-induced emission active aggregates through intermolecular hydrogen bonds in an aqueous medium. The designed and synthesized CDP-naphthalimide (CDP-NI) conjugate provides sensitive detection of doxorubicin and rifampin antibiotics with LODs of 18 nM and 202 nM (Fig. 8a) [116].
Fig. 8.
a) Schematic illustration of the design strategy of cyclic dipeptide (CDP)-conjugated ACQphoric naphthalic anhydrides (CDP-NI conjugates) [116]. b) Schematic illustration of the sensing platform for detecting Van [119]. c) An illustrative description of the development of a PL peptide hydrogel through the self-assembly of Fmoc-FF building blocks and their PL quenching associated with the enzymatic detection of analytes [125]. d) A model representation of the PNA sphere lattice before (left) and following (right) the addition of salt (The highly organized array is geometrically altered, resulting in a visible shift in the reflected wavelengths) [127].
The current research shows that a fluorescent aptamer biosensor with a specific binding ability to the target has been used to detect antibiotics effectively. Aptamers are usually synthetic oligonucleotide sequences that have high activity and specificity in binding to target molecules. For example, Zhao's team used gold nanoparticles as a fluorescence dynamic quenching source and a nucleic acid aptamer with a FAM fluorophore as a specific binding carrier. A fluorescence sensor constructed by this method can realize the microdetection of kanamycin, and the corresponding LOD is 0.1 pM [117]. Generally, the binding affinity of the target molecule is inversely proportional to the dissociation constant (Kd) of the aptamer; that is, the larger the Kd value is, the worse the detection performance of the sensor system for the target antibiotic. To improve the sensitivity of the corresponding sensing system, adopt an appropriate signal amplification strategy is very conducive to the sensitive detection of antibiotics. Recently, an integrated aptamer sensor has been designed, synthesized, and applied to detect antibiotics in food. Interestingly, the sensor is based on an enzyme-driven three-dimensional DNA walker. By anchoring the high-density substrate chain on the walking interface, the signal amplification efficiency is accelerated, effectively overcoming the time-consuming shortcomings of the past. The functions of target recognition, signal amplification, and signal output are designed to be integrated into a single probe. The addition of kanamycin causes the fluorescence intensity of the solution to increase, and the effective detection of target molecules with a LOD of 1.23 pM is completed within 40 min. When applied to spiked milk samples, a good recovery rate of 97.76–105.33% can be obtained [118]. Fluorescence sensors used in the quantitative analysis of antibiotics have improved feasibility, selectivity, and sensitivity. The addition of biological factors such as peptides has been shown to improve better the recognition affinity of the sensing system to target molecules. For example, a peptide coupled with aggregation-induced emission luminogen (AIEgen) with blue luminescence and gold nanoclusters modified with aptamer (AuNCs-apt) with red luminescence was developed, and a dual-emission fluorescent biosensor was constructed based on this peptide. The aptamer and peptide can recognize vancomycin with high affinity, resulting in changes in the fluorescence intensity at 470 nm and 650 nm (Fig. 8b). The fluorescence response has an excellent linear correlation with the target molecule in a particular concentration range, and the LOD is 2.79 ng/mL. A fact that cannot be ignored is that this type of newly developed biosensor can detect 1 μg/mL vancomycin by the naked eye [119].
The establishment of a simple and sensitive method for the detection of antibiotics is of great significance to food safety. Peptide units have begun to be used in the construction of some fluorescent aptamer biosensors, but these applications often benefit from the excellent biocompatibility and structural controllability of individual peptides. The photoluminescence properties of short peptide self-assembly have not been fully explored and applied thus far. According to the existing research foundation, organic light-emitting nanomaterials have gradually attracted the attention of researchers. In terms of meeting higher recognition affinity and the synthesis of greener fluorescent nanomaterials, self-assembled peptide nanostructures are excellent candidates for constructing optical sensors for detecting antibiotics in food.
Self-assembled peptide-based nanostructures in nutrient analysis
Nutrients are organic compounds that can prevent diseases such as high blood pressure, cardiovascular disease, thyroid disease, or cancer. They play an important role in human biological processes. Most nutrients may need to be taken from external sources because they cannot be produced directly in the body. Some foods, such as grains, vegetables, and fruits, have been used as natural nutrients and play an important role in maintaining the nutrients required for human health. However, the lack of nutrients in food is becoming increasingly severe, and food producers, consumers, and quality control agencies have begun to pay attention to this problem. Therefore, developing a highly sensitive and efficient analytical detection method that can be used to analyse various nutrients in food is urgent because it helps food testing laboratories more quickly and accurately evaluate the nutritional value of food. Current related detection mostly relies on advanced analysis tools. Considering the high diversity and wide dynamic range of some nutritional molecules in food samples, optical detection methods with cost advantages should also be more applied to nutritional component detection in food.
Recently, conjugated polymer nanoparticles (CPNs) with excellent optical properties and biocompatibility have attracted the attention of researchers. The rigid structure and conjugate delocalization of the main chain of the conjugated polymer give it excellent fluorescence performance. The large π-electron delocalization skeleton on the conjugated polymer structure gives it a higher fluorescence quantum yield and better absorption coefficient. Therefore, Zhao's team designed and synthesized an Fe3+, poly [3-{2,5-bis (2-ethyl-hexyloxy)]-phenyl}-vinyl}-9-octyl-carbazole] (PBOC) and polystyrene-maleic anhydride (PSMA) fluorescence sensing platform (CPNsPBOC-COOH-Fe3+) for the quantitative detection of ascorbic acid (AA). Fe3+ quenches its fluorescence by polymerizing CPNsPBOC-COOH, and the addition of AA reduces aggregation and restores fluorescence. Based on this, the platform achieves highly selective and sensitive detection of AA in actual samples, with an LOD of 10 nM [120]. Good optical performance and chemical stability are always the primary considerations for choosing optical nanomaterials for optical sensing platforms. Recently, Du and colleagues developed a new type of carbon nanodot, polyethyleneimine (PEI), as the precursor for synthesizing carbon nanodots. PEI is an ideal nitrogen source for the surface functionalization of nanomaterials and improving optical properties, which is mainly due to the inherent multiple active amine groups of PEI itself. Based on the designed carbon nanodots, a unique ratio fluorescence resonance energy transfer probe was constructed to detect VB2. The fluorescence resonance energy transfer is attributed to the strong interaction between the —C O and —NH— groups of VB2 and the functional groups on the surface of the carbon nanodots. This platform has been used for the detection of VB2 in food samples with an LOD of 37.2 nM and satisfactory performance [121].
Some peptide-based fluorescence sensing systems with excellent biological properties are gradually being developed to detect biologically active ingredients. Schmuck's team reported on a ratiometric fluorescence chemical sensor set (AP 1·CB) composed of pyrene-based amphiphilic peptide beacons (AP 1) and cucurbit urea (CB). Among them, AP 1 has two symmetrical amphiphilic peptide arms, both of which contain cationic lysine as a positively charged head group. The peptide arm is connected to the middle lysine spacer through the C-terminus. The ends of the peptide arms are connected to the pyrene moieties through lipophilic γ-aminobutyric acid linkers to provide amphiphilicity. After the hydrophobic CB cavity wraps the pyrene end, the pyrene excimer becomes a monomer to emit, and AP 1 is unfolded. The substrate with a higher affinity to the CB cavity replaces AP 1 overall. AP 1 can be folded again to form a pyrene excimer, achieving ratiometric fluorescence detection of substrates, including amino acid derivatives, proteins, and specific peptides [122]. Biosensing analysis platforms usually rely on the structure and function of fixed probes. Self-assembled peptide hydrogels can achieve local restriction of biomolecules based on attracting semi-wet systems while maintaining their structures and functions. They also exhibit changes in response to external stimuli such as pH or temperature and are beneficial for long-term storage [123]. King et al. obtained a self-assembled three-dimensional peptide hydrogel using basic peptides containing antiparallel β-sheets. The basic peptides on the surface were fixed with oligonucleotides via conjugation and hybridized with molecular beacon (MB) fluorescent probes to achieve DNA molecular recognition and detection of binding events [124]. To further optimize the functional optical properties and controllability of peptide self-assembled nanostructures, chemical conjugation with functional parts has been explored. A hydrophobic 9-fluorenylmethyloxycarbonyl (Fmoc) group was introduced into the N-terminus of FF to increase its hydrophobicity. Fmoc-FF encapsulated fluorescent signal molecules QDs (CdTe and CdSe) and enzyme bioreceptors (i.e., horseradish peroxidase or glucose oxidase) through self-assembly of peptide hydrogel. The peptide hydrogel was studied for biosensing. By simply mixing the aqueous solution containing QDs and enzyme with Fmoc-FF solution, QDs and enzymes were successfully immobilized in the hydrogel matrix. The prepared self-assembled peptide hydrogel has a physical three-dimensional nanofibre network with approximately 70–90 nm diameter. The hydrogel was used to detect glucose. It was found that doped QDs retained their photoluminescence properties, and enzymatic conversion of the analyte acted as an electron acceptor on the surface of QDs while also acting as a quencher to absorb the excited electrons of QDs, causing the photoluminescence of QDs to be quenched (Fig. 8c). The degree of quenching is related to the concentration of the analyte. These results show that the self-assembled peptides nanostructure successfully achieved high-efficiency encapsulation of biological receptors and fluorescent signal molecules, having great potential as an optical biosensing platform for enzymatic detection of analytes [125]. In addition, a novel structural molecule named peptide nucleic acid (PNA) was obtained through the combination of peptide units and nucleic acid molecules. The extended structural unit diPNA has excellent self-assembly capabilities. Under directional and extensive noncovalent interactions, diPNA-guanine-cytosine has integrated excitation-dependent fluorescence from 440 nm to 684 nm, and the entire visible light region is almost completely covered. Another interesting finding is that this self-assembled structure can achieve controllable electroluminescence voltage through the application of alternating current, showing potential in various light-emitting applications, such as organic light-emitting diodes and optosensing [126]. Additionally, PNA-G modified with Fmoc and benzhydryloxycarbonyl (Bhoc) can self-assemble into uniform photonic crystals with a diameter of approximately 1.7 mm under the drive of noncovalent forces. It is worth noting that guanine-based PNA balls can easily obtain a coloured film layer in the solution. Agglomeration of the PNA crystal ball occurs due to NaCl addition, causing a change in the colour of the film layer and a change in the reflection wavelength visible to the naked eye accordingly (Fig. 8d) [127]. In brief, PNA self-assembled balls show promising potential for use as optical coating sensors.
The complexity of food matrices and the chemical heterogeneity of some nutrients make it challenging to develop detection methods for the rapid and sensitive detection of single nutrients. Optical sensors involving self-assembled peptide nanostructures have begun to be applied to detect small nutrient molecules such as amino acids but mostly rely on the conjugation of fluorescent carriers. Short peptide self-assembly with its excellent fluorescence performance can achieve convenient detection and better specificity through specific structural control.
Summary and outlook
Different peptide self-assembly strategies provide a wide range of nanomaterial geometries and morphologies. By fine-tuning the type and structure of peptides, the morphology and function of the assemblies can be controlled at the molecular level. Peptide nanostructures with excellent physical and chemical properties can then be constructed under the action of noncovalent bonding. Current challenges and future perspectives in the fabrication of self-assembled peptide nanostructures include the large-scale fabrication of self-assembled peptide nanostructures that generate intense optical activity, such as peptide QDs. Self-assembled peptide nanostructures with good fabrication yield, stability, dispersity in a complex sample matrix, biocompatibility, and environmental friendliness are ideal development goals in the future. Owing to its flexible and unique optical properties, some short peptide self-assemblies can possibly be used to achieve the purpose of rapid and sensitive detection of composition in food, agriculture, and the environment, expanding the understanding and application of peptide-based optics in analytical chemistry. Self-assembled peptide nanostructures can also be used as promising alternatives to inorganic counterparts that can improve the association between biology and chemistry, materials science, and optics, providing promising technological tools for nanobiotechnology research. However, before we can develop the optical application of self-assembled peptide nanostructures, we need to urgently explore the relationship between the molecular structure, mechanism, and functionality of nanoassemblies, allowing precise design and fabrication of self-assembled peptide systems with desirable biological and physicochemical properties. Unfolding the potential of self-assembled peptide systems in optical applications would advance interdisciplinary technologies and expand their application range.
Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 31822040), the National Key R&D Program of China (No. 2018YFC1602300), the Young Top-Notch Talent of High-Level Innovation and Entrepreneurs Support Program (2017000026833ZK28), School Level Cultivation Fund of Beijing Technology and Business University for Distinguished and Excellent Young Scholars (BTBUYP2020).
Biographies

Xuecheng Zhu is now an M.S. student at the Beijing Technology and Business University, Beijing, supervised by Professor Huilin Liu. She has four international publications in journals including Elsevier & others. She is a keen observer, and has curiosity for research in scientific field. Her research interest lies in the synthesis of optical nanomaterials, analytical chemistry, and rapid trace detection of agricultural products.

Ruixue Duan received bachelor’s degree in biology from Shanxi university in 2008, and master’s degree in biophysics from South China Normal University in 2011. After two years of research assistant training at Institute of Chemistry of Chinese Academy of Sciences, and Huazhong University of Science and Technology, she joined Prof. Xia group for PhD study and obtained the degree in 2016. Then she joined Prof. Xiaogang Liu group at national university of Singapore for postdoctoral fellow training for four years. Now she is an associate professor of Soochow University. Her research focuses on developing smart nanoprobes for the applications in cancer metabolism, cancer diagnosis and therapy.

Siew Yin Chan received her BSc in Food Science and Technology with First Class Honours (2013) and subsequently obtained her PhD in Chemical Science (2018) from Monash University. She is currently a scientist in the Institute of Materials and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR). She was previously an Assistant Professor in the Institute of Flexible Electronics at Northwestern Polytechnical University, China. Her research interests include biomaterials and sustainable functional materials.

Luxuan Han is now an M.S. student at the Beijing Technology and Business University, Beijing, supervised by Professor Huilin Liu. She has published a research artical in Analytica Chimica Acta. She is a diligent seeker and explorer, and has great interests in the detection of pesticide residues in food. Her current research focuses on rapid trace detection of hazardous substances in alcohol, particularly ethyl carbamate.

Huilin Liu received her Ph.D. from the Tianjin University of Science and Technology in 2014. She was appointed as a lecturer (2014), associate professor (2017), and professor (2019) at the Beijing Advanced Innovation Center for Food Nutrition and Human Health, Beijing Technology and Business University, Beijing, China. She worked as a visiting scholar at the Faculty of Science, National University of Singapore, in 2019. She has 47 international publications in journals including Springer & others and 14 publications in national journals. Her current research interests are focused on the self-assembly of nanoparticles for biological separation and detection.

Baoguo Sun received his Ph.D. in the Department of Chemical Engineering of Tsinghua University. He is now holding a Professorship in Beijing Technology and Business University, and recognized as a Fellow of Chinese Academy of Engineering since Dec. of 2009. His research interests include constructed a molecular characteristic structural unit model of meat flavor sulfur compounds, manufacture of 3-furan sulfide series and asymmetric disulfide food spices, meat flavor manufacturing technology using livestock and poultry meat, bone, and fat as the main raw materials, research on Functional Factors in Baijiu, and research on the Flavor of Baijiu.
Footnotes
Peer review under responsibility of Cairo University.
References
- 1.Reddy S.M.M., Raßlenberg E., Sloan‐Dennison S., Hesketh T., Silberbush O., Tuttle T., et al. Proton‐conductive melanin‐like fibers through enzymatic oxidation of a self‐assembling peptide. Adv Mater. 2020;32(46):2003511. doi: 10.1002/adma.v32.46. [DOI] [PubMed] [Google Scholar]
- 2.Bong D.T., Clark T.D., Granja J.R., Ghadiri M.R. Self-assembling organic nanotubes. Angew Chem Int Ed. 2001;40(6):988–1011. [PubMed] [Google Scholar]
- 3.Hiew S.H., Mohanram H., Ning L., Guo J., Sánchez‐Ferrer A., Shi X., et al. A short peptide hydrogel with high stiffness induced by 310-Helices to β-Sheet transition in water. Adv Sci. 2019;6(21):1901173. doi: 10.1002/advs.v6.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartgerink J.D., Beniash E., Stupp S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 5.Yu Y.-C., Berndt P., Tirrell M., Fields G.B. Self-assembling amphiphiles for construction of protein molecular architecture. J Am Chem Soc. 1996;118(50):12515–12520. [Google Scholar]
- 6.Lakshmanan A., Zhang S., Hauser C.A.E. Short self-assembling peptides as building blocks for modern nanodevices. Trends Biotechnol. 2012;30(3):155–165. doi: 10.1016/j.tibtech.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Habibi N., Kamaly N., Memic A., Shafiee H. Self-assembled peptide-based nanostructures: smart nanomaterials toward targeted drug delivery. Nano Today. 2016;11(1):41–60. doi: 10.1016/j.nantod.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21(10):1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 9.Lou S., Wang X., Yu Z., Shi L. Peptide tectonics: encoded structural complementarity dictates programmable self-assembly. Adv Sci. 2019;6(13):1802043. doi: 10.1002/advs.v6.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qi G.-B., Gao Y.-J., Wang L., Wang H. Self-assembled peptide-based nanomaterials for biomedical imaging and therapy. Adv Mater. 2018;30(22):1703444. doi: 10.1002/adma.v30.22. [DOI] [PubMed] [Google Scholar]
- 11.Xu L.-P., Liu Y., Zhang X. Interfacial self-assembly of amino acids and peptides: scanning tunneling microscopy investigation. Nanoscale. 2011;3(12):4901. doi: 10.1039/c1nr11070e. [DOI] [PubMed] [Google Scholar]
- 12.Bancroft J.B., Hills G.J., Markham R. A study of the self-assembly process in a small spherical virus: formation of organized structures from protein subunits in vitro. Virology. 1967;31(2):354–379. doi: 10.1016/0042-6822(67)90180-8. [DOI] [PubMed] [Google Scholar]
- 13.Rajagopal K., Schneider J.P. Self-assembling peptides and proteins for nanotechnological applications. Curr Opin Struc Biol. 2004;14(4):480–486. doi: 10.1016/j.sbi.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Hartgerink J.D. Covalent capture: a natural complement to self-assembly. Curr Opin Chem Biol. 2004;8(6):604–609. doi: 10.1016/j.cbpa.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Chen H., Shou K., Chen S.i., Qu C., Wang Z., Jiang L., et al. Smart self-assembly amphiphilic cyclopeptide-dye for near-infrared window-II imaging. Adv Mater. 2021;33(16):2006902. doi: 10.1002/adma.v33.16. [DOI] [PubMed] [Google Scholar]
- 16.Ghadiri M.R., Granja J.R., Milligan R.A., McRee D.E., Khazanovich N. Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature. 1993;366(6453):324–327. doi: 10.1038/366324a0. [DOI] [PubMed] [Google Scholar]
- 17.Amdursky N., Molotskii M., Gazit E., Rosenman G. Self-assembled bioinspired quantum dots: optical properties. Appl Phys Lett. 2009;94(26):261907. doi: 10.1063/1.3167354. [DOI] [Google Scholar]
- 18.Tao K., Fan Z., Sun L., Makam P., Tian Z., Ruegsegger M., et al. Quantum confined peptide assemblies with tunable visible to near-infrared spectral range. Nat Commun. 2018;9(1) doi: 10.1038/s41467-018-05568-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li C., Jin X., Zhao T., Zhou J., Duan P. Optically active quantum dots with induced circularly polarized luminescence in amphiphilic peptide dendron hydrogel. Nanoscale Adv. 2019;1(2):508–512. doi: 10.1039/c8na00216a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCluskey J.B., Clark D.S., Glover D.J. Functional applications of nucleic acid–protein hybrid nanostructures. Trends Biotechnol. 2020;38(9):976–989. doi: 10.1016/j.tibtech.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Song N., Zhang Z., Liu P., Yang Y.-W., Wang L., Wang D., et al. Nanomaterials with supramolecular assembly based on AIE luminogens for theranostic applications. Adv Mater. 2020;32(49):2004208. doi: 10.1002/adma.v32.49. [DOI] [PubMed] [Google Scholar]
- 22.Ma H., Fei J., Li Q.i., Li J. Photo-induced reversible structural transition of cationic diphenylalanine peptide self-assembly. Small. 2015;11(15):1787–1791. doi: 10.1002/smll.201402140. [DOI] [PubMed] [Google Scholar]
- 23.Tao K., Levin A., Adler-Abramovich L., Gazit E. Fmoc-modified amino acids and short peptides: simple bio-inspired building blocks for the fabrication of functional materials. Chem Soc Rev. 2016;45(14):3935–3953. doi: 10.1039/c5cs00889a. [DOI] [PubMed] [Google Scholar]
- 24.Liu X., Fei J., Wang A., Cui W., Zhu P., Li J. Transformation of dipeptide-based organogels into chiral crystals by cryogenic treatment. Angew Chem. 2017;56(10):2660–2663. doi: 10.1002/anie.201612024. [DOI] [PubMed] [Google Scholar]
- 25.Wang J., Liu K., Xing R., Yan X. Peptide self-assembly: thermodynamics and kinetics. Chem Soc Rev. 2016;45(20):5589–5604. doi: 10.1039/c6cs00176a. [DOI] [PubMed] [Google Scholar]
- 26.Toksoz S., Acar H., Guler M.O. Self-assembled one-dimensional soft nanostructures. Soft Matter. 2010;6(23):5839. doi: 10.1039/c0sm00121j. [DOI] [Google Scholar]
- 27.Hu K., Jiang Y., Xiong W., Li H.u., Zhang P.-Y., Yin F., et al. Tuning peptide self-assembly by an in-tether chiral center. Sci Adv. 2018;4(5) doi: 10.1126/sciadv.aar5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan T., Yu X., Shen B., Sun L. Peptide self-assembled nanostructures for drug delivery applications. J Nanomater. 2017;2017:1–16. [Google Scholar]
- 29.Mondal S., Gazit E. The self-assembly of helical peptide building blocks. ChemNanoMat. 2016;2(5):323–332. [Google Scholar]
- 30.Zhao X., Zhang S. Fabrication of molecular materials using peptide construction motifs. Trends Biotechnol. 2004;22(9):470–476. doi: 10.1016/j.tibtech.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Kim D., Han S.A., Kim J.H., Lee J.-H., Kim S.-W., Lee S.-W. Biomolecular piezoelectric materials: from amino acids to living tissues. Adv Mater. 2020;32(14):1906989. doi: 10.1002/adma.v32.14. [DOI] [PubMed] [Google Scholar]
- 32.Eisenberg D. The discovery of the α-helix and β-sheet, the principal structural features of proteins. Proc Natl Acad Sci U S A. 2003;100:11207–11210. doi: 10.1073/pnas.2034522100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Z., Cai Z., Chen Q., Liu M., Ye L., Ren J., et al. Engineering β-sheet peptide assemblies for biomedical applications. Biomater Sci. 2016;4(3):365–374. doi: 10.1039/c5bm00472a. [DOI] [PubMed] [Google Scholar]
- 34.Pugliese R., Maleki M., Zuckermann R.N., Gelain F. Self-assembling peptides cross-linked with genipin: resilient hydrogels and self-standing electrospun scaffolds for tissue engineering applications. Biomater Sci. 2019;7(1):76–91. doi: 10.1039/c8bm00825f. [DOI] [PubMed] [Google Scholar]
- 35.Amit M., Yuran S., Gazit E., Reches M., Ashkenasy N. Tailor-made functional peptide self-assembling nanostructures. Adv Mater. 2018;30(41):1707083. doi: 10.1002/adma.v30.41. [DOI] [PubMed] [Google Scholar]
- 36.Gribbon C., Channon K.J., Zhang W., Banwell E.F., Bromley E.H.C., Chaudhuri J.B., et al. MagicWand: a single, designed peptide that assembles to stable, ordered alpha-helical fibers. Biochemistry. 2008;47:10365–10371. doi: 10.1021/bi801072s. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y., Gong X. Special oleophobic and hydrophilic surfaces: approaches, mechanisms, and applications. J Mater Chem A. 2017;5(8):3759–3773. [Google Scholar]
- 38.Cheetham A.G., Zhang P., Lin Y.-a., Lock L.L., Cui H. Supramolecular nanostructures formed by anticancer drug assembly. J Am Chem Soc. 2013;135(8):2907–2910. doi: 10.1021/ja3115983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L., Yang P.-P., Zhao X.-X., Wang H. Self-assembled nanomaterials for photoacoustic imaging. Nanoscale. 2016;8(5):2488–2509. doi: 10.1039/c5nr07437a. [DOI] [PubMed] [Google Scholar]
- 40.Ohmura H., Tabata Y., Kimura S., Uji H. Piezoelectric properties reflecting nanostructures of tetrathiafulvalene and chloranil complexes using cyclic peptide nanotube scaffolds. Peptide Sci. 2021;113(2) doi: 10.1002/pep2.v113.2. [DOI] [Google Scholar]
- 41.Seo M.J., Song J., Kantha C., Khazi M.I., Kundapur U., Heo J.-M., et al. Reversibly thermochromic cyclic dipeptide nanotubes. Langmuir. 2018;34(28):8365–8373. doi: 10.1021/acs.langmuir.8b00743. [DOI] [PubMed] [Google Scholar]
- 42.Vauthey S., Santoso S., Gong H., Watson N., Zhang S. Molecular self-assembly of surfactant-like peptides to form nanotubes and nanovesicles. Proc Natl Acad Sci. 2002;99(8):5355–5360. doi: 10.1073/pnas.072089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reches M., Gazit E. Formation of closed-cage nanostructures by self-assembly of aromatic dipeptides. Nano Lett. 2004;4(4):581–585. [Google Scholar]
- 44.Adler-Abramovich L., Reches M., Sedman V.L., Allen S., Tendler S.J.B., Gazit E. Thermal and chemical stability of diphenylalanine peptide nanotubes: implications for nanotechnological applications. Langmuir. 2006;22:1313–1320. doi: 10.1021/la052409d. [DOI] [PubMed] [Google Scholar]
- 45.Adler-Abramovich L., Aronov D., Beker P., Yevnin M., Stempler S., Buzhansky L., et al. Self-assembled arrays of peptide nanotubes by vapour deposition. Nat Nanotechnol. 2009;4(12):849–854. doi: 10.1038/nnano.2009.298. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg J. Cyclic peptide antibiotics; self-assembly required. Trends Biotechnol. 2001;19(10):379. doi: 10.1016/S0167-7799(01)01816-9. [DOI] [Google Scholar]
- 47.Khavani M., Izadyar M., Housaindokht M.R. The effects of amino acid sequence and solvent polarity on the self-assembling of cyclic peptide nanotubes and molecular channel formation inside the lipid bilayer. J Mol Liq. 2020;314:113660. doi: 10.1016/j.molliq.2020.113660. [DOI] [Google Scholar]
- 48.Danial M., My-Nhi Tran C., Young P.G., Perrier S., Jolliffe K.A. Janus cyclic peptide–polymer nanotubes. Nat Commun. 2013;4(1) doi: 10.1038/ncomms3780. [DOI] [PubMed] [Google Scholar]
- 49.Qin S.-Y., Jiang H.-F., Liu X.-J., Pei Y.i., Cheng H., Sun Y.-X., et al. High length-diameter ratio nanotubes self-assembled from a facial cyclopeptide. Soft Matter. 2014;10(7):947. doi: 10.1039/c3sm52730a. [DOI] [PubMed] [Google Scholar]
- 50.Fujimura F., Fukuda M., Sugiyama J., Morita T., Kimura S. Parallel assembly of dipolar columns composed of a stacked cyclic tri-β-peptide. Org Biomol Chem. 2006;4(10):1896–1901. doi: 10.1039/b600407e. [DOI] [PubMed] [Google Scholar]
- 51.Tabata Y., Takagaki K., Uji H., Kimura S. Piezoelectric property of bundled peptide nanotubes stapled by bis-cyclic-β-peptide. J Peptide Sci. 2019;25(1):e3134. doi: 10.1002/psc.v25.1. [DOI] [PubMed] [Google Scholar]
- 52.Gudipati S., Zhang K.e., Rouge J.L. Towards self-transfecting nucleic acid nanostructures for gene regulation. Trends Biotechnol. 2019;37(9):983–994. doi: 10.1016/j.tibtech.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Yang J.-M., Hu X.-W., Liu Y.-X., Zhang W. Fabrication of a carbon quantum dots-immobilized zirconium-based metal-organic framework composite fluorescence sensor for highly sensitive detection of 4-nitrophenol. Micropor Mesopor Mat. 2019;274:149–154. [Google Scholar]
- 54.Fontana F., Gelain F. Probing mechanical properties and failure mechanisms of fibrils of self-assembling peptides. Nanoscale Adv. 2020;2(1):190–198. doi: 10.1039/c9na00621d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chakraborty P., Tang Y., Yamamoto T., Yao Y., Guterman T., Zilberzwige‐Tal S., et al. Unusual two-step assembly of a minimalistic dipeptide-based functional hypergelator. Adv Mater. 2020;32(9):1906043. doi: 10.1002/adma.v32.9. [DOI] [PubMed] [Google Scholar]
- 56.Chen Y., Gan H.X., Tong Y.W. pH-controlled hierarchical self-assembly of peptide amphiphile. Macromolecules. 2015;48(8):2647–2653. [Google Scholar]
- 57.Tantakitti F., Boekhoven J., Wang X., Kazantsev R., Yu T., Li J., et al. Energy landscapes and function of supramolecular systems. Nat Mater. 2016;15:469–476. doi: 10.1038/nmat4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu I.W., Markegard C.B., Nguyen H.D. Solvent effects on kinetic mechanisms of self-assembly by peptide amphiphiles via molecular dynamics simulations. Langmuir. 2015;31(1):315–324. doi: 10.1021/la503399x. [DOI] [PubMed] [Google Scholar]
- 59.Li J., Chen Z., Zhou M., Jing J., Li W., Wang Y., et al. Polyoxometalate-driven self-assembly of short peptides into multivalent nanofibers with enhanced antibacterial activity. Angew Chem. 2016;128(7):2638–2641. doi: 10.1002/anie.201511276. [DOI] [PubMed] [Google Scholar]
- 60.Yang Z., Xu H., Zhao X. Designer self-assembling peptide hydrogels to engineer 3D cell microenvironments for cell constructs formation and precise oncology remodeling in ovarian cancer. Adv Sci. 2020;7(9):1903718. doi: 10.1002/advs.v7.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Okuno N., Otsuki S., Aoyama J.o., Nakagawa K., Murakami T., Ikeda K., et al. Feasibility of a self-assembling peptide hydrogel scaffold for meniscal defect: an in vivo study in a rabbit model. J Orthop Res. 2021;39(1):165–176. doi: 10.1002/jor.24841. [DOI] [PubMed] [Google Scholar]
- 62.Jiang L., Xu D., Sellati T.J., Dong H.e. Self-assembly of cationic multidomain peptide hydrogels: supramolecular nanostructure and rheological properties dictate antimicrobial activity. Nanoscale. 2015;7(45):19160–19169. doi: 10.1039/c5nr05233e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pochan D.J., Schneider J.P., Kretsinger J., Ozbas B., Rajagopal K., Haines L. Thermally reversible hydrogels via intramolecular folding and consequent self-assembly of a de novo designed peptide. J Am Chem Soc. 2003;125(39):11802–11803. doi: 10.1021/ja0353154. [DOI] [PubMed] [Google Scholar]
- 64.Haines L.A., Rajagopal K., Ozbas B., Salick D.A., Pochan D.J., Schneider J.P. Light-activated hydrogel formation via the triggered folding and self-assembly of a designed peptide. J Am Chem Soc. 2005;127(48):17025–17029. doi: 10.1021/ja054719o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moore A.N., Hartgerink J.D. Self-assembling multidomain peptide nanofibers for delivery of bioactive molecules and tissue regeneration. Accounts Chem Res. 2017;50(4):714–722. doi: 10.1021/acs.accounts.6b00553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shaikh H., Rho J.Y., Macdougall L.J., Gurnani P., Lunn A.M., Yang J., et al. Hydrogel and organogel formation by hierarchical self-assembly of cyclic peptides nanotubes. Chem-Eur J. 2018;24(71):19066–19074. doi: 10.1002/chem.201804576. [DOI] [PubMed] [Google Scholar]
- 67.Xing P., Zhao Y. Multifunctional nanoparticles self-assembled from small organic building blocks for biomedicine. Adv Mater. 2016;28(34):7304–7339. doi: 10.1002/adma.201600906. [DOI] [PubMed] [Google Scholar]
- 68.Eskandari S., Guerin T., Toth I., Stephenson R.J. Recent advances in self-assembled peptides: implications for targeted drug delivery and vaccine engineering. Adv Drug Deliver Rev. 2017;110-111:169–187. doi: 10.1016/j.addr.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 69.Gudlur S., Sukthankar P., Gao J., Avila L.A., Hiromasa Y., Chen J., et al. Peptide nanovesicles formed by the self-assembly of branched amphiphilic peptides. PLoS ONE. 2012;7(9):e45374. doi: 10.1371/journal.pone.0045374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fatouros D.G., Lamprou D.A., Urquhart A.J., Yannopoulos S.N., Vizirianakis I.S., Zhang S., et al. Lipid-like self-assembling peptide nanovesicles for drug delivery. ACS Appl Mater Inter. 2014;6(11):8184–8189. doi: 10.1021/am501673x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang C., Chen X.u., Lu Y., Yang H., Yang W. Electrogenerated chemiluminescence behavior of peptide nanovesicle and its application in sensing dopamine. Biosens Bioelectron. 2015;63:478–482. doi: 10.1016/j.bios.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 72.Tong L.u., Wang Z., Xia C., Yang Y., Yuan S., Sun D.i., et al. Self-assembly of peptide-polyoxometalate hybrid sub-micrometer spheres for photocatalytic degradation of methylene blue. J Physical Chem B. 2017;121(46):10566–10573. doi: 10.1021/acs.jpcb.7b07100. [DOI] [PubMed] [Google Scholar]
- 73.Scelsi A., Bochicchio B., Smith A., Saiani A., Pepe A. Nanospheres from the self-assembly of an elastin-inspired triblock peptide. RSC Adv. 2015;5(115):95007–95013. [Google Scholar]
- 74.Matsuura K., Mizuguchi Y., Kimizuka N. Peptide nanospheres self-assembled from a modified β-annulus peptide of Sesbania mosaic virus. Peptide Sci. 2016;106:470–475. doi: 10.1002/bip.22774. [DOI] [PubMed] [Google Scholar]
- 75.Bissette A.J., Fletcher S.P. Mechanisms of autocatalysis. Angew Chem Int Ed. 2013;52(49):12800–12826. doi: 10.1002/anie.201303822. [DOI] [PubMed] [Google Scholar]
- 76.Brea R.J., Devaraj N.K. Continual reproduction of self-assembling oligotriazole peptide nanomaterials. Nat Commun. 2017;8:730. doi: 10.1038/s41467-017-00849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang H., Feng Z., Xu B. D-amino acid-containing supramolecular nanofibers for potential cancer therapeutics. Adv Drug Deliver Rev. 2017;110-111:102–111. doi: 10.1016/j.addr.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X., Zhou L., Wei Y., El-Toni A.M., Zhang F., Zhao D. Anisotropic encapsulation-induced synthesis of asymmetric single-hole mesoporous nanocages. J Am Chem Soc. 2015;137(18):5903–5906. doi: 10.1021/jacs.5b03207. [DOI] [PubMed] [Google Scholar]
- 79.Wang J., Shen G., Ma K., Jiao T., Liu K., Yan X. Dipeptide concave nanospheres based on interfacially controlled self-assembly: from crescent to solid. Phys Chem Chem Phys. 2016;18(45):30926–30930. doi: 10.1039/c6cp06150h. [DOI] [PubMed] [Google Scholar]
- 80.Gilboa B., Lafargue C., Handelman A., Shimon L.J.W., Rosenman G., Zyss J., et al. Strong electro-optic effect and spontaneous domain formation in self-assembled peptide structures. Adv Sci. 2017;4(9):1700052. doi: 10.1002/advs.201700052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tao K., Hu W., Xue B., Chovan D., Brown N., Shimon L.J.W., et al. Bioinspired stable and photoluminescent assemblies for power generation. Adv Mater. 2019;31(12):1807481. doi: 10.1002/adma.v31.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sun B., Tao K., Jia Y.i., Yan X., Zou Q., Gazit E., et al. Photoactive properties of supramolecular assembled short peptides. Chem Soc Rev. 2019;48(16):4387–4400. doi: 10.1039/c9cs00085b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tao K., Makam P., Aizen R., Gazit E. Self-assembling peptide semiconductors. Science. 2017;358(6365) doi: 10.1126/science:aam9756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amdursky N., Molotskii M., Aronov D., Adler-Abramovich L., Gazit E., Rosenman G. Blue luminescence based on quantum confinement at peptide nanotubes. Nano Lett. 2009;9(9):3111–3115. doi: 10.1021/nl9008265. [DOI] [PubMed] [Google Scholar]
- 85.Tao K., Yoskovitz E., Adler-Abramovich L., Gazit E. Optical property modulation of Fmoc group by pH-dependent self-assembly. RSC Adv. 2015;5(90):73914–73918. [Google Scholar]
- 86.Hauser C.A.E., Zhang S. Nanotechnology: peptides as biological semiconductors. Nature. 2010;468(7323):516–517. doi: 10.1038/468516a. [DOI] [PubMed] [Google Scholar]
- 87.Amdursky N., Molotskii M., Gazit E., Rosenman G. Elementary building blocks of self-assembled peptide nanotubes. J Am Chem Soc. 2010;132(44):15632–15636. doi: 10.1021/ja104373e. [DOI] [PubMed] [Google Scholar]
- 88.Apter B., Lapshina N., Handelman A., Fainberg B.D., Rosenman G. Peptide nanophotonics: from optical waveguiding to precise medicine and multifunctional biochips. Small. 2018;14(34):1801147. doi: 10.1002/smll.v14.34. [DOI] [PubMed] [Google Scholar]
- 89.Yan X., Li J., Möhwald H. Self-assembly of hexagonal peptide microtubes and their optical waveguiding. Adv Mater. 2011;23(25):2796–2801. doi: 10.1002/adma.201100353. [DOI] [PubMed] [Google Scholar]
- 90.Li Q.i., Ma H., Wang A., Jia Y.i., Dai L., Li J. Self-assembly of cationic dipeptides forming rectangular microtubes and microrods with optical waveguiding properties. Adv Opti Mater. 2015;3(2):194–198. [Google Scholar]
- 91.Yan X., Su Y., Li J., Früh J., Möhwald H. Uniaxially oriented peptide crystals for active optical waveguiding. Angew Chem Int Ed. 2011;50(47):11186–11191. doi: 10.1002/anie.201103941. [DOI] [PubMed] [Google Scholar]
- 92.Zou Q., Liu K., Abbas M., Yan X. Peptide-modulated self-assembly of chromophores toward biomimetic light-harvesting nanoarchitectonics. Adv Mater. 2016;28(6):1031–1043. doi: 10.1002/adma.201502454. [DOI] [PubMed] [Google Scholar]
- 93.Zhao C., Chen Z., Shi R., Yang X., Zhang T. Recent advances in conjugated polymers for visible-light-driven water splitting. Adv Mater. 2020;32(28):1907296. doi: 10.1002/adma.v32.28. [DOI] [PubMed] [Google Scholar]
- 94.Charalambidis G., Georgilis E., Panda M.K., Anson C.E., Powell A.K., Doyle S., et al. A switchable self-assembling and disassembling chiral system based on a porphyrin-substituted phenylalanine-phenylalanine motif. Nat Commun. 2016;7(1) doi: 10.1038/ncomms12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zou Q., Zhang L.u., Yan X., Wang A., Ma G., Li J., et al. Multifunctional porous microspheres based on peptide-porphyrin hierarchical co-assembly. Angew Chem Int Ed. 2014;53(9):2366–2370. doi: 10.1002/anie.201308792. [DOI] [PubMed] [Google Scholar]
- 96.Ko J.W., Choi W.S., Kim J., Kuk S.K., Lee S.H., Park C.B. Self-assembled peptide-carbon nitride hydrogel as a light-responsive scaffold material. Biomacromolecules. 2017;18(11):3551–3556. doi: 10.1021/acs.biomac.7b00889. [DOI] [PubMed] [Google Scholar]
- 97.Tao K., Xue B., Frere S., Slutsky I., Cao Y.i., Wang W., et al. Multiporous supramolecular microspheres for artificial photosynthesis. Chem Mater. 2017;29(10):4454–4460. doi: 10.1021/acs.chemmater.7b00966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Subash S.P., Chand P., Pavithra S., Balaji S., Pal S. Pesticide use in Indian agriculture: trends. Market Struct Policy Issues. 2017 [Google Scholar]
- 99.Fang R., Chen G.-H., Yi L.-X., Shao Y.-X., Zhang L.i., Cai Q.-H., et al. Determination of eight triazine herbicide residues in cereal and vegetable by micellar electrokinetic capillary chromatography with on-line sweeping. Food Chem. 2014;145:41–48. doi: 10.1016/j.foodchem.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 100.Luo Q.J., Li Z.G., Lai J.H., Li F.Q., Qiu P., Wang X.L. An on–off–on gold nanocluster-based fluorescent probe for sensitive detection of organophosphorus pesticides. RSC Adv. 2017;7(87):55199–55205. [Google Scholar]
- 101.Fahimi-Kashani N., Rashti A., Hormozi-Nezhad M.R., Mahdavi V. MoS2 quantum-dots as a label-free fluorescent nanoprobe for the highly selective detection of methyl parathion pesticide. Anal Method. 2017;9(4):716–723. [Google Scholar]
- 102.Xu X.-Y., Yan B., Lian X. Wearable glove sensor for non-invasive organophosphorus pesticide detection based on a double-signal fluorescence strategy. Nanoscale. 2018;10(28):13722–13729. doi: 10.1039/c8nr03352h. [DOI] [PubMed] [Google Scholar]
- 103.Korram J., Dewangan L., Karbhal I., Nagwanshi R., Vaishanav S.K., Ghosh K.K., et al. CdTe QD-based inhibition and reactivation assay of acetylcholinesterase for the detection of organophosphorus pesticides. RSC Adv. 2020;10(41):24190–24202. doi: 10.1039/d0ra03055d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bala R., Dhingra S., Kumar M., Bansal K., Mittal S., Sharma R.K., et al. Detection of organophosphorus pesticide-malathion in environmental samples using peptide and aptamer based nanoprobes. Chem Eng J. 2017;311:111–116. [Google Scholar]
- 105.Wang J., Zhang J., Wang J., Fang G., Liu J., Wang S. Fluorescent peptide probes for organophosphorus pesticides detection. J Hazard Mater. 2020;389:122074. doi: 10.1016/j.jhazmat.2020.122074. [DOI] [PubMed] [Google Scholar]
- 106.Lu H., Xu S., Liu J. One pot generation of blue and red carbon dots in one binary solvent system for dual channel detection of Cr3+ and Pb2+ based on ion imprinted fluorescence polymers. ACS Sensors. 2019;4(7):1917–1924. doi: 10.1021/acssensors.9b00886. [DOI] [PubMed] [Google Scholar]
- 107.Wang D., Wang Z., Wang X., Zhuang X., Tian C., Luan F., et al. Functionalized copper nanoclusters-based fluorescent probe with aggregation-induced emission property for selective detection of sulfide ions in food additives. J Agric Food Chem. 2020;68(40):11301–11308. doi: 10.1021/acs.jafc.0c04275. [DOI] [PubMed] [Google Scholar]
- 108.Gui S., Huang Y., Zhu Y., Jin Y., Zhao R. Biomimetic sensing system for tracing Pb2+ distribution in living cells based on the metal-peptide supramolecular assembly. ACS Appl Mater Inter. 2019;11(6):5804–5811. doi: 10.1021/acsami.8b19076. [DOI] [PubMed] [Google Scholar]
- 109.Neupane L.N., Oh E.-T., Park H.J., Lee K.-H. Selective and sensitive detection of heavy metal ions in 100% aqueous solution and cells with a fluorescence chemosensor based on peptide using aggregation-induced emission. Anal Chem. 2016;88(6):3333–3340. doi: 10.1021/acs.analchem.5b04892. [DOI] [PubMed] [Google Scholar]
- 110.Hwang G.W., Jeon J., Neupane L.N., Lee K.H. Sensitive ratiometric detection of Al(iii) ions in a 100% aqueous buffered solution using a fluorescent probe based on a peptide receptor. New J Chem. 2018;42:1437–1445. [Google Scholar]
- 111.Donadio G., Di Martino R., Oliva R., Petraccone L., Del Vecchio P., Di Luccia B., et al. A new peptide-based fluorescent probe selective for zinc(ii) and copper(ii) J Mater Chem B. 2016;4:6979–6988. doi: 10.1039/c6tb00671j. [DOI] [PubMed] [Google Scholar]
- 112.Pang X., Wang L., Gao L., Feng H., Kong J., Li L. Multifunctional peptide-based fluorescent chemosensor for detection of Hg2+, Cu2+ and S2− ions. Luminescence. 2019;34(6):585–594. doi: 10.1002/bio.3641. [DOI] [PubMed] [Google Scholar]
- 113.Ma Y., Jin X., Xing Y., Ni G., Peng J. Construction of an NAND logic gate based on molecularly imprinted dual-emission quantum dot composites for the detection of antibiotics. Anal Method. 2019;11(15):2033–2040. [Google Scholar]
- 114.Chen C.-X., Li Y.-H., Zhou Y.-L., Zhang J.-H., Wei Q.-Z., Dai T., et al. Rapidly detecting antibiotics with magnetic nanoparticle coated CdTe quantum dots. RSC Adv. 2020;10(4):1966–1970. doi: 10.1039/c9ra09894a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao N., Liu J.-M., Yang F.-E., Lv S.-W., Wang J., Wang S. Easy green construction of a universal sensing platform based on crystalline polyimide covalent organic frameworks with sensitive fluorescence response to metal ions and antibiotics. ACS Appl Bio Mater. 2021;4(1):995–1002. [Google Scholar]
- 116.Balachandra C., Govindaraju T. Cyclic dipeptide-guided aggregation-induced emission of naphthalimide and its application for the detection of phenolic drugs. J Org Chem. 2020;85(3):1525–1536. doi: 10.1021/acs.joc.9b02580. [DOI] [PubMed] [Google Scholar]
- 117.Sun Y., Qi T., Jin Y., Liang L., Zhao J. A signal-on fluorescent aptasensor based on gold nanoparticles for kanamycin detection. RSC Adv. 2021;11(17):10054–10060. doi: 10.1039/d0ra10602j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ye T., Zhang Z., Yuan M., Cao H., Yin F., Wu X., et al. An all-in-one aptasensor integrating enzyme powered three-dimensional DNA machine for antibiotic detection. J Agric Food Chem. 2020;68(9):2826–2831. doi: 10.1021/acs.jafc.9b08143. [DOI] [PubMed] [Google Scholar]
- 119.Mu F., He J., Fan F., Shi G. Dual-emission fluorescence biosensing of vancomycin based on AIEgen-peptide conjugates and aptamer-modified Au nanoclusters. Anal Chim Acta. 2021;1150:238177. doi: 10.1016/j.aca.2020.12.052. [DOI] [PubMed] [Google Scholar]
- 120.Wang Q.-b., Zhang C.-J., Yu H., Zhang X., Lu Q., Yao J.-S., et al. The sensitive “Turn-on” fluorescence platform of ascorbic acid based on conjugated polymer nanoparticles. Anal Chim Acta. 2020;1097:153–160. doi: 10.1016/j.aca.2019.10.076. [DOI] [PubMed] [Google Scholar]
- 121.Du F., Cheng Z., Wang G., Li M., Lu W., Shuang S., et al. Carbon nanodots as a multifunctional fluorescent sensing platform for ratiometric determination of vitamin B2 and “turn-off” detection of pH. J Agric Food Chem. 2021;69(9):2836–2844. doi: 10.1021/acs.jafc.0c07019. [DOI] [PubMed] [Google Scholar]
- 122.Khatayevich D., Page T., Gresswell C., Hayamizu Y., Grady W., Sarikaya M. Selective detection of target proteins by peptide-enabled graphene biosensor. Small. 2014;10(8):1505–1513. doi: 10.1002/smll.201302188. [DOI] [PubMed] [Google Scholar]
- 123.Gagni P., Romanato A., Bergamaschi G., Bettotti P., Vanna R., Piotto C., et al. A self-assembling peptide hydrogel for ultrarapid 3D bioassays. Nanoscale Adv. 2019;1(2):490–497. doi: 10.1039/c8na00158h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.King P.J.S., Saiani A., Bichenkova E.V., Miller A.F. A de novo self-assembling peptide hydrogel biosensor with covalently immobilised DNA-recognising motifs. Chem Commun. 2016;52(40):6697–6700. doi: 10.1039/c6cc01433j. [DOI] [PubMed] [Google Scholar]
- 125.Kim J.H., Lim S.Y., Nam D.H., Ryu J., Ku S.H., Park C.B. Self-assembled, photoluminescent peptide hydrogel as a versatile platform for enzyme-based optical biosensors. Biosens Bioelectron. 2011;26(5):1860–1865. doi: 10.1016/j.bios.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 126.Berger O.r., Adler-Abramovich L., Levy-Sakin M., Grunwald A., Liebes-Peer Y., Bachar M., et al. Light-emitting self-assembled peptide nucleic acids exhibit both stacking interactions and Watson-Crick base pairing. Nat Nanotechnol. 2015;10(4):353–360. doi: 10.1038/nnano.2015.27. [DOI] [PubMed] [Google Scholar]
- 127.Berger O.r., Yoskovitz E., Adler-Abramovich L., Gazit E. Spectral transition in bio-inspired self-assembled peptide nucleic acid photonic crystals. Adv Mater. 2016;28(11):2195–2200. doi: 10.1002/adma.201504160. [DOI] [PubMed] [Google Scholar]