Abstract
It is widely accepted that some types of learning involve structural and functional changes of hippocampal synapses. Cell adhesion molecules neural cell adhesion molecule (NCAM), its polysialylated form polysialic acid to NCAM (PSA-NCAM), and L1 are prominent modulators of those changes. On the other hand, trace eyeblink conditioning, an associative motor learning task, requires the active participation of hippocampal circuits. However, the involvement of NCAM, PSA-NCAM, and L1 in this type of learning is not fully known. Here, we aimed to investigate the possible time sequence modifications of such neural cell adhesion molecules in the hippocampus during the acquisition of a trace eyeblink conditioning. To do so, the hippocampal expression of NCAM, PSA-NCAM, and L1 was assessed at three different time points during conditioning: after one (initial acquisition), three (partial acquisition), and six (complete acquisition) sessions of the conditioning paradigm. The conditioned stimulus (CS) was a weak electrical pulse separated by a 250-ms time interval from the unconditioned stimuli (US, a strong electrical pulse). An acquisition-dependent regulation of these adhesion molecules was found in the hippocampus. During the initial acquisition of the conditioning eyeblink paradigm (12 h after 1 and 3 days of training), synaptic expression of L1 and PSA-NCAM was transiently increased in the contralateral hippocampus to the paired CS-US presentations, whereas, when the associative learning was completed, such increase disappeared, but a marked and bilateral upregulation of NCAM was found. In conclusion, our findings show a specific temporal pattern of hippocampal CAMs expression during the acquisition process, highlighting the relevance of NCAM, PSA-NCAM, and L1 as learning-modulated molecules critically involved in remodeling processes underlying associative motor-memories formation.
Keywords: classical eyeblink conditioning, synaptosomes, L1, NCAM, PSA-NCAM, hippocampus, associative motor learning
1 Introduction
Acquired motor abilities are believed to be stored in the form of structural and/or functional changes in synaptic efficiency (Hebb, 1949; Kandel, 2001). The neural cell adhesion molecule (NCAM) and the L1, two members of the immunoglobulin superfamily of cell adhesion molecules (CAM), are important constituents of synapses that mediate neuron-neuron adhesion and shape the formation of neuronal networks during brain development and synaptic plasticity throughout adulthood (Duncan et al., 2021). Both molecules also play a key role in regulating the structural synaptic changes underlying learning acquisition and memory formation (Sandi, 2004; Bisaz et al., 2009; Conboy et al., 2010; Hartz and Rønn, 2010; Duncan et al., 2021). In addition, post-translational attachment of polysialic acid to NCAM (PSA-NCAM), a reaction shown to downregulate NCAM adhesion properties (Rutishauser and Landmesser, 1996; Colley, 2010; Hildebrandt et al., 2010), has also been implicated in synaptic remodeling after learning experiences (O’Malley et al., 2000; Kiss et al., 2001; El Maarouf and Rutishauser, 2010; Gascon et al., 2010; Saini et al., 2020).
The role of NCAM, its polysialylated form, and L1 in synaptic plasticity and cognitive processes has been based on several lines of evidence. First, interference with the expression and/or function of these molecules in knock-out mice models or pharmacologically by the administration of antibodies, peptides, enzymes, or antisense oligonucleotides results in impaired long term potentiation (LTP) (Cremer et al., 2000; Eckhardt et al., 2000; Duncan et al., 2021) and learning and memory deficits (Cremer et al., 1994; Arami et al., 1996; Becker et al., 1996; Cambon et al., 2003; Seymour et al., 2008; Bisaz et al., 2009). Second, the converse evidence, i.e., improved LTP and enhanced learning abilities, has been achieved by mimicking NCAM function (Cambon et al., 2004) or by L1 or PSA-NCAM over-expression (Wolfer et al., 1998; Rendeiro et al., 2014; Saini et al., 2020). Third, mRNA and protein expression of NCAM, PSA-NCAM, and L1 in different brain areas is regulated by learning and LTP (Ronn et al., 2000; Hartz and Rønn, 2010; Saini et al., 2020; Duncan et al., 2021). Specifically, the expression of these CAMs is modulated in the hippocampus 1–24 h after LTP induction, by training in a memory task (Fazeli et al., 1994; Schuster et al., 1998; Ronn et al., 2000), or after a variety of hippocampal-dependent training experiences including passive avoidance training (Doyle et al., 1992a; Fox et al., 1995, 2000; Brandewiede et al., 2014), contextual fear conditioning (Merino et al., 2000; Sandi et al., 2003; Maćkowiak et al., 2009), and olfactory (Knafo et al., 2005a,b), and spatial (Sandi, 2004; Venero et al., 2006; Yamagishi et al., 2018) learning tests. Furthermore, pathologies characterized by learning and memory deficits, such as Alzheimer’s disease, major depression, or drug addiction, are related to a decreased expression of these CAMs (Maćkowiak et al., 2009; Gilabert-Juan et al., 2012; Murray et al., 2018).
Classical conditioning of eyelid responses is a well-known and widely used associative motor learning task. In trace eyeblink conditioning paradigms, the conditioned stimulus (CS) is separated from the unconditioned stimulus (US) presentation by a stimulus-free interval (trace), and the hippocampus is thought to play a major role in the formation of this memory trace (Solomon et al., 1986; Gruart et al., 2006, 2015). In fact, acquisition and retention of trace conditioning requires an intact hippocampus (Weiss et al., 1999; Gruart et al., 2015) and it has been demonstrated that hippocampal synaptic strength is modulated by this type of associative learning (Gruart et al., 2006, 2015).
Given the critical role of the hippocampus in associative learning (Gruart et al., 2015), and the established involvement of NCAM, PSA-NCAM, and L1 in some learning processes (Duncan et al., 2021), we aimed to investigate the time sequence of such neural cell-adhesion molecules modifications during the acquisition of a trace eyeblink conditioning in alert behaving rats.
2 Materials and methods
2.1 Animals
Fifty-seven adult male Wistar rats (250–300 g before surgery; Iffa-Credo, France), group housed three per cage, were used for all experiments. Animals were kept in their home cages at 22 ± 2°C and with a 12 h light-dark cycle (lights on at 7:00 a.m.). Food and water were provided ad libitum. All experimental procedures were reviewed and approved by the Ethical Committee for Use of Laboratory Animals of the National Distance Education University (UNED) and conducted according to the European Union guidelines (2010/63/EU) and the Spanish regulations for the use of laboratory animals in chronic experiments (RD 53/2013 on the care of experimental animals: BOE 08/02/2013). All efforts were made to minimize animal suffering.
2.2 Surgery
All surgical procedures were carried out under sodium pentobarbital anesthesia (40 mg/kg i.p.) and using aseptic surgical techniques. Four teflon-coated stainless-steel wires (No. 7910; A-M Systems, US) were implanted in the subcutaneous tissue of the right upper eyelid to be used as bipolar stimulating electrodes of the right supraorbital branch of the trigeminal nerve and as bipolar electromyographic (EMG) recording electrodes in the ipsilateral orbicularis oculi (OO) muscle (Figure 1A). Electrode tips were cleaned of the coating cover for ∼0.5 mm and bent as a hook to facilitate stable insertion in the eyelid tissue. Electrodes were soldered to a four-pin connector that was secured to the skull with stainless-steel screws and a dental acrylic resin. Details of this surgical preparation have been described elsewhere (Jiménez-Díaz et al., 2006; Valenzuela-Harrington et al., 2007). A recovery period of 7–9 days was allowed before any behavioral experiment.
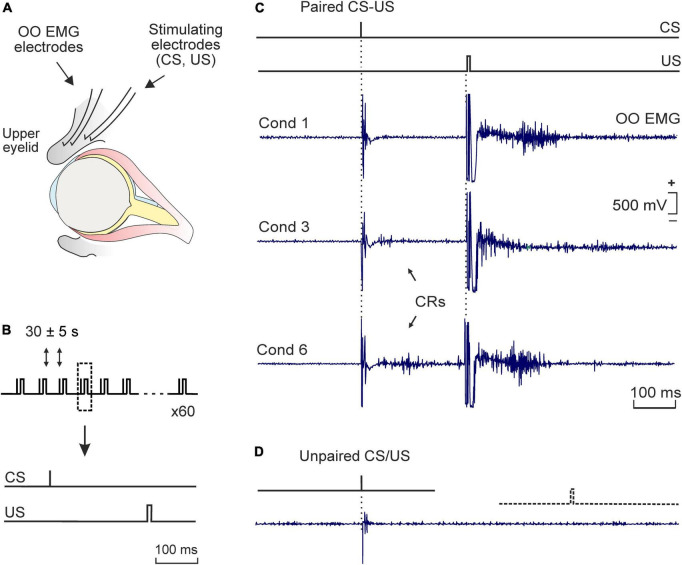
FIGURE 1.
Experimental design. (A) Bipolar hook electrodes were implanted on the right supraorbital nerve for electrical stimulation of this sensory nerve. The electromyographic activity of the ipsilateral orbicularis oculi muscle [orbicularis oculi (OO) electromyographic (EMG)] was also recorded. (B) Each conditioning session consisted of 6 blocks comprising 10 trails per block. The conditioned (CS) and unconditioned stimuli (US) consisted of square, cathodic pulses presented to the supraorbital nerve, lasting 0.05 ms (1.5X threshold) and 0.5 ms (3X threshold), respectively. The CS was followed by the US with an interval of 250 ms. The CS was presented alone the first trial of each block. (C) Representative examples of OO EMG profiles of eyelid responses (in mV) recorded during the 1st, 3rd, and 6th conditioning sessions (cond 1, 3, and 6). Conditioned responses (CRs) are indicated by arrows. (D) A typical eyelid response collected during pseudo-conditioning (i.e., unpaired presentation of the CS and the US). Calibration in panel (C) also applies to panel (D).
2.3 Classical eyeblink conditioning training
Classical conditioning training was developed using an experimental design that allowed the animals to freely move during acquisition sessions (i.e., movement restrictions were avoided and animals freely moved during CS-US presentations in each training session). For this purpose, electrical stimulation of the supraorbital sensory nerve was used as conditioned (CS) and unconditioned (US) stimuli, respectively (Figure 1). Electrical stimulation elicited reflex eyelid movements associated with the presence of reflex responses in the OO EMG that were recorded for learning quantification purposes [i.e., for conditioned responses (CRs) measurement]. For recordings, each rat was placed in a plastic cage (30 × 20 × 20 cm). Prior to behavioral training, animals were handled daily for 2 min during 3 consecutive days and habituated for 2 more min to the specific recording procedure. A tether, containing a low-weight, 4-channel wire (to relay OO EMG activity and to deliver the CS and US stimuli), was connected to the socket implanted on the animal’s head and attached to a 360° rotatory electrical commutator (electrical swivel, Plastics One, US) which allowed the rat to move freely during training.
Classical conditioning was achieved using a trace paradigm (Figure 1B). Briefly, a short (50 μs), weak (∼1.5 × threshold, defined as the minimum intensity able to elicit a small eyelid movement associated with the presence of a reflex response in the OO EMG), square, cathodal pulse was presented as CS. The US consisted of a long (500 μs), strong (∼2–3 × threshold, able to consistently elicit a complete and immediate eyelid closure), square, cathodal pulse. The US started 250 ms after the end of the CS. CS and US intensities (in mA) were experimentally determined for each subject before the beginning of the training and remained unchanged along the conditioning program. In total, two habituation and six conditioning sessions were carried out per animal. During habituation, the CS was presented alone. A conditioning session consisted of 60 paired CS-US presentations, and lasted ∼30 min. In 10% of the cases, the CS was presented alone. CS-US presentations were separated at random by 30 ± 5 s. For control purposes, pseudo-conditioning and context-exposed groups were also included in the experimental design. For pseudo-conditioning, unpaired CS and US presentations were carried out for 6 sessions (60 CS and 60 US per session, presented at random). For context-exposure, rats were placed into the recording chamber for 30 min, in the absence of any stimulation. Further details of this experimental preparation are presented elsewhere (Fontan-Lozano et al., 2005; Inda et al., 2005).
2.4 Electromyography recording
The EMG activity of the OO muscle was recorded using a GRASS P511 differential amplifier with a bandwidth of 1 Hz to 10 kHz (Grass-Telefactor, USA). Data was stored directly on a computer with an analog/digital converter (1401 Plus; Cambridge Electronic Design, UK), at a sampling frequency of 11–22 kHz and an amplitude resolution of 12 bits. Data was analyzed off-line for quantification CRs. The presence of EMG activity during the CS-US period which lasted >10 ms and was initiated >50 ms after CS onset was considered a CR; thus, we avoided including putative alpha responses in the quantification of true CRs. Those recordings presenting EMG activity in the 250 ms preceding CS presentation (change in baseline), or with an evident startle response (response beginning 4 ms after CS and expanded until 100 ms) or artifactual recordings were excluded from the quantitative analysis. The percentage of trials containing a CR in a given session was calculated based on the number of valid trials.
2.5 Tissue preparation
Animals were anesthetized with an overdose of 2,2,2-tribromoethanol (Sigma-Aldrich, US) 12 h after the end of the 1st, 3rd, or 6th training session and decapitated. A total of 4–9 animals per group were used. Immediately after decapitation, brains were kept on ice and both right and left hippocampi (ipsi- and contralateral to stimuli presentation, respectively) were dissected. Tissue samples were coded and stored at −80°C until use. Crude synaptosomal pellets were obtained according to a modified protocol from Lynch and Voss (1991). In brief, tissue was homogenized in ten volumes of ice-cold sucrose (0.32 M) and HEPES (5 mm) buffer that contained a cocktail of protease inhibitors (Complete TM; Boehringer Mannheim, UK) with 16 strokes, and centrifuged at 1,000 g for 5 min. The supernatant was then centrifuged at 15,000 g for 15 min, and the pellet was resuspended in Krebs buffer, containing protease inhibitors, for further use. Protein concentration for each sample was measured by the Bradford method (Bradford, 1976).
2.6 ELISAs
Total NCAM (including all NCAM isoforms), PSA-NCAM, and L1 were quantified by enzyme-linked immunosorbent assays (ELISAs) according to a previously described protocol (Merino et al., 2000). In brief, flat bottom 96 well microplates were allowed to adsorb a coating solution (Na2CO3 0.1 M/NaHCO3, 0.1 M) for 2 h at room temperature (RT). The solution was removed and 50 μl of pellet samples were added at a concentration of 10 μg/ml to each well of polystyrene flat-bottom ELISA plates. Plates were incubated overnight at 4°C and then washed three times with 1 M phosphate-buffered saline (PBS) containing 0.05% Tween 20, pH 7.4. Additional binding sites were blocked with BSA (3%) for 2 h at RT. Wells were rinsed three times as described above, and incubated with 50 μl aliquots of the corresponding first antibody for 20–24 h at 4°C. Then, wells were washed and 50 μl aliquots of peroxidase-conjugated secondary antibody were added for a 2 h incubation period. Afterward, 50 μl of citrate buffer (50 mm Na2HPO4, 25 mm citric acid, pH 4.5) containing 1 mg/ml o-phenylene diamine and 0.06% H2O2, added just before use, were placed in each well, and the peroxidase allowed to react for 10 min at RT. The reaction was terminated by the addition of 50 μl of 10 M H2SO4 to each well. Optical density was determined by measuring absorbance at 492 nm with a Microplate Reader (DigiScan Reader V3.0 and DigiWIN software; ASYS Hitech GmbH, Austria).
The following primary antibodies were used: a polyclonal rabbit anti-rat NCAM IgG (1:300; from Prof. Elisabeth Bock, University of Copenhagen, Denmark); a monoclonal mouse IgM antibody that recognizes specifically a-2–8-linked PSA with chain length superior to 12 residues, and binds with high specificity to PSA on NCAM (1:2 dilution of ascites fluid; diluted 1:300 for the ELISA; Men B, clone B1.2; generous gift from Prof. Genevieve Rougon, CNRS Marseille, France); and a monoclonal rat anti-rat L1 IgG (1:150; Chemicon International, US). As secondary antibodies, an anti-rabbit IgG peroxidase conjugate (whole molecule conjugate; 1:2000; Jackson Immunoresearch, US), an IgM anti-mouse peroxidase conjugate (l chain; 1:1000; Sigma-Aldrich, US), and an anti-rat Ig-POD Fab fragments (from sheep immunoglobulin) to IgG-rat peroxidase conjugated (1:500; Boehringer, UK) were used, respectively.
2.7 Statistical analysis
Data was expressed as mean ± SEM. Statistical differences between percentages of CRs were determined by repeated measures two-way ANOVA, followed by Tukey’s post-hoc analysis. Biochemical data were analyzed with three-way ANOVAs (using training, sessions, and hippocampal side as fixed factors), followed by Tukey’s post-hoc analysis. Statistical significance was set at p < 0.05. If the Levene’s test for normal distribution was significant then data were normalized by square root transformation (percentage of conditioned responses). All analyses were performed using the SPSS software v.28 (RRID:SCR_002865; IBM, US) and final figures were prepared using CorelDraw X8 Software (RRID:SCR_014235; Corel Corporation, Canada).
3 Results
3.1 Classical eyeblink conditioning acquisition
Animals were randomly assigned to one of the following three experimental paradigms: classical eyeblink conditioning (paired group, n = 26), pseudo-conditioning (unpaired group, n = 18), or context exposure without stimulation (context group, n = 13). Animals were sacrificed after the 1st (paired group n = 8; unpaired group n = 6; context group n = 4), 3rd (paired group n = 9; unpaired group n = 6; context group n = 5), or 6th (paired group n = 9; unpaired group n = 6; context group n = 4) training sessions.
For conditioned and pseudo-conditioned animals, we first confirmed that electrode implantation in the upper eyelid did not disturb reflexively evoked eyeblink responses. We found that the electrical stimulation (2X threshold) of the supraorbital nerve evoked an early (6–7 ms) activation of the OO muscle, followed by a second, more variable (15–25 ms) EMG activation (not illustrated). These successive muscle activations corresponded to the R1 and R2 components previously described in rats (Valenzuela-Harrington et al., 2007), as well as humans (Kugelberg, 1952), cats (Gruart et al., 1995), and mice (Dominguez-Del-Toro et al., 2004).
Then the learning capabilities of conditioned and pseudo-conditioned rats were compared using a trace conditioning paradigm. As illustrated in Figure 1C, conditioned eyelid responses reaching criterion were evident by the 3rd conditioning session, although they were already frequently observed during the second session (Figure 2A). Data showed that conditioned animals presented the normal learning curve previously described in rodents when using a similar trace conditioning procedure (Gruart et al., 2006; Valenzuela-Harrington et al., 2007; Fernandez-Lamo et al., 2009). Animals sacrificed after one conditioning session (initial acquisition) presented a mean of 14 ± 3% CRs, while those sacrificed after the third session (partial acquisition) had already reached a mean of 38 ± 5% CRs. Animals trained for six sessions (complete acquisition) had reached asymptotic values of 74 ± 6% CRs (Figure 2A). By contrast, pseudo-conditioned animals (i.e., those receiving the unpaired presentation of the stimuli used as CS and US; Figure 1D) presented extremely poor learning performances, showing a low rate (<8%) of eyelid responses that fulfilled the criterion set for OO EMG to be considered as CRs (Figures 1D, 2A). When comparing the percentage of CRs obtained from conditioned versus pseudo-conditioned control animals, a two-way ANOVA showed a significant training difference [F(1,8) = 318.802; p < 0.001; Figure 2A], and post-hoc analysis revealed that the different was present in all training sessions except the first one.
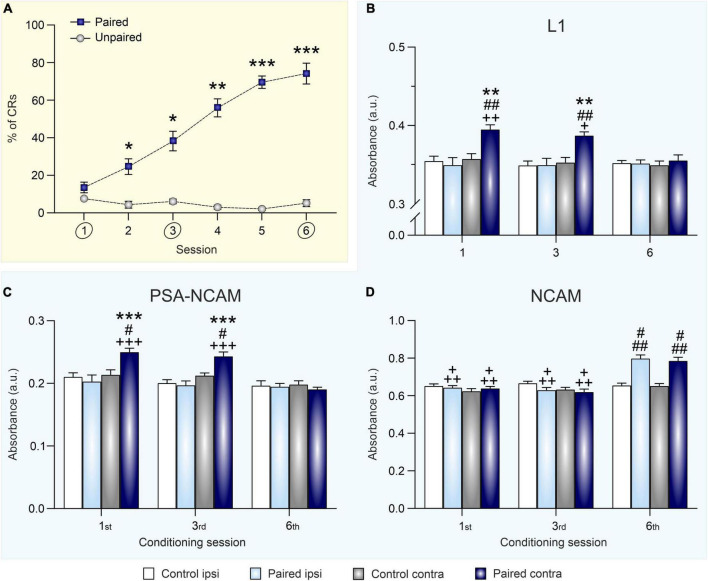
FIGURE 2.
Eyeblink conditioning acquisition modulates synaptic expression of L1, PSA-NCAM, and NCAM in the hippocampus. (A) Percentage of conditioned eyelid responses [conditioned responses (CRs)] across six conditioning sessions. Data is expressed as mean ± SEM of animals sacrificed at the end of the sixth session. *p < 0.05, **p < 0.01, ***p < 0.001 vs. unpaired. (B–D) Optical density measurements of L1 (B), PSA-NCAM (C), and NCAM (D) expression, after the 1st, 3rd, and 6th training sessions, in hippocampal synaptosomes obtained from right (ipsilateral to CS and US presentation) and left (contralateral) hippocampi of conditioned and control (merged from unpaired and context groups) animals. Data is expressed as mean ± SEM for each session (1st: paired group n = 8 and control group n = 10; 3rd: paired group n = 9 and control group n = 11; 6th: paired group n = 9, and control group n = 10). a.u., arbitrary units. **p < 0.01, ***p < 0.001 vs. ipsi; #p < 0.05, ##p < 0.01 vs. control; +p < 0.05, + +p < 0.01, + + +p < 0.001 vs. session 6.
3.2 Hippocampal synaptic NCAM, PSA-NCAM, and L1 expression during classical eyeblink conditioning is acquisition-dependent
In order to evaluate the effect of classical eyeblink conditioning on the expression of neural cell adhesion molecules in the hippocampus, expression levels of NCAM, PSA-NCAM, and L1 in hippocampal crude synaptosomes were determined 12 h after the 1st (initial acquisition), 3rd (partial acquisition), and 6th conditioning sessions (complete acquisition) (Figures 2B–D).
The two control groups submitted to either pseudo-conditioning (unpaired CS/US training) or context exposure (non CS or US stimulation) did not significantly differ in neither their synaptic ipsi- nor the contralateral hippocampal NCAM [F(1,42) = 1.967; p = 0.168], PSA-NCAM [F(1,50) = 0.553; p = 0.461], or L1 [F(1,48) = 0.066; p = 0.79] expression levels at any of the training session studied (Supplementary Table 1). Thus, for presentation and statistical purposes, Figures 2B–D show the merge data from pseudo-conditioned and context groups, as data obtained from both control groups were equivalent at all testing times.
Regarding L1 levels (Figure 2B), a three-way ANOVA showed a significant training effect [F(1,98) = 11.00; p = 0.0013] and post-hoc analysis indicated that it was specifically an upregulation of L1 expression in the contralateral hippocampus of conditioned rats after sessions one and three. Moreover, there was also an hippocampal side effect [F(1,98) = 16.92; p < 0.0001], since that increase in the contralateral hippocampus was not present in the ipsilateral area, and a session effect [F(2,98) = 3.671; p = 0.029] as the difference between groups was lost in the last session. Thus, L1 levels seems to be regulated by conditioning learning in an acquisition-dependent way in the contralateral hippocampus, being overexpressed specifically in this side during initial and partial learning acquisition but not when the conditioning was completely acquired.
A similar pattern of learning-induced modulation was found for synaptic PSA-NCAM levels in the paired (i.e., conditioned) group (Figure 2C). Conditioning significantly upregulated PSA-NCAM expression [training effect: F(1,102) = 4.113; p = 0.0452] in the contralateral hippocampus after one and three training sessions. Once again, there was a significant side effect [F(1,102) = 21.51; p < 0.0001], specifically in the contralateral hippocampus. After one and three conditioning session PSA-NCAM values were enhanced, but they did not differ to control ones after six sessions [session effect: F(2,102) = 14.59; p < 0.0001]. As it happened with L1, PSA-NCAM was overexpressed in the contralateral hippocampus during the initial and partial acquisition sessions but decreased to normal values after complete conditioning acquisition.
Finally, total NCAM expression in hippocampal crude synaptosomes, as assessed by ELISA using an antibody that recognizes all NCAM isoforms, was bilaterally increased 12 h after submitting rats to six conditioning sessions [Figure 2D; session effect: F(2,91) = 47.53; p < 0.0001]. The regulation was not only session-dependent, but also training-dependent. Hence, synaptic NCAM levels from conditioned rats were higher than those of both pseudo-conditioned and context-exposed control groups after the sixth conditioning session, when asymptotic learning levels had been reached [training effect: F(1,91) = 21.23; p < 0.0001]. As shown in Figure 2D, NCAM levels from conditioned rats were equivalent between both hippocampi across conditioning [side effect: F(1,91) = 3.195; p = 0.0772]. Therefore, NCAM seems to be bilaterally upregulated by conditioning learning only after complete acquisition of the association.
4 Discussion
4.1 Cell adhesion molecules upregulation is specific to conditioned rats
The hippocampus has been one of the brain areas tightly related to the acquisition of new motor abilities. Many studies have proven that this area facilitates, reinforces, and organizes the proper achievement of CRs. According to available information, the full integrity of the hippocampus is needed in order to acquire the conditioning (Weiss et al., 1999; Gruart et al., 2015).
In the present work, classical eyeblink conditioning in rats was achieved using a trace paradigm in which both the CS and the US were electric pulses. Although rats in both paired (conditioned) and unpaired (pseudo-conditioned) groups were presented with CS and US electrical stimuli, only rats assigned to the paired group did establish an association between both stimuli and therefore learned the task (Figures 1C, D, 2A), achieving a CRs ratio over 80% from the fifth session on, slightly faster than in other earlier reports (Weiss et al., 1999). This accelerated acquisition may be related to the type of stimulus chosen, since most studies use a sound as CS, and we have used an electric pulse. The use of sound instead of proprioceptive stimulus seems to delay CR acquisition (Múnera et al., 2001). Moreover, our data showed a gradual decrease in the latencies to start the CRs, along with an increase in the duration and amplitude of the response, in line with others (Weiss et al., 1999; Stanton, 2000; Rogers et al., 2001).
Then, to evaluate the effect of classical eyeblink conditioning on the expression of cell adhesion molecules in the hippocampus, levels of L1, PSA-NCAM, and NCAM in hippocampal crude synaptosomes were determined. A few rodent studies have evaluated the possible regulation on NCAM, its polysialylation and/or L1 by hippocampal-dependent training experiences (Sandi et al., 2003; Maćkowiak et al., 2009; Brandewiede et al., 2014; Yamagishi et al., 2018) but none of them included associative motor learning protocols, despite the great attention devoted by neurobiological studies to this type of learning. Our results indicate that L1, NCAM, and PSA-NCAM expression was only modulated when exposure to electrical stimuli was accompanied by associative motor learning. Although stress have been shown to modulate these molecules (Sandi, 2004; Bisaz et al., 2009; Pesarico et al., 2019), as no differences were observed between context-exposed and pseudo-trained groups, the observed expression changes cannot be attributed to context nor to the stress that may accompany eyelid electric stimulation or motor activity within the arena.
4.2 Transient nature of hippocampal learning-induced cell adhesion molecules upregulation
Additionally, to determine the evolution of the expression of these molecules throughout the learning curve, the measurement was carried out after 1st (initial acquisition), 3rd (partial acquisition), and 6th (complete acquisition) conditioning sessions.
We report here that L1 and PSA-NCAM upregulation is transient in the hippocampus. Thus, both CAMs were overexpressed after first and third sessions in the contralateral hippocampus of the conditioned animals compared to both its ipsilateral hippocampus and the hippocampi of the control group. However, after the 6th conditioning session both L1 and PSA-NCAM returned to basal levels. This finding is in accordance with other studies relating learning to changes in cell adhesion molecules (Foley et al., 2003a,b; Knafo et al., 2005a,b) or the number of neurons expressing these molecules (Fox et al., 1995, 2000). It has been suggested that the post-translational attachment of PSA-NCAM may contribute to decrease synaptic adhesion, which allows for the synaptic remodeling observed during learning and memory formation (Benson et al., 2000; Ronn et al., 2000). Furthermore, the deletion of PSA in the hippocampus is deleterious for spatial learning in rats (Becker et al., 1996), hence, it is essential for proper memory formation. Our result is in accordance with other studies that had shown a transitory upregulation of hippocampal PSA after different types of learning (Doyle et al., 1992b; Fox et al., 1995; Murphy et al., 1996; O’Connell et al., 1997; Lopez-Fernandez et al., 2007; Rendeiro et al., 2014). Regarding L1, this CAM has a role in different processes such as neurite growing, axonal fasciculation, cell migration, and CNS repair (Jucker et al., 1996; Schachner, 1997). Likewise, after a fear conditioning training, L1 expression is enhanced in the hippocampus (Merino et al., 2000; Sandi et al., 2001), suggesting a relationship between this molecule and some types of learning (Scholey et al., 1993; Arami et al., 1996; Knafo et al., 2005b) as shown by our results. Interestingly, during initial acquisition, the hippocampus showed alterations of these CAMs in the contralateral side to where the US was presented. This finding agrees with the fact that conditioned responses are preferentially formed on the side of US presentation (Gruart et al., 1995; Jiménez-Díaz et al., 2006) and molecular/cellular/synaptic changes induced by conditioning are found mainly in the contralateral (to the US) brain region studied, including the hippocampus (Gruart et al., 2000, 2015; Jiménez-Díaz et al., 2006).
On the other hand, our data showed a completely different pattern for NCAM expression. During the initial and partial conditioning, there were no alterations of its levels, while after conditioning completion (six sessions), a bilateral enhancement of NCAM was observed in the paired group compared to control animals. Previous studies have related NCAM with the progression from a labile memory to a stable, consolidated memory (Doyle et al., 1992a; Scholey et al., 1993; Sandi et al., 1995; Merino et al., 2000). Our results are in agreement with that and, being that this molecule main role is to stabilize synapses, this upregulation after the acquisition of the conditioned response may be related to the structural changes induced in the hippocampus by this type of associative learning (Geinisman et al., 2000, 2001), including an increase of the post-synaptic density and of the number of synaptic boutons (Sytnyk et al., 2006), although further experiments are required to fully prove this hypothesis.
Moreover, NCAM is composed of three molecular components with relative molecular masses of 120, 140, and 180 kD. NCAM-120 does not appear to be expressed in synapses, whereas NCAM-140 is located on both pre- and post-synaptic membranes and NCAM-180 is restricted mostly to post-synaptic sites (Persohn et al., 1989; Persohn and Schachner, 1990; Schuster et al., 2001). In the present work, all forms of NCAM were detected, and no differences in any particular component were found (data not shown).
5 Conclusion
In conclusion, the temporal evolution of the three studied adhesion molecules in the hippocampus along the conditioning eyeblink paradigm (i.e., side-specific increase of PSA-NCAM and L1 expression during early/partial acquisition followed by bilateral increase of NCAM levels when the learned response had been acquired) could account for initial facilitation of structural changes during learning acquisition (by PSA-NCAM and L1) and late stabilization of previously restructured synapses to progress from a labile to a stable motor memory (by NCAM). These results also suggest that CAMs are learning-modulated molecules whose expression needs to be orchestrated in a coordinated manner in the hippocampus for the proper acquisition of hippocampal-dependent associative motor learning.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This animal study was reviewed and approved by Ethical Committee for Use of Laboratory Animals of the National Distance Education University (UNED).
Author contributions
LJ-D, JD-G, AG, and CS were responsible for the initial conceptualization. LJ-D, AH, KT, KC, and CV performed the experiments. LJ-D, JN-L, and AC analyzed the data, did the writing—review and editing, and were responsible for writing the original draft. CS contributed to the materials. LJ-D, JN-L, JD-G, and AG were responsible for funding acquisition. All authors contributed to the article and approved the submitted version.
Acknowledgments
We acknowledge S. M. Geranton for her enlightening comments.
Abbreviations
NCAM, neural cell adhesion molecule; LTP, long term potentiation; CS, conditioned stimulus; US, unconditioned stimulus; PSA, polysialic acid; EMG, electromyographic; OO, orbicularis oculi; CR, conditioned response; Ipsi, ipsilateral; Contra, contralateral.
Funding
This work was supported by grants PID2020-115823-GB100 funded by MCIN/AEI/10.13039/501100011033 and SBPLY/21/180501/000150 funded by JCCM and ERDF/A way of making Europe, to LJ-D and JN-L; PID2021-122446NB-100 to AG and JD-G. AC held a Margarita Salas Post-doctoral Research Fellow funded by European Union NextGenerationEU/PRTR.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnana.2022.1082701/full#supplementary-material
References
- Arami S., Jucker M., Schachner M., Welzl H. (1996). The effect of continuous intraventricular infusion of L1 and NCAM antibodies on spatial learning in rats. Behav. Brain Res. 81 81–87. 10.1016/s0166-4328(96)00046-0 [DOI] [PubMed] [Google Scholar]
- Becker C. G., Artola A., Gerardy-Schahn R., Becker T., Welzl H., Schachner M. (1996). The polysialic acid modification of the neural cell adhesion molecule is involved in spatial learning and hippocampal long-term potentiation. J. Neurosci. Res. 45 143–152. [DOI] [PubMed] [Google Scholar]
- Benson D. L., Schnapp L. M., Shapiro L., Huntley G. W. (2000). Making memories stick: cell-adhesion molecules in synaptic plasticity. Trends Cell. Biol. 10 473–482. 10.1016/s0962-8924(00)01838-9 [DOI] [PubMed] [Google Scholar]
- Bisaz R., Conboy L., Sandi C. (2009). Learning under stress: a role for the neural cell adhesion molecule NCAM. Neurobiol. Learn. Mem. 91 333–342. 10.1016/j.nlm.2008.11.003 [DOI] [PubMed] [Google Scholar]
- Bradford M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 248–254. [DOI] [PubMed] [Google Scholar]
- Brandewiede J., Stork O., Schachner M. (2014). NCAM deficiency in the mouse forebrain impairs innate and learned avoidance behaviours. Genes Brain Behav. 13 468–477. 10.1111/gbb.12138 [DOI] [PubMed] [Google Scholar]
- Cambon K., Hansen S. M., Venero C., Herrero A. I., Skibo G., Berezin V., et al. (2004). A synthetic neural cell adhesion molecule mimetic peptide promotes synaptogenesis, enhances presynaptic function, and facilitates memory consolidation. J. Neurosci. 24 4197–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambon K., Venero C., Berezin V., Bock E., Sandi C. (2003). Post-training administration of a synthetic peptide ligand of the neural cell adhesion molecule, C3d, attenuates long-term expression of contextual fear conditioning. Neuroscience 122 183–191. 10.1016/s0306-4522(03)00597-9 [DOI] [PubMed] [Google Scholar]
- Colley K. J. (2010). Structural basis for the polysialylation of the neural cell adhesion molecule. Adv. Exp. Med. Biol. 663 111–126. [DOI] [PubMed] [Google Scholar]
- Conboy L., Bisaz R., Markram K., Sandi C. (2010). Role of NCAM in emotion and learning. Adv. Exp. Med. Biol. 663 271–296. [DOI] [PubMed] [Google Scholar]
- Cremer H., Chazal G., Lledo P. M., Rougon G., Montaron M. F., Mayo W., et al. (2000). PSA-NCAM: an important regulator of hippocampal plasticity. Int. J. Dev. Neurosci. 18 213–220. [DOI] [PubMed] [Google Scholar]
- Cremer H., Lange R., Christoph A., Plomann M., Vopper G., Roes J., et al. (1994). Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature 367 455–459. 10.1038/367455a0 [DOI] [PubMed] [Google Scholar]
- Dominguez-Del-Toro E., Rodriguez-Moreno A., Porras-Garcia E., Sanchez-Campusano R., Blanchard V., Laville M., et al. (2004). An in vitro and in vivo study of early deficits in associative learning in transgenic mice that over-express a mutant form of human APP associated with Alzheimer’s disease. Eur. J. Neurosci. 20 1945–1952. 10.1111/j.1460-9568.2004.03643.x [DOI] [PubMed] [Google Scholar]
- Doyle E., Nolan P. M., Bell R., Regan C. M. (1992a). Hippocampal NCAM180 transiently increases sialylation during the acquisition and consolidation of a passive avoidance response in the adult rat. J. Neurosci. Res. 31 513–523. 10.1002/jnr.490310315 [DOI] [PubMed] [Google Scholar]
- Doyle E., Nolan P. M., Bell R., Regan C. M. (1992b). Intraventricular infusions of anti-neural cell adhesion molecules in a discrete posttraining period impair consolidation of a passive avoidance response in the rat. J. Neurochem. 59 1570–1573. 10.1111/j.1471-4159.1992.tb08477.x [DOI] [PubMed] [Google Scholar]
- Duncan B. W., Murphy K. E., Maness P. F. (2021). Molecular mechanisms of L1 and NCAM adhesion molecules in synaptic pruning, plasticity, and stabilization. Front. Cell Dev. Biol. 9:625340. 10.3389/fcell.2021.625340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhardt M., Bukalo O., Chazal G., Wang L., Goridis C., Schachner M., et al. (2000). Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J. Neurosci. 20 5234–5244. 10.1523/JNEUROSCI.20-14-05234.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maarouf A., Rutishauser U. (2010). Use of PSA-NCAM in repair of the central nervous system. Adv. Exp. Med. Biol. 663 137–147. [DOI] [PubMed] [Google Scholar]
- Fazeli M. S., Breen K., Errington M. L., Bliss T. V. (1994). Increase in extracellular NCAM and amyloid precursor protein following induction of long-term potentiation in the dentate gyrus of anaesthetized rats. Neurosci. Lett. 169 77–80. 10.1016/0304-3940(94)90360-3 [DOI] [PubMed] [Google Scholar]
- Fernandez-Lamo I., Montero-Pedrazuela A., Delgado-Garcia J. M., Guadano-Ferraz A., Gruart A. (2009). Effects of thyroid hormone replacement on associative learning and hippocampal synaptic plasticity in adult hypothyroid rats. Eur. J. Neurosci. 30 679–692. 10.1111/j.1460-9568.2009.06862.x [DOI] [PubMed] [Google Scholar]
- Foley A. G., Hedigan K., Roullet P., Moricard Y., Murphy K. J., Sara S. J., et al. (2003a). Consolidation of memory for odour-reward association requires transient polysialylation of the neural cell adhesion molecule in the rat hippocampal dentate gyrus. J. Neurosci. Res. 74 570–576. 10.1002/jnr.10758 [DOI] [PubMed] [Google Scholar]
- Foley A. G., Rønn L. C., Murphy K. J., Regan C. M. (2003b). Distribution of polysialylated neural cell adhesion molecule in rat septal nuclei and septohippocampal pathway: transient increase of polysialylated interneurons in the subtriangular septal zone during memory consolidation. J. Neurosci. Res. 74 807–817. 10.1002/jnr.10820 [DOI] [PubMed] [Google Scholar]
- Fontan-Lozano A., Troncoso J., Munera A., Carrion A. M., Delgado-Garcia J. M. (2005). Cholinergic septo-hippocampal innervation is required for trace eyeblink classical conditioning. Learn. Mem. 12 557–563. 10.1101/lm.28105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. B., Fichera G., Barry T., O’Connell A. W., Gallagher H. C., Murphy K. J., et al. (2000). Consolidation of passive avoidance learning is associated with transient increases of polysialylated neurons in layer II of the rat medial temporal cortex. J. Neurobiol. 45 135–141. [PubMed] [Google Scholar]
- Fox G. B., O’Connell A. W., Murphy K. J., Regan C. M. (1995). Memory consolidation induces a transient and time-dependent increase in the frequency of neural cell adhesion molecule polysialylated cells in the adult rat hippocampus. J. Neurochem. 65 2796–2799. 10.1046/j.1471-4159.1995.65062796.x [DOI] [PubMed] [Google Scholar]
- Gascon E., Vutskits L., Kiss J. Z. (2010). The role of PSA-NCAM in adult neurogenesis. Adv. Exp. Med. Biol. 663 127–136. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., Berry R. W., Disterhoft J. F., Power J. M., Van Der Zee E. A. (2001). Associative learning elicits the formation of multiple-synapse boutons. J. Neurosci. 21 5568–5573. 10.1523/JNEUROSCI.21-15-05568.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y., Disterhoft J. F., Gundersen H. J., Mcechron M. D., Persina I. S., Power J. M., et al. (2000). Remodeling of hippocampal synapses after hippocampus-dependent associative learning. J. Comp. Neurol. 417 49–59. [PubMed] [Google Scholar]
- Gilabert-Juan J., Varea E., Guirado R., Blasco-Ibáñez J. M., Crespo C., Nácher J. (2012). Alterations in the expression of PSA-NCAM and synaptic proteins in the dorsolateral prefrontal cortex of psychiatric disorder patients. Neurosci. Lett. 530 97–102. 10.1016/j.neulet.2012.09.032 [DOI] [PubMed] [Google Scholar]
- Gruart A., Blazquez P., Gado-Garcia J. M. (1995). Kinematics of spontaneous, reflex, and conditioned eyelid movements in the alert cat. J. Neurophysiol. 74 226–248. 10.1152/jn.1995.74.1.226 [DOI] [PubMed] [Google Scholar]
- Gruart A., Leal-Campanario R., López-Ramos J. C., Delgado-García J. M. (2015). Functional basis of associative learning and its relationships with long-term potentiation evoked in the involved neural circuits: lessons from studies in behaving mammals. Neurobiol. Learn. Mem. 124 3–18. [DOI] [PubMed] [Google Scholar]
- Gruart A., Morcuende S., Martinez S., Delgado-Garcia J. M. (2000). Involvement of cerebral cortical structures in the classical conditioning of eyelid responses in rabbits. Neuroscience 100 719–730. [DOI] [PubMed] [Google Scholar]
- Gruart A., Munoz M. D., Delgado-Garcia J. M. (2006). Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J. Neurosci. 26 1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartz B. P., Rønn L. C. (2010). NCAM in long-term potentiation and learning. Adv. Exp. Med. Biol. 663 257–270. [DOI] [PubMed] [Google Scholar]
- Hebb D. O. (1949). The Organization of Behaviour. New York, NY: Wiley. [Google Scholar]
- Hildebrandt H., Mühlenhoff M., Gerardy-Schahn R. (2010). Polysialylation of NCAM. Adv. Exp. Med. Biol. 663 95–109. [DOI] [PubMed] [Google Scholar]
- Inda M. C., Delgado-Garcia J. M., Carrion A. M. (2005). Acquisition, consolidation, reconsolidation, and extinction of eyelid conditioning responses require de novo protein synthesis. J. Neurosci. 25 2070–2080. 10.1523/JNEUROSCI.4163-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Díaz L., Sancho-Bielsa F., Gruart A., Lopéz-García C., Delgado-García J. M. (2006). Evolution of cerebral cortex involvement in the acquisition of associative learning. Behav. Neurosci. 120 1043–1056. [DOI] [PubMed] [Google Scholar]
- Jucker M., D’amato F., Mondadori C., Mohajeri H., Magyar J., Bartsch U., et al. (1996). Expression of the neural adhesion molecule L1 in the deafferented dentate gyrus. Neuroscience 75 703–715. 10.1016/0306-4522(96)00276-x [DOI] [PubMed] [Google Scholar]
- Kandel E. R. (2001). The molecular biology of memory storage: a dialogue between genes and synapses. Science 294 1030–1038. 10.1126/science.1067020 [DOI] [PubMed] [Google Scholar]
- Kiss J. Z., Troncoso E., Djebbara Z., Vutskits L., Muller D. (2001). The role of neural cell adhesion molecules in plasticity and repair. Brain Res. Brain Res. Rev. 36 175–184. [DOI] [PubMed] [Google Scholar]
- Knafo S., Barkai E., Herrero A. I., Libersat F., Sandi C., Venero C. (2005a). Olfactory learning-related NCAM expression is state, time, and location specific and is correlated with individual learning capabilities. Hippocampus 15 316–325. 10.1002/hipo.20052 [DOI] [PubMed] [Google Scholar]
- Knafo S., Barkai E., Libersat F., Sandi C., Venero C. (2005b). Dynamics of olfactory learning-induced up-regulation of L1 in the piriform cortex and hippocampus. Eur. J. Neurosci. 21 581–586. 10.1111/j.1460-9568.2005.03862.x [DOI] [PubMed] [Google Scholar]
- Kugelberg E. (1952). [Facial reflexes.]. Brain 75 385–396. [DOI] [PubMed] [Google Scholar]
- Lopez-Fernandez M. A., Montaron M. F., Varea E., Rougon G., Venero C., Abrous D. N., et al. (2007). Upregulation of polysialylated neural cell adhesion molecule in the dorsal hippocampus after contextual fear conditioning is involved in long-term memory formation. J. Neurosci. 27 4552–4561. 10.1523/JNEUROSCI.0396-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch M. A., Voss K. L. (1991). Presynaptic changes in long-term potentiation: elevated synaptosomal calcium concentration and basal phosphoinositide turnover in dentate gyrus. J. Neurochem. 56 113–118. 10.1111/j.1471-4159.1991.tb02569.x [DOI] [PubMed] [Google Scholar]
- Maćkowiak M., Chocyk A., Dudys D., Wedzony K. (2009). Activation of CB1 cannabinoid receptors impairs memory consolidation and hippocampal polysialylated neural cell adhesion molecule expression in contextual fear conditioning. Neuroscience 158 1708–1716. 10.1016/j.neuroscience.2008.11.037 [DOI] [PubMed] [Google Scholar]
- Merino J. J., Cordero M. I., Sandi C. (2000). Regulation of hippocampal cell adhesion molecules NCAM and L1 by contextual fear conditioning is dependent upon time and stressor intensity. Eur. J. Neurosci. 12 3283–3290. 10.1046/j.1460-9568.2000.00191.x [DOI] [PubMed] [Google Scholar]
- Múnera A., Gruart A., Muñoz M. D., Fernández-Mas R., Delgado-García J. M. (2001). Hippocampal pyramidal cell activity encodes conditioned stimulus predictive value during classical conditioning in alert cats. J. Neurophysiol. 86 2571–2582. 10.1152/jn.2001.86.5.2571 [DOI] [PubMed] [Google Scholar]
- Murphy K. J., O’Connell A. W., Regan C. M. (1996). Repetitive and transient increases in hippocampal neural cell adhesion molecule polysialylation state following multitrial spatial training. J. Neurochem. 67 1268–1274. 10.1046/j.1471-4159.1996.67031268.x [DOI] [PubMed] [Google Scholar]
- Murray H. C., Swanson M. E. V., Dieriks B. V., Turner C., Faull R. L. M., Curtis M. A. (2018). Neurochemical characterization of PSA-NCAM(+) cells in the human brain and phenotypic quantification in Alzheimer’s disease entorhinal cortex. Neuroscience 372 289–303. 10.1016/j.neuroscience.2017.12.019 [DOI] [PubMed] [Google Scholar]
- O’Connell A. W., Fox G. B., Barry T., Murphy K. J., Fichera G., Foley A. G., et al. (1997). Spatial learning activates neural cell adhesion molecule polysialylation in a corticohippocampal pathway within the medial temporal lobe. J. Neurochem. 68 2538–2546. 10.1046/j.1471-4159.1997.68062538.x [DOI] [PubMed] [Google Scholar]
- O’Malley A., O’Connell C., Murphy K. J., Regan C. M. (2000). Transient spine density increases in the mid-molecular layer of hippocampal dentate gyrus accompany consolidation of a spatial learning task in the rodent. Neuroscience 99 229–232. 10.1016/s0306-4522(00)00182-2 [DOI] [PubMed] [Google Scholar]
- Persohn E., Pollerberg G. E., Schachner M. (1989). Immunoelectron-microscopic localization of the 180 kD component of the neural cell adhesion molecule N-CAM in postsynaptic membranes. J. Comp. Neurol. 288 92–100. 10.1002/cne.902880108 [DOI] [PubMed] [Google Scholar]
- Persohn E., Schachner M. (1990). Immunohistological localization of the neural adhesion molecules L1 and N-CAM in the developing hippocampus of the mouse. J. Neurocytol. 19 807–819. 10.1007/BF01186812 [DOI] [PubMed] [Google Scholar]
- Pesarico A. P., Bueno-Fernandez C., Guirado R., Gomez-Climent M. A., Curto Y., Carceller H., et al. (2019). Chronic stress modulates interneuronal plasticity: effects on PSA-NCAM and perineuronal nets in cortical and extracortical regions. Front. Cell Neurosci. 13:197. 10.3389/fncel.2019.00197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendeiro C., Foley A., Lau V. C., Ring R., Rodriguez-Mateos A., Vauzour D., et al. (2014). A role for hippocampal PSA-NCAM and NMDA-NR2B receptor function in flavonoid-induced spatial memory improvements in young rats. Neuropharmacology 79 335–344. 10.1016/j.neuropharm.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R. F., Britton G. B., Steinmetz J. E. (2001). Learning-related interpositus activity is conserved across species as studied during eyeblink conditioning in the rat. Brain Res. 905 171–177. 10.1016/s0006-8993(01)02532-x [DOI] [PubMed] [Google Scholar]
- Ronn L. C., Berezin V., Bock E. (2000). The neural cell adhesion molecule in synaptic plasticity and ageing. Int. J. Dev. Neurosci. 18 193–199. [DOI] [PubMed] [Google Scholar]
- Rutishauser U., Landmesser L. (1996). Polysialic acid in the vertebrate nervous system: a promoter of plasticity in cell-cell interactions. Trends Neurosci. 19 422–427. 10.1016/0166-2236(96)10041-2 [DOI] [PubMed] [Google Scholar]
- Saini V., Kaur T., Kalotra S., Kaur G. (2020). The neuroplasticity marker PSA-NCAM: insights into new therapeutic avenues for promoting neuroregeneration. Pharmacol. Res. 160:105186. 10.1016/j.phrs.2020.105186 [DOI] [PubMed] [Google Scholar]
- Sandi C. (2004). Stress, cognitive impairment and cell adhesion molecules. Nat. Rev. Neurosci. 5 917–930. [DOI] [PubMed] [Google Scholar]
- Sandi C., Merino J. J., Cordero M. I., Kruyt N. D., Murphy K. J., Regan C. M. (2003). Modulation of hippocampal NCAM polysialylation and spatial memory consolidation by fear conditioning. Biol. Psychiatry 54 599–607. [DOI] [PubMed] [Google Scholar]
- Sandi C., Merino J. J., Cordero M. I., Touyarot K., Venero C. (2001). Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience 102 329–339. 10.1016/s0306-4522(00)00484-x [DOI] [PubMed] [Google Scholar]
- Sandi C., Venero C., Guaza C. (1995). Decreased spontaneous motor activity and startle response in nitric oxide synthase inhibitor-treated rats. Eur. J. Pharmacol. 277 89–97. 10.1016/0014-2999(95)00068-v [DOI] [PubMed] [Google Scholar]
- Schachner M. (1997). Neural recognition molecules and synaptic plasticity. Curr. Opin. Cell Biol. 9 627–634. [DOI] [PubMed] [Google Scholar]
- Scholey A. B., Rose S. P., Zamani M. R., Bock E., Schachner M. (1993). A role for the neural cell adhesion molecule in a late, consolidating phase of glycoprotein synthesis six hours following passive avoidance training of the young chick. Neuroscience 55 499–509. 10.1016/0306-4522(93)90519-l [DOI] [PubMed] [Google Scholar]
- Schuster T., Krug M., Hassan H., Schachner M. (1998). Increase in proportion of hippocampal spine synapses expressing neural cell adhesion molecule NCAM180 following long-term potentiation. J. Neurobiol. 37 359–372. [DOI] [PubMed] [Google Scholar]
- Schuster T., Krug M., Stalder M., Hackel N., Gerardy-Schahn R., Schachner M. (2001). Immunoelectron microscopic localization of the neural recognition molecules L1, NCAM, and its isoform NCAM180, the NCAM-associated polysialic acid, beta1 integrin and the extracellular matrix molecule tenascin-R in synapses of the adult rat hippocampus. J. Neurobiol. 49 142–158. 10.1002/neu.1071 [DOI] [PubMed] [Google Scholar]
- Seymour C. M., Foley A. G., Murphy K. J., Regan C. M. (2008). Intraventricular infusions of anti-NCAM PSA impair the process of consolidation of both avoidance conditioning and spatial learning paradigms in Wistar rats. Neuroscience 157 813–820. 10.1016/j.neuroscience.2008.09.041 [DOI] [PubMed] [Google Scholar]
- Solomon P. R., Vander Schaaf E. R., Thompson R. F., Weisz D. J. (1986). Hippocampus and trace conditioning of the rabbit’s classically conditioned nictitating membrane response. Behav. Neurosci. 100 729–744. [DOI] [PubMed] [Google Scholar]
- Stanton M. E. (2000). Multiple memory systems, development and conditioning. Behav. Brain Res. 110 25–37. [DOI] [PubMed] [Google Scholar]
- Sytnyk V., Leshchyns’ka I., Nikonenko A. G., Schachner M. (2006). NCAM promotes assembly and activity-dependent remodeling of the postsynaptic signaling complex. J. Cell Biol. 174 1071–1085. 10.1083/jcb.200604145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela-Harrington M., Gruart A., Delgado-García J. M. (2007). Contribution of NMDA receptor NR2B subunit to synaptic plasticity during associative learning in behaving rats. Eur. J. Neurosci. 25 830–836. 10.1111/j.1460-9568.2007.05325.x [DOI] [PubMed] [Google Scholar]
- Venero C., Herrero A. I., Touyarot K., Cambon K., Lopez-Fernandez M. A., Berezin V., et al. (2006). Hippocampal up-regulation of NCAM expression and polysialylation plays a key role on spatial memory. Eur. J. Neurosci. 23 1585–1595. 10.1111/j.1460-9568.2006.04663.x [DOI] [PubMed] [Google Scholar]
- Weiss C., Bouwmeester H., Power J. M., Disterhoft J. F. (1999). Hippocampal lesions prevent trace eyeblink conditioning in the freely moving rat. Behav. Brain Res. 99 123–132. 10.1016/s0166-4328(98)00096-5 [DOI] [PubMed] [Google Scholar]
- Wolfer D. P., Mohajeri H. M., Lipp H. P., Schachner M. (1998). Increased flexibility and selectivity in spatial learning of transgenic mice ectopically expressing the neural cell adhesion molecule L1 in astrocytes. Eur. J. Neurosci. 10 708–717. 10.1046/j.1460-9568.1998.00089.x [DOI] [PubMed] [Google Scholar]
- Yamagishi T., Yoshitake K., Kamatani D., Watanabe K., Tsukano H., Hishida R., et al. (2018). Molecular diversity of clustered protocadherin-alpha required for sensory integration and short-term memory in mice. Sci. Rep. 8:9616. 10.1038/s41598-018-28034-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.