Abstract
Regulatory T (Treg) cells show promise for treating autoimmune diseases, but their induction to elevated potency has been problematic when the most optimally derived cells are from diseased animals. To circumvent reliance on autoantigen-reactive Treg cells, stimulation to myelin-independent Ags may offer a viable alternative while maintaining potency to treat experimental autoimmune encephalomyelitis (EAE). The experimental Salmonella vaccine expressing colonization factor Ag I possesses anti-inflammatory properties and, when applied therapeutically, reduces further development of EAE in SJL mice. To ascertain Treg cell dependency, a kinetic analysis was performed showing increased levels of FoxP3+ CD25+CD4+ T cells. Inactivation of these Treg cells resulted in loss of protection. Adoptive transfer of the vaccine-induced Treg cells protected mice against EAE with greater potency than naive or Salmonella vector-induced Treg cells, and cytokine analysis revealed enhanced production of TGF-β, not IL-10. The development of these Treg cells in conjunction with immune deviation by Th2 cells optimally induced protective Treg cells when compared those induced in the absence of Th2 cells. These data show that Treg cells can be induced to high potency to non-disease-inducing Ags using a bacterial vaccine.
Multiple sclerosis (MS)3 is a human inflammatory disease of the CNS that remains problematic because of no curative treatment (1). This neurodegenerative disease is characterized by perivascular inflammatory lesions, demyelination, and axonal damage (2). Experimental autoimmune encephalomyelitis (EAE) has many features similar to MS and can be induced by active immunization with specific myelin Ags, including myelin basic protein (3, 4), proteolipid protein (PLP) (5-7), and myelin oligodendrocyte glycoprotein (8, 9) or passively by adoptive transfer with encephalitogenic CD4+ Th1 cells (10). These encephalitogenic T cells secrete the proinflammatory cytokines, IFN-γ, TNF-α, and IL-2, resulting in macrophage and microglial activation, infiltration of inflammatory cells into the CNS, and eventual demyelination (11, 12). Protection against EAE can be induced by adoptive transfer of myelin-specific Th2 cells or by immunization with altered peptide ligands bearing reactive T cell epitopes that reverse the development of Th1 cells. Thus, promotion of Th2 cell induction can neutralize the development of proinflammatory encephalitogenic T cells.
Regulatory T (Treg) cells were originally described in neonatally thymectomized mice that showed increased susceptibility to autoimmune diseases (13, 14). Consequently, these cells have been shown to be important for maintenance of peripheral tolerance and ultimately protect against colitis (15), arthritis (16), and EAE (17-23). In contrast, some viruses have subverted Treg cells to dampen host responses to permit viral persistence (24, 25). Nonetheless, the expectation is that these Treg cells can serve as a potential therapeutic for treating autoimmunity. In particular, these cells have been shown to effectively reverse EAE, but depending upon their source, innate vs inducible Treg cells (26), variable amounts are required (17, 18, 22, 27). Thus, the issue of potency becomes essential in attempting to stimulate Treg cells with sufficient potency that can be readily induced and not require their expansion from diseased animals (18).
To address this feasibility, we tested whether our Salmonella vaccines could be adapted for stimulating Treg cells, which could readily address the demand for stimulating Treg cells against defined, irrelevant Ags and not against autoantigens. Because Treg cells share anti-inflammatory properties with Th2 cells, it is possible that the Salmonella-colonization factor Ag I (CFA/I) vaccine could possess the ability to elicit Treg cells. Immunization with innocuous or vaccine Ags as subunits or as shown here, with a live vaccine, could provide an alternative for Treg cell stimulation. We hypothesize the presence of a Th2 cell-promoting environment (28-31) favors the development of Treg cells as a consequence of vaccination and can allow further development of myelin-specific Treg cells. In the present work, we tested whether Salmonella-CFA/I could orally treat EAE, and we can demonstrate the critical role of Treg cells induced by Salmonella vaccines. Although only partial protection was observed after adoptive transfer of Treg cells obtained from mice immunized with the Salmonella vector, our results clearly demonstrate that Treg cells induced by immunization with Salmonella-CFA/I provide optimal protection.
Materials and Methods
Mice
Female SJL/J mice (6 wk old) were obtained from The Jackson Laboratory. All mice were maintained at Montana State University Animal Resources Center under pathogen-free conditions in individual ventilated cages under HEPA-filtered barrier conditions and were fed sterile food and water ad libitum. All animal care and procedures were in accordance with institutional policies for animal health and well-being.
PLP139–151 challenge and oral Salmonella vaccination
The encephalitogenic PLP peptide (PLP139–151; HSLGKWLGHPDKF) was synthesized by Global Peptide Services and HPLC purified to >90%. For each experiment, female SJL mice (five per group) were challenged s.c. with 200 μl of PLP139–151 (200 μg; Ref. 28). On days 0 and 2 postchallenge, mice received i.p. 200 ng of Bordetella pertussis toxin (PT; List Biological Laboratories). Six days after PLP139–151 challenge, mice were given a single oral dose of 5 × 109 CFU of the Salmonella-CFA/I vaccine (ΔaroA Salmonella enterica serovar Typhimurium-CFA/I vector vaccine, strain H696, expressing functional CFA/I fimbriae from Escherichia coli; Ref. 32) or its isogenic control strain H647 (Salmonella vector; Ref. 32). Fimbriae expression was maintained by a plasmid bearing a functional asd gene to complement the lethal chromosomal Δasd mutation in the parent Salmonella strain. Control groups were treated with PBS. Mice were monitored and scored daily for disease progression (28, 33): 0, normal; 1, a limp tail; 2, hind limb weakness; 3, hind limb paresis; 4, quadriplegia; and 5, death.
Histological evaluation of spinal cords
For histological evaluation of tissue pathology, spinal cords were removed 14 days after challenge and fixed with neutral-buffered formalin (VWR International), embedded into paraffin, and sectioned at 5 μm. Transverse sections of spinal cords were stained with H&E for pathological changes and inflammatory cell infiltration. Adjacent sections were stained with luxol fast blue (LFB) and examined for loss of myelin. Pathological manifestations were scored separately for cell infiltrates and demyelination. Each H&E section was scored from 0 to 4: 0, normal; 1, cell infiltrate into the meninges; 2, one to four small focal perivascular infiltrates; 3, five or more small focal perivascular infiltrates and/or one or more large infiltrates invading the parenchyma; and 4, extensive cell infiltrates involving ≥20% of the white matter (28). In each LFB-stained section, myelin was also scored from 0 to 4: 0, normal; 1, one small focal area of demyelination; 2, two or three small focal areas of demyelination; 3, one to two large areas of demyelination; and 4, extensive demyelination involving ≥20% of white matter (28).
Cytokine ELISA
Spleens and cervical lymph nodes (CLN) were aseptically removed 14 days after challenge from PBS-, H647-, and H696-treated groups of mice. Lymphocytes were prepared, as previously described, and resuspended in complete medium (CM): RPMI 1640 medium supplemented with 1 mM sodium pyruvate, 1 mM nonessential amino acids, penicillin/streptomycin (10 U/ml), and 10% FBS (Atlanta Biologicals). Lymphocytes were cultured in 24-well tissue culture plates at 5 × 106 cells/ml in CM alone or in the presence of OVA (10 μg/ml; Sigma-Aldrich), CFA/I fimbriae (10 μg/ml), or PLP139–151 peptide (30 μg/ml) in a total volume of 1 ml for 60 h at 37°C. The supernatants were collected by centrifugation and stored at −80°C. Capture ELISA was used to quantify, on duplicate sets of samples, the levels of IFN-γ, IL-4, IL-10, and IL-13 produced by lymphocytes, as described previously (28). For the TGF-β ELISA, wells were coated with 10 μg/ml anti-TGF-β mAb (clone 1D11; R&D Systems) and, for IL-17 ELISA, 2 μg/ml anti-IL-17 mAb (clone TC11-18H10; BD Pharmingen) overnight at 4°C. After blocking with PBS plus 1% BSA for 2 h at 37°C, washed wells were incubated with cell culture supernatants at 4°C for 24 h. After washing, 5.0 μg/ml biotinylated chicken anti-human TGF-β1 Ab (RD Systems) or 0.5 μg/ml biotinylated rat anti-mouse IL-17 Ab (clone TC11-8H4; BD Pharmingen) were added for 2 h at 37°C. Following washing, 1/1000 HRP-goat anti-biotin Ab (Vector Laboratories) was added for 1 h at room temperature. After washing, ABTS peroxidase substrate (Moss) was added to develop the reaction.
FACS analysis
Lymphocytes from the CLN, submaxillary gland LN (SMLN), Peyer’s patches (PP), mesenteric LN (MLN), and spleens were isolated 14 days after challenge and single cell preparations were prepared, as described previously (28). To obtain lymphocytes from spinal cords, mice were perfused through the left ventricle with 20 ml of cold PBS, and spinal cords were removed by flushing the vertebral canal with medium. Spinal cords were forced through 70-μm nylon mesh (BD Falcon). The single suspensions were incubated 75 min (37°C) in HEPES-buffered medium containing collagenase (300 U/ml; Sigma-Aldrich). The homogenates were resuspended in 20% Percoll (Sigma-Aldrich) and underlaid with 80% Percoll. The gradients were centrifuged at 2500 × g at 20°C for 20 min. Lymphocytes were collected from the Percoll interface, washed, and resuspended in FACS buffer.
Cells were stained for FACS analysis using conventional methods. To distinguish between neutrophils, monocytes/macrophages, and lymphocytes, staining for CD45 and MHC class II was done as described previously (34-37). Leukocyte gates were set within the forward and side scatter profiles to exclude resting microglia cells from the spinal cord preparations. To detect neutrophils, cells were stained with SK208 mAb (28), followed by FITC-donkey anti-rat anti-IgG (Jackson ImmunoResearch Laboratories) and fluorochrome-conjugated anti-CD11b (BD Pharmingen) and, for macrophages, fluorochrome-conjugated CD45 (clone 30-F11; BD Pharmingen), fluorochrome-conjugated I-As (clone 10-3.6; BD Pharmingen), and FITC-anti-CD11b mAb and PE-F4/80 mAb (Serotec). T cells were analyzed using fluorochrome-conjugated mAbs (BD Pharmingen) for CD4, CD25, TCRβ, and CD8. Intracellular staining for FoxP3 was accomplished using PE-anti-FoxP3 mAb (clone FJK-16s; eBioscience). Bound fluorescence was analyzed with a FACSCalibur (BD Biosciences).
In vivo inactivation of Treg cells
Mice were orally immunized 7 days before EAE challenge with PLP139–151 and PT. To deplete/inactivate CD25+CD4+ T cells, the same mice were given i.p. 1.0 mg of anti-CD25 mAb (clone PC 61.5.3; ATCC TIB-222) on days 5 and 2 before EAE challenge. As a control group, vaccinated mice received 1.0 mg of purified rat IgG Ab on the same days before EAE challenge. A separate control group was immunized with PBS 7 days before EAE challenge. All mice were monitored daily for development of EAE.
Adoptive transfer studies
Fourteen days after oral immunization with H696 or H647, CD4+ T cells from spleens, head and neck LN (HNLN), and MLN were obtained (negative CD4+ T cell isolation kit; Dynal Biotech). CD25+CD4+ cells and CD25−CD4+ T cells were isolated to >94 and 99%, respectively, by cell sorting (FACSVantage with Turbo-Sort; BD Biosciences) of stained T cells. To test Treg cell efficacy, 6 × 105 CD25−CD4+ T cells or CD25+CD4+ T cells were i.v. injected into naive recipients. Separate groups of mice were orally dosed with Salmonella-CFA/I, the Salmonella vaccine vector, or PBS 7 days before PLP139–151 challenge. One day after the adoptive transfer of T cells, mice were challenged with PLP139–151. Naive CD25+CD4+ and CD25−CD4+ T cells were also tested in a PLP139–151 challenge.
In vitro T cell assays
To assess Treg cell activity, 1 × 105 responder (CD25−CD4+) T cells were cocultured in triplicate with 5 × 104 Treg cells. Feeder cells (T cell-depleted mitomycin C-treated), splenocytes prepared from naive SJL mice (32), were added at 1 × 105 cells/well. Cells were cocultured with or without 10 μg/ml purified CFA/I fimbriae (32). Cells were incubated at 37°C in 5% of CO2 for 72 h before pulsed with 1 μCi of [3H]TdR for 18 h at 37°C with a 5% of CO2. [3H]TdR incorporated was measured by scintillation counting.
To assess cytokine production by Treg and effector T cells, CD25+CD4+ T cells and CD25−CD4+ T cells (2 × 105) were stimulated in vitro with anti-CD3 mAb-coated wells (10 μg/ml; BD Pharmingen) plus a soluble anti-CD28 mAb (5.0 μg/ml; BD Pharmingen) for 5 days in CM (final volume of 300 μl in a 48-well plate). Capture ELISA was used to quantify triplicate sets of samples to measure cytokines.
Statistical analysis
The ANOVA followed by a post hoc Tukey test was applied to show differences in clinical scores in treated vs PBS mice and in the Treg cell kinetic experiments. The Student t test was used to evaluate the differences between variations in cytokine level production.
Results
Resolution of EAE after oral treatment with Salmonella-CFA/7 (H696) in SJL mice
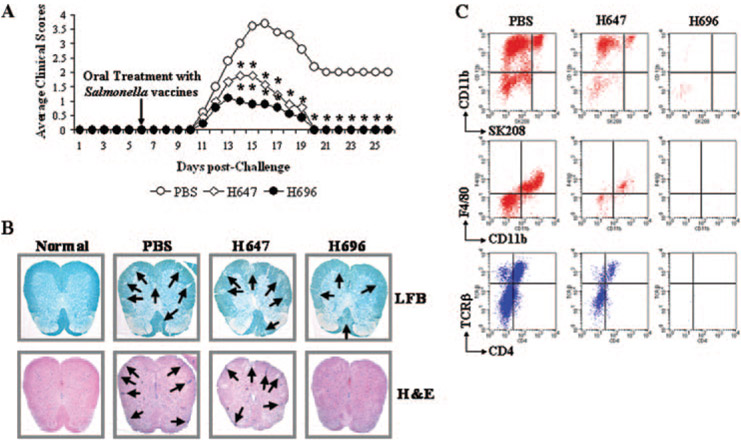
SJL mice were subjected to conventional PLP139–151 challenge, and 6 days later, mice were either treated with PBS, the isogenic Salmonella vaccine vector (strain H647), or the anti-inflammatory vaccine, Salmonella-CFA/I (strain H696). While the mean day onset among the three treatment groups was not different, peaking between days 13 and 15 after PLP139–151 challenge (Table I), mice treated with strain H696 showed reduced clinical scores and reduced disease duration (p < 0.001; Table I and Fig. 1A) when compared with the PBS-treated group. To a lesser degree, the H647 also showed a reduction in the maximum clinical score (Table I) and significant differences (p < 0.001) in the disease kinetics (Fig. 1A). Yet, disease in these mice was more severe than in the H696-treated group, as evidenced by the cumulative clinical scores: 28.5, 11.3, and 6.8 for PBS-, H647-, and H696-treated groups, respectively (Table I).
Table I.
Therapeutic treatment with Salmonella vaccines after PLP139–151 challenge protects SJL/J mice from EAEa
| Treatmentb | EAE/Totalc | Onsetd | Maximum Scoree | Cumulative Scoresf | Inflammationg | Demyelinationh | Percentage of CD4+ T Cellsi |
|---|---|---|---|---|---|---|---|
| PBS | 20/20 | 10.4 ± 0.6 | 5 | 30.6 | 2.2 ± 0.8 | 3.3 ± 0.3 | 4.9 ± 1.3 |
| H647 | 19/19 | 11.1 ± 0.1 | 3 | 11.75* | 2.0 ± 0.4 | 2.7 ± 0.2 | 0.6 ± 0.1* |
| H696 | 19/19 | 11.2 ± 1.2 | 2 | 6.6*,* | 0.7 ± 0.6*,* | 1.1 ± 0.6*,* | 0.0 ± 0.0*,* |
SJL/J mice were challenged s.c. with 200 μg of PLP139–151 in CFA plus 200 ng of PT i.p. on days 0 and 2.
Mice were immunized 6 days postchallenge with PBS or 5 × 109 CFU of S. enterica Typhimurium H647 (vector) or H696 (CFA/I fimbriae).
Number of mice with EAE/total in group.
Mean day ± SD of clinical disease onset.
Maximum daily clinical score.
Cumulative scores were calculated as the sum of all scores from disease onset to day 25 postchallenge and divided by the number of mice in each group. *, p < 0.001 for PBS vs H696, PBS vs H647, and H647 vs H696.
Mean score ± SEM of inflammation: the infiltration of nucleated cells into spinal cords was scored from 0 to 4 in each mouse separately, and the mean score and SEM were calculated. *, p < 0.001 for PBS vs H696 and H647 vs H696.
Mean score ± SEM of demyelination: the demyelination in spinal cords was scored from 0 to 4 in each mouse separately, and the mean score and SEM were calculated. *, p < 0.001 for PBS vs H696 and H647 vs H696.
Percentage of CD4+TCRβ+ T cells from the total cells in spinal cords and analyzed by FACS (Fig. 1C). *, p < 0.001 for PBS vs H647, PBS vs H696, and H647 vs H696.
FIGURE 1.
Oral Salmonella-CFA/I (H696) treatment reduced EAE clinical scores in PLP139–151-challenged SJL/J mice (A) and decreased spinal cord demyelination (B) and inflammation (B and C). A, Mice therapeutically treated with H696 showed a significant reduction in their clinical scores with all mice recovering from EAE. Mice treated with Salmonella vector (strain H647) also recovered, but presented greater clinical scores when compared with PBS-treated mice. Depicted are the combined results from four separate experiments for a total of 20 mice/group: *, p < 0.001 for PBS vs H647 and PBS vs H696. B, H696-treated group showed minimal demyelination (LFB) and inflammatory (H&E stain) cell infiltration (indicated by arrows) at 14 days postchallenge when compared with PBS-dosed group; the H647-treated mice showed increased demyelination and inflammation. Representative samples from six mice are depicted. C, FACS analysis of spinal cord leukocytes was performed 14 days after challenge to assess the types of inflammatory cells: neutrophils, CD11b+SK208+; macrophages, CD11b+SK208−F4/80+; and T cells, TCRβ+. Minimal leukocytes were detected in H696-treated mice unlike that observed for PBS- or H647-treated mice; representative samples of five mice per group are depicted.
Treatment with Salmonella-CFA/I reduces inflammatory cell infiltration into the CNS
During EAE, autoaggressive myelin-reactive T lymphocytes migrate into the CNS where they recognize their target Ag and initiate an inflammatory response, thus provoking tissue damage. Histopathological analyses performed on spinal cords from the different treatment groups revealed that mice treated with Salmonella-CFA/I showed minimal inflammatory cell infiltration and demyelination when compared with the PBS-treated group (p < 0.001; Fig. 1B and Table I). Mice treated with the Salmonella vector showed no significant differences when compared with the PBS-treated group, suggesting that treatment with the Salmonella vector was insufficient to diminish inflammation and prevent tissue damage.
FACS analysis of inflammatory cells obtained from spinal cords from treated mice was performed. Consistent with the histopathology analysis, Salmonella-CFA/I-treated mice at 14 days after challenge (8 days after treatment) failed to show inflammatory cell infiltration (Fig. 1C). In contrast, both PBS- and Salmonella vector-treated mice showed marked infiltration by neutrophils (CD11b+SK208+), macrophages (CD11b+F4/80+), and T cells (CD4+TCRβ+ and CD4−TCRβ+). Although the H647 showed a reduced clinical score, it failed to prevent inflammatory cell infiltration and demyelination.
Salmonella-CFA/7 reduces inflammatory PLP139–151-specific CNS damage
PLP139–151-specific immune deviation from Th1-type to Th2-type responses by Salmonella-CFA/I was previously shown for prophylactic vaccination of SJL mice against EAE (28). We tested whether the reduction of the EAE clinical scores in H696-treated mice was relevant to the observed protection (Fig. 1 and Table I). Ag restimulation cultures were conducted to ascertain the cytokine profiles in response to PLP139–151 peptide (Fig. 2). Following in vitro Ag pulsing, lymphocytes from H696-treated mice spleens and CLN showed an enhanced production of IL-4 and IL-13 with concomitant reduction in IFN-γ when compared with PBS (p < 0.001)- or H647-treated (p < 0.001) mice. The CLN from H696-treated mice showed significant reduction in the production of the proinflammatory cytokine, IL-17 (p < 0.001), when compared with PBS-treated mice (Fig. 2B). An increase in IL-10 production was only evident in the CLN from H696 (p < 0.001)- and to a lesser degree by H647-treated mice (p < 0.05; Fig. 2B). PBS- and H647-treated mice showed mostly a Th1-type dominant response with minimal Th2-type cytokines. Thus, the oral Salmonella-CFA/I vaccine can cause immune deviation, particularly in the CLN, a major site for the production of PLP139–151-specific T cell activation.
FIGURE 2.
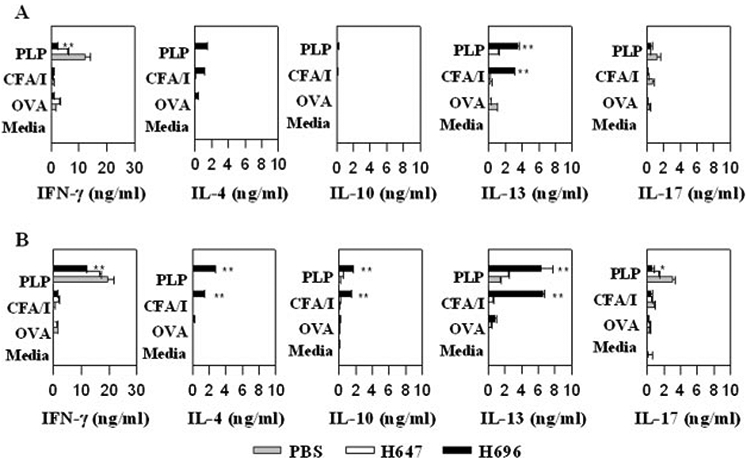
Elevated Th2-type cytokines contribute to the therapeutic treatment by H696 vaccine by splenic (A) and CLN (B) lymphocytes with reductions in IFN-γ. Splenic (A) and CLN (B) lymphocytes were collected 14 days after EAE challenge (8 days after oral Salmonella vaccination) and cultured in the presence of OVA, purified CFA/I fimbriae, PLP139–151, or medium. Data depict the mean of three different experiments ± SEM. *, p < 0.001 for H696 vs PBS and H696 vs H647.
Salmonella-CFA/I vaccination elicits Treg cells and protect against EAE
To ascertain whether Treg cells are induced as a result of Salmonella-CFA/I vaccination, CLN from challenged, then vaccinated, mice were evaluated for the presence of Treg cells. Although it was evident that all treatment groups stimulated Treg cell induction (Fig. 3A), the percentages of Treg cells in nearly all tissues examined showed an enhanced number of Treg cells in the H696-treated mice when compared with PBS-treated mice (Fig. 3B). Subsequent analysis revealed that >90% of the Treg cells were FoxP3+, and these FoxP3+ Treg cells increased after PLP139–151 challenge (Fig. 3A). The observed increase of Treg cells in the PBS-treated group was similar to that previously reported (18) and might explain the natural recovery from EAE observed in SJL mice. In addition, we analyzed whether the Salmonella-CFA/I vaccine by itself could induce Treg cells independent of PLP139–151 challenge (Fig.3, C and D). Although the magnitude of induced Treg cells of the total lymphocytes was less, the percentage of FoxP3+ remained elevated, >90%. Thus, oral vaccination with H696 can elicit Treg cells.
FIGURE 3.
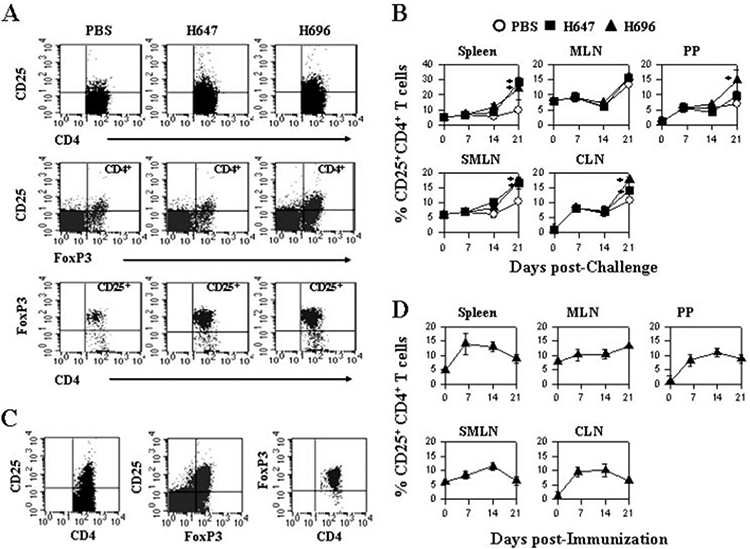
Oral Salmonella vaccination enhances the percentage of Treg cells expressing FoxP3 in both the mucosal and systemic compartments. A, CLN lymphocytes taken from SJL mice 14 days after EAE challenge (treated with H696, H647, or PBS 6 days postchallenge) and were examined by FACS for the presence of CD25+CD4+ T cells. Each group showed stimulation of Treg cells, but mice treated with H647 or H696 had increased numbers, and these were >90% FoxP3+. B, A kinetic analysis was performed on spleen, MLN, PP, SMLN, and deep CLN. Increases in FoxP3+ Treg cells were observed for Salmonella-treated groups. *, p < 0.001 for PBS vs H647 and PBS vs H696. C and D, Oral vaccination with H696 only (no PLP139–151 challenge) results in FoxP3+ Treg cells in spleen, MLN, PP, SMLN, and (C and D) CLN peaking ~2 wk after immunization (mean of five mice per group ± SEM).
To assess the functionality of the observed Treg cells and whether these could suppress CD4+ T cell responses, CD25− CD4+ and CD25+CD4+ T cells were purified from spleens, MLN, and HNLN 14 days after oral immunization with Salmonella-CFA/I. An anti-CFA/I proliferation assay was performed and showed the inhibitory effect of the CD25+CD4+ T cells in each of the tissues, and 60, 75, and 50% inhibition was observed with Treg cells isolated from spleen, MLN, and HNLN, respectively (Fig. 4A). These results demonstrated that indeed oral immunization with the Salmonella-CFA/I vaccine induces the expansion of CD25+CD4+ T cells, and these Treg cells could contribute to the observed recovery from EAE.
FIGURE 4.
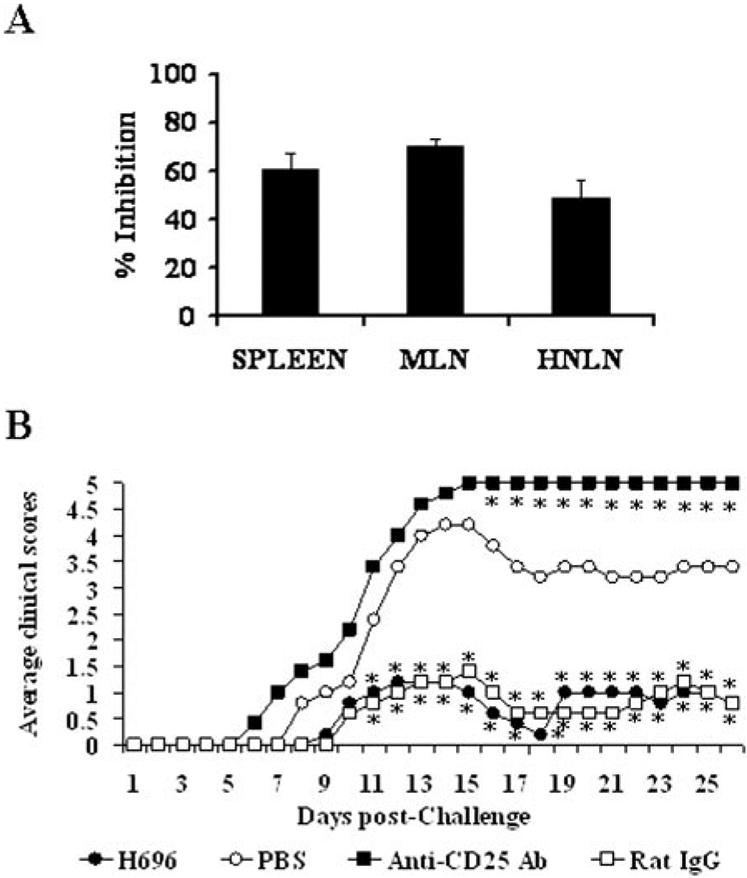
The Treg cells induced by Salmonella-CFA/I vaccination suppress effector T cells, and their in vivo inactivation results in enhanced clinical disease. A, CD25−CD4+ and CD25+CD4+ T cells from spleens, MLN, and HNLN were cell sorted from mice orally immunized with H696 for 2 wk. Teff (1 × 105) cells with feeder (1 × 105) cells were cultured with CFA/I fimbriae and with or without Treg (5 × 104) cells for 4 days. During the last 18 h of culture, cells were pulsed with [3H]TdR. Proliferation in the absence of Treg cells was set at zero inhibition. Depicted is the percent inhibition upon coculture with splenic, MLN, or HNLN Treg cells. One of three experiments is depicted. B, One week before PLP139–151 challenge, mice (five per group) orally immunized with H696 were treated in vivo with anti-CD25 mAb, rat IgG, or PBS on days −5 and −2 before challenge. Inactivation of Treg cells provoked greater EAE than the PBS-treated group. Normal rat IgG-treated mice vaccinated with H696 showed minimal EAE. *, p < 0.001 for anti-CD25-treated group vs H696-vaccinated or H696-vaccinated plus normal rat IgG-treated mice.
The in vivo role of CD25+CD4+ T cells in the recovery of EAE after the immunization with Salmonella-CFA/I was analyzed in CD25-blocked mice. SJL mice were orally immunized with H696 vaccine 7 days before PLP139–151 challenge (day 0). On days −5 and −2, they were treated with anti-CD25 mAb or rat IgG. This treatment paradigm with the anti-CD25 mAb was designed to ascertain how the loss of the induced Treg cell function subsequent to oral immunization with Salmonella-CFA/I would impact the recovery from EAE. As a result, the inactivation of CD25+ T cells provoked an increased disease severity in which all of the mice succumbed to EAE within 14 days after challenge (Fig. 4B). The clinical scores of the treated mice were significantly greater (p < 0.001) than mice treated with the rat IgG or the PBS-treated mice, suggesting that Treg cells are essential in the recovery from EAE, and these cells supercede or contribute to the immune deviation by Salmonella-CFA/I vaccination.
Salmonella-induced, not innate Treg cells are protective against EAE
Studies have shown that Treg cells obtained from EAE-diseased mice and adoptively transferred into naive mice protected against subsequent EAE induction (17, 18, 23). Such results suggest that Treg cells can reduce the severity in an inflammatory disease. While these past studies focused on anti-encephalitogenic Treg cells, the studies from Fig. 3 suggest that the protective Treg cells are independent of myelin T cell epitopes. To directly determine the protective capacity of the vaccine-induced Treg cells, CD25+ CD4+ T cells were allowed to develop 14 days after oral vaccination with Salmonella-CFA/I or the Salmonella vaccine vector to become activated and expanded in vivo. CD25+CD4+ T cells and CD25−CD4+ T cells were isolated by cell sorting from the H696- and H647-vaccinated mice and adoptively transferred (6 × 105 cells/mouse) into naive recipients. One day after the adoptive transfer, mice were challenged with PLP139–151, and mice were monitored for normal course of disease. Beginning with adoptive transfers with cells from H696-dosed mice, the PBS-treated group developed the expected EAE disease (Fig. 5A). The mice receiving the CD25+CD4+ T cells showed considerable potency with minimal to no EAE developing when compared with PBS-treated mice (p < 0.001) and even compared with control mice vaccinated with H696 (p < 0.001; Fig. 5A and Table II). Mice adoptively transferred with CD25−CD4+ T cells developed a more severe EAE, but these mice still exhibited a substantially reduced EAE when compared with the PBS-treated mice. These data suggest that the H696 confers protection via both immune deviation by Th2 cells and via the induction of Treg cells. Adoptive transfer of immune CD4+ T cells obtained from Salmonella vector-vaccinated mice showed a different outcome. The induced Treg cells were protective against EAE challenge, but these mice still developed EAE, unlike those Treg cells induced with the Salmonella-CFA/I vaccine (Fig. 5B and Table II). Similar to H696-induced Treg cells, all the mice recovered from EAE (Fig. 5B). However, adoptive transfer of CD25−CD4+ T cells from H647-vaccinated mice failed to confer any protection, suggesting a requirement for Th2 cells either for the development or coinduction. Moreover, for these studies, it was essential that the Treg cells come from vaccinated mice because the adoptive transfer of naive CD25+CD4+ T cells had minimal impact. Naive CD25+CD4+ T cells and CD25−CD4+ T cells were sorted from normal SJL mice and adoptively transferred into naive SJL mice. Mice were challenged with PLP139–151 and monitored for disease course (Fig. 5C and Table II). The CD25−CD4+ T cells failed to delay EAE onset and showed a similar disease course as did the PBS-treated mice. While there was a 4-day delay of EAE onset (Table II), the mice given the Treg cells still eventually developed EAE with similar maximum clinical scores, suggesting that their potency is not as great as the vaccine-induced Treg cells. These data show that innate Treg cells are insufficient for protection against EAE.
FIGURE 5.
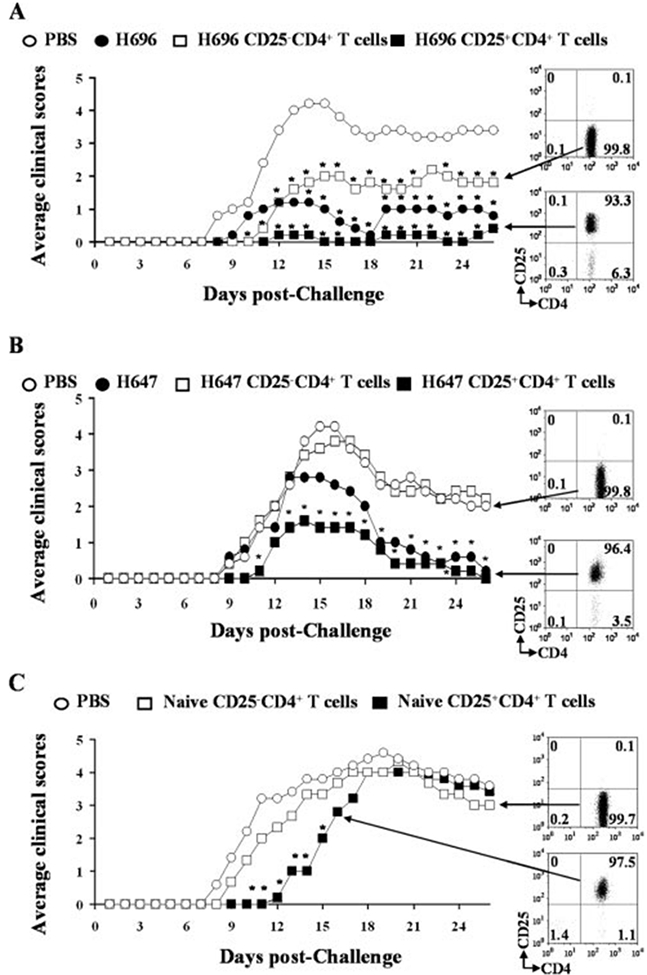
Adoptive transfer of Treg cells obtained from Salmonella-CFA/I-immunized mice confers greater protection against EAE challenge than Salmonella vector-induced Treg cells whereas those from naive mice failed to protect; protection is TGF-β dependent. Immune CD25+CD4+ T cells and CD25−CD4+ T cells from 2-wk H696 (A)- and H647 (B)-immunized SJL mice were obtained from pooled spleens, MLN, and HNLN, purified by cell sorting, and 6 × 105 Treg or Teff cells were injected i.v. into naive SJL mice. PBS-, H696-, and H647-immunized mice were used as negative and positive controls, respectively. Recipient mice given immune H696 Treg cells conferred total protection against EAE, whereas mice given Teff cells showed only partial protection with greater clinical disease than H696-vaccinated mice. *, p < 0.001 for PBS vs Treg cell group, CD25−CD4+ T cell group, or H696-vaccinated group. Treg cells obtained from H647-vaccinated mice were less protective, and Teff cells failed to protect against EAE. C, Naive SJL Treg and Teff cells were purified by cell sorting of lymphocytes from MLN, HNLN, and spleens. Treg or CD25−CD4+ T cells (6 × 105) were injected i.v. into naive recipients 1 day before PLP139–151 challenge. While adoptive transfer of naive Treg cells delayed EAE onset, these failed to protect against EAE development. *, p < 0.001 for PBS vs naive Treg cell group or naive CD25−CD4+ T cell group.
Table II.
Adoptive transfer of immune CD25+CD4+ T cells from Salmonella vaccines confers protection against EAEa but not naive Treg cells
| Treatment | EAE/Totalb | Onsetc | Maximum Scored | Cumulative Scoree |
|---|---|---|---|---|
| PBS | 5/5 | 8.4 ± 9.5 | 5 | 58.8 |
| H69f | 5/5 | 12.2 ± 4.0 | 3 | 14.6* |
| H647f | 5/5 | 9.6 ± 0.9 | 5 | 27.9† |
| H696 CD25+CD4+ T cellsg | 3/5 | 19.0 ± 5.7*,* | 1 | 1.6*,* |
| H696 CD25−CD4+ T cellsg | 5/5 | 11.6 ± 0.5* | 5 | 25.2* |
| H647 CD25+CD4+ T cellsg | 5/5 | 12.1 ± 0.7† | 3 | 15.4†,† |
| H647 CD25−CD4+ T cellsg | 5/5 | 9.8 ± 0.8 | 3 | 48.2 |
| Naive CD25+CD4+ T cellsg | 5/5 | 12.8 ± 0.4* | 5 | 40.2* |
| Naive CD25−CD4+ T cellsg | 5/5 | 9.2 ± 0.4 | 5 | 43.0* |
SJL/J mice were challenged as per Table I.
Number of mice with clinical EAE per group.
Mean day onset ± SEM of clinical disease. *, p < 0.001 for PBS vs H696 CD25+CD4+ T cells, PBS vs H696 CD25−CD4+ T cells, and H696 vs H696 CD25+CD4+ T cells. †, p < 0.001 for PBS vs H647.
Maximum daily clinical score.
Cumulative clinical scores were calculated as the sum of all clinical scores from disease onset to day 25 postchallenge and divided by the number of mice in each group. *, p < 0.001 for PBS vs H696, PBS vs H696 CD25+CD4+ T cells, PBS vs H696 CD25−CD4+ T cells, and H696 vs H696 CD25+CD4+ T cells. †, p < 0.001 for PBS vs H647, PBS vs H647 CD25+CD4+ T cells, and H647 vs H647 CD25+CD4+ T cells.
Mice were vaccinated 7 days before challenge with 5 × 109 CFU of S. Typhimurium H696 or S. Typhimurium H647.
CD25−CD24+ T cells or CD25+CD4+ T cells from SJL/J mice previously immunized with H696 or H647 or from naive SJL/J mice were injected i.v 1 day before the induction of EAE with PLP139–151.
To discern what attributes contributed to protection, cytokine production for the immune CD25+CD4+ T cells and CD25−CD4+ T cells was analyzed (Fig. 6). Following in vitro restimulation of the purified CD25+CD4+ T cells and CD25−CD4+ T cells from H696- and H647-vaccinated mice, the IFN-γ production was mostly derived from CD25−CD4+ T cells rather than CD25+CD4+ T cells (p < 0.001), and in fact, the amount of IFN-γ was greater from CD25−CD4+ T cells obtained from H647-vaccinated mice (p < 0.001; Fig. 6). IL-4, IL-10, and IL-13 production was enhanced in H696-induced CD25−CD4+ T cells (p < 0.001). As expected, based on results shown in Fig. 2 and in previous reports (28), CD25−CD4+ T cells from of H647-vaccinated mice produced minimal to no Th2-type cytokines. Notably, production of TGF-β was enhanced in H696-induced CD25+CD4+ T cells when compared with CD25−CD4+ T cells (p < 0.001), as well as when compared with production by H647-induced CD25+CD4+ or CD25−CD4+ T cells by (p < 0.001), suggesting that Treg cells mediated their suppression via TGF-β. The observed protective effect by H647-induced Treg cells may have come from the combined protection of regulatory cytokines, IL-4, IL-10, IL-13, and TGF-β. Interestingly, IL-17 was only produced by these H647-induced Treg cells, which may contribute or lessen the impact by the regulatory cytokines (Fig. 6).
FIGURE 6.
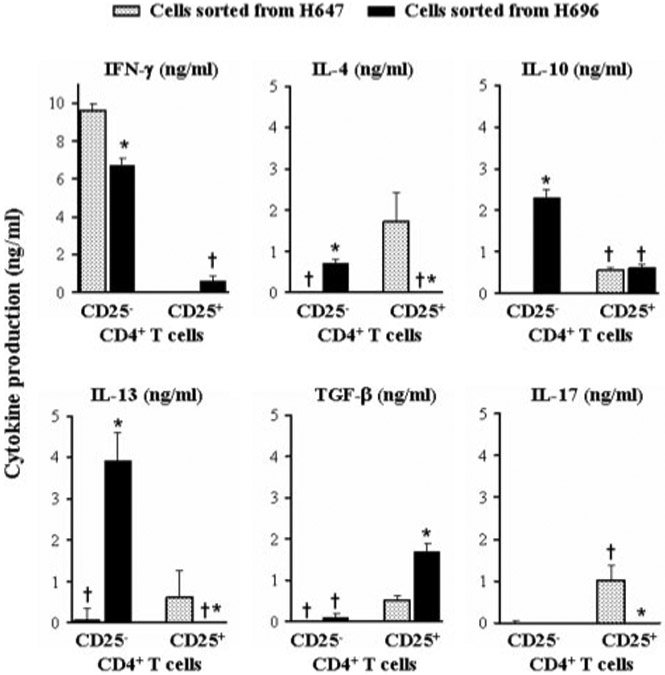
Oral vaccination with Salmonella-CFAH elicits TGF-β-producing Treg cells and IL-4-, IL-10-, and IL-13-producing Teff cells in contrast to IL-17-producing, but no Th2-type cytokine-producing, Teff cells upon vaccination with Salmonella vector. Cell-sorted CD25+CD4+ and CD25−CD4+ T cells from mice orally immunized with H696 or H647 were evaluated for cytokine production following anti-CD3 and anti-CD28 costimulation. Treg cells had reduced IFN-γ production but elevated TGF-β when compared with Teff cells. IL-4, IL-10, and IL-13 segregated with the Teff cells induced by vaccination with H696. Teff cells from H647-vaccinated mice did not produce any Th2-type cytokines but rather produced elevated levels of IFN-γ. H647-induced Treg cells did produce TGF-β (although less than H696-vaccinated mice) and IL-17. Thus, protection conferred by Salmonella-CFA/I-induced Treg cells is because of reduced IFN-γ production and increased TGF-β, as well as in part supported by immune deviation by the Teff cells. *, p < 0.001 represents differences in cytokine production between CD25+CD4+ and CD25−CD4+ T cells, and †, p < 0.001 represents differences in cytokine production between H696- and H647-sorted cells.
Discussion
Encephalitogenic T cells secrete Th1-type cytokines inducing the activation of macrophages and microglial cells and the infiltration of inflammatory cells from peripheral lymphoid tissues into CNS (11, 12). Studies to date have explored the potential for developing anti-encephalitogenic Treg cells as a therapeutic for autoimmune diseases (17, 18, 23). Cells producing anti-inflammatory cytokines, IL-4, IL-10 (23, 24, 30), or IL-13 (28), down-regulate these inflammatory responses, inhibiting autoimmune damage. Such efforts involve the stimulation of myelin-based epitopes by vaccinating with altered peptide ligands (23, 31) or isolating Treg cells from diseased animals (18). Resorting to the latter may have limited the clinical value. Alternatively, stimulating Treg cells in vitro (22) or, as evidenced in this study, using Treg cells induced by Ags or systems independent of myelin has the advantage of developing a therapeutic unrestricted by the specificity of the encephalitogenic T cells involved. As such, treating EAE with Salmonella-CFA/I-generated FoxP3+CD25+CD4+ T cells that could resolve disease. In fact, in vivo depletion/inactivation (38) of Treg cells in Salmonella-CFA/I-vaccinated mice resulted in a more severe disease than those mice treated with rat IgG or even the PBS-treated mice. The anti-CD25 mAb-treated mice developed clinical disease 2 days prior the PBS-treated group, reaffirming the importance of Treg cells induced by Salmonella-CFA/I.
This approach of using an oral vaccine specific for Ags unrelated to myelin is unique in that it stimulated an environment conducive for the development of Treg cells. When used as a therapeutic, it did appear to stimulate anti-encephalitogenic Treg cells because the numbers of these Treg cells increased during the treatment period when compared with Treg cells induced in the absence of PLP139–151 challenge. Partial protection was also conferred by the stimulation of Th2-type cytokines, resulting via immune deviation. Anti-encephalitogenic CD4 T cells produced IL-4 and IL-13 in response to PLP139–151 in contrast to lymphocytes from Salmonella vector-immunized mice that did not produce Th2-type cytokines. Responses from unprotected mice showed elevations in IFN-γ and IL-17 production, whereas these cytokines were reduced in H696-vaccinated mice. Thus, both myelin-specific and myelin-independent Treg and Th2 cells were induced by oral vaccination with Salmonella-CFA/I, whereas the vaccination with Salmonella vaccine vector induced Treg cells, but in the absence of effector Th2 cells.
Another clear distinction between these two Salmonella vaccines is the level of Treg cell potency produced. Therapeutic treatment with the Salmonella vector (strain H647) conferred limited protection, and the magnitude of this protection was similar to that observed when used prophylactically (28). However, the extent of demyelination and inflammation in the spinal cords was not significantly different from PBS-treated control mice, although fewer macrophages were observed after PLP139–151 challenge. Studies evaluating cytokine production showed no significant differences in IFN-γ or IL-17 levels between H647- and PBS-treated mice, as well as a lack of Th2-type cytokines. One possible explanation of these findings is that the Salmonella vector was able to induce a competing proinflammatory response against that provoked by PLP139–151 challenge, although the magnitude of the PLP139–151-specific IFN-γ and IL-17 responses was not significantly different from the PBS-treated control group. Recent reports (39-45) show that IL-17 is a major contributor to the development of EAE. Our results show that H696 vaccination reduces IL-17 production; however, H647 vaccination did not significantly diminish IL-17 production when compared with the PBS-treated mice. An alternative explanation supported by the data suggests that infection with the Salmonella vector can stimulate increases in FoxP3+CD25+CD4+ T cells. In fact, many of the tissues examined had minimal differences in the percentages of FoxP3+CD25+CD4+ T cells between H647- and H696-immunized mice. Yet, the potency of incurred protection by the Salmonella-induced Treg cells was not as great as those induced by Salmonella-CFA/I vaccine.
What became evident from this study is the importance of Th2 cells upon Treg cell development as implicated from the adoptive transfer of CD25+ and CD25−CD4+ T cells. Partial protection was observed, as denoted by the reduction in EAE after adoptive transfer of Salmonella vector-induced Treg cells, yet this was unmatched to the potency obtained with the Salmonella-CFA/I-induced Treg cells in which minimal to no disease was observed. Moreover, adoptive transfer of effector CD4+ T cells induced by the Salmonella vaccine vector did not protect against EAE, whereas adoptive transfer of the H696-induced effector Teff cells conferred partial protection of similar magnitude to that obtained with the Salmonella vector-induced Treg cells. Not only were there clear distinctions in the functional outcomes of these collective CD4+ T cells from the two vaccine groups, differences in the cytokine profiles for both the CD25+CD4+ and CD25−CD4+ T cells were observed. Examination of the effector CD25−CD4+ T cells revealed that the mice vaccinated with H696 showed lower IFN-γ production, but greater IL-10 and IL-13 when compared with Salmonella vector-immunized effector CD25−CD4+ T cells. Interestingly, the H696-induced Treg cells, for the most part, produced TGF-β whereas the CD25−CD4+ T cells did not. The role of IL-10 in this present study was less prominent than TGF-β. In contrast, the H647-induced CD25+CD4+ T cells produced less TGF-β but more IL-4 and IL-17. This latter observation is of interest because IL-17 development is TGF-β dependent and has antagonist properties to Th1 and Th2 cells (46), suggesting that perhaps neutralization of this cytokine may make the H647-induced Treg cells more potent. The effector CD25−CD4+ T cells from the H647-vaccinated mice showed no Th2- nor Th17-type cytokine production.
Other works have shown that protective Treg cells preferentially produce IL-10 (23, 47-49). Clearly, this was not the case here, although some IL-10 was produced by CD25−CD4+ T cells. Our finding with TGF-β+CD25+CD4+ T cells was consistent with what others have found for Treg cells’ role in oral tolerance (50, 51) and protection to autoimmune diseases (52, 53). In this regard, TGF-β appears to be critical for the expression of FoxP3 in CD25+CD4+ T cells (54), conferring the regulatory role to these cells (15, 55). TGF-β has important immunosuppressive properties on lymphocytes and has also been implicated in the conversion of CD25−CD4+ T cells into CD25+CD4+ T cells by the induction of FoxP3. Moreover, it seems to be an important factor for the expansion of CD25+CD4+ T cells in inflammatory diseases, such as colitis (15), and has been associated with the recovery from experimental colitis in mice (53). These reports and our data suggest that TGF-β plays a critical role in the recovery from autoinflammatory diseases.
It was interesting to learn that infection with an attenuated Salmonella strain could elicit Treg cells. Such a finding had not been reported previously for Salmonella infections. The difference in the mechanism of action between the two Salmonella strains may be due to the absence of inducing immune deviation by the Salmonella vector, as affirmed by the lack of effector Th2 cells by H647-vaccinated mice. While the induced Th2 cells by Salmonella-CFA/I vaccination were not as effective as purified Treg cells (but this effect may be dose dependent) from the same vaccinated mice, the addition of immune deviation may enhance Treg cell development (56) and ultimately its potency. This became further evident upon the evaluation of the relative potency among the induced Treg cells compared with naive CD25+CD4+ T cells. When the naive CD25+CD4+ T cells were isolated, adoptively transferred, and tested for their ability to inhibit EAE, these failed to prevent EAE development. Previous studies have shown that at least 2–3 million naive Treg cells are required to show some level of protection (29, 31, 55, 57). Since we used a lesser amount, the absence of protection may be because of insufficient numbers. Nonetheless, the Salmonella-CFAJI vaccine-induced Treg cells were clearly more potent.
The feasibility of using adoptively transferred Treg cells remains problematic because of insufficient levels. While others have addressed this issue by in vitro activation with cognate Ag (22), which clearly expands Treg cell numbers, such an approach may be hampered if the TCR specificity is unknown. Alternatively, polyclonal activation methods using anti-CD3 and IL-2 (57-59) can successfully increase Treg cell numbers, but not knowing the specificity of polyclonally activated Treg cells may have other unknown or adverse consequences (60). To address these concerns, one option could be the induction of Treg cells with a defined specificity but unrelated to autoantigens. As in the case for Salmonella-CFA/I, vaccination can be readily accomplished to elicit Treg cells that ultimately protect or reduce EAE. To our knowledge, this is the first work describing such an approach of vaccinating against an irrelevant Ag, thus not relying on naive Treg cells and, to a lesser extent, relying on disease-induced Treg cells.
In summary, we demonstrated that therapeutic treatment with Salmonella-CFAJI is able to protect SJL mice against EAE by preventing encephalitogenic T cells to enter the CNS. Although immune deviation plays a significant role in diminishing EAE, its participation may also enhance the development of Treg cells. Eliminating Treg cell function in mice immunized with Salmonella-CFA/I provoked a more severe EAE, clearly indicating that vaccination stimulates Treg cell formation. Adoptive transfer with Salmonella-CFA/I-induced Treg cells, but not naive Treg cells, and to a lesser degree with Salmonella vaccine vector-induced Treg cells, protected against EAE challenge. The suppressive FoxP3+ Treg cells producing TGF-β conferred protection. To our knowledge, this is the first work that reports the protective effects of immune Treg cells induced and expanded by immunization with an irrelevant, non-self Ag, such as CFA/I fimbriae.
Acknowledgments
We thank Dr. Mark A. Jutila (Veterinary Molecular Biology, Montana State University, Bozeman, MT) for providing the SK208 mAb and Nancy Kommers for her assistance in preparing this manuscript.
This work was supported by Public Health Service Grant AI-41123 and in part by Montana Agricultural Station and U.S. Department of Agriculture Formula Funds. The Veterinary Molecular Biology flow cytometry facility was in part supported by National Institutes of Health/National Center for Research Resources Centers of Biomedical Research Excellence P20 RR-020185.
Footnotes
Abbreviations used in this paper: MS, multiple sclerosis; CFA/I, colonization factor Ag I; CLN, cervical lymph node; CM, complete medium; EAE, experimental autoimmune encephalomyelitis; HNLN, head and neck lymph node; LFB, luxol fast blue; MLN, mesenteric lymph node; PLP, protein proteolipid; PP, Peyer patches; PT, Bordetella pertussis toxin; SMLN, submaxillary gland lymph node; Teff, effector T; Treg, regulatory T.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Sospedra M, and Martin R. 2005. Immunology of multiple sclerosis. Annu. Rev. Immunol 23: 683–747. [DOI] [PubMed] [Google Scholar]
- 2.Steinman L 1996. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell 85: 299–302. [DOI] [PubMed] [Google Scholar]
- 3.Zeine R, Heath D, and Owens T. 1993. Enhanced response to antigen within lymph nodes of SJL/J mice that were protected against experimental allergic encephalomyelitis by T cell vaccination. J. Neuroimmunol 44: 85–94. [DOI] [PubMed] [Google Scholar]
- 4.Shaw MK, Kim C, Hao HW, Chen F, and Tse HY. 1996. Induction of myelin basic protein-specific experimental autoimmune encephalomyelitis in C57BL/6 mice: mapping of T cell epitopes and T cell receptor Vβ gene segment usage. J. Neurosci. Res 45: 690–699. [DOI] [PubMed] [Google Scholar]
- 5.Greer JM, Sobel RA, Sette A, Southwood S, Lees MB, and Kuchroo VK. 1996. Immunogenic and encephalitogenic epitope clusters of myelin proteolipid protein. J. Immunol 156: 371–379. [PubMed] [Google Scholar]
- 6.Encinas JA, Lees MB, Sobel RA, Symonowicz C, Greer JM, Shovlin CL, Weiner HL, Seidman CE, Seidman JG, and Kuchroo VK. 1996. Genetic analysis of susceptibility to experimental autoimmune encephalomyelitis in a cross between SJL/J and B10.S mice. J. Immunol 157: 2186–2192. [PubMed] [Google Scholar]
- 7.Tuohy VK, Lu Z, Sobel RA, Laursen RA, and Lees MB. 1989. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J. Immunol 142: 1523–1527. [PubMed] [Google Scholar]
- 8.Mendel I, Kerlero de Rosbo N, and Ben-Nun A. 1995. A myelin oligodendrocyte glycoprotein peptide induces typical chronic experimental autoimmune encephalomyelitis in H-2b mice: fine specificity and TCRVβ expression of encephalitogenic T cells. Eur. J. Immunol 25: 1951–1959. [DOI] [PubMed] [Google Scholar]
- 9.Adelmann M, Wood J, Benzel I, Fiori P, Lassmann H, Matthieu JM, Gardinier MV, Dornmair K, and Linington C. 1995. The N-terminal domain of the myelin oligodendrocyte glycoprotein (MOG) induces acute demyelinating experimental autoimmune encephalomyelitis in the Lewis rat. J. Neuroimmunol 63: 17–27. [DOI] [PubMed] [Google Scholar]
- 10.Linington C, Berger T, Perry L, Weerth S, Hinze-Selch D, Zhang Y, Lu HC, Lassmann H, and Wekerle H. 1993. T cells specific for the myelin oligodendrocyte glycoprotein mediate an unusual autoimmune inflammatory response in the central nervous system. Eur. J. Immunol 23: 1364–1372. [DOI] [PubMed] [Google Scholar]
- 11.Juedes AE, Hjelmstrom P, Bergman CM, Neild AL, and Ruddle NH. 2000. Kinetics and cellular origin of cytokines in the central nervous system: insight into mechanisms of myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis. J. Immunol. 164: 419–426. [DOI] [PubMed] [Google Scholar]
- 12.Karpus WJ, and Ransohoff RM. 1998. Chemokine regulation of experimental autoimmune encephalomyelitis: temporal and spatial expression patterns govern disease pathogenesis. J. Immunol 161: 2667–2671. [PubMed] [Google Scholar]
- 13.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, and Toda M. 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol 155: 1151–1164. [PubMed] [Google Scholar]
- 14.Asano M, Toda M, Sakaguchi N, and Sakaguchi S. 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med 184: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, Protschka M, Galle PR, Neurath MF, and Blessing M. 2004. Cutting edge: TGF-β signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J. Immunol 173: 6526–6531. [DOI] [PubMed] [Google Scholar]
- 16.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, de Vries RR, and Toes RE. 2005. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 52: 2212–2221. [DOI] [PubMed] [Google Scholar]
- 17.Kohm AP, Carpentier PA, Anger HA, and Miller SD. 2002. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol 169: 4712–4716. [DOI] [PubMed] [Google Scholar]
- 18.McGeachy MJ, Stephens LA, and Anderton SM. 2005. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J. Immunol 175: 3025–3032. [DOI] [PubMed] [Google Scholar]
- 19.Hori S, Nomura T, and Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science 299: 1057–1061. [DOI] [PubMed] [Google Scholar]
- 20.Khattri R, Cox T, Yasayko SA, and Ramsdell F. 2003. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol 4: 337–342. [DOI] [PubMed] [Google Scholar]
- 21.Reddy J, Illes Z, Zhang X, Encinas J, Pyrdol J, Nicholson L, Sobel RA, Wucherpfennig KW, and Kuchroo VK. 2004. Myelin proteolipid protein-specific CD4+CD25+ regulatory cells mediate genetic resistance to experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 101: 15434–15439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu P, Gregg RK, Bell JJ, Ellis JS, Divekar R, Lee HH, Jain R, Waldner H, Hardaway JC, Collins M, et al. 2005. Specific T regulatory cells display broad suppressive functions against experimental allergic encephalomyelitis upon activation with cognate antigen. J. Immunol 174: 6772–6780. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, and Weiner HL. 2004. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int. Immunol 16: 249–256. [DOI] [PubMed] [Google Scholar]
- 24.Dittmer U, He H, Messer RJ, Schimmer S, Olbrich AR, Ohlen C, Greenberg PD, Stromnes IM, Iwashiro M, Sakaguchi S, et al. 2004. Functional impairment of CD8+ T cells by regulatory T cells during persistent retroviral infection. Immunity 20: 293–303. [DOI] [PubMed] [Google Scholar]
- 25.Robertson SJ, Messer RJ, A. B., Carmody, and K. J. Hasenkrug. 2006. In vitro suppression of CD8+ T cell function by friend virus-induced regulatory T cells. J. Immunol 176: 3342–3349. [DOI] [PubMed] [Google Scholar]
- 26.Bluestone JA, and Abbas AK. 2003. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol 3: 253–257. [DOI] [PubMed] [Google Scholar]
- 27.Zhang GX, Xiao BG, Bakhiet M, van der Meide P, Wigzell H, Link H, and Olsson T. 1996. Both CD4+ and CD8+ T cells are essential to induce experimental autoimmune myasthenia gravis. J. Exp. Med 184: 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jun S, Gilmore W, Callis G, Rynda A, Haddad A, and Pascual DW. 2005. A live diarrheal vaccine imprints a Th2 cell bias and acts as an anti-inflammatory vaccine. J. Immunol 175: 6733–6740. [DOI] [PubMed] [Google Scholar]
- 29.Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, and Glimcher LH. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 80: 707–718. [DOI] [PubMed] [Google Scholar]
- 30.Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, and Kuchroo VK. 1998. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J. Immunol 161: 3299–3306. [PubMed] [Google Scholar]
- 31.Young DA, Lowe LD, Booth SS, Whitters MJ, Nicholson L, Kuchroo VK, and Collins M. 2000. IL-4, IL-10, IL-13, and TGF-β from an altered peptide ligand-specific Th2 cell clone down-regulate adoptive transfer of experimental autoimmune encephalomyelitis. J. Immunol 164: 3563–3572. [DOI] [PubMed] [Google Scholar]
- 32.Pascual DW, Hone DM, Hall S, van Ginkel FW, Yamamoto M, Walters N, Fujihashi K, Powell RJ, Wu S, VanCott JL, et al. 1999. Expression of recombinant enterotoxigenic Escherichia coli colonization factor antigen I by Salmonella typhimurium elicits a biphasic T helper cell response. Infect. Immun 67: 6249–6256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.St. Louis J, Uniyal S, Chan E, Singh B, Chan BM, and Strejan GH. 2001. Tolerance induction by acylated peptides: suppression of EAE in the mouse with palmitoylated PLP peptides. J. Neuroimmunol 115: 79–90. [DOI] [PubMed] [Google Scholar]
- 34.Carson MJ, Sutcliffe JG, and Campbell IL. 1999. Microglia stimulate naive T cell differentiation without stimulating T cell proliferation. J. Neurosci. Res 55: 127–134. [DOI] [PubMed] [Google Scholar]
- 35.Carson MJ 2002. Microglia as liaisons between the immune and central nervous systems: functional implications for multiple sclerosis. Glia 40: 218–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juedes AE, and Ruddle NH. 2001. Resident and infiltrating central nervous system APCs regulate the emergence and resolution of experimental autoimmune encephalomyelitis. J. Immunol 166: 5168–5175. [DOI] [PubMed] [Google Scholar]
- 37.Ponomarev ED, Shriver LP, Maresz K, and Dittel BN. 2005. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res 81: 374–389. [DOI] [PubMed] [Google Scholar]
- 38.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, Ziegler SF, and Miller SD. 2006. Cutting edge: anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J. Immunol 176: 3301–3305. [DOI] [PubMed] [Google Scholar]
- 39.Uyttenhove C, and Van Snick J. 2006. Development of an anti-IL17A auto-vaccine that prevents experimental auto-immune encephalomyelitis. Eur. J. Immunol 36: 2857–2867. [DOI] [PubMed] [Google Scholar]
- 40.Rohn TA, Jennings GT, Hernandez M, Grest P, Beck M, Zou Y, Kopf M, and Bachmann MF. 2006. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur. J. Immunol 36: 2857–2867. [DOI] [PubMed] [Google Scholar]
- 41.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, and Ghilardi N. 2006. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat. Immunol 7: 929–936. [DOI] [PubMed] [Google Scholar]
- 42.Sutton C, Brereton C, Keogh B, Mills KH, and Lavelle EC. 2006. A crucial role of interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med 203: 1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishgame H, Kakuta S, Sudo K, and Iwakura Y. 2006. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol 177: 566–573. [DOI] [PubMed] [Google Scholar]
- 44.Hofstetter HH, Ibrahim SM, Koczan D, Kruse N, Weishaupt A, Toyka KV, and Gold R. 2005. Therapeutic efficacy of IL-17 neutralization in murine experimental autoimmune encephalomyelitis. Cell. Immunol 237: 123–130. [DOI] [PubMed] [Google Scholar]
- 45.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, and Dong C. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol 6: 1069–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, and Murphy KM. 2006. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity 24: 677–688. [DOI] [PubMed] [Google Scholar]
- 47.Mekala DJ, Alli RS, and Geiger TL. 2005. IL-10-dependent suppression of experimental allergic encephalomyelitis by Th2-differentiated, anti-TCR redirected T lymphocytes. J. Immunol 174: 3789–3797. [DOI] [PubMed] [Google Scholar]
- 48.Mekala DJ, Alli RS, and Geiger TL. 2005. IL-10-dependent infectious tolerance after the treatment of experimental allergic encephalomyelitis with redirected CD4+CD25+ T lymphocytes. Proc. Natl. Acad. Sci. USA 102: 11817–11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Reddy J, Ochi H, Frenkel D, Kuchroo VK, and Weiner HL. 2006. Recovery from experimental allergic encephalomyelitis is TGF-β dependent and associated with increases in CD4+LAP+ and CD4+CD25+ T cells. Int. Immunol 18: 495–503. [DOI] [PubMed] [Google Scholar]
- 50.Faria AM, and Weiner HL. 2005. Oral tolerance. Immunol. Rev 206: 232–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chung Y, Lee SH, Kim DH, and Kang CY. 2005. Complementary role of CD4+CD25+ regulatory T cells and TGF-β in oral tolerance. J. Leukocyte Biol 77: 906–913. [DOI] [PubMed] [Google Scholar]
- 52.Huang X, Zhu J, and Yang Y. 2005. Protection against autoimmunity in non-lymphopenic hosts by CD4+CD25+ regulatory T cells is antigen-specific and requires IL-10 and TGF-β. J. Immunol 175: 4283–4291. [DOI] [PubMed] [Google Scholar]
- 53.Neurath MF, Fuss I, Kelsall BL, Presky DH, Waegell W, and Strober W. 1996. Experimental granulomatous colitis in mice is abrogated by induction of TGF-β-mediated oral tolerance. J. Exp. Med 183: 2605–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marie JC, Letterio JJ, Gavin M, and Rudensky AY. 2005. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med 201: 1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, and Wahl SM. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med 198: 1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skapenko A, Kalden JR, Lipsky PE, and Schulze-Koops H. 2005. The IL-4 receptor α-chain binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25 +CD4+ regulatory T cells from CD25−CD4+ precursors. J. Immunol 175: 6107–6116. [DOI] [PubMed] [Google Scholar]
- 57.Taylor PA, Lees CJ, and Blazar BR. 2002. The infusion of ex vivo activated and expanded CD4+CD25+ immune regulatory cells inhibits graft-versus-host disease lethality. Blood 99: 3493–3499. [DOI] [PubMed] [Google Scholar]
- 58.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, and O’Garra A. 2002. In vitro generation of interleukin 10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med 195: 603–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vieira PL, Christensen JR, Minaee S, O’Neill EJ, Barrat FJ, Boonstra A, Barthlott T, Stockinger B, Wraith DC, and O’Garra A. 2004. IL-10-secreting regulatory T cells do not express Foxp3 but have comparable regulatory function to naturally occurring CD4+CD25+ regulatory T cells. J. Immunol 172: 5986–5993. [DOI] [PubMed] [Google Scholar]
- 60.Marshall E 2006. Violent reaction to monoclonal antibody therapy remains a mystery. Science 311: 1688–1689. [DOI] [PubMed] [Google Scholar]