Abstract
Signaling pathways mediated by corticotropin-releasing factor and its receptor 1 (CRF1) play a central role in stress responses. Dysfunction of the CRF system has been associated with neuropsychiatric disorders. However, dynamic changes in the CRF system during brain development and aging are not well investigated. In this study, we characterized CRF1, CRF, and corticotropin-releasing factor binding protein (CRFBP) expression in different brain regions in both male and female C57BL/6J mice from 1 to 18 months of age under basal conditions as well as after an acute 2-hr-restraint stress. We found that CRF and CRF1 levels tended to increase in the hippocampus and hypothalamus, and to decrease in the prefrontal cortex with aging, especially at 18 months of age, whereas CRFBP expression followed an opposite direction in these brain areas. We also observed area-specific sex differences in the expression of these three proteins. For example, CRF expression was lower in females than in males in all the brain regions examined except the prefrontal cortex. After acute stress, CRF and CRF1 were up-regulated at 1, 6, and 12 months of age, and down-regulated at 18 months of age. Females showed more robust changes compared to males of the same age. CRFBP expression either decreased or remained unchanged in most of the brain areas following acute stress. Our findings suggest that brain CRF1, CRF, and CRFBP expression changes dynamically across the lifespan and under stress condition in a sex- and regional-specific manner. Sex differences in the CRF system in response to stress may contribute to the etiology of stress-related neuropsychiatric disorders.
Keywords: aging, corticotropin-releasing factor, CRF1, CRFBP, sex differences, stressed mice
1 ∣. INTRODUCTION
Corticotropin-releasing factor (CRF) signaling via CRF receptor 1 (CRF1) is involved in endocrine and behavioral responses to stress (Smith et al., 1998; Subbannayya et al., 2013). CRF1 and corticotropin-releasing factor receptor 2 (CRF2) belong to the same family and are characterized by their similar structure, consisting of seven transmembrane α-helical proteins with binding in the interior of the helical protein cone stimulating signal transduction via G proteins (Hillhouse & Grammatopoulos, 2006). CRF1 expression in the brain is higher than that of CRF2, particularly in the corticolimbic brain regions including prefrontal cortex, hippocampus, amygdala, and hypothalamus (Van Pett et al., 2000). Moreover, CRF has a greater binding affinity to CRF1 than to CRF2, implicating profound differences not only in the expression but also in the physiological function between these two receptors (Perrin et al., 1995). However, studies aiming to map CRF1 expression in rodents have mostly been conducted on rats (Avishai-Eliner, Yi, & Baram, 1996; Justice, Yuan, Sawchenko, & Vale, 2008; Tan, Vaughan, Perrin, Rivier, & Sawchenko, 2017; Van Pett et al., 2000), and are mainly limited to mRNA expression level. Much less is known about CRF1 expression and distribution in the mouse brain (Rosinger, Jacobskind, Park, Justice, & Zuloaga, 2017). Whether CRF1 expression displays dynamic changes with sex and regional differences during brain development and aging is still obscure.
In spite of the uncertainty about the mechanisms regulating CRF1 expression, various studies conducted mostly on rats indicated a strong transcriptional activation of the gene encoding CRF1 in the hypothalamus following different genetic manipulations or exposure to systemic stressors. More specifically, acute stress induces a marked and transient alteration of CRF1 mRNA levels in the hypothalamic paraventricular and the supraoptic nuclei (Bonaz & Rivest, 1998; Imaki et al., 2001; Imaki, Nahan, Rivier, Sawchenko, & Vale, 1991). Furthermore, CRF1 mRNA expression is reduced in the frontal cortex and increased in the hippocampus and in the hypothalamic paraventricular nucleus after chronic stress (Brunson, Grigoriadis, Lorang, & Baram, 2002; Iredale, Terwilliger, Widnell, Nestler, & Duman, 1996). CRF1 mRNA levels are increased by early-life stressors, namely maternal separation, in the dentate gyrus of Wistar–Kyoto versus Sprague–Dawley male rats (Bravo, Dinan, & Cryan, 2011). Studies in mice have shown that exposure to acute stress alters CRF1 mRNA expression in the hypothalamus and prefrontal cortex (Makino et al., 2005; Uribe-Mariño et al., 2016). However, most of the studies only included only few brain sub-regions with one or two time points. The mechanisms behind stress-dependent modulation of the CRF and related receptor signaling still need to be further investigated.
The hypothalamus plays a crucial role in stress response and it is considered the first station of the hypothalamic-pituitary-adrenal (HPA) axis; in fact, hypothalamus stimulates the hypophysis through CRF (McEwen, 1998). Once activated, the hypophysis secretes the adrenocorticotropic hormone which, stimulates the adrenal cortical gland responsible for the production of cortisol in humans and corticosterone in rodents (Chrousos, 1995). CRF binding protein (CRFBP) also plays an important role in stress responses, since it modulates the availability of CRF at CRFRs (Owens & Nemeroff, 1991). CRFBP is a 37-kDa secreted glycoprotein that binds CRF with high affinity; by inhibiting CRF activity, CRFBP is actively involved in the regulation of CRF function and homeostasis (Potter et al., 1991). The corticolimbic system also plays an essential role in the regulation of the stress response through negative feedback from the HPA axis (Vachon-Presseau, 2018). Both the prefrontal cortex and the hippocampus directly modulate the HPA axis (Radley & Sawchenko, 2011). For example, lesions in the prefrontal cortex facilitate HPA activity after exposure to acute stress (Diorio, Viau, & Meaney, 1993). Likewise, lesions of the hippocampus increase peripheral corticosteroid levels, whereas electrical stimulation of the hippocampus generates an opposite effect by reducing corticosterone (Jacobson & Sapolsky, 1991). The hippocampus exerts a constant basal tonic inhibition on the HPA axis (Fendler, Karmos, & Telegdy, 1961; Jacobson & Sapolsky, 1991). Also, a study indicated that glutamatergic and GABAergic neuronal fibers directly project from the amygdala, prefrontal cortex, and hippocampus to the hypothalamus (de Kloet, Joëls, & Holsboer, 2005), suggesting a fine balance in the modulation of stress response carried by these three regions. Despite this evidence, little is known about the expression of CRF1, CRF, and CRFBP in these brain regions in different physiological life stages as well as following stress. Bearing in mind the deep involvement of these brain regions and CRF system in stress response, understanding the potential dynamic changes and distribution of CRF1, CRF, and CRFBP is essential.
Previous studies suggested sex differences in the CRF signaling pathways and their response to stress (Bangasser et al., 2017; Bangasser, Wiersielis, & Khantsis, 2016). Sex differences in CRF1 expression seem vary both with brain region and species. For instance, CRF-positive cells are more numerous in the central amygdala of male rats (Karanikas, Lu, & Richardson, 2013), and in the preoptic area and BNST of female rats (Funabashi, Kawaguchi, Furuta, Fukushima, & Kimura, 2004; Lim, Nair, & Young, 2005; McDonald, Mascagni, & Wilson, 1994). Moreover, CRF1 expression is higher in the nucleus accumbens, olfactory tubercle, anterior cingulate and piriform cortex of adult female compared to male rats (Weathington, Hamki, & Cooke, 2014). Finally, some different studies on mice have shown a sexually dimorphic distribution of both CRF1 (Rosinger et al., 2017) and CRFBP (Speert, McClennen, & Seasholtz, 2002) as well as sex-specific differences in CRF1 cells in the hypothalamus (Rosinger et al., 2020; Rosinger, Jacobskind, Bulanchuk, et al., 2019; Rosinger, Jacobskind, De Guzman, Justice, & Zuloaga, 2019). However, sex differences in CRF, CRF1, and CRFBP expressions in the brain and how they respond to stress still need to be examined in more detail. Evaluation of sex differences in the distribution of CRF1 is fundamental to understanding and conceptualizing the observed differences in a variety of stress-related behavioral and hormonal responses reported in both rats and mice (Jasnow, Schulkin, & Pfaff, 2006; Pisu et al., 2016; Porcu & Morrow, 2014; Zuloaga, Puts, Jordan, & Breedlove, 2008). Sex differences in both CRF1 expression and function may contribute to the etiology of stress-related neurological and psychiatric disorders including anxiety, depression, and Alzheimer's disease (Valentino, Reyes, Van Bockstaele, & Bangasser, 2012; Yan, Dominguez, Fisher, & Dong, 2018), which are more prevalent in women than in men (Fisher, Bennett, & Dong, 2018; Seney & Sibille, 2014).
In this study, we first quantified CRF, CRF1, and CRFBP levels in different brain regions that are linked to stress regulation, namely prefrontal cortex, hippocampus, amygdala, and hypothalamus, at different ages including 1 month (youth), 6 months (adult), 12 months (post-menopausal), and 18 months (elderly), and investigated potential sex differences in CRF, CRF1, and CRFBP expression following acute-restraint stress. Our results demonstrate that CRF, CRF1, and CRFBP expression in response to stress is dynamic in the mouse brain, with sex- and age-dependent differences.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Animals
A total of 80 mice (C57BL/6J, Research Resource Identifier, RRID:IMSR_JAX:000664; 40 males and 40 females) at 1, 6, 12, or 18 months of age (n = 5 per group) from the Jackson Laboratory were used for this study (Figure 1a). Animals were housed in groups of 5 on a 12-hr light/dark cycle (lights on at 8:00 a.m.) and given food and water ad libitum. The vivarium temperature was kept at 25°C and the humidity near 65%. All procedures were performed according to NIH guidelines for the treatment of animals and the Current Guide for the Care and Use of Laboratory Animals (2011, 8th edition) under the protocol # IS0000543 approved by the Northwestern University Animal Care and Use Committee.
FIGURE 1.
Experimental design. (a) Total number of mice used in this study their distribution in each experimental group. (b) Time course of the experiments. m CO, male controls; f CO, female controls; m ST, male stress; f ST, female stress
2.2 ∣. Acute-restraint stress
Mice were habituated to the animal facility for at least 1 week prior to experiments. Before the experimental day, our animal care included an index assessment of health deficits across the integument, musculoskeletal system, ocular system, digestive/urogenital systems, respiratory system as well as assessment of discomfort, body weight, temperature, and food intake. Any age-related changes in our colony was consulted with a veterinarian. On the experimental day, mice assigned to stress groups were exposed to restraint stress for 2 hr. Stress sessions were conducted from 8 a.m. to 10 a.m. Briefly, stressed mice were placed in a well-ventilated Falcon 50 ml polypropylene conical tube (catalog # 14-959-49A, 114.4 mm long, 29.1 mm outer diameter; Fisher Scientific, 2019). The restrained mice were placed in their home cage during the 2-hr stress session. The stress procedure was performed in a sound-attenuated room adjacent to the housing room. Stressed mice were monitored every 30 min for the whole duration of the stress protocol. Non-stressed control mice were left undisturbed in their home cages and were allowed free access to food and water during the 2-hr stress session. For this study, we did not monitor the estrous cycle in females’ pre-mortem to avoid a bias because of the vaginal smear which could have interfered with the restraint stressor and, importantly, increased stress levels in non-stressed control mice. However, we evaluated the estrous cycle post mortem and we found the following results: (a) 1 month: no distinguishable phases detected; (b) 6 months: 1 female in estrous, 2 females in diestrous 1, 1 female in diestrous 2 and 1 female in proestrous in both non-stressed and stressed group; (c) 12 months: post-menopausal-like estrous cycle, with no distinguishable estrous phases detected; (d) 18 months: similar to what observed at 12 months, no distinguishable estrous phases detected. No exclusion criteria were applied nor animals died during the experiments; in addition, no randomization was performed to allocate subjects in the study. Based on previous evidence in the literature (Imaki et al., 2002; Rivest, Laflamme, & Nappi, 1995), we killed stressed mice 1.5 hr after the ending of restraint stress session to be able to measure stress-induced changes in the expression of CRF1. By following an alternating order, both stressed and non-stressed mice were sacrifice by injection of Euthasol® solution to induce painless pentobarbital death (catalog # 200-071; Virbac, 2019) followed by decapitation. As previously described by our group (Locci & Pinna, 2019; Rodríguez et al., 2017), brains were rapidly removed from the skull using fine surgical tools including iris scissors and narrow pattern forceps (Fine Science Tools). The prefrontal cortex, hippocampus, amygdala, and hypothalamus were dissected on ice using curved serrated forceps and a scalpel (Fine Science Tools). An electric fluorescent magnifier (Lighting Specialties) with cold light pointed toward the brain was used during the entire procedure to facilitate brain dissection. After being separated from the rest of the brain, regions were immediately transferred into 2 ml sterile tubes (Fisher Scientific) and frozen at −80°C until analyses (Figure 1b).
2.3 ∣. Western blot
The abundance of CRF1 was determined in lysates of prefrontal cortex, hippocampus, amygdala, and hypothalamus. Protein extraction was performed by homogenizing brain tissues in a mix of ice-cold RIPA buffer (catalog # R0278; Sigma-Aldrich, 2019) and protease inhibitor cocktail solution (catalog # Pl78410; Fisher Scientific, 2019). Tissues were processed first using a cordless motor connected to a Teflon pestle (20 s; catalog # 12-141-362; Fisher Scientific, 2017) followed by rapid sonication with Branson 450 Digital Sonifier (amplitude 70%, 2–3 s; catalog # B450; Marshall Scientific, 2018). Samples were then centrifuged at 20,000 g for 10 min at 4°C and supernatants were collected for determination of total protein concentration. Protein content was measured using the Pierce™ BCA protein assay kit (catalog # PIA53226; Fisher Scientific, 2019). An equal amounts of proteins (20 μg) were loaded and resolved through electrophoresis in 10% Criterion™ TGX Stain-Free™ Precast Gels at 100 V for 1.5 hr (catalog # 5671035; Biorad, 2019). Proteins were transferred onto a polyvinylidenedifluoride membrane using TransBlot® Semi-Dry Electrophoretic Transfer Cell at 15 V for 1.5 hr. Blots were exposed to 5% non-fat dry milk as b locking solution for 1 hr at room temperature (22°C) and immunostained overnight at 4°C with primary antibodies against CRF1 (1:1,000; catalog # NBP2-16010; Novus Biologicals, 2019), CRF (1:2000; RRID:AB_572228; Immunostar, 2020), CRFBP (1:2000; catalog # LS-B15599-50; LSBio2020), and β-actin (1:1,000; RRID:AB_2714189; Santa Cruz Biotechnology, 2019). Additionally, we confirmed the absence of effects of aging, sex, and stress on β-actin expression, by conducting parallel experiments using GAPDH primary antibody (1:1,000; RRID:AB_627679; Santa Cruz Biotechnology, 2019) as protein of control (data not shown). Membranes were incubated with secondary goat anti-rabbit (1:3,000; RRID:AB_11125345; Biorad, 2019) or anti-mouse (1:10,000; RRID:AB_11125936; Biorad, 2019) HRP-conjugated antibodies for 2 hr at room temperature. After applying SuperSignal™ West Dura HRP substrate (Fisher Scientific) onto the membranes, blotted proteins were detected by Gel Doc EQ System Universal Hood II (Biorad), and the densitometric signal were finally quantified by ImageJ software (https://imagej.nih.gov/ij/download.html). The levels of CRF1 were normalized to β-actin. Importantly, all the experiments were repeated twice to confirm the first results obtained. Custom-made materials will be shared upon reasonable request. This study was exploratory and no blinding nor sample calculation was performed. The sample size of each group was determined by referring to a previous key publication (Rosinger et al., 2017), which shows sex differences in brain CRF1 expression.
2.4 ∣. Statistical analysis
Graphpad Prism 7 software (RRID:SCR_002798, San Diego, CA, 2016) was used for statistical analyses. The significant difference between the experimental groups was assessed using a two-way analysis of variance (ANOVA), followed by Tukey's post hoc test. Data represent mean values ± SEM. No samples were excluded for data analysis. Verification of normal data distribution was performed using the free tool http://www.statskingdom.com/320ShapiroWilk. html that applies the Shapiro–Wilk test. Then, to identify potential outliers, we adopted the free tool https://www.graphpad.com/quick calcs/Grubbs1.cfm that applies the Grubbs test. This study was not pre-registered.
3 ∣. RESULTS
3.1 ∣. CRF1, CRF, and CRFBP expression changes in the corticolimbic system across the lifespan
3.1.1 ∣. Prefrontal cortex
Two-way ANOVA showed an effects of age (F3,32 = 22.32, p < .001), and sex (F1,32 = 7.65, p = .009) in CRF1 expression in the prefrontal cortex of C57BL/6J mice (Figure 2a). Post hoc analysis showed that its levels were the highest at 1 and 6 months of age, then were significantly reduced in both male and female mice at 12 (−59%, p = .027; −62%, p < .0001, respectively), and 18 months (−58%, p = .003; −51%, p = .0006, respectively) compared to 1-month-old group. We also observed no significant differences in the expression of CRF1 in the prefrontal cortex of females compared to males.
FIGURE 2.
Fluctuations of CRF1, CRF, and CRFBP expression in different corticolimbic regions of unstressed male and female mice across the lifespan. (a) As reported here, CRF1 is significantly down-regulated in the prefrontal cortex during the late stages of lifespan in both male (black bars) and female (white bars) mice. On the other hand, CRF1 expression is increased in the hippocampus of both males and females at 6, 12, and 18 months. The expression of CRF1 was found unaltered during the lifespan in the amygdala of C57BL/6J mice. Finally, CRF1 is up-regulated in the hypothalamus of 18-month-old mice. (b) CRF is down-regulated in the prefrontal cortex of both males and females at 6 and 12 months of age compared to 1-month-old mice, and, interestingly, it is more expressed in the prefrontal cortex of females at 1 and 18 months compared to males at same age. CRF expression is higher in the hippocampus of 6-month-old female mice compared to females at 1 month, and it is less abundant in females at 12 months compared to 12-month-old males. Moreover, CRF levels are lower in the amygdala of both males and females at 6, 12, and 18 months of age, whereas 1-, 6-, and 18-month-old female mice exhibit lower CRF levels compared to males at same age. Finally, CRF expression is higher in the hypothalamus of male mice at 6 and 18 months, and in females at 6, 12, and 18 months of age. Interestingly, hypothalamic CRF shows sex differences at 6 months. (c) CRFBP is up-regulated in the prefrontal cortex of both males and females at 12 and 18 months of age. On the other hand, CRFBP levels are reduced in the late life stages of both males and females in the hippocampus and amygdala. Finally, hypothalamic CRFBP is up-regulated in 12-month-old males, and down-regulated in 18-month-old females. Of note, both amygdala and hypothalamus are characterized by sex differences in CRFBP abundance at 18 months of age. CRF1: 51 kDa; CRF: 18 kDa; CRFBP: 37 kDa; β-actin: 43 kDa. Data represent the mean ± SEM of 5 mice. *p < .05, **p < .01, and ***p < .001 when compared with 1-month-old male mice; #p < .05, ##p < .01, and ###p < .001 when compared with 1-month-old female mice; °p < .05, °°p < .01, and °°°p < .001 when compared with male mice at same age. Two-way ANOVA followed by Tukey's post hoc analysis
CRF levels were strongly altered across the lifespan in the prefrontal cortex of C57BL/6J mice by age (F3,32 = 32.7, p < .0001), sex (F1,32 = 26.23, p < .0001), and age x sex interaction (F3,32 = 2.94, p = .047) (Figure 2b). Post hoc test suggested that the highest CRF levels were at 1 month of age, then were significantly reduced in both male and female mice at 6 (−60%, p = .003; −69%, p < .0001, respectively), and 12 months (−57%, p = .002; −64%, p < .0001, respectively). Interestingly, we found higher levels of CRF1 in the prefrontal cortex of females compared to males at 1 (+65% p = .0004), and 18 months (+60%, p = .005).
Finally, we found that age (F3,32 = 16.41, p < .0001) induced marked changes in CRFBP expression across different life stages in the prefrontal cortex (Figure 2c). Specifically, post hoc analysis revealed that CRFBP abundance gradually increased across the lifespan in both male and female mice, however, differences were significant only in 12 (+85%, p = .03; +124%, p = .01, respectively), and 18-month-old mice (+157%, p = .0004; +147%, p = .003, respectively). CRFBP expression did not change between males and females at any time point.
3.1.2 ∣. Hippocampus
Hippocampal CRF1 expression varied with age (F3,32 = 37.47, p < .0001), and sex (F1,32 = 11.95, p = .002) (Figure 2a). A post hoc test revealed a significant increase in CRF1 expression in both male and female mice at 6 (+97%, p = .002; +74%, p = .0013, respectively), 12 (+94%, p = .003; +67%, p = .004, respectively), and 18 months of age (+178%, p < .0001; +137%, p < .0001, respectively) when compared with the 1-month-old mice group. In line with the results obtained in the prefrontal cortex, hippocampal CRF1 expression did not exhibit significant sex differences.
When we evaluated CRF expression across the lifespan in the hippocampus of male and female mice, we found an effect of age (F3,32 = 4.79, p = .007), and age x sex interaction (F3,32 = 5.39, p = .004) (Figure 2b). Post hoc test showed that CRF levels increased at 6 months of age in female mice (+45%, p = .048). In terms of sex differences, we observed a slight trend toward differences between males and females at 6 months (+40%, p = .07); moreover, we observed a significant difference at 12 months of age (−36%, p = .039).
CRFBP expression showed marked dynamic changes at different life stages in C57BL/6J mice. A two-way ANOVA showed an effect of age (F3,32 = 43.49, p < .0001), and a trend toward an age x sex interaction (F3,32 = 2.85, p = .052) (Figure 2c). In fact, CRFBP was significantly down-regulated in 6-month-old males (−51%, p < .0001) but not females; moreover, CRFBP levels were lower in both male and female mice at 12 (−61%, p < .0001; −35%, p = .015, respectively), and 18 months (−81%, p < .0001; −75%, p < .0001, respectively). Similar to what observed in the prefrontal cortex, CRFBP expression was not different between males and females at any time point.
3.1.3 ∣. Amygdala
Surprisingly, the levels of CRF1 expression were not significantly altered across the lifespan in the amygdala within either male or female mice (Figure 2a), nor was CRF1 differentially expressed in the amygdala between male versus female mice.
On the other hand, CRF levels showed marked changes at different time points in the amygdala of C57BL/6J mice. A Two-way ANOVA suggested an effect of age (F3,32 = 84.48, p < .0001), and sex (F1,32 = 20.11, p < .0001) (Figure 2b). A post hoc test showed the highest CRF expression at 1 month of age, then significantly reduced levels in both male and female mice at either 6 (−54%, p < .0001; −61%, p < .0001, respectively), 12 (−66%, p < .0001; −66%, p < .0001, respectively), and 18 (−71%, p < .0001; −72%, p < .0001) months of age. Notably, we found lower levels of CRF in the amygdala of females compared to males; differences were statistically significant at any time point except 12 months (1 month: −24% p = .002; 6 months; −35%, p < .0001; 12 months: −24%, p = .094; 18 months: −27%, p = .049).
CRFBP expression was changed in the amygdala by age (F3,32 = 22.29, p < .0001), and sex (F1,32 = 4.94, p = .033), with a trend toward age x sex interaction (F3,32 = 2.75, p = .059) (Figure 2c). A post hoc analysis demonstrated that CRFBP levels slightly decreased across the lifespan in both male and female mice, although differences were significant exclusively in 12- and 18-month-old male (−31%, p = .028; −74%, p < .0001, respectively), and in 18-month-old females (−48%, p = .006). CRFBP expression significantly differed between males and females at 18 months of age (+52%, p = .034).
3.1.4 ∣. Hypothalamus
A two-way ANOVA indicated an age effect on CRF1 expression also in the hypothalamus (F3,32 = 8.33, p = .0003) and a trend of sex (F1,32 = 3.97, p = .054) (Figure 2a). CRF1 levels were higher in both male and female mice at 18 months (+49%, p = .027; +50%, p = .006, respectively) when compared with 1-month-old group. The hypothalamus did not show significant sex differences in terms of CRF1 abundance.
Hypothalamic CRF levels were strongly affected by age (F3,32 = 19.91, p < .0001), and by age x sex interaction (F3,32 = 7.35, p < .0001), as schematized by Figure 2b. More specifically, Tukey's analysis showed that CRF levels raised in both males and females mice at 6 months (+129%, p < .0001; +98%, p = .027, respectively), and 18 months (+79%, p = .012; +228%, p < .0001, respectively); furthermore, CRF expression was significantly higher in females (+152%, p = .0003) but not in males (+51%, p = .17) at 12 months. Of note, we observed significant sex differences at 6 months of age (−38%, p = .004), and only a trend at 18 months of age (+33%, p = .07).
Finally, we found that age (F3,32 = 10.21, p < .0001) significantly affected CRFBP expression in the hypothalamus, effect associated with an age x sex significant interaction (F3,32 = 3.85, p = .02) (Figure 2c). Indeed, post hoc test revealed that CRFBP abundance increased in 12-month-old males (+60%, p = .006), and decreased in 18-month-old females (−51%, p = .0008) when compared with the sex-respective control groups at 1 month of age. Moreover, CRFBP expression was lower in females than males at 18 months (−30%, p = .035). The trend toward an increase observed in 1-month-old females was not statistically significant (+44%, p = .058).
Taken together, these data suggest that CRF1, CRF, and CRFBP expression is subject to dynamic sex-specific fluctuations in different corticolimbic brain regions across the lifespan in mice.
3.2 ∣. Acute-restraint stress alters brain CRF1, CRF, and CRFBP expression in a sex-specific manner
Next, we wanted to evaluate whether CRF1, CRF, and CRFBP levels are altered by acute stress (a) at different ages, (b) in different brain sub-regions, and (c) in a sex-specific manner.
3.2.1 ∣. Prefrontal cortex
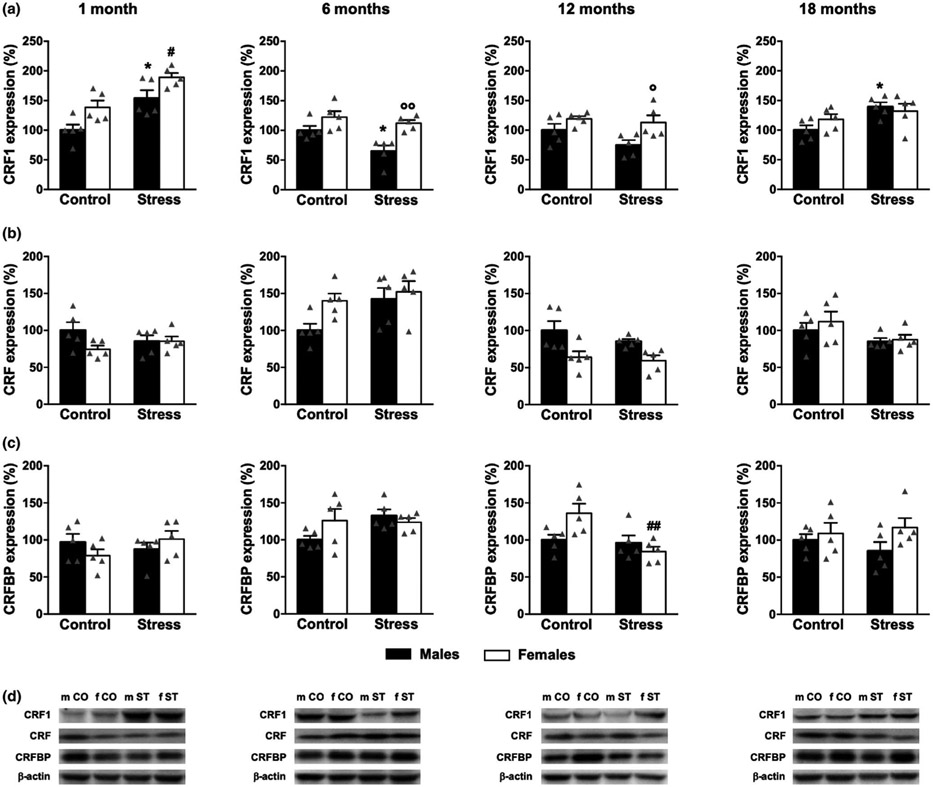
Two-way ANOVA indicated a significant sex effect induced by 2-hr-restraint stress on CRF1 levels at 1 (F1,16 = 6.24, p = .024), and 6 months (F1,16 = 4.58, p = .048). Moreover, stress had a strong effect of stress on CRF1 expression (1 month: F1,16 = 58.04, p < .0001; 6 months: F1,16 = 20.49, p = .0003; 12 months: F1,16 = 17.90, p = .0006; 18 months: F1,16 = 46.47, p < .0001) (Figure 3a). Specifically, Tukey's post hoc test confirmed that CRF1 was up-regulated in the prefrontal cortex of 1-, 6-, 12-, and 18-month-old male (+129%, p = .001; +74%, p = .016; +62%, p = .034; +167%, p = .0003, respectively) as well as female mice (+120, p = .0001; +47%, p = .041; +49%, p = .044; +102%, p = .003, respectively).
FIGURE 3.
Effect of acute stress on CRF1, CRF, and CRFBP expression in the prefrontal cortex of male and female mice. Mice were exposed to 2-hr-restraint stress and sacrificed 1.5 hr after the end of the stress procedure. (a) As reported in this graph, CRF1 expression significantly increases in both males (black bars) and females (white bars) at the age of 1, 6, 12, and 18 months following acute stress. (b) CRF levels significantly increase exclusively in 1-month-old stressed males. (c) CRFBP expression changes only in 18-month-old male mice under stress conditions. (d) Representative bands for CRF1, CRF, CRFBP, and β-actin. m CO, male controls; f CO, female controls; m ST, male stress; f ST, female stress. Values are reported in percentage; average of control male mice is considered as 100%. CRF1: 51 kDa; CRF: 18 kDa; CRFBP: 37 kDa; β-actin: 43 kDa. Data represent the mean ± SEM of 5 mice. *p < .05, **p < .01, and ***p < .001 when compared with control male mice; #p < .05, ##p < .01, and ###p < .001 when compared with control female mice. Two-way ANOVA followed by Tukey's post hoc analysis
There was a trend toward a sex effect (F1,16 = 3.99, p = .06), and sex × stress interaction (F1,16 = 17.12, p = .0008) in CRF expression in the prefrontal cortex at 1 month of age. CRF expression did not significantly change at 12 months of age. Finally, in 6-and 18-month-old mice, two-way ANOVA indicated a significant sex × stress interaction (F1,16 = 1.89, p = .019; F1,16 = 13.16, p = .002, respectively). Post hoc analysis suggested that CRF levels were significant higher only in the prefrontal cortex of 1-month-old males compared to non-stressed control group (+56%, p = .009). Instead, CRF expression was not significantly altered in the late life stages by acute stress.
We lastly studied the effects induced by 2-hr-restraint stress on CRFBP expression. A two-way ANOVA revealed a significant effect of stress (F1,16 = 4.96, p = .041), and sex × stress interaction (F1,16 = 4.54, p = .048) exclusively in 18-month-old mice (Figure 3c). Post hoc analysis revealed that CRFBP expression was significantly down-regulated by stress only in 18-month-old male males (−41%, p = .033). CRFBP expression did not differ between males and females under stress condition at any time point.
3.2.2 ∣. Hippocampus
Figure 4a shows the effects of acute-restraint stress on hippocampal CRF1 levels. We found an effect of sex (F1,16 = 11.93, p = .003), and stress (F1,16 = 23.63, p < .001) at 1 month. CRF1 expression was also affected by sex (F1,16 = 17.63, p = .003), and stress (F1,16 = 7.45, p = .015) at 6 months of age. Furthermore, at 12 months, sex (F1,16 = 9.32, p = .008) had a significant effect on CRF1 expression. Finally, an effect of stress (F1,16 = 7.79, p = .013) on CRF1 expression was observed in 18-month-old mice. A more detailed post hoc analysis revealed increased CRF1 expression following stress in both 1-month-old males and females (+54%, p = .013; +36%, p = .02, respectively). CRF1 was significantly altered by acute stress in the hippocampus of males at 6 and 18 months of age (−35%, p = .038; +39%, p = .044, respectively), an effect not observed in females. CRF1 expression did not significantly change in the hippocampus of 12-month-old males and females. Importantly, we found sex differences in stress response comparing CRF1 levels of stressed female versus stressed male mice at 6 and 12 months (+73%, p = .005; +49%, p = .047, respectively).
FIGURE 4.
Effect of acute stress on CRF1, CRF, and CRFBP expression in the hippocampus of male and female mice. Mice were exposed to 2-hr-restraint stress and killed 1.5 hr after the end of the stress procedure. (a) As shown here, CRF1 levels change in both males (black bars) and females (white bars) at the age of 1 month after exposure to acute stress. Moreover, stress alters CRF1 expression only in males at 6 and 18 months of age. Importantly, these findings suggest sex difference in CRF1 levels between males-stressed and females-stressed mice at the age of 6 and 12 months. (b) Surprisingly, CRF levels are not affected by acute-restraint stress. (c) CRFBP expression changes only in the hippocampus of 12-month-old female mice under stress conditions. (d) Representative bands for CRF1, CRF, CRFBP, and β-actin. m CO, male controls; f CO, female controls; m ST, male stress; f ST, female stress. Values are reported in percentage; average of control male mice is considered as 100%. CRF1: 51 kDa; CRF: 18 kDa; CRFBP: 37 kDa; β-actin: 43 kDa. Data represent the mean ± SEM of 5 mice. *p < .05 when compared with control male mice; #p < .05, and ##p < .01 when compared with control female mice; °p < .05, and °°p < .01 when compared with stressed male mice. Two-way ANOVA followed by Tukey's post hoc analysis
As shown in Figure 4b, acute stress induced just mild effects on hippocampal CRF expression. In fact, at 6 months of age, two-way ANOVA revealed a trend toward an effect of sex (F1,16 = 4.01, p = .06), and a significant effect of stress (F1,16 = 4.91, p = .041). Moreover, CRF levels were altered by sex (F1,16 = 13.21, p = .002) at 12 months. Finally, we observed only a trend for stress effect (F1,16 = 4.42, p = .052) in 18-month-old mice. Post hoc analysis indicated that the trends observed in 6-month old male mice was not statistically significant (+42%, p = .11). On the other hand, we did not observe stress-related sex differences in the hippocampal expression of CRF.
Significant changes induced by 2-hr-restraint stress (F1,16 = 8.57, p = .009), and sex × stress interaction (F1,16 = 6.25, p = .024) on CRFBP expression were specifically observed in 12-month-old mice. At 6 months, two-way ANOVA suggested only a trend toward sex × stress interaction (F1,16 = 3.25, p = .09) (Figure 4c). Concomitantly, a post hoc analysis confirmed that CRFBP was specifically down-regulated in the female hippocampus exposed to acute stress at 12 months of age (−38%, p = .007). We did not find significant sex differences in CRFBP expression under stress conditions.
3.2.3 ∣. Amygdala
Figure 5a summarizes the results obtained in the amygdala on CRF1 expression. We found no significant effects in 1-month-old mice. Instead, at 6 months, CRF1 expression was influenced by sex (F1,16 = 23.64, p = .0002), stress (F1,16 = 32.49, p < .001), and sex × stress interaction (F1,16 = 7.38, p = .015). Notably, two-way ANOVA indicated an effect of sex (F1,16 = 13.16, p = .002), and a trend toward a sex × stress interaction (F1,16 = 3.07, p = .09) at 12 months. Finally, CRF1 levels were affected by sex (F1,16 = 8.64, p = .009) at 18 months of age, whereas stress showed only a slight trend (F1,16 = 2.83, p = .11). By performing post hoc analysis, we observed that restraint stress drastically increased CRF1 expression exclusively in 6-month-old female mice (+91%, p = .0001). In agreement with the results obtained in the hippocampus, here we observed sex differences in stress response, as demonstrated by the higher levels of CRF1 in the amygdala of females versus males at the age of 6, 12, and 18 months (+75%, p = .0003; +76%, p = .008; +43%, p = .047, respectively).
FIGURE 5.
Effect of acute stress on CRF1, CRF, and CRFBP expression in the amygdala of male and female mice. Mice were exposed to 2-hr-restraint stress and killed 1.5 hr after the end of the stress procedure. (a) As shown in this graph, CRF1 expression changes in females (white bars) but not in males (black bars) at the age of 6 months after exposure to acute stress. Of note, data show a specific sex difference in CRF1 expression between males-stressed and females-stressed mice at the age of 6, 12, and 18 months. (b) CRF abundance is higher only in 6-month-old stressed male mice. Of note, amygdala shows stress-related sex differences at 6 and 12 months of age. (c) CRFBP expression does not change between males and females after acute stress at any time point. (d) Representative bands for CRF1, CRF, CRFBP, and β-actin. m CO, male controls; f CO, female controls; m ST, male stress; f ST, female stress. Values are reported in percentage; average of control male mice is considered as 100%. CRF1: 51 kDa; CRF: 18 kDa; CRFBP: 37 kDa; β-actin: 43 kDa. Data represent the mean ± SEM of five mice. ***p < .001 when compared withcontrol male mice; ###p < .001 when compared with control female mice; °p < .05, °°p < .01, and °°°p < .001 when compared with stressed male mice. Two-way ANOVA followed by Tukey's post hoc analysis
We explored the effect of acute-restraint stress on amygdala CRF expression. Two-way ANOVA showed a significant sex × stress interaction (F1,16 = 5.95, p = .03) at 1 month, as well as a significant effect of sex (F1,16 = 110.6, p < .0001), stress (F1,16 = 15.37, p = .012), and sex × stress interaction (F1,16 = 10.83, p = .005) at 6 months. In addition, 12-month-old mice were affected by sex (F1,16 = 36.09, p < .0001). Finally, 18-month-old mice were influenced by sex (F1,16 = 11.85, p = .003), and sex × stress interaction (F1,16 = 8.21, p = .011) (Figure 5b). More in details, post hoc analysis suggested that CRF was up-regulated in the amygdala of 6-month-old male mice (+35%, p = .02). Moreover, we observed stress-induced sex differences at 6 and 12 months, as demonstrated by the lower CRF levels in females compared to males (−35%, p < .0001; −24%, p = .0001, respectively).
As reported in Figure 5c, sex (F1,16 = 5.04 p = .04) seemed to affect amygdalar CRFBP expression at 1 month. At 6 months, two-way ANOVA suggested a significant sex × stress interaction (F1,16 = 5.54, p = .03). Furthermore, sex had a significant effect on amygdalar CRFBP expression at 12 months (F1,16 = 4.82, p = .04). We observed an effect of sex (F1,16 = 6.21, p = .02), and a trend toward sex × stress interaction (F1,16 = 3.31, p = .09) at 18 months of age. However, Tukey's post hoc test indicated only a trend toward a decrease in 6-month-old stressed males compared to control males (−34%, p = .09). In addition, post hoc analysis confirmed the absence of stress-related sex differences in CRFBP expression at each time point.
3.2.4 ∣. Hypothalamus
Lastly, we evaluated the effect of acute stress on hypothalamic CRF1 expression. Whereas a two-way ANOVA showed no effects at 1 month, stress significantly altered CRF1 in 6-month-old mice (F1,16 = 5.09, p = .038). Sex (F1,16 = 14.01, p = .002), stress (F1,16 = 9.78, p = .007), and sex × stress interaction (F1,16 = 5.98, p = .027) had a significant effect at 12 months of age. Moreover, at 18 months, two-way ANOVA suggested a significant sex × stress interaction (F1,16 = 8.21, p = .011) (Figure 6a). Specifically, stress altered CRF1 levels only in female mice at 12 (+48%, p = .006), and 18 (−25%, p = .036) months. Intriguingly, we observed a significant increase in CRF1 expression in 12-month old females versus males at the same time point (+56%, p = .002).
FIGURE 6.
Effect of acute stress on CRF1, CRF, and CRFBP expression in the hypothalamus of male and female mice. Mice were exposed to 2-hr-restraint stress and killed 1.5 hr after the end of the stress procedure. (a) As reported here, CRF1 levels are significantly altered in females (white bars) but not in males (black bars) at the age of 12 and 18 months after exposure to acute stress. Interestingly, these results suggest a sex difference in CRF1 expression between males-stressed and females-stressed mice at the age of 12 months. (b) CRF levels change after acute stress in 1-month-old males as well as 1-, 6-, and 18-month-old females. Interestingly, 12-month-old female mice show higher levels of hypothalamic CRF then stressed males at same age. (c) CRFBP expression is affected by acute stress in both males and females at 1 month of age. (d) Representative bands for CRF1, CRF, CRFBP, and β-actin. m CO, male controls; f CO, female controls; m ST, male stress; f ST, female stress. Values are reported in percentage; average of male control mice is considered as 100%. CRF1: 51 kDa; CRF: 18 kDa; CRFBP: 37 kDa; β-actin: 43 kDa. Data represent the mean ± SEM of 5 mice. *p < .05, and ***p < .001 when compared with control male mice; #p < .05, and ##p < .01, and ###p < .001 when compared with control female mice; °°p < .01 when compared with stressed male mice. Two-way ANOVA followed by Tukey's post hoc analysis
We also analyzed the effect induced by restraint stress on CRF levels. Two-way ANOVA showed a significant effect of stress (F1,16 = 41.41, p < .0001), and a trend towards a sex effect (F1,16 = 3.75, p = .07), at 1 month of age. A significant sex effect (F1,16 = 8.75, p = .009) was likewise present at 6 months, although we found no significant effect of stress (F1,16 = 2.63, p = .12), and sex × stress interaction (F1,16 = 3.35, p = .08). At 12 months of age, we found an effect of sex (F1,16 = 11.83, p = .003), stress (F1,16 = 20.7, p = .0003), and a trend toward sex × stress interaction (F1,16 = 3.45, p = .08). Also at 18 months of age, CRF levels were affected by stress (F1,16 = 13.41, p = .002), with a significant sex × stress interaction (F1,16 = 6.58, p = .02) (Figure 6b). Specifically, CRF expression was altered in the hypothalamus of 1-month-old males (−67%, p = .0003) as well as 1-, 12-, and 18-month-old females (−62%, p = .011; +70%, p = .0017; −47%, p = .002, respectively). In addition, we observed sex differences in stress response only at 12 months of age (+52%, p = .009).
As suggested by the results reported in Figure 6c, hypothalamic CRFBP expression was affected by sex (F1,16 = 4.87, p = .04), and stress (F1,16 = 30.72, p < .0001) at 1 month. On the other hand, two-way ANOVA only showed no significant stress effects in 6-month-old mice. Similarly, at 12 months, stress did not induce any significant effect (F1,16 = 3.49, p = .08). In addition, two-way ANOVA indicated an effect of sex (F1,16 = 11.91, p = .003) at 18 months of age. Post hoc analysis confirmed that CRFBP was down-regulated by stress in both males (−52%, p = .04) and females (−59%, p = .0009) at 1 month. No sex differences under stress conditions were observed at any time point.
4 ∣. DISCUSSION
As summarized in Table 1, the main goal of this study was to systemically characterize CRF1, CRF, and CRFBP protein expression in corticolimbic regions of the mouse brain that are functionally linked to stress regulation, during brain development and aging. We found that CRF1 levels change in certain brain regions during specific life stages (Figure 2). In particular, CRF1 levels decreased in the prefrontal cortex at 12 and 18 months of age and increased in the hippocampus of 6-, 12-, and 18-month-old males and females compared to 1-month-old group. However, we did not observe changes in amygdalar CRF1 levels during aging, whereas a significant increase in CRF1 levels was found in both males and females at 18 months of age versus 1-month-old group. These results represent the first systematic characterization of CRF1 levels in the mouse at different ages including 1, 6, 12, and 18 months of age, which is analog to human life stages, namely youth, adult, post-menopausal period, and elderly. We also measured the expression of both CRF and CRFBP, two crucial components of the CRF system. Similar to what observed for CRF1, our results suggest that their levels change area-specifically across the lifespan (Figure 2). Indeed, CRF expression was lower in the prefrontal cortex of both males and females at 6 and 12 months as well as in the amygdala at 6, 12, and 18 months of age. On the other hand, CRF levels were higher in the hypothalamus during the late life stages in both males and females, and only in the hippocampus of 6-month-old females. CRFBP expression followed a peculiar trend across the lifespan. In fact, its levels gradually increased from the early to late stages in the prefrontal cortex of both males and females, whereas decreased in the hippocampus and amygdala. In the hypothalamus, CRFBP was up-regulated in male mice at 12 months of age, and, oppositely, down-regulated in 18-month-old females. Furthermore, it is important to underlie that we observed sex differences in CRF expression at different time points in all these four brain regions. Finally, CRFBP expression showed sex differences in both amygdala and hypothalamus at 18 months of age.
TABLE 1.
Schematic summary of the findings on CRF1, CRF, and CRFBP expression in the mouse prefrontal cortex (PFC), hippocampus (HIPPO), amygdala (AMY), and hyoothalam (HYPO)
| AGE (↓↑compared to 1 m of age) | SEXa | STRESS (↓↑compared to controls) | SEXb | |
|---|---|---|---|---|
| PFC | ||||
| CRF1 | ♂&♀ (12 m, 18 m) ↓ | = | ♂&♀ (1 m, 6 m, 12 m, 18 m) ↑ | = |
| CRF | ♂&♀ (6 m, 12 m) ↓ | ♀<♂ (1 m, 18 m) | ♂ (1 m) ↑ | = |
| CRFBP | ♂&♀ (12 m, 18 m) ↑ | = | ♂ (18 m) ↓ | = |
| HIPPO | ||||
| CRF1 | ♂&♀ (6 m, 12 m, 18 m) ↑ | = | ♂&♀ (1 m) ↑; ♂ (6 m) ↓; ♂ (18 m) ↑ | ♀>♂ (6 m, 12 m) |
| CRF | ♀ (6 m) ↑ | ♀<♂ (12 m) | = | = |
| CRFBP | ♂ (6 m, 12 m, 18 m) ↓; ♀ (12 m, 18 m) ↓ | = | ♀ (12 m) ↓ | = |
| AMY | ||||
| CRF1 | = | = | ♀ (6 m) ↑ | ♀>♂ (6 m, 12 m, 18 m) |
| CRF | ♂&♀ (6 m, 12 m, 18 m) ↓ | ♀<♂ (1 m, 6 m, 18 m) | ♂ (6 m) ↑ | ♀<♂ (6 m, 12 m) |
| CRFBP | ♂ (12 m, 18 m) ↓; ♀ (18 m) ↓ | ♀>♂ (18 m) | = | = |
| HYPO | ||||
| CRF1 | ♂&♀ (18 m) ↑ | = | ♀ (12 m) ↑; ♀ (18 m) ↓ | ♀ >♂ (12 m) |
| CRF | ♂&♀ (6 m, 18 m) ↑; ♀ (12 m) ↑ | ♀<♂ (6 m) | ♂&♀ (1 m) ↓; ♀ (12 m) ↑; ♀ (18 m) ↓ | ♀>♂ (12 m) |
| CRFBP | ♂ (12 m) ↑; ♀ (18 m) ↓ | ♀<♂ (18 m) | ♂&♀ (1 m) ↓ | = |
Note: AGE = changes across the lifespan; SEXa = sex differences across the lifespan; STRESS = changes in response to 2-hr-restraint stress; SEXb = sex differences in response to 2-hr-restraint stress.
Our results suggest a sub-brain region-dependent plasticity of CRF1 across the lifespan; exploring the regional-specific function of CRF1 may be particularly important in the optic of discovering an unknown pathophysiological axis CRF1-related. Interestingly, we observed opposite trends in the levels of CRF1 in the prefrontal cortex compared to the hippocampus. This may suggest a distinguished physiological function of CRF1 in these two different corticolimbic regions during life span. For example, it might be possible that higher levels of CRF1 are necessary in the early life stages in the prefrontal cortex to guarantee a correct physiological regulation of brain function, whereas the increased CRF1 expression in the hippocampus with aging in response to environment stressors. Although the physiological meanings of CRF1 levels in regional- and age-specific manner remain unknown, it is possible that perturbations in the physiological corticolimbic distribution of CRF1 may drive aberrant brain functions which, ultimately, may be associated with abnormal stress response and, in sensitive subjects, the onset of stress-related neuropsychopathologies such as anxiety and depression.
We also found a general trend of increased CRF1 basal levels in females at any age and region compared to males (Figure 2). Although this statement might oversimplify the physiological role of CRF in the brain, a plausible hypothesis is that higher CRF1 levels ‘ in the female brain may contribute to rendering them more sensitive to stress. However, this speculation needs to be confirmed by other techniques and future studies using different approaches such as immunohistochemistry and RT-PCR will be performed to confirm such sex-biased CRF1 expression. Indeed, sex differences in CRF receptor abundance have been identified in several brain regions studied of rats under basal physiological conditions (Weathington et al., 2014). In particular, CRF1 binding is greater in adult female than male rats in regions implicated in depression such as the cortex, amygdala, and nucleus accumbens (Weathington et al., 2014). The functional and physiological relevance of the cited sex differences still remain largely obscure and surely need further exploration.
In addition to CRF1, here we report evidence for brain CRF and CRFBP protein expression being subject to time-dependent dynamic fluctuations in the mouse corticolimbic system (Figure 2). However, only few previous studies described fluctuations in CRF expression in the rat brain across the lifespan. For instance, Kasckow and colleagues suggested that CRF mRNA levels are significantly lower at 24 months in the hypothalamus, at 11,17, and 24 months in the amygdala, and at 17 and 24 months in the BNST compared to 3-month-old male rats (Kasckow, Regmi, Mulchahey, Plotsky, & Hauger, 1999). The same group also demonstrated that the number of CRFBP-containing cells was lower in the basolateral and lateral nucleus of the amygdala of 24-month-old rats compared to 4-month-old rats (Xiao et al., 2006). Moreover, both 4- and 12-month-old rats showed greater CRF immunoreactivity in the central amygdala compared to 24-month-old rats (Xiao et al., 2006). One study reported higher CRFBP mRNA levels in the pituitary of 2-month-old females than males of the same age (Speert et al., 2002). In said study, CRFBP mRNA expression dynamically changed during estrous cycle, effect dependent on estrogen regulation (Speert et al., 2002). From this perspective, sex differences observed by our group during the late stages (“post-menopausal period”) may be partially explained by the drop in the circulating levels of sex hormones. Additional studies are necessary to determine factors and molecular mechanisms behind sex differences in CRF system.
In this study, in addition to the comparison of the sex differences in CRF system across the lifespan, we also demonstrate the changes in the CRF1, CRF, and CRFBP expression in response to acute-restraint stress in a sex-specific manner depending on the brain regions in mice (Figures 3-6). Specifically, following acute-restraint stress, CRF1 expression was increased in the prefrontal cortex of both males and females at any age. Acute stress also increased CRF1 expression in the male hippocampus in the young (1 month of age) and aged groups (18 months of age), suggesting an enhanced sensitivity of these two ages in response to stress in both sexes. Interestingly, stressed-exposed 6-month-old males showed CRF1 down-regulation in the hippocampus, an effect not observed in females. Moreover, CRF1 was sex-specifically up-regulated by stress in the amygdala of females particularly at 6 months of age and in the hypothalamus of females at 12 months of age, whereas it was down-regulated in the hypothalamus of female of at 18 months of age. Overall, these results are in line with previous studies indicating that CRF and CRF1 expression in response to stress displayed different patterns between the regions examined. For instance, it has been demonstrated that CRF1 mRNA expression is reduced in the frontal cortex and increased in the hippocampus and in the hypothalamic paraventricular nucleus after stress (Iredale et al., 1996). Also Ramot and colleagues demonstrated that CRF1 is essential for HPA axis regulation following chronic stress in the paraventricular nucleus of the hypothalamus that prepares the organism for successive exposure to stressful stimuli (Ramot et al., 2017). Acute stress may induce higher levels of CRF1 mRNA expression, whereas CRF mimicked acute stress-induced dysfunctions in the prefrontal cortex of adult male mice (Uribe-Marino et al., 2016). However, this particular study was conducted in male adult rats exposed to chronic unpredictable stress. Iredale and colleagues measured the expression of CRF1 mRNA instead of the protein levels as we did. Despite the divergence described earlier, our results are in line with previous studies in what concerns the opposite direction followed by CRF1 expression in the prefrontal cortex versus hippocampus of 6-month-old males. This observation is particularly interesting because it may underlie a different regulation of stress response by the CRF system in these two corticolimbic areas that are significantly involved in the modulation of the HPA axis function (Radley & Sawchenko, 2011). Additionally, we observed that CRF1 expression did not change in the hippocampus of 6-month-old females after acute stress which suggests the existence of sex differences in CRF1 expression and function within the hippocampus.
Our results added evidence supporting sex differences in stress sensitivity (Heck & Handa, 2019; Sze & Brunton, 2019). For instance, female rodents typically show an enhanced neuroendocrine response to acute stress compared to males, as demonstrated by the increased corticosterone and adrenocorticotropic hormone levels after being exposed to several stressful stimuli and different experimental protocols (Handa et al., 1994; Viau, Bingham, Davis, Lee, & Wong, 2005). The expression of HPA axis-related genes is sexually dimorphic, as implied by the greater expression of CRF mRNA in the hypothalamus of female rats (Babb, Masini, Day, & Campeau, 2012; Seale et al., 2004). Rosinger and colleagues demonstrated marked sex differences in terms of CRF1 distribution in the rostral periventricular hypothalamus of mice during the postnatal period (Rosinger et al., 2017) as well as aging (Rosinger, Jacobskind, De Guzman, et al., 2019). It is also noteworthy that both stress (Rosinger, Jacobskind, De Guzman, et al., 2019) and hormonal treatments (Rosinger, Jacobskind, Bulanchuk, et al., 2019, 2000) induce a peculiar sexually dimorphic pattern of neural activation in hypothalamic CRF1 cells. In addition to sex-specific differences in CRF1 expression, sex differences in CRF1 receptor coupling and signaling also have been well established. For example, female rats have greater cortical CRF1 coupling to the Gs protein compared to males (Bangasser et al., 2010). This evidence suggests that in females, CRF1 is more likely linked to cAMP-PKA signaling, thus, sex differences at the level of receptor signaling translates into sex differences in stress physiology. It has been well established that CRF1 is internalized, and, thus, inactivated, in response to saturating concentrations of CRF (Hauger, Smith, Braun, Dautzenberg, & Catt, 2000). This represents a key point toward understanding the molecular mechanisms behind CRF system. Recent findings have shown that internalization is started by β-arrestin binding to CRF1 in the cortex of males but not in females (Hauger, Risbrough, Oakley, Olivares-Reyes, & Dautzenberg, 2009; Bangasser et al., 2010). Moreover, in males, β-arrestin is strongly connected to a peculiar intracellular cascade which is distinguished from the cAMP-PKA signaling (DeWire, Ahn, Lefkowitz, & Shenoy, 2007) that, ultimately, suggests a sex-specific CRFl-related intracellular pathway (Bangasser & Valentino, 2012; Valentino, Bangasser, & Van Bockstaele, 2013). Altogether, the combination of differences in CRF1 expression, stress sensitivity, and sex-biased CRF1 receptor signaling may contribute to elicit sex differences in CRF responses both in physiological and pathological conditions.
Besides CRF1, we observed that stress induced CRF up-regulation only in the prefrontal cortex of 1-month-old males as well as in the amygdala of 6-month-old males. Oppositely, hypothalamic CRF was down-regulated by stress in both males and females at 1 month. Finally, CRF levels were affected by stress in female hypothalamus, as suggested by the increase at 12 months and the decrease at 18 months. CRFBP levels were moderately affected by acute stress as well. In fact, we found that stress down-regulated CRFBP in the prefrontal cortex of 18-months-old male mice as well as hippocampus of 12-month-old female mice. Furthermore, acute stress down-regulated hypothalamic CRFBP in both males and females at 1 month of age. Our results suggest different trends in CRF and CRFBP response to acute stress depending on life stage and brain region. However, the physiological meanings of the observed alterations of CRF and CRFBP expression after stress remain unknown and need to be investigated in the future. We also found some sex difference in CRF expression after stress. Particularly, we found stress-related sex differences in CRF expression exclusively in the amygdala at 6 and 12 months of age as well as in the hypothalamus at 12 months of age. Surprisingly, we did not observe sex differences in CRFBP distribution. The lack of these effects may be related to the time of sacrifice (90 min after the end of 2-hr-restraint stress) which may be appropriate to study CRF1 expression but maybe “too late” to broadly evaluate CRF and/or CRFBP. It may be possible that the expression of these two proteins changes in the “early phase” of stress response and, in our experiments, we just could measure the “late phase” of their fluctuations. Future studies based on specific time courses will be conducted to better characterize the effect of acute stress on CRF and CRFBP in a sex-difference point of view.
Our findings may inspire and facilitate future studies aimed to dissect molecular mechanisms and cellular pathways linked to CRF signaling and stress in this particular brain regions, and, ultimately, to increase the knowledge of gender-specific predisposition to stress-related psychiatric disorders. In line with that, CRF1, CRF, and CRFBP are involved in the pathogenesis of anxiety and depression (Binder & Nemeroff, 2010; Nemeroff & Vale, 2005) that are characterized by differential prevalence depending on the sex: Women are twice as likely to suffer from depression as men (Kessler et al., 2003). Given the link between CRF1 and depression, one interesting idea is that differences in stress regulation throughout the lifespan, as CRF1 expression changes, may be a key-factor to the link between biological sex and diagnosis prevalence. Clinical studies increasingly back up the hypothesis that the risk of developing Alzheimer disease is higher in women, although the mechanisms underlying this sexual discrepancy remain unknown (Alzheimer's Association, 2020). Risk factors, including differences in life expectancy, hormones, genetics, and brain structure, have been considered as possible factor contributing in the higher risk of Alzheimer’s disease in women, but results have been discrepant (Altmann, Tian, Henderson, & Greicius, 2014; Lin & Doraiswamy, 2015; Morrison, Brinton, Schmidt, & Gore, 2006; Riedel, Thompson, & Brinton, 2016). Our group demonstrated a strong link between CRF1 and chronic stress on the one hand, and aging and neurodegenerative disorders such as Alzheimer disease on the other hand. Specifically, genetic enhancement of brain CRF expression, by mimicking chronic stress conditions, induced both neurochemical and behavioral impairment in a mouse model of Alzheimer disease, reflected in an increase in beta amyloid deposition, neurodegeneration, and memory deficits, an effect prevented by CRF1 antagonism (Dong et al., 2012, 2014, 2018). Overall, understanding the sex differences of CRF system in the lifespan may provide insight into how CRF signaling can impact brain development and function under both physiological and stress-related neuropathological conditions.
Our findings support the idea that females respond to stress differently than males; however, because of the complexity of CRF system in brain, we still need to improve our understanding of the physiology of CRF1 signaling throughout the corticolimbic system, and its interaction with stress and sex. Of note, despite the substantial efforts and strong preclinical rationale, targeting CRF1 for the treatment of stress-related neuropsychiatric disorders has been unsuccessful (Spierling & Zorrilla, 2017). Negative clinical outcomes have been linked to (i) positive preclinical results obtained using specific models and conditions with dynamic CRF/CRF1 activation that, ultimately, are not translatable to tested patients; (ii) low efficacy of the current drug candidates because of inadequate pharmacokinetic and physicochemical properties; (iii) lack of understanding of the whole CRF system, with a particular need to address constitutive CRF1 expression and activity, or the involvement of CRFBP, CRF2, or molecules that modulate agonist-independent activity. Future mechanistic studies focused on exploring cellular and molecular pathways are required to reach final conclusions. Therefore, our group will next evaluate (a) CRF1 plasticity under chronic stress conditions, (b) CRF1 expression in the brain of specific mouse models of Alzheimer’s disease in a sex-specific perspective, and, (c) alterations of CRF1 function in the specific brain regions of the Alzheimer disease mouse model.
In conclusion, this study revealed a sex- and age-dependent pattern in CRF1, CRF, and CRFBP expression in discrete murine corticolimbic regions, and how it responds to acute-restraint stress. Our results also enhance our understanding of how CRF signaling might affect brain function in a sex-specific manner during lifespan. We hope that the presented results will aid future studies aimed at evaluating age, regional, and sex differences in the CRF system involved in acute and chronic stress, and the link to neuropsychiatric disorders.
ACKNOWLEDGMENT
This study was supported by RF1AG057884 and R56AG053491 to Hongxin Dong.
All experiments were conducted in compliance with the ARRIVE guidelines.
Abbreviations:
- CRF
corticotropin-releasing factor
- CRF1
corticotropin-releasing factor receptor 1
- CRF2
corticotropin-releasing factor receptor 2
- CRFBP
corticotropin-releasing factor binding protein
- HPA
hypothalamic-pituitary-adrenal
- mRNA
messenger ribonucleic acid
- RRID
Research Resource Identifier (see https://scicrunch.org)
Footnotes
CONFLICT OF INTEREST
The authors declare no competing interests.
REFERENCES
- Altmann A, Tian L, Henderson VW, & Greicius MD; Alzheimer's Disease Neuroimaging Initiative Investigators. Sex modifies the APOE-related risk of developing Alzheimer disease. Annals of Neurology, 75(4), 563–573. 10.1002/ana.24135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer's Association (2020). 2020 Alzheimer's disease facts and figures. Alzheimer's and Dementia, 16, 391–460. 10.1002/alz.12068 [DOI] [PubMed] [Google Scholar]
- Avishai-Eliner S, Yi SJ, & Baram TZ (1996). Developmental profile of messenger RNA for the corticotropin-releasing hormone receptor in the rat limbic system. Developmental Brain Research, 91(2), 159–163. 10.1016/0165-3806(95)00158-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb JA, Masini CV, Day HE, & Campeau S (2012). Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience, 234, 40–52. 10.1016/j.neuroscience.2012.12.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, … Valentino RJ (2010). Sex differences in corticotropin-releasing factor receptor signaling and trafficking: Potential role in female vulnerability to stress-related psychopathology. Molecular Psychiatry, 15(9), 896–904. 10.1038/mp.2010.66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Dong H, Carroll J, Plona Z, Ding H, Rodriguez L, … Valentino RJ (2017). Corticotropin-releasing factor overexpression gives rise to sex differences in Alzheimer's disease-related signaling. Molecular Psychiatry, 22(8), 1126–1133. 10.1038/mp.2016.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, & Valentino RJ (2012). Sex differences in molecular and cellular substrates of stress. Cellular and Molecular Neurobiology, 32(5), 709–723. 10.1007/s10571-012-9824-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Wiersielis KR, & Khantsis S (2016). Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Research, 1641(Pt B), 177–188. 10.1016/j.brainres.2015.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, & Nemeroff CB (2010). The CRF system, stress, depression and anxiety-insights from human genetic studies. Molecular Psychiatry, 15(6), 574–588. 10.1038/mp.2009.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaz B, & Rivest S (1998). Effect of a chronic stress on CRF neuronal activity and expression of its type 1 receptor in the rat brain. American Journal of Physiology, 275(5), R1438–R1449. 10.1152/ajpregu.1998.275.5.R1438 [DOI] [PubMed] [Google Scholar]
- Bravo JA, Dinan TG, & Cryan JF (2011). Alterations in the central CRF system of two different rat models of comorbid depression and functional gastrointestinal disorders. International Journal of Neuropsychopharmacology, 14(5), 666–683. 10.1017/S1461145710000994 [DOI] [PubMed] [Google Scholar]
- Brunson KL, Grigoriadis DE, Lorang MT, & Baram TZ (2002). Corticotropin-releasing hormone (CRH) downregulates the function of its receptor (CRF1) and induces CRF1 expression in hippocampal and cortical regions of the immature rat brain. Experimental Neurology, 176(1), 75–86. 10.1006/exnr.2002.7937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP (1995). The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. New England Journal of Medicine, 332(20), 1351–1362. 10.1056/NEJM199505183322008 [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joëls M, & Holsboer F (2005). Stress and the brain: From adaptation to disease. Nature Reviews Neuroscience, 6(6), 463–475. 10.1038/nrn1683 [DOI] [PubMed] [Google Scholar]
- DeWire SM Ahn S Lefkowitz RJ, & Shenoy SK (2007). Betaarrestins and cell signaling. Annual Review of Physiology, 69, 483–510. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, & Meaney MJ (1993). The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. Journal of Neuroscience, 13(9), 3839–3847. 10.1523/JNEUROSCI.13-09-03839.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Keegan JM, Hong E, Gallardo C, Montalvo-Ortiz J, Wang B, … Csernansky J (2018). Corticotrophin releasing factor receptor 1 antagonists prevent chronic stress-induced behavioral changes and synapse loss in aged rats. Psychoneuroendocrinology., 90, 92–101. 10.1016/j.psyneuen.2018.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Murphy KM, Meng L, Montalvo-Ortiz J, Zeng Z, Kolber BJ, … Csernansky JG (2012). Corticotrophin releasing factor accelerates neuropathology and cognitive decline in a mouse model of Alzheimer's disease. Journal of Alzheimer's Disease, 28(3), 579–592. 10.3233/JAD-2011-111328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Wang S, Zeng Z, Li F, Montalvo-Ortiz J, Tucker C, … Csernansky JG (2014). Effects of corticotrophin-releasing factor receptor 1 antagonists on amyloid-β and behavior in Tg2576 mice. Psychopharmacology (Berl), 231(24), 4711–4722. 10.1007/s00213-014-3629-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendler K, Karmos G, & Telegdy G (1961). The effect of hippocampal lesion on pituitary-adrenal function. Acta Physiologica Academiae Scientiarum Hungaricae, 20, 293–297. [PubMed] [Google Scholar]
- Fisher DW, Bennett DA, & Dong H (2018). Sexual dimorphism in predisposition to Alzheimer's disease. Neurobiology of Aging, 70, 308–324. 10.1016/j.neurobiolaging.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabashi T, Kawaguchi M, Furuta M, Fukushima A, & Kimura F (2004). Exposure to bisphenol A during gestation and lactation causes loss of sex difference in corticotropin-releasing hormone-immunoreactive neurons in the bed nucleus of the stria terminalis of rats. Psychoneuroendocrinology, 29(4), 475–485. 10.1016/S0306-4530(03)00055-6 [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, & Bollnow MR (1994). Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiology and Behavior, 55(1), 117–124. 10.1016/0031-9384(94)90018-3 [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, & Dautzenberg FM (2009). Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Annals of the New York Academy of Sciences, 1179, 120–143. 10.1111/j.1749-6632.2009.05011.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauger RL, Smith RD, Braun S, Dautzenberg FM, & Catt KJ (2000). Rapid agonist-induced phosphorylation of the human CRF receptor, type 1: A potential mechanism for homologous desensitization. Biochemical and Biophysical Research Communications, 268(2), 572–576. 10.1006/bbrc.2000.2183 [DOI] [PubMed] [Google Scholar]
- Heck AL, & Handa RJ (2019). Sex differences in the hypothalamic-pituitary-adrenal axis' response to stress: An important role for gonadal hormones. Neuropsychopharmacology, 44(1), 45–58. 10.1038/s41386-018-0167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse EW, & Grammatopoulos DK (2006). The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: Implications for physiology and pathophysiology. Endocrine Reviews, 27(3), 260–286. 10.1210/er.2005-0034 [DOI] [PubMed] [Google Scholar]
- Imaki T, Katsumata H, Miyata M, Naruse M, Imaki J, & Minami S (2001). Expression of corticotropin releasing factor (CRF), urocortin and CRF type 1 receptors in hypothalamic-hypophyseal systems under osmotic stimulation. Journal of Neuroendocrinology, 13(4), 328–338. 10.1046/j.1365-2826.2001.00629.x [DOI] [PubMed] [Google Scholar]
- Imaki T, Nahan JL, Rivier C, Sawchenko PE, & Vale W (1991). Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. Journal of Neuroscience, 11(3), 585–599. 10.1523/JNEUROSCI.11-03-00585.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaki T, Naruse M, Harada S, Chikada N, Nakajima K, Yoshimoto T, & Demura H (2002). Stress-induced changes of gene expression inthe paraventricular nucleus are enhanced in spontaneously hypertensive rats. Journal of Neuroendocrinology, 10(8), 635–643. 10.1046/j.1365-2826.1998.00249.x [DOI] [PubMed] [Google Scholar]
- Iredale PA, Terwilliger R, Widnell KL, Nestler EJ, & Duman RS (1996). Differential regulation of corticotropin-releasing factor1 receptor expression by stress and agonist treatments in brain and cultured cells. Molecular Pharmacology, 50(5), 1103–1110. [PubMed] [Google Scholar]
- Jacobson L, & Sapolsky R (1991). The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocrine Reviews, 12(2), 118–134. 10.1210/edrv-12-2-118 [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, & Pfaff DW (2006). Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Hormones and Behavior, 49(2), 197–205. 10.1016/j.yhbeh.2005.06.005 [DOI] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, & Vale W (2008). Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: Implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. The Journal of Comparative Neurology, 511(4), 479–496. 10.1002/cne.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanikas CA, Lu YL, & Richardson HN (2013). Adolescent drinking targets corticotropin-releasing factor peptide-labeled cells in the central amygdala of male and female rats. Neuroscience, 249, 98–105. 10.1016/j.neuroscience.2013.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasckow JW, Regmi A, Mulchahey JJ, Plotsky PM, & Hauger RL (1999). Changes in brain corticotropin-releasing factor messenger RNA expression in aged Fischer 344 rats. Brain Research, 822(1–2), 228–230. 10.1016/s0006-8993(98)01365-1 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, … National Comorbidity Survey Replication. (2003). The epidemiology of major depressive disorder. JAMA, 289(23), 3095. 10.1001/jama.289.23.3095 [DOI] [PubMed] [Google Scholar]
- Lim MM, Nair HP, & Young LJ (2005). Species and sex differences in brain distribution of corticotropin-releasing factor receptor subtypes 1 and 2 in monogamous and promiscuous vole species. The Journal of Comparative Neurology, 487(1), 75–92. 10.1002/cne.20532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KA, & Doraiswamy PM (2015). When mars versus venus is not a Cliché: Gender differences in the neurobiology of Alzheimer's disease. Frontiers in Neurology, 5, 288. 10.3389/fneur.2014.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locci A, & Pinna G (2019). Stimulation of peroxisome proliferator-activated receptor-α by N-palmitoylethanolamine engages allopregnanolone biosynthesis to modulate emotional behavior. Biological Psychiatry, 85(12), 1036–1045. 10.1016/j.biopsych.2019.02.006 [DOI] [PubMed] [Google Scholar]
- Makino S, Tanaka Y, Nazarloo HP, Noguchi T, Nishimura K, & Hashimoto K (2005). Expression of type 1 corticotropin-releasing hormone (CRH) receptor mRNA in the hypothalamic paraventricular nucleus following restraint stress in CRH-deficient mice. Brain Research, 1048(1–2), 131–137. 10.1016/j.brainres.2005.04.065 [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, & Wilson MA (1994). A sexually dimorphic population of CRF neurons in the medial preoptic area. NeuroReport, 5(5), 653–656. 10.1097/00001756-199401000-00031 [DOI] [PubMed] [Google Scholar]
- McEwen BS (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- Morrison JH, Brinton RD, Schmidt PJ, & Gore AC (2006). Estrogen, menopause, and the aging brain: How basic neuroscience can inform hormone therapy in women. Journal of Neuroscience, 26(41), 10332–10348. 10.1523/JNEUROSCI.3369-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, & Vale WW (2005). The neurobiology of depression: Inroads to treatment and new drug discovery. Journal of Clinical Psychiatry, 66(Suppl. 7), 5–13. [PubMed] [Google Scholar]
- Owens MJ, & Nemeroff CB (1991). Physiology and pharmacology of corticotropin-releasing factor. Pharmacological Reviews, 43(4), 425–473. [PubMed] [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, … Vale W (1995). Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proceedings of the National Academy of Sciences of the United States of America, 92(7), 2969–2973. 10.1073/pnas.92.7.2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisu MG, Garau A, Boero G, Biggio F, Pibiri V, Dore R, … Serra M (2016). Sex differences in the outcome of juvenile social isolation on HPA axis function in rats. Neuroscience, 320, 172–182. 10.1016/j.neuroscience.2016.02.009 [DOI] [PubMed] [Google Scholar]
- Porcu P, & Morrow AL (2014). Divergent neuroactive steroid responses to stress and ethanol in rat and mouse strains: Relevance for human studies. Psychopharmacology (Berl), 231(17), 3257–3272. 10.1007/s00213-014-3564-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, & Vale WW (1991). Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature, 349(6308), 423–426. [DOI] [PubMed] [Google Scholar]
- Radley JJ, & Sawchenko PE (2011). A common substrate for prefrontal and hippocampal inhibition of the neuroendocrine stress response. Journal of Neuroscience, 31(26), 9683–9695. 10.1523/JNEUROSCI.6040-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramot A, Jiang Z, Tian JB, Nahum T, Kuperman Y, Justice N, & Chen A (2017). Hypothalamic CRFR1 is essential for HPA axis regulation following chronic stress. Nature Neuroscience, 20(3), 385–388. 10.1038/nn.4491 [DOI] [PubMed] [Google Scholar]
- Riedel BC, Thompson PM, & Brinton RD (2016). Age, APOE and sex: Triad of risk of Alzheimer's disease. Journal of Steroid Biochemistry and Molecular Biology, 160, 134–147. 10.1016/j.jsbmb.2016.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S, Laflamme N, & Nappi RE (1995). Immune challenge and immobilization stress induce transcription of the gene encoding the CRF receptor in selective nuclei of the rat hypothalamus. Journal of Neuroscience, 15(4), 2680–2695. 10.1523/JNEUROSCI.15-04-02680.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez G, Neugebauer NM, Yao KL, Meltzer HY, Csernansky JG, & Dong H (2017). Δ9-tetrahydrocannabinol (Δ9-THC) administration after neonatal exposure to phencyclidine potentiates schizophrenia-related behavioral phenotypes in mice. Pharmacology, Biochemistry and Behavior, 159, 6–11. 10.1016/j.pbb.2017.06.010 [DOI] [PubMed] [Google Scholar]
- Rosinger ZJ, De Guzman RM, Jacobskind JS, Saglimbeni B, Malone M, Fico D, … Zuloaga DG (2020). Sex-dependent effects of chronic variable stress on discrete corticotropin-releasing factor receptor 1 cell populations. Physiology and Behavior, 219, 112847. 10.1016/j.physbeh.2020.112847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger ZJ, Jacobskind JS, Bulanchuk N, Malone M, Fico D, Justice NJ, & Zuloaga DG (2019). Characterization and gonadal hormone regulation of a sexually dimorphic corticotropin-releasing factor receptor 1 cell group. The Journal of Comparative Neurology, 527(6), 1056–1069. 10.1002/cne.24588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger ZJ, Jacobskind JS, De Guzman RM, Justice NJ, & Zuloaga DG (2019). A sexually dimorphic distribution of corticotropin-releasing factor receptor 1 in the paraventricular hypothalamus. Neuroscience, 409, 195–203. 10.1016/j.neuroscience.2019.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinger ZJ, Jacobskind JS, Park SG, Justice NJ, & Zuloaga DG (2017). Distribution of corticotropin-releasing factor receptor 1 in the developing mouse forebrain: A novel sex difference revealed in the rostral periventricular hypothalamus. Neuroscience, 361, 167–178. 10.1016/j.neuroscience.2017.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, Ingram CD, … Harbuz MS (2004). Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. Journal of Neuroendocrinology, 16(6), 516–524. 10.1111/j.1365-2826.2004.01195.x [DOI] [PubMed] [Google Scholar]
- Seney ML, & Sibille E (2014). Sex differences in mood disorders: Perspectives from humans and rodent models. Biology of Sex Differences, 5(1), 17. 10.1186/s13293-014-0017-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, … Lee KF (1998). Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron, 20(6), 1093–1102. 10.1016/S0896-6273(00)80491-2 [DOI] [PubMed] [Google Scholar]
- Speert DB, McClennen SJ, & Seasholtz AF (2002). Sexually dimorphic expression of corticotropin-releasing hormone-binding protein in the mouse pituitary. Endocrinology, 143(12), 4730–4741. 10.1210/en.2002-220556 [DOI] [PubMed] [Google Scholar]
- Spierling SR, & Zorrilla EP (2017). Don't stress about CRF: Assessing the translational failures of CRF1antagonists. Psychopharmacology (Berl), 234(9–10), 1467–1481. 10.1007/s00213-017-4556-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbannayya T, Balakrishnan L, Sudarshan G, Advani J, Kumar S, Mahmood R, … Prasad TS (2013). An integrated map of corticotropin-releasing hormone signaling pathway. Journal of Cell Communication and Signaling, 7(4), 295–300. 10.1007/S12079-013-0197-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze Y, & Brunton PJ (2019). Sex, stress and steroids. European Journal of Neuroscience, 10.1111/ejn.14615 [DOI] [PubMed] [Google Scholar]
- Tan LA, Vaughan JM, Perrin MH, Rivier JE, & Sawchenko PE (2017). Distribution of corticotropin-releasing factor (CRF) receptor binding in the mouse brain using a new, high-affinity radioligand, [125 I]-PD-Sauvagine. The Journal of Comparative Neurology, 525(18), 3840–3864. 10.1002/cne.24307 [DOI] [PubMed] [Google Scholar]
- Uribe-Mariño A, Gassen NC, Wiesbeck MF, Balsevich G, Santarelli S, Solfrank B, … Schmidt MV (2016). Prefrontal cortex corticotropin-releasing factor receptor 1 conveys acute stress-induced executive dysfunction. Biological Psychiatry, 80(10), 743–753. 10.1016/j.biopsych.2016.03.2106 [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E (2018). Effects of stress on the corticolimbic system: Implications for chronic pain. Progress in NeuroPsychopharmacology and Biological Psychiatry, 87(Pt B), 216–223. 10.1016/j.pnpbp.2017.10.014 [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Bangasser D, & Van Bockstaele EJ (2013). Sex-biased stress signaling: The corticotropin-releasing factor receptor as a model. Molecular Pharmacology, 83(4), 737–745. 10.1124/mol.112.083550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino RJ, Reyes B, Van Bockstaele E, & Bangasser D (2012). Molecular and cellular sex differences at the intersection of stress and arousal. Neuropharmacology, 62(1), 13–20. 10.1016/j.neuropharm.2011.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, … Sawchenko PE (2000). Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. The Journal of Comparative Neurology, 428(2), 191–212. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, & Wong M (2005). Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology, 146(1), 137–146. 10.1210/en.2004-0846 [DOI] [PubMed] [Google Scholar]
- Weathington JM, Hamki A, & Cooke BM (2014). Sex- and region-specific pubertal maturation of the corticotropin-releasing factor receptor system in the rat. The Journal of Comparative Neurology, 522(6), 1284–1298. 10.1002/cne.23475 [DOI] [PubMed] [Google Scholar]
- Xiao C, Sartin J, Mulchahey JJ, Segar T, Sheriff S, Herman JP, & Kasckow JW (2006). Aging associated changes in amygdalar corticotropin-releasing hormone (CRH) and CRH-binding protein in Fischer 344 rats. Brain Research, 1073-1074, 325–331. 10.1016/j.brainres.2005.12.063 [DOI] [PubMed] [Google Scholar]
- Yan Y, Dominguez S, Fisher DW, & Dong H (2018). Sex differences in chronic stress responses and Alzheimer's disease. Neurobiology of Stress, 8, 120–126. 10.1016/j.ynstr.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuloaga DG, Puts DA, Jordan CL, & Breedlove SM (2008). The role of androgen receptors in the masculinization of brain and behavior: What we've learned from the testicular feminization mutation. Hormones and Behavior, 53(5), 613–626. 10.1016/j.yhbeh.2008.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]