Abstract
Background
Janus kinase (JAK) inhibitors and secukinumab have been demonstrated to be effective treatments for ankylosing spondylitis (AS). However, there have been no head-to-head trials comparing the effectiveness and safety characteristics of JAK inhibitors with secukinumab. This study aimed to evaluate the relative effectiveness and safety of JAK inhibitors and secukinumab in patients with active AS.
Summary
A Bayesian network meta-analysis was conducted using direct and indirect data from randomized controlled trials (RCTs) that examined the efficacy and safety of tofacitinib 5 mg, upadacitinib 15 mg, filgotinib 200 mg, and secukinumab 150 mg in patients with active AS who had a poor response or intolerance to nonsteroidal anti-inflammatory drugs (NSAIDs) and were tumor necrosis factor (TNF) inhibitor-naïve. Data from six RCTs comprising 937 patients were analyzed. The Assessment of SpondyloArthritis International Society 20 (ASAS20) response rates were significantly higher in the JAK inhibitors and secukinumab groups than in the placebo group. The surface under the cumulative ranking curve (SUCRA)-based ranking probability based on the ASAS20 response rate suggested that tofacitinib 5 mg had the highest likelihood of being the best treatment for achieving the ASAS20 response rate, followed by filgotinib 200 mg, upadacitinib 15 mg, secukinumab 150 mg, and placebo. The SUCRA-based ranking probability based on the ASAS20 response rate suggested that tofacitinib 5 mg had the highest likelihood of being the best treatment for achieving the ASAS40 response rate, followed by upadacitinib 15 mg, secukinumab 150 mg, filgotinib 200 mg, and placebo.
Key Messages
Tofacitinib 5 mg was the most effective treatment for AS, whereas JAK inhibitors and secukinumab 150 mg were effective treatments in patients with active AS who had a poor response or intolerance to NSAIDs and were TNF inhibitor-naïve.
Keywords: Ankylosing spondylitis, JAK inhibitors, Secukinumab, Network meta-analysis, Arthritis
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory disease characterized by entheses and inflammation of the spinal and sacroiliac joints, which leads to bone and joint erosion and eventually to new bone formation, syndesmophytes, and ankylosis, resulting in increased structural damage, weakness, and decreased quality of life [1, 2, 3]. Nonsteroidal anti-inflammatory drugs (NSAIDs) are used as first-line pharmacological therapy for AS. For patients with AS who do not react well to NSAIDs, biological disease-modifying antirheumatic drugs such as anti-tumor necrosis factor (TNF) treatments and secukinumab, an interleukin-17A (IL-17A) inhibitor, are used [4]. However, in some patients, the lack or failure of response to existing therapies remains a significant concern [5].
IL-17A has an important role in the inflammatory reactions associated with AS [33]. IL-17A and its receptor are expressed in target tissues in patients with AS and may modulate biological activities, resulting in inflammation, damage, and tissue remodeling of joints and entheses [6]. Secukinumab, the first monoclonal IL-17 antibody used for AS treatment, is a completely human recombinant anti-IL-17A IgG1 monoclonal antibody [7]. Secukinumab is an effective treatment for active AS with a tolerable safety profile. Several cytokines, including those implicated in the IL-23/IL-17 axis, communicate through the Janus kinase (JAK) family of tyrosine kinases. As a result, the JAK pathway may be a therapeutic target for AS. Tofacitinib, an oral JAK inhibitor [8], selectively inhibits JAK1, JAK2, and JAK3, with a preference for JAK1 and JAK3 over JAK2. Upadacitinib was designed to be more selective for JAK1 than for JAK2, JAK3, or Tyk2 [9]. Similarly, filgotinib, a JAK1 inhibitor, was designed to be more selective for JAK1 than for the others.
JAK inhibitors and secukinumab have been demonstrated to be effective treatments for AS in several studies [10, 11, 12, 13, 14, 15]. However, there have been no head-to-head trials comparing the effectiveness and safety characteristics of JAK inhibitors with secukinumab. A network meta-analysis may include both direct and indirect data generated from relative treatment effects throughout a network of randomized controlled trials (RCTs) that can determine the effectiveness of various therapies, even in the absence of direct comparative investigations [16, 17, 18]. As a result, this study used a network meta-analysis to assess the efficacy and safety of JAK inhibitors and secukinumab in patients with active AS who had poor response or intolerance to NSAIDs and were TNF inhibitor-naïve.
Materials and Methods
Identification of Eligible Studies and Data Collection
A systematic literature search was performed using Medline, Embase, and the Cochrane Controlled Trials Database to find available research articles on the efficacy and safety of JAK inhibitors and secukinumab in patients with active AS, published until February 2022. The keywords and subject terms used in the analysis included “JAK inhibitor,” “secukinumab,” and “ankylosing spondylitis.” All the references in the research articles were verified to find relevant studies that were not included in the online repositories. The inclusion criteria for the selection of an RCT were as follows: (1) comparison of JAK inhibitor or secukinumab with placebo for the treatment of patients with active AS exhibiting inadequate response or intolerance to NSAIDs and being TNF inhibitor-naïve, and (2) reporting of the clinical effectiveness and safety endpoints of JAK inhibitors or secukinumab at 12–16 weeks. The exclusion criteria included (1) duplicate data and (2) lack of data needed for inclusion. The efficacy endpoint was the number of patients who met either the Assessment of SpondyloArthritis International Society (ASAS) 20% (ASAS20) or 40% (ASAS40) of the response requirements. The safety outcome was based on the number of patients with serious adverse events (SAEs) [19]. The results were collected from the original studies by two independent reviewers. Any discrepancies were resolved by consensus between the reviewers. Data from each publication included the first author's name, publication year, doses of JAK inhibitors and secukinumab, and efficacy and safety outcomes. The network meta-analysis was performed in compliance with the recommendations of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement [20].
Evaluation of Statistical Associations for Network Meta-Analysis
The findings were simultaneously analyzed for RCTs that compared JAK inhibitors and secukinumab in different arms. The efficacy and safety of JAK inhibitors and secukinumab in different arms were ordered based on the likelihood of being rated as the best performing regimen. Using NetMetaXL [21] and WinBUGS version 1.4.3 (MRC Biostatistics Unit, Institute of Public Health, Cambridge, UK), a Bayesian fixed-effect model was applied for the network meta-analysis. The Monte Carlo Markov chain approach was used to estimate the size of the pooled effect [16]. All chains were run with 10,000 iterations of burn-in and 10,000 iterations. The relative effect information was transformed into a prediction of the best performance of a drug. The rating of each intervention, expressed as a percentage, was also calculated as the surface under the cumulative ranking curve (SUCRA). SUCRA was 100% when the best treatment was certain and 0% when the worst treatment was certain. The league table presents the overview estimates by rating the treatments in order, starting with the highest outcome effect, as calculated by SUCRA [22]. A 95% confidence interval was recorded along with the pairwise odds ratio (OR) and Bayesian credible interval (CrI). Trial outcomes for the different treatment arms were modified. Pooled tests were deemed statistically significant unless a value of 1 was included in the confidence interval.
Tests for Inconsistency and Sensitivity
Inconsistency refers to the degree to which the direct and indirect data differ [23]. In the inconsistencies model, to assess network inconsistencies between direct and indirect estimates in each loop, the posterior mean deviation of the individual data points against the posterior mean deviation in the consistency model was defined [24]. A sensitivity test was conducted using a random and fixed-effect model comparison.
Results
Meta-Analysis Studies
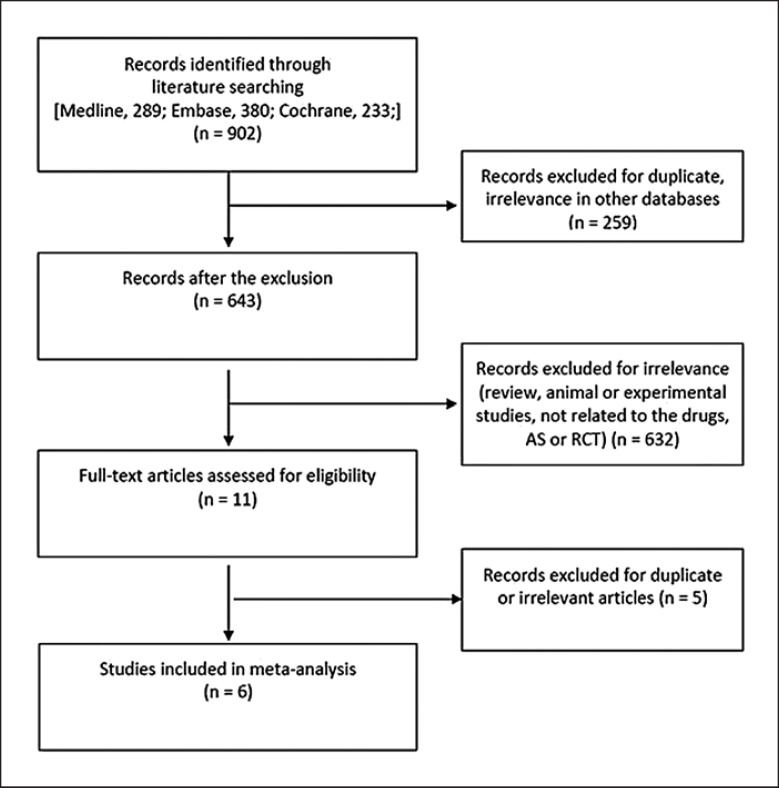
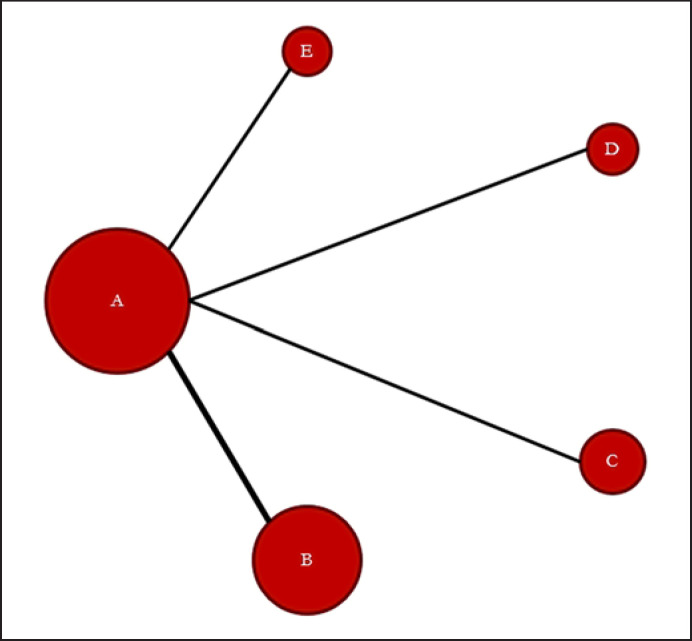
Through an electronic or manual search, a total of 902 studies were identified; of these, based on the title and abstract information, 11 were selected for a full-text review. Subsequently, five studies were omitted because of duplicate results or a non-RCT study design (Fig. 1). Eventually, six RCTs comprising 937 patients that met the criteria inclusion were analyzed [10, 11, 12, 13, 14, 15] (Table 1). There were ten pairs of contrasts, including four direct comparisons and five treatments comprising placebo and upadacitinib, filgotinib, tofacitinib, and secukinumab 150 mg (Table 1; Fig. 2). JAK inhibitors were administered at the following dosages: upadacitinib 15 mg once daily, filgotinib 200 mg once daily, and tofacitinib 5 mg twice daily. Patients received subcutaneous injections of secukinumab 150 mg at weeks 0, 1, 2, 3, and 4 and were administered subcutaneous secukinumab injections every 4 weeks. Table 1 presents the related characteristics of the studies included in the meta-analysis.
Fig. 1.
Flow diagram of the study selection process.
Table 1.
Characteristics of individual studies included in the network meta-analysis
| Study | Drugs | N | Drugs | Patients, n | ASAS20 | ASAS40 |
|---|---|---|---|---|---|---|
| Van der Heijde et al. [15] | JAK inhibitor | 187 | Upadacitinib 15 mg | 93 | 60 | 48 |
| Placebo | 94 | 38 | 24 | |||
|
| ||||||
| Van der Heijde et al. [14] | JAK inhibitor | 116 | Filgotinib 200 mg | 58 | 44 | 22 |
| Placebo | 58 | 23 | 11 | |||
|
| ||||||
| Van der Heijde et al. [12] | JAK inhibitor | 103 | Tofacitinib 5 mg | 52 | 42 | 24 |
| Placebo | 51 | 21 | 10 | |||
|
| ||||||
| Kivitz et al. [13] | Anti-IL-17A | 168 | Secukinumab 150 mg | 85 | 51 | 34 |
| Placebo | 83 | 41 | 25 | |||
|
| ||||||
| Pavelka et al. [11] | Anti-IL-17A | 116 | Secukinumab 150 mg | 57 | 36 | 25 |
| Placebo | 59 | 23 | 14 | |||
|
| ||||||
| Baeten et al. [10] | Anti-IL-17A | 247 | Secukinumab 150 mg | 125 | 76 | 52 |
| Placebo | 122 | 35 | 16 | |||
|
| ||||||
| Comparison | Study number | Patients, n | ||||
|
| ||||||
| Placebo | 6 | 467 | ||||
| Secukinumab 150 mg | 3 | 267 | ||||
| Upadacitinib 15 mg | 1 | 93 | ||||
| Filgotinib 200 mg | 1 | 58 | ||||
| Tofacitinib 5 mg | 1 | 52 | ||||
ASAS20 or 40, Assessment of SpondyloArthritis International Society 20 or 40 response criteria (improvement of ≥20% and absolute improvement of ≥1 unit [on a 10-unit scale] in at least three of the four main ASAS domains, with no worsening by ≥20% in the remaining domain) [19]; anti-IL-17A, anti-interleukin-17A monoclonal antibody.
Fig. 2.
Evidence network of comparisons for network meta-analysis. In the evidence network, the width of each edge is proportional to the number of RCTs comparing each pair of treatments, and the size of each treatment node is proportional to the number of randomized participants (sample size). a Placebo. b Secukinumab 150 mg. c Upadacitinib 15 mg. d Filgotinib 200 mg. e Tofacitinib 5 mg.
Network Meta-Analysis of the Efficacy of JAK Inhibitors and Secukinumab
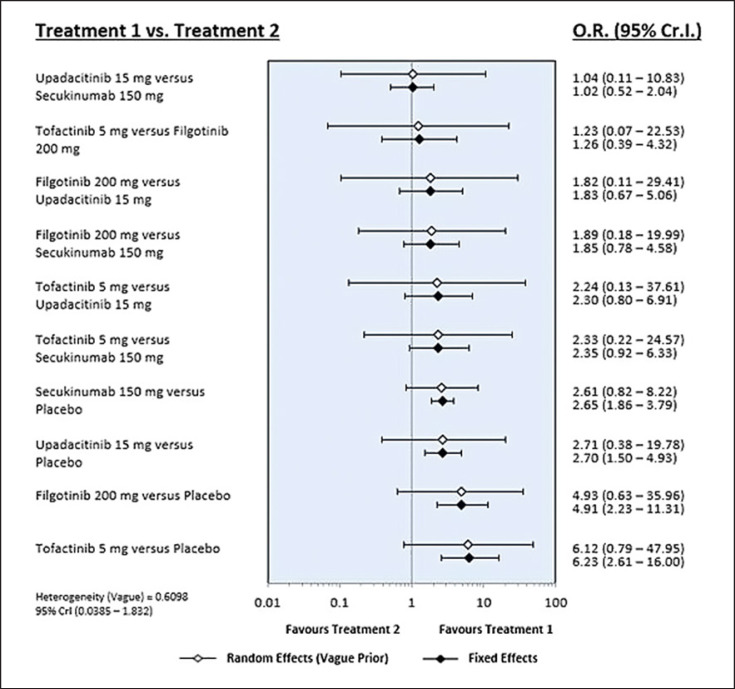
Tofacitinib 5 mg was listed at the top left of the league table diagonal (Table 2) because it was associated with the most favorable ASAS20 response rate SUCRA, whereas the placebo was listed at the bottom right of the league table diagonal because it was associated with the least favorable results. The ASAS20 response rate was significantly higher in the tofacitinib 5 mg group than in the placebo group (OR 6.23, 95% CrI: 2.61–16.00) (Table 2; Fig. 2). Similarly, the ASAS20 response rates were significantly higher in the filgotinib 200 mg, upadacitinib 315 mg, and secukinumab 150 mg groups than in the placebo group (Table 2; Fig. 3). The SUCRA-based ranking probability based on the ASAS20 response rate suggested that tofacitinib 5 mg had the highest probability of being the best treatment for achieving the ASAS20 response rate, followed by filgotinib 200 mg, upadacitinib 15 mg, secukinumab 150 mg, and placebo (Table 3). The response rate of ASAS40 showed a pattern of distribution similar to that of ASAS20; tofacitinib 5 mg was associated with the most favorable ASAS40 response rate (Table 2). SUCRA-based ranking probability (Table 3) suggested that tofacitinib 5 mg had the highest likelihood of being the best treatment to achieve the ASAS40 response rate, followed by upadacitinib 15 mg, secukinumab 150 mg, filgotinib 200 mg, and placebo (Table 3).
Table 2.
Network meta-analyses comprising the effects for all contrasts along with ORs and 95% CrIs
| A. ASAS20. OR > 1 means the treatment in top left is better | ||||
| Tofacitinib 5 mg | ||||
| 1.26 (0.39–4.32) | Filgotinib 200 mg | |||
| 2.30 (0.80–6.91) | 1.83 (0.67–5.06) | Upadacitinib 15 mg | ||
| 2.35 (0.92–6.33) | 1.85 (0.78–4.58) | 1.02 (0.52–2.04) | Secukinumab 150 mg | |
| 6.23 (2.61–16.00) | 4.91 (2.23–11.31) | 2.70 (1.50–4.93) | 2.65 (1.86–3.79) | Placebo |
|
| ||||
| B. ASAS40 | ||||
| Tofacitinib 5 mg | ||||
| 1.15 (0.39–3.53) | Upadacitinib 15 mg | |||
| 1.32 (0.51–3.57) | 1.15 (0.55–2.40) | Secukinumab 150 mg | ||
| 1.36 (0.39–4.73) | 1.19 (0.40–3.35) | 1.03 (0.39–2.60) | Filgotinib 200 mg | |
| 3.62 (1.52–9.08) | 3.16 (1.71–5.94) | 2.75 (1.88–4.10) | 2.67 (1.16–6.43) | Placebo |
|
| ||||
| C. Safety. OR < 1 means that the treatment in the top left block is better | ||||
| Tofacitinib 5 mg | ||||
| 0.51 (0.01–7.82) | Secukinumab 150 mg | |||
| 0.36 (0.00–34.96) | 0.73 (0.02–32.37) | Filgotinib 200 mg | ||
| 0.35 (0.00–32.10) | 0.72 (0.02–30.51) | 0.99 (0.01–163.20) | Upadacitinib 15 mg | |
| 0.39 (0.01–5.23) | 0.76 (0.31–1.76) | 1.03 (0.02–38.44) | 1.05 (0.03–43.12) | Placebo |
Fig. 3.
Results of the Bayesian network meta-analysis of randomized controlled studies assessing the relative efficacy (ASAS20) of JAK inhibitors and secukinumab.
Table 3.
Rank probability in terms of efficacy based on the number of patients that achieved an ASAS20 or ASAS40 response, and the safety based on the number of SAEs
| Treatment | SUCRA |
|---|---|
| A. Efficacy: ASAS20 | |
| Tofacitinib 5 mg | 0.887 |
| Filgotinib 200 mg | 0.788 |
| Upadacitinib 15 mg | 0.426 |
| Secukinumab 150 mg | 0.399 |
| Placebo | <0.001 |
|
| |
| B. Efficacy: ASAS40 | |
| Tofacitinib 5 mg | 0.751 |
| Upadacitinib 15 mg | 0.668 |
| Secukinumab 150 mg | 0.543 |
| Filgotinib 200 mg | 0.535 |
| Placebo | 0.003 |
|
| |
| C. Safety | |
| Tofacitinib 5 mg | 0.700 |
| Secukinumab 150 mg | 0.553 |
| Filgotinib 200 mg | 0.437 |
| Upadacitinib 15 mg | 0.431 |
| Placebo | 0.379 |
SUCRA, surface under the cumulative ranking curve.
Network Meta-Analysis of the Safety of JAK Inhibitors and Secukinumab
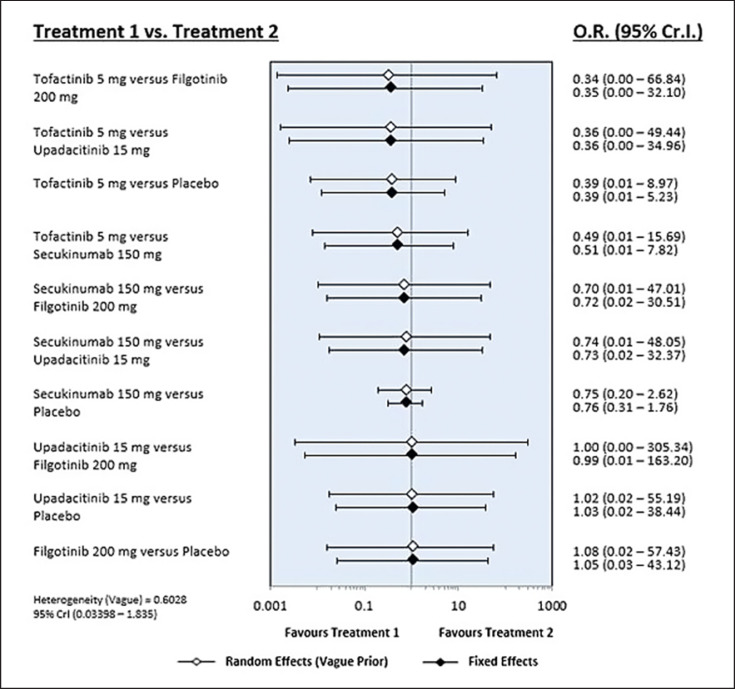
The number of SAEs with tofacitinib 5 mg was numerically smaller than those observed with the other JAK inhibitors, secukinumab, and placebo; however, the results were not statistically significant (Table 3; Fig. 4). There was no significant difference in the number of SAEs among the seven treatments (Table 3; Fig. 4).
Fig. 4.
Results of the Bayesian network meta-analysis of randomized controlled studies assessing the relative safety (SAE) of JAK inhibitors and secukinumab.
Inconsistency and Sensitivity Analysis
Inconsistency plots, evaluating network inconsistencies between direct and indirect estimates, showed low potential for differences that could significantly affect the results of the network meta-analysis. This was verified by the random and fixed-effect model comparison, thus indicating reliable meta-analysis findings from this network (Fig. 3, 4).
Discussion
Tofacitinib 5 mg was shown to be the most effective therapy for AS, whereas JAK inhibitors and secukinumab were beneficial in patients with active AS who had poor response or intolerance to NSAIDs and were TNF inhibitor-naïve. There was no statistically significant difference in ASAS response rates between the JAK inhibitors and secukinumab. In terms of safety, there was no significant difference in the number of SAEs across the seven treatment groups, suggesting that the JAK inhibitors, secukinumab, and placebo groups were all safe. Our findings support the efficacy of JAK inhibitors and secukinumab in the treatment of AS.
IL-17A plays a significant role in the pathophysiology of AS [25]. Secukinumab is the first monoclonal anti-IL-17A antibody to demonstrate the efficacy of a non-TNF-targeted AS therapy as a potential therapeutic option for AS [7]. TNF inhibitors and secukinumab are currently the only options for individuals with AS who do not react well to NSAIDs [4]. Our results indicate that JAK inhibitors may be an effective and safe treatment regimen for individuals with active AS who do not respond well to NSAIDs; these findings may stimulate additional research into the use of JAK inhibitors in the management of AS.
Given the following limitations, the results of this study should be interpreted with caution. First, a relatively short treatment duration (12–16 weeks) was chosen as the time point for follow-up. Consequently, the follow-up period was insufficient to establish the long-term effects of the treatment. Second, because the chosen study designs and patient characteristics were diverse, the results of this network meta-analysis may have been affected by inter-study differences [26, 27]. Third, our study did not adequately examine the effectiveness and safety outcomes of JAK inhibitors and secukinumab in patients with AS. We focused exclusively on treatment efficacy based on the number of patients who attained ASAS20 and ASAS40. We focused on safety based on the number of SAEs. The number of SAEs, in particular, may not be suitable as a safety outcome indicator owing to their low frequency. Finally, this meta-analysis included only a small number of trials. As a result, this study was underpowered to investigate the relative efficacy and safety of the test drugs.
A Bayesian network meta-analysis compares all treatment choices to a traditional meta-analysis, allowing for simultaneous comparisons of different treatment options when direct head-to-head comparisons are not possible [6, 28]. This is the first meta-analysis to use Bayesian network analysis to evaluate the effectiveness and safety of JAK inhibitors and secukinumab in active AS. This may be the best evidence available in this area until conclusive RCTs are conducted [18, 29, 30].
In conclusion, we conducted a Bayesian network meta-analysis of six RCTs and found that tofacitinib 5 mg was the most effective treatment based on the ASAS20 and ASAS40 response rates, whereas JAK inhibitors and secukinumab 150 mg were effective in patients with active AS who had poor response or intolerance to NSAIDs and were TNF inhibitor-naïve. JAK inhibitors and secukinumab were not associated with an increased risk of SAEs. Long-term studies are required to compare the effectiveness and safety of JAK inhibitors and secukinumab in larger number of patients with active AS.
Statement of Ethics
Ethical review and approval was not required as the study is based exclusively on published literature.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There were no funding sources.
Author Contributions
Young Ho Lee was involved in conception and design of study, acquisition of data, analysis and/or interpretation of data, drafting the manuscript, and revising the manuscript critically for important intellectual content.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Funding Statement
There were no funding sources.
References
- 1.Inman RD. Axial spondyloarthritis: current advances, future challenges. J Rheum Dis. 2021;28((2)):55–9. doi: 10.4078/jrd.2021.28.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeong H, Bae E-K, Hwang J, Park E-J, Lee J, Jeon CH, et al. The effects of sex and estrogen on radiographic progression of ankylosing spondylitis in Korean patients. J Rheum Dis. 2021;28((2)):76–84. doi: 10.4078/jrd.2021.28.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, Koh JH, Choi SJ, Jeon CH, Kwok SK, Kim SK, et al. KOBIO, the first web-based Korean biologics registry operated with a unified platform among distinct disease entities. J Rheum Dis. 2021;28((4)):176–182. doi: 10.4078/jrd.2021.28.4.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Heijde D, Ramiro S, Landewé R, Baraliakos X, Van den Bosch F, Sepriano A, et al. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 20162017;76((6)):978–991. doi: 10.1136/annrheumdis-2016-210770. [DOI] [PubMed] [Google Scholar]
- 5.Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, et al. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013 Nov 23;382((9906)):1705–1713. doi: 10.1016/S0140-6736(13)61134-4. [DOI] [PubMed] [Google Scholar]
- 6.Song GG, Bae SC, Lee YH. Association between vitamin D intake and the risk of rheumatoid arthritis: a meta-analysis. Clin Rheumatol. 2012;31((12)):1733–1739. doi: 10.1007/s10067-012-2080-7. [DOI] [PubMed] [Google Scholar]
- 7.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis: results of two phase 3 trials. N Engl J Med. 2014 Jul 24;371((4)):326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 8.Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003 Oct 31;302((5646)):875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- 9.Nakase T, Wada H, Minamikawa K, Wakita Y, Shimura M, Hiyoyama K, et al. Increased activated protein C-protein C inhibitor complex level in patients positive for lupus anticoagulant. Blood Coagul Fibrinolysis. 1994 Apr;5((2)):173–177. doi: 10.1097/00001721-199404000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015 Dec 24;373((26)):2534–2548. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 11.Pavelka K, Kivitz A, Dokoupilova E, Blanco R, Maradiaga M, Tahir H, et al. Efficacy, safety, and tolerability of secukinumab in patients with active ankylosing spondylitis: a randomized, double-blind phase 3 study, MEASURE 3. Arthritis Res Ther. 2017;19((1)):285. doi: 10.1186/s13075-017-1490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Heijde D, Deodhar A, Wei JC, Drescher E, Fleishaker D, Hendrikx T, et al. Tofacitinib in patients with ankylosing spondylitis: a phase II 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis. 2017;76((8)):1340–1347. doi: 10.1136/annrheumdis-2016-210322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kivitz AJ, Wagner U, Dokoupilova E, Supronik J, Martin R, Talloczy Z, et al. Efficacy and safety of secukinumab 150 mg with and without loading regimen in ankylosing spondylitis: 104-week results from MEASURE 4 study. Rheumatol Ther. 2018;5((2)):447–462. doi: 10.1007/s40744-018-0123-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Heijde D, Baraliakos X, Gensler LS, Maksymowych WP, Tseluyko V, Nadashkevich O, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active ankylosing spondylitis (TORTUGA): results from a randomised, placebo-controlled, phase 2 trial. Lancet. 2018;392((10162)):2378–2387. doi: 10.1016/S0140-6736(18)32463-2. [DOI] [PubMed] [Google Scholar]
- 15.van der Heijde D, Song I-H, Pangan AL, Deodhar A, van den Bosch F, Maksymowych WP, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet. 2019;394((10214)):2108–2117. doi: 10.1016/S0140-6736(19)32534-6. [DOI] [PubMed] [Google Scholar]
- 16.Caldwell DM, Ades A, Higgins J. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331((7521)):897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YH, Bae SC, Song GG. The association between the functional PTPN22 1858 C/T and MIF -173 C/G polymorphisms and juvenile idiopathic arthritis: a meta-analysis. Inflamm Res. 2012;61((5)):411–415. doi: 10.1007/s00011-012-0447-5. [DOI] [PubMed] [Google Scholar]
- 18.Young Ho LMD, PD, Gwan Gyu S, MD, PD Associations between circulating interleukin-17 levels and systemic lupus erythematosus and between interleukin-17 gene polymorphisms and disease susceptibility: a meta-analysis. J Rheum Dis. 2020;27((1)):37–44. [Google Scholar]
- 19.Anderson JJ, Baron G, van der Heijde D, Felson DT, Dougados M. Ankylosing spondylitis assessment group preliminary definition of short-term improvement in ankylosing spondylitis. Arthritis Rheum. 2001 Aug;44((8)):1876–1886. doi: 10.1002/1529-0131(200108)44:8<1876::AID-ART326>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009 Aug 18;339((4)):b2535w64. [PMC free article] [PubMed] [Google Scholar]
- 21.Brown S, Hutton B, Clifford T, Coyle D, Grima D, Wells G, et al. A microsoft-excel-based tool for running and critically appraising network meta-analyses: an overview and application of NetMetaXL. Syst Rev. 2014;3((1)):110. doi: 10.1186/2046-4053-3-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011 Feb;64((2)):163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making. 2013 Jul;33((5)):641–656. doi: 10.1177/0272989X12455847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods. 2012 Dec;3((4)):285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 25.Shen H, Goodall JC, Hill Gaston JS. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum. 2009 Jun;60((6)):1647–1656. doi: 10.1002/art.24568. [DOI] [PubMed] [Google Scholar]
- 26.Byun S, Jung SM, Song JJ, Park YB, Lee SW. Hemoglobin A1c, not glycated albumin, can independently reflect the ankylosing spondylitis disease activity score. J Rheum Dis. 2018;25((2)):131–139. [Google Scholar]
- 27.Hahn YS. Enthesitis-related arthritis. J Rheum Dis. 2018;25((4)):221–230. [Google Scholar]
- 28.Young Ho L. Overview of network meta-analysis for a rheumatologist. J Rheum Dis. 2016;23((1)):4–10. [Google Scholar]
- 29.Lee YH. An overview of meta-analysis for clinicians. Korean J Intern Med. 2018;33((2)):277. doi: 10.3904/kjim.2016.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee YH, Song GG. Circulating interleukin-37 levels in rheumatoid arthritis and systemic lupus erythematosus and their correlations with disease activity: a meta-analysis. J Rheum Dis. 2020;27((3)):152–158. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.