Abstract
The prevalence of gestational diabetes parallels the prevalence of type 2 diabetes mellitus and is associated with adverse pregnancy outcomes. However, these data are not available for many parts of the world. We assessed the prevalence of gestational diabetes and pregnancy outcomes in Tajikistan. This cohort study included 2438 consecutively recruited representative pregnant women from 8 locations in two cities in Tajikistan, in whom an oral glucose tolerance test (75 g, fasting, 1 h, 2 h) was performed during gestational weeks 24–28. Women with known diabetes and twin pregnancies were excluded. Associations between glucose tolerance test results and pregnancy outcomes were examined. According to the WHO 2013 thresholds, 32.4% of women qualified as having gestational diabetes, the vast majority (29.7%) based on an elevated fasting glucose level (5.1–5.6 mmol/L), while only 2.8% had elevated 1- or 2-hour values or met more than one threshold. Women with only elevated fasting glucose (impaired gestational fasting glycemia) had no evidence of adverse pregnancy outcomes, while those with elevated 1- and/or 2-hour values (impaired gestational glucose tolerance) had more pregnancy complications (infection of urinary tract 1.8 vs. 8.8% p<0.001; preeclampsia 0.7 vs. 10.3% p<0.001) and emergency cesarean sections (4.4 vs. 13.2% p=0.002). Neonates from pregnancies with impaired gestational glucose tolerance had lower APGARs, lower birth weights, lower 30 min glucose levels, and a lower probability of being discharged alive (all p<0.05). In conclusion, the formal prevalence of gestational diabetes is high in Tajikistan; however, this does not translate into adverse pregnancy outcomes for women with impaired gestational fasting glycemia.
Key words: Central Asia, oral glucose tolerance test, maternal outcome, neonatal outcome, neonatal hypoglycemia
Introduction
The prevalence of type 2 diabetes is increasing globally, however, with considerable variation in different countries and regions worldwide 1 . This increase reflects urbanization, the aging of populations and also reductions in the prevalence of undernutrition and infectious diseases, which may at least partly explain the variation in trends in different regions of the world. The increased prevalence of type 2 diabetes is paralleled by increased rates of gestational diabetes, which affects 6–25% of pregnant women 2 3 . However, for many countries, the true prevalence of gestational diabetes is not known [ https://www.diabetesatlas.org ]. Since gestational diabetes puts affected mothers and their children at short-term and long-term risk 4 5 6 7 , WHO and professional societies recommend screening strategies to identify women with gestational diabetes and treatment of those affected.
Based on the original recommendations by the International Association of Diabetes and Pregnancy Groups (IADPSG), many professional societies and WHO recommend a one-step screening of all pregnant women during weeks 24–28 of pregnancy, using an oral glucose tolerance test (OGTT) with 75 g of glucose following an overnight fast 8 9 . Gestational diabetes is diagnosed if any of the following thresholds is met (fasting≥5.1 mmol/L, 1-hour ≥ 10.0 mmol/L, 2-hours≥8.5 mmol/L) 8 . Although widely accepted as a concept, this strategy is not generally implemented in many countries and has attracted criticism, as it may inappropriately label women as having gestational diabetes who are at a low absolute risk of pregnancy complications and may even lead to unnecessary interventions 10 11 12 .
In central Asia, the prevalence of diabetes is increasing rapidly, but data on the prevalence of gestational diabetes are only available for selected populations 1 13 . We, therefore, implemented a screening program in two cities in Tajikistan, in Dushanbe, the capital, and Qurghonteppa (renamed Bokhtar), a regional city in the south, close to the Afghan border. In both cities, the Reproductive Health Centers (antenatal care) and the city hospitals (delivery) are accessible (and used) by the general population and are free of charge to the women. We included seven Reproductive Health Centers located throughout the city of Dushanbe and one in a more rural area to evaluate a representative sample of the population for the prevalence of gestational diabetes in Tajikistan and relate the results of the glucose tolerance test to pregnancy outcomes.
Materials and Methods
The study was registered at ClinicalTrials.gov (NCT02436551) and performed between September 2015 and November 2017, during which time we screened 2643 pregnant women for gestational diabetes. A total of 1718 women were recruited from seven different Reproductive Health Centers in Dushanbe (located in different sections of the city). For delivery, women from all seven Reproductive Health Centers come to the City Medical Center Karim Akhmedov, which manages approximately 8000 deliveries per year (the total number of deliveries per year in Tajikistan is approximately 190000). A total of 925 women were recruited from the Reproductive Health Center in Qurghonteppa (Bokhtar) and women who delivered in the regional hospital (approximately 2000 deliveries per year). In each Reproductive Health Center, up to five subsequent women per day were informed about the study during the initial visit (usually before pregnancy week 12) and asked to participate. Although more than 20000 children were born during the study period in these maternity hospitals, only 2643 women were recruited due to logistic (handling of glucose tolerance tests) and budget reasons. Women with any chronic condition (thyroid disease, autoimmune disease) or those with twin pregnancies were excluded from the study. After obtaining written informed consent, an OGTT was scheduled between weeks 24 and 28 of gestation.
On the day of the OGTT, women arrived fasting (8 hours minimum) at the health center between 7:00 am and 9:00 am. Weight, height, and blood pressure were measured and a questionnaire (family history, history of previous pregnancies, etc.) was completed; then, the OGTT (75 g of glucose and venous glucose measurements at 0, 60, and 120 minutes) was performed. Glucose levels were determined using a photometer (Photoelectric colorimeter CPC-2 model; presumably 1980). Gestational diabetes was diagnosed if one or more values were at or above the following thresholds: 0-minute:≥5.1 mmol/L; 60-minutes:≥10.0 mmol/L; 120-minutes:≥8.5 mmol/L). All pregnant women were followed until delivery and infant (APGAR, 30 min glucose level, survival) and maternal (mode of delivery, complications such as preeclampsia, infections, and bleeding, and survival) data were collected. Complications observed during pregnancy were noted at the time of delivery. Furthermore, the discharging physician indicated whether the women were healthy or sick (at discharge). Patients with gestational diabetes were managed according to local standards.
Based on recent publications indicating that women with slightly elevated glucose levels (in particular fasting values between 5.1–5.6 mmol/L) have no adverse pregnancy outcome 14 15 , we reclassified women with gestational diabetes as either “impaired gestational fasting glycemia” if fasting glucose levels were 5.1–5.6 mmol/L) or as “impaired gestational glucose tolerance” if fasting values were>5.6 mmol/L and/or 1-hour and/or 2-hour values were elevated. This subclassification was introduced during the study period and was thus, not part of the sample size calculation.
The sample size calculation and statistical analysis were based on data from a previous study performed in Turkmenistan (showing a gestational diabetes rate of 6.3%) 13 and the assumption that at least 100 cases of gestational diabetes are needed to meaningfully relate data from the glucose tolerance to pregnancy outcome; we thus included a sample size of 2500 pregnancies (which allows a drop-out rate of 20% at a prevalence of 5%). The sample size calculation was based on the presumed overall rate of gestational diabetes and did not take the subclassifications (“impaired gestational fasting glycaemia” and “impaired gestational glucose tolerance”) into account. All data were noted on paper and then entered into an Excel file for further analysis. Statistical analysis was performed using the SPSS Statistics 23 software package (IBM, USA). Analysis of variance (ANOVA) for independent continuous variables was performed using ANOVA (H – Kruskal-Wallis test) for multiple comparisons and Mann-Whitney U-test for comparisons between two groups. Comparisons of distinct variables were carried out using a contingency table according to the χ2 criterion for the compared quantities over 10, according to the χ2 criterion with Yates’ correction for the compared quantities over 5, and according to Fisher’s exact criterion for the compared quantities less than 5. A p-value of<0.05 was considered significant.
The study was performed in accordance with the Helsinki Declaration II and approved by the Ethics Committee of the Medical Faculty of the University of Munich and by the Medical Ethics Committee (MEC), Ministry of Health and Social Protection of the Republic of Tajikistan. Written informed consent was obtained from all participants.
Results
The characteristics of the women included in the study are shown in Table 1 . As shown in Fig. 1 , 205 women were excluded from the analysis (n=65 did not complete OGTT; n=20 were lost to follow-up; n=1 had diabetes mellitus type 1; n=9 had diabetes mellitus type 2; n=7 had implausible glucose levels (<2.5 mmol/L); n=65 had implausible gestational age; n=38 had twin pregnancy).
Table 1 Characteristics of study participants.
| General characteristics | Overall | No gestational diabetes | Gestational diabetes | p-value† | No gestational diabetes | Impaired gestational fasting glycaemia | Impaired gestational glucose tolerance | p-value‡ |
|---|---|---|---|---|---|---|---|---|
| Sample size n (%) | 2438 (100) | 1647 (100) | 791 (100) | 1647 (100) | 723 (100) | 68 (100) | ||
| Age (y)* | 24.8±5.1 | 24.6±4.9 | 25.3±5.3 | 0.001 | 24.6±4.9 | 25.4±5.3§ | 24.3±5.2 | 0.001 |
| Body mass index (kg*m −2 )* | 23.4±4.1 | 23.2±4.0 | 23.8±4.3 | 0.002 | 23.2±4.0 | 23.9±4.3§ | 23.0±4.2 || | 0.001 |
| Mean art. pressure (mm Hg)* | 75.9±7.8 | 75.8±7.6 | 76.2±8.3 | ns | 75.8±7.6 | 75.6±7.5 | 81.7±13.0|§ | <0.001 |
| Gestational age at OGTT (wk)* | 26.3±2.5 | 26.3±2.8 | 26.3±2.7 | ns | 26.3±2.8 | 26.3±2.8 | 26.4±2.4 | ns |
| Parity n (%) | 0.005 | 0.006 | ||||||
| 1 | 812 (33.3) | 560 (34.0) | 252 (31.9) | 560 (34.0) | 222 (30.7) | 30 (44.1) || | ||
| 2 | 691 (28.3) | 487 (29.6) | 204 (25.8) | 487 (29.6) | 189 (26.1) | 15 (22.1) | ||
| ≥3 | 935 (38.4) | 600 (36.4) | 335 (42.4) § | 600 (36.4) | 312 (43.2) | 23 (33.8) | ||
| Previous pregnancy complications n (%) | 844 (34.6) | 576 (35.0) | 268 (33.9) | ns | 576 (35.0) | 247 (34.2) | 21 (30.9) | ns |
| Family history for diabetes mellitus n (%) | 248 (10.2) | 167 (10.1) | 81 (10.2) | ns | 167 (10.1) | 72 (10.0) | 9 (13.2) | ns |
| Consanguinity n (%) | 377 (15.5) | 255 (15.5) | 122 (15.4) | ns | 255 (15.5) | 111 (15.4) | 11 (16.2) | ns |
* Mean±SD and n (%); † comparison of p-values of no gestational diabetes and gestational diabetes; ‡ comparison of p-values of no gestational diabetes, impaired gestational fasting glycaemia, and impaired gestational glucose tolerance; § p<0.01 compared to no gestational diabetes; | p<0.01 compared to impaired gestational fasting glycemia; || p<0.05 compared to impaired gestational fasting glycemia.
Fig. 1.
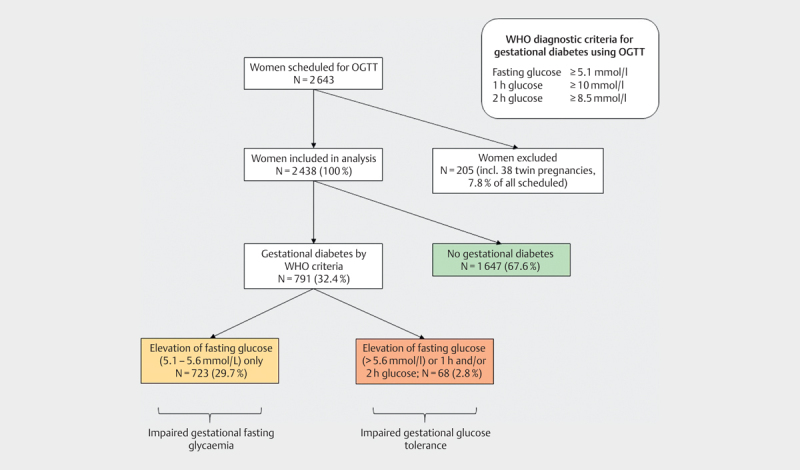
Flow diagram of women included in the study. OGTT, oral glucose tolerance test.
Fig.1 indicates a very high rate of gestational diabetes in Tajikistan (32.4%), mostly based on elevated fasting glucose levels (women with impaired gestational fasting glycemia). The rate of gestational diabetes is much lower (2.8%) when the diagnosis was limited to women with either more than one elevated glucose level or severely elevated fasting glucose (5.6–7.0 mmol/l) or elevated 1 h or 2 h glucose levels (women with impaired gestational glucose tolerance),.
Compared to women without gestational diabetes, those with gestational diabetes were older and had a higher BMI and parity ( Table 1 ). A similar result was seen when women with impaired gestational fasting glycemia are compared to women without gestational diabetes, while women with impaired gestational glucose tolerance were slimmer, had higher blood pressure, and were more likely to be parity 1.
Maternal and neonate outcomes of women without gestational diabetes, those with impaired gestational fasting diabetes, and those with impaired glucose tolerance are mentioned in Tables 2 3 . No clinically significant difference in outcome was observed between women without gestational diabetes and those with gestational diabetes under fasting only (impaired gestational fasting glycemia), while women with impaired gestational glucose tolerance had more complications, infections, preeclampsia, and emergency cesarean sections. Similarly, in the neonates, offspring from mothers with impaired gestational fasting glycemia had a similar outcome as those from mothers without gestational diabetes. Offspring from women with impaired gestational glucose tolerance had a poor prognosis with lower APGAR values, more hypoglycemia, and a much higher perinatal mortality rate. As expected, the rate of neonatal hypoglycemia was much higher in women with impaired gestational glucose tolerance compared to those with impaired gestational fasting glycemia and no gestational diabetes. Only a few children with glucose levels below 2 mmol/L received intra-venous glucose.
Table 2 Pregnancy and maternal outcome according to gestational diabetes status in this study.
| No gestational diabetes (n=1647) | Impaired gestational fasting glycaemia (n=723) | Impaired gestational glucose tolerance (n=68) | p-value* | ||
|---|---|---|---|---|---|
| Pregnancy | |||||
| Any complication | 461 (28.0%) | 111 (15.4%)† | 39 (57.4%)†‡ | <0.001 | |
| Threatening miscarriage | 278 (16.9%) | 56 (7.7%)† | 21 (30.9%†)‡ | <0.001 | |
| Preeclampsia | 26 (1.6%) | 5 (0.7%) | 7 (10.3%)†‡ | <0.001 | |
| Urinary tract infection | 80 (4.9%) | 13 (1.8%)† | 6 (8.8%)‡ | <0.001 | |
| Delivery | |||||
| Gestational weeks at delivery | 39.2±2.5 | 39.3±2.2 | 37.2±4.6†‡ | 0.001 | |
| <37 weeks | 146 (8.9%) | 62 (8.6%) | 17 (25.0%)†‡ | <0.001 | |
| Spontaneous delivery | 1504 (91.3%) | 659 (91.1%) | 58 (85.3%) | ns | |
| Cesarean section | 142 (8.6%) | 63 (8.7%) | 9 (13.2%) | ns | |
| Emergency Cesarean s. | 102 (6.2%) | 32 (4.4%) | 9 (13.2%)†§ | <0.01 | |
| Mother | |||||
| Healthy | 1623 (98.5%) | 702 (97.1%) | 68 (100%) | ns | |
| Sick | 24 (1.5%) | 21 (2.9%) | 0 | ns | |
| Dead | 0 | 0 | 0 | ||
* refers to significant differences between groups; † p<0.01 compared to no gestational diabetes; ‡ p<0.01 compared to impaired gestational fasting glycemia; § p<0.05 compared to no gestational diabetes.
Table 3 Neonatal outcome according to gestational diabetes status.
| No gestational diabetes (n=1647) | Impaired gestational fasting glycaemia (n=723) | Impaired gestational glucose tolerance (n=68) | p-value* | ||
|---|---|---|---|---|---|
| Neonate | |||||
| Alive at birth | 1624 (98.6%) | 719 (99.4%) | 61 (89.7%)†‡ | <0.001 | |
| Antenatal death | 23 (1.3%) | 4 (0.6%) | 4 (5.9%)†‡ | <0.001 | |
| Postnatal death | 45 (2.7%) | 13 (1.8%) | 11 (16.2%)†‡ | <0.001 | |
| Discharged alive | 1599 (97.1%) | 710 (98.2%) | 57 (83.8%)†‡ | <0.001 | |
| Birth weight | 3229±607 | 3246±587 | 2899±1003§|| | <0.05 | |
| >4000 g | 135 (8.2%) | 65 (9.0%) | 6 (8.8%) | ns | |
| <1500 g | 40 (2.4%) | 15 (2.1%) | 12 (17.6%)†‡ | <0.001 | |
| APGAR 1 min | 7.21±0.91 | 7.28±0.91† | 6.68±1.30†‡ | <0.001 | |
| <7 | 111 (6.7%) | 49 (6.8%) | 11 (16.2%) | ns | |
| APGAR 5 min | 7.91±0.76 | 7.86±0.71 | 7.68±1.30 | ns | |
| <7 | 150 (9.1%) | 73 (10.1%) | 11 (16.2%)|| | ns | |
| APGAR 10 min | 8.65±0.94 | 8.47±0.86† | 8.65±1.49§‡ | <0.001 | |
| <7 | 36 (2.2%) | 14 (1.9%) | 4 (5.9%) | ns | |
| 30 min glucose (mmol/L) | 3.38±0.76 | 3.41±0.64 | 2.97±1.24 | ns | |
| <2.0 mmol/L | 60 (3.6%) | 14 (1.9%)† | 9 (13.8%)†‡ | <0.001 | |
| IV treatment for children with glucose<2.0 mmol/L | 6 (10%) | 2 (14%) | 2 (22%)|| | <0.05 | |
| Deceased children with glucose<2.0 mmol/L | 2 (3.3%) | 1 (7.1%) | 1 (11%)|| | ns | |
* refers to significant differences between groups; † p<0.01 compared to no gestational diabetes; ‡ p<0.01 compared to impaired gestational fasting glycemia; § p<0.05 compared to no gestational diabetes; || p<0.05 compared to impaired gestational fasting glycemia.
Discussion
In this study, we evaluated the prevalence of gestational diabetes in Tajikistan and studied the association between glucose levels during OGTT with pregnancy outcomes. The data shown in Table 1 indicate that our cohort is representative of pregnant women in Tajikistan with respect to age and parity ( https://dhsprogram.com/pubs/pdf/SR203/SR203.pdf ). Since we included women from different parts of Dushanbe (seven large Reproductive Health Centers) and a rural city, we believe that the cohort is also representative of the socio-economic status.
Based on the WHO criteria, we found a very high rate of gestational diabetes in Tajikistan (32.4%). In most patients, the diagnosis was based on a slightly elevated fasting glucose value, while the severe elevation of fasting glucose (>5.6 mmol/L) and/or elevated 1-hour and/or 2-hour values were present only in 2.8% of the participants. As expected, women with impaired gestational fasting glycemia were older and had a higher BMI than women without gestational diabetes; surprisingly women with impaired gestational glucose tolerance did not differ from women without gestational diabetes with respect to age and BMI. When the clinical outcome in mothers and newborns were evaluated, we found no difference between women with impaired gestational fasting glycemia and those with all normal values (no gestational diabetes). On the other hand, those with impaired gestational glucose tolerance characterized by severely elevated fasting values and/or elevated 1-hour and/or 2-hour values had a much higher complication rate, higher emergency caesarian section rate, and their neonates had more complications. This finding can be interpreted in multiple ways.
First, women classified as having impaired gestational fasting glycemia may have no gestational diabetes because the obtained value may not represent a true and adequately obtained fasting value. Although the women were instructed to fast before coming for the OGTT (and were asked about their feeding status at the beginning of the test), this may not have always been the case as these women sometimes have to leave the house very early to reach the Health Center. Also, these women may have been relatively nervous about the upcoming OGTT, which may have increased cortisol levels and thus glucose values. On the other hand, long ways to the Health Care Centers and thus more physical activity could also decrease glucose levels and thus result in false negative results. In addition, measurements of glucose levels may not have been very precise as photometric results were directly read by a technician from the photometer (slightly swinging needle indicator) (Photoelectric colorimeter CPC-2 model; presumably 1980). All centers are now (since 2018) equipped with more modern techniques, which allow a more precise determination of glucose levels.
Second, these women may have a very mild form of gestational diabetes, which does not translate into adverse pregnancy outcome. In this sense, our study corroborates with recently published studies also confirming that a diagnosis of gestational diabetes only based on elevated fasting glucose levels does not translate into adverse clinical outcomes 14 15 16 17 . For example, a recent observational study in Denmark in 1,516 women revealed a gestational diabetes rate of>40% (based on elevated fasting glucose levels) with normal pregnancy outcomes in those having fasting glucose levels between 5.1 and 5.6 mmol/L 15 . Similarly, a recent evaluation in the US indicates that a two-step screening approach (compared to a one-step screening) results in a considerably lower prevalence of gestational diabetes with similar clinical outcomes in mothers and offspring, again indicating that some forms of gestational diabetes may not translate into clinical problems 14 .
Finally, our observations are also consistent with the interpretation that the diagnosis of gestational diabetes induced a therapeutic action (lifestyle modification), which normalized glucose levels and led to a normal pregnancy outcome. This, however, is unlikely as gestational diabetes was largely “unknown” in the Reproductive Health Centers until our project started. To our knowledge, no formal diabetes counseling was performed, and very few (if any) patients received insulin. This makes it unlikely that mild fasting gestational diabetes was treated at all.
Considering these aspects and the data from previous studies performed in the USA and Denmark, it is most likely that some forms of gestational diabetes do not translate into adverse pregnancy outcome, although the HAPO study indicated that increasing fasting glucose levels are linearly associated with adverse pregnancy outcomes without apparent threshold 4 . This is highly relevant, because diagnosing gestational diabetes puts a burden on pregnant women (psychologically and economically in many societies) and on the healthcare system and may result in an increased rate of cesarean sections 12 . On the other hand, even impaired gestational fasting glycemia may be a risk factor for childhood obesity, but it is unclear if treating this condition can prevent obesity and other metabolic consequences in the offspring 18 . While it is known that gestational diabetes is associated with an increased risk of type 2 diabetes and metabolic diseases later in life, it is unclear whether elevated fasting glucose (which does not translate into adverse short-term pregnancy outcomes) leads to metabolic consequences later in life (in the mother and/or the offspring).
If our findings, that an elevated fasting glucose level does not translate into adverse pregnancy outcomes, are confirmed by other studies, then a strategy that propagates general screening using WHO criteria in countries such as Tajikistan, where most pregnant women are young and slim, should be questioned. In that case, different screening strategies may be more appropriate to identify women with clinically significant gestational diabetes. Options include a two-step screening 19 , or only measuring 2-hour glucose level after ingesting 75 g glucose in the non-fasting state as suggested by the “Diabetes In Pregnancy Study group of India (DIPSI)” 20 , or using a 75 g glucose test in the fasting state but applying different cut-off values as suggested by the International Federation of Obstetrics and Gynecology (FIGO) 21 . Finally, screening only women with risk factors could be a further option. However, these alternatives have limitations, and none is validated for Tajikistan or any other central Asian country 22 23 . Interestingly, a recent report by the US Preventive Services Task Force indicates that many aspects of screening for gestational diabetes, remain unclear also in developed countries 24 .
Our study also shows that severe elevation of glucose levels during the OGTT is associated with a very high rate of complications in the mother and the offspring. This probably reflects the fact that gestational diabetes, even when diagnosed, is not managed appropriately. Unfortunately, we could not collect data on diabetes management in affected women.
In a previous study, we used a two-step approach to determine the prevalence of gestational diabetes in Turkmenistan, revealing a rate of 6.3% 13 . This is more than double the prevalence of impaired gestational glucose tolerance in Tajikistan described in this study, although Turkmenistan and Tajikistan share many similarities. This difference in prevalence is most likely because in Turkmenistan, our study was performed in a private hospital with women coming from a privileged socio-economic background and thus being older and more obese (age: 27.6±5.2 vs. 24.8±5.1 years; BMI: 26.6±4.8 vs. 23.4±4.1 kg/m²).
The strengths of our study relate to the size of the cohort, the first study to report data on the prevalence of gestational diabetes in Tajikistan in a representative sample of women, and present the prevalence data in relation to outcome.
Our study has some limitations. We did not collect data on diabetes management and therefore cannot make any statement on why the outcome of those with impaired gestational glucose tolerance is so poor. Similarly, as discussed above, we cannot exclude that the excellent outcome of women with impaired gestational fasting glycemia relates to a treatment effect.
Although we included data from several centers in Tajikistan, we cannot make any statement on women living in more rural areas such as the Pamir mountains. In more remote areas, women have to travel to get to their Reproductive Health Center, which is challenging if a fasting glucose level is required. In addition, our results refer only to the ethnic group of Tajiks, which represent approximately 84% of the Tajik population. [ https://en.wikipedia.org/wiki/Demographics_of_Tajikistan#Ethnic_groups ]
Although we studied a large number of women, the absolute number of women with impaired gestational glucose tolerance was low, preventing any further analysis of this subgroup (for example, to show predictors for poor outcome).
In this study, we confirmed very high perinatal mortality, approximately 10-times higher than in European countries. The project also highlights that many aspects of the health sector, that can be taken for granted in Europe, are not widely available in Tajikistan (such as easy-to-use and exact glucose measurement devices or treatment options for gestational diabetes). This indicates that Tajikistan must develop strategies to better manage high-risk pregnancies and decrease the high perinatal mortality. This should include guideline-oriented management of women with gestational diabetes. In that context, it would be interesting to repeat the screening project after introducing diabetes education, self-measurement of glucose levels, and insulin therapy for women with gestational diabetes, if necessary.
In our trial, we found a high rate of gestational diabetes in Tajikistan, of which, however, only a minority were clinically relevant. This finding is compatible with the fact that the women did not receive any therapy and that a small increase in fasting glucose in this group did not lead to adverse outcomes even without therapy under the local health care system.
Author contribution
DP analyzed the data and wrote the manuscript. MD analyzed the data and reviewed the manuscript. UH contributed to the discussion and reviewed/edited the manuscript. AWF contributed to the discussion and reviewed/edited the manuscript. ZA researched the data and reviewed the manuscript. KS researched the data and reviewed the manuscript. NS researched the data and reviewed the manuscript. SR researched the data and reviewed the manuscript. KGP designed the study and wrote the manuscript.
Acknowledgment
We thank Dr. Renee G. Stark, MD, MPH for statistical guidance and review.
Funding Statement
Funding This study was supported by a grant from the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung) to Klaus G. Parhofer (Förderkennzeichen 01DK14022).
Footnotes
Conflict of Interest The authors declare that the research was conducted with no commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Collaboration NCDRF . Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho N H, Shaw J E, Karuranga S et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 3.Sacks D A, Hadden D R, Maresh M et al. Frequency of gestational diabetes mellitus at collaborating centers based on IADPSG consensus panel-recommended criteria: the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 2012;35:526–528. doi: 10.2337/dc11-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Group HSCR . Metzger B E, Lowe L P, Dyer A R et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 5.Lowe W L, Scholtens D M, Kuang A et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): Maternal gestational diabetes mellitus and childhood glucose metabolism. Diabetes Care. 2019;42:372–380. doi: 10.2337/dc18-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholtens D M, Kuang A, Lowe L P et al. Hyperglycemia and adverse pregnancy outcome follow-up study (HAPO FUS): Maternal glycemia and childhood glucose metabolism. Diabetes Care. 2019;42:381–392. doi: 10.2337/dc18-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillier T A, Pedula K L, Schmidt M M et al. Childhood obesity and metabolic imprinting: The ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–2292. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res Clin Pract. 2014;103:341–363. doi: 10.1016/j.diabres.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 9.International Association of D, Pregnancy Study Groups Consensus P . Metzger B E, Gabbe S G, Persson B et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung N W, Jiang S, Athayde N. Impact of the IADPSG criteria for gestational diabetes, and of obesity, on pregnancy outcomes. Aust N Z J Obstet Gynaecol. 2018;58:553–559. doi: 10.1111/ajo.12772. [DOI] [PubMed] [Google Scholar]
- 11.Cheung N W, Moses R G. Gestational diabetes mellitus: Is it time to reconsider the diagnostic criteria? Diabetes Care. 2018;41:1337–1338. doi: 10.2337/dci18-0013. [DOI] [PubMed] [Google Scholar]
- 12.Feldman R K, Tieu R S, Yasumura L. Gestational diabetes screening: The International Association of the Diabetes and Pregnancy Study Groups compared with Carpenter-Coustan Screening. Obstet Gynecol. 2016;127:10–17. doi: 10.1097/AOG.0000000000001132. [DOI] [PubMed] [Google Scholar]
- 13.Parhofer K G, Hasbargen U, Ulugberdiyewa A et al. Gestational diabetes in Turkmenistan: Implementation of a screening program and first results. Arch Gynecol Obstet. 2014;289:293–298. doi: 10.1007/s00404-013-2961-2. [DOI] [PubMed] [Google Scholar]
- 14.Hillier T A, Pedula K L, Ogasawara K K et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021;38:895–904. doi: 10.1056/NEJMoa2026028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIntyre H D, Jensen D M, Jensen R C et al. Gestational diabetes mellitus: Does one size fit all? A challenge to uniform worldwide diagnostic thresholds. Diabetes Care. 2018;41:1339–1342. doi: 10.2337/dc17-2393. [DOI] [PubMed] [Google Scholar]
- 16.Kong J M, Lim K, Thompson D M. Evaluation of the International Association of the Diabetes in pregnancy study group new criteria: Gestational diabetes project. Can J Diabetes. 2015;39:128–132. doi: 10.1016/j.jcjd.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Meloncelli NJ L, Barnett A G, D’Emden M et al. Effects of changing diagnostic criteria for gestational diabetes mellitus in Queensland, Australia. Obstet Gynecol. 2020;135:1215–1221. doi: 10.1097/AOG.0000000000003790. [DOI] [PubMed] [Google Scholar]
- 18.Gillman M W, Oakey H, Baghurst P A et al. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care. 2010;33:964–968. doi: 10.2337/dc09-1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ACOG Practice Bulletin No. 190Gestational Diabetes Mellitus Obstet Gynecol 2018131e49–e64. [DOI] [PubMed] [Google Scholar]
- 20.Tripathi R, Verma D, Gupta V K et al. Evaluation of 75 g glucose load in non-fasting state [Diabetes in Pregnancy Study group of India (DIPSI) criteria] as a diagnostic test for gestational diabetes mellitus. Indian J Med Res. 2017;145:209–214. doi: 10.4103/ijmr.IJMR_1716_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapur A, McIntyre H D, Divakar H et al. Towards a global consensus on GDM diagnosis: Light at the end of the tunnel? Int J Gynaecol Obstet. 2020;149:257–261. doi: 10.1002/ijgo.13149. [DOI] [PubMed] [Google Scholar]
- 22.Benhalima K, Van Crombrugge P, Moyson C et al. The sensitivity and specificity of the glucose challenge test in a universal two-step screening strategy for gestational diabetes mellitus using the 2013 World Health Organization criteria. Diabetes Care. 2018;41:e111–e112. doi: 10.2337/dc18-0556. [DOI] [PubMed] [Google Scholar]
- 23.McIntyre H D, Oats JJ N, Kihara A B et al. Update on diagnosis of hyperglycemia in pregnancy and gestational diabetes mellitus from FIGO’s Pregnancy & Non-Communicable Diseases Committee. Int J Gynaecol Obstet. 2021;154:189–194. doi: 10.1002/ijgo.13764. [DOI] [PubMed] [Google Scholar]
- 24.Pillay J, Donovan L, Guitard S et al. Screening for gestational diabetes: Updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;326:539–562. doi: 10.1001/jama.2021.10404. [DOI] [PubMed] [Google Scholar]


