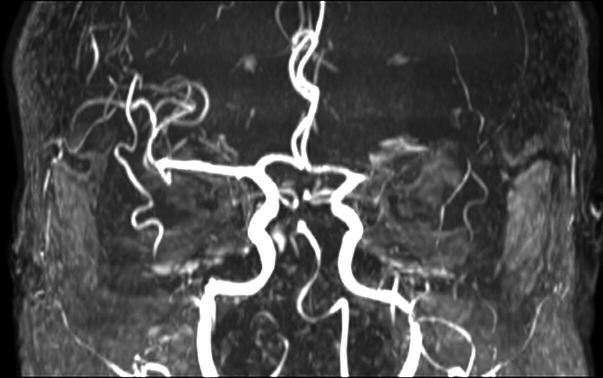
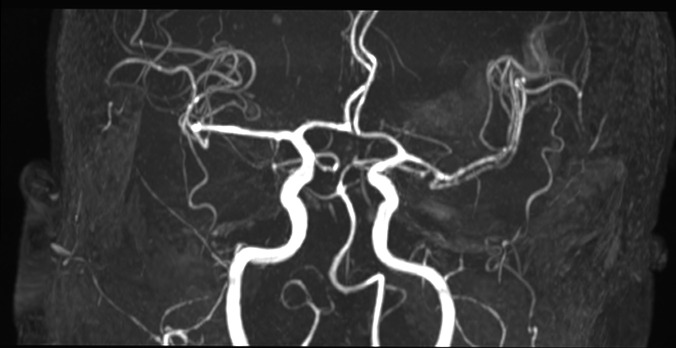
Abstract
Background
The ischaemic stroke of the territory of the middle cerebral artery represents an event burdened by high mortality and severe morbidity. The proposed medical treatments do not always prove effective. Decompressive craniectomy allows the ischaemic tissue to shift through the surgical defect rather than to the unaffected regions of the brain, thus avoiding secondary damage due to increased intracranial pressure. In this study, we propose a novel treatment for these patients characterised by surgical fenestration of the cisterns of the skull base.
Methods
We have treated 16 patients affected by malignant middle cerebral artery ischaemia and treated with cisternostomy between August 2018 and December 2019. The clinical history, neurological examination findings and neuroradiological studies (brain CT, CT angiography, MRI) were performed to diagnose stroke. Clinical examination was recorded on admission and preoperatively using the Glasgow Coma Scale and the National Institutes of Health Stroke Scale.
Results
The study included 16 patients, 10 males and 6 females. The mean age at surgery was 60.1 years (range 19–73). Surgical procedure was performed in all patients. The patients underwent immediate postoperative CT scan and were in the early hours evaluated in sedation window. In total, we recorded two deaths (12.5%). A functional outcome between mRS 0–3, defined as favourable, was observed in 9 (64.2%) patients 9 months after discharge. A functional outcome between mRS 4–6, defined as poor, was observed in 5 (35.7%) patients 9 months after discharge.
Conclusions
The obtained clinical results appear, however, substantially overlapping to decompressive craniectomy. Cisternostomy results in a favourable functional outcome after 9 months. This proposed technique permits that the patient no longer should be undergone cranioplasty thus avoiding the possible complications related to this procedure. The results are certainly interesting but higher case numbers are needed to reach definitive conclusions.
Keywords: Stroke, Magnetic Resonance Imaging, Intracranial Pressure
What is already known on this topic
The malignant middle cerebral artery infarction identifies a severe pathological condition characterised by the infarction of the territory of the cerebral artery and burdened by high mortality. Decompressive craniectomy represents a life-saving surgical procedure that increasing the chances of survival. Patients undergoing decompressive craniectomy must necessarily undergo cranioplasty, which is burdened by a high frequency of complications.
What this study adds
Fenestration of the basal cisterns results in a reverse cerebrospinal fluid flow from brain parenchyma, to cisterns, with reduction of intracranial pressure and brain oedema. We highlighted the absence of postoperative seizures, hydrocephalus and no need to perform cranioplasty.
How this study might affect research, practice or policy
Cisternostomy can represent a safe surgical procedure. It is mandatory to promote multicentric prospective studies to confirm our hypotheses in the treatment of these patients being able to compare data with a group of patients underwent decompressive craniectomy.
Introduction
The term ‘malignant middle cerebral artery (MMCA) infarction’ identifies a severe pathological condition characterised by a complete infarction of the middle cerebral artery (MCA) territory accompanied by space-occupying mass effect that develops during the first 5 days.1 This event shows a rapid and progressive evolution with more than 80% mortality in conservatively managed patients.2 Ischaemia involving a wide portion of cerebral hemisphere may lead to progressive neurological deterioration due to the onset of cytotoxic and vasogenic oedema, midline structures shift with subsequent trans-tentorial herniation, brainstem compression and death.3 No medical therapy has proven effective in preventing brain herniation and improving patient outcome. Although MMCA infarction constitute a small subgroup (1%–10%) of supratentorial ischaemic strokes, their huge impact on mortality, morbidity and health-related quality of life has led researchers to develop new therapeutic strategies.
Decompressive craniectomy (DC) represents a life-saving surgical procedure that reduces the mean intracranial pressure decreasing the mortality rate.4 The purpose of this treatment is to create space to reduce intracranial pressure, prevent brain tissue herniation and safeguard cerebral blood flow to prevent secondary brain damage. However, the utility of surgery in older patients is still debated. Recent clinical trials show that surgical decompression in older patients is lifesaving; however, often this group survives with severe disability.5
Lately, traumatic brain injury scenario has undergone promising changes with publications by Cherian et al, which, applying principles of microvascular surgery have shown the effectiveness of cisternostomy in the treatment of selected patients affected by severe brain injury.6 Rationale for cisternostomy is based on the new concept of cerebrospinal fluid (CSF) pathway. Altered CSF flow between the CSF system and the brain parenchyma depends on pathophysiological conditions of the various compartments. Iliff et al described a ‘glymphatic system’ mainly consists of paravascular spaces that connect subarachnoid spaces with the interstitial fluid system (ISF).7 Cisternostomy opens the basal cisterns resulting in a reverse CSF flow from brain parenchyma, through Virchow-Robin (VR) spaces, to cisterns, with reduction of intracranial pressure and brain oedema.8 This novel procedure may be also suitable to treat intraoperative malignant brain oedema, oedema of intracerebral haemorrhage and subarachnoid bleeding, but at our knowledge, a detailed description of cisternostomy for MMCA infarction has not yet been reported in the literature.
In the present study, we report our preliminary results of this technique, applied to patients affected by MMCA infarction. Furthermore, we propose pathophysiological mechanisms underlying MMCA infarction, focusing on impairment of the glymphatic system.
Methods
Patients selection
From August 2018 to December 2019, 16 patients affected by MMCA infarction were treated with cisternostomy at our institution (Unit of Neurosurgery, University of Messina, Italy). All patients were admitted to our Stroke Unit (Department of Neurology). The clinical history, neurological examination findings and neuroradiological studies (brain CT, CT angiography, MRI (MRI) also with angiographic sequences) were performed to diagnose stroke. Local ethics committee approval and written informed consent were obtained by patients’ relatives.
The admitted patients were treated according to the standardised stroke protocol, which followed the recommendations for early management of patients with acute ischaemic stroke of the European Stroke Organisation.9 A standard medical therapy with antiedema agents (mannitol, furosemide, steroids), and/or hypertonic saline infusion was attempted in all cases. Clinical examination was recorded on admission and preoperatively using the Glasgow Coma Scale (GCS) and the National Institutes of Health Stroke Scale (NIHSS).
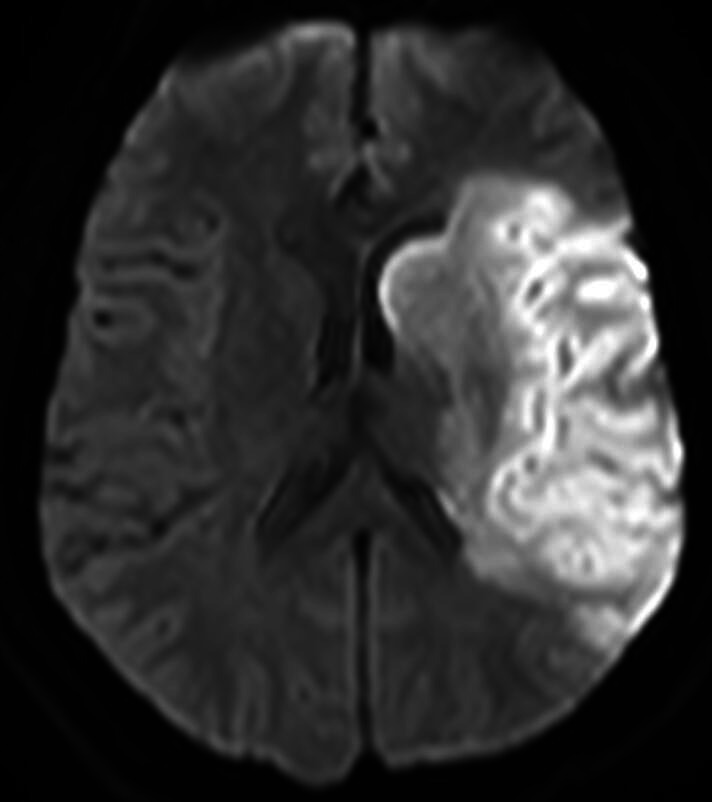
Suitable patients were subjected to intravenous thrombolysis if the time between onset and confirmation of the infarct was within 4.5 hours with subsequent intra-arterial thrombectomy, if a treatable clot was seen on CT angiography within 6 hours of onset.10 The clinical course and overall condition of each patient were discussed by the neurologists, neuroradiologists, and neurosurgeons. Indication for surgery was placed after a comprehensive evaluation based on radiological and clinical data. We considered inclusion criteria for surgical treatment: (1) a volume equal to or greater than 145 cm3 of ischaemic area demonstrated on diffusion-weighted MRI within 14 hours (figure 1); (2) brain CT ischaemic signs involving >50% of the MCA territory; (3) an NIHSS score ≥16, including a score ≥1 for item 1a (level of consciousness).
Figure 1.
The diffusion-weighted MRI study shows hyperintensity from left middle cerebral artery infarction with a volume of 155 cm3 of ischaemic area.
The patients underwent immediate postoperative CT scan and were in the early hours evaluated in sedation window. The outcome was analysed during outpatient department visits in the Stroke Unit at 3, 6 and 9 months. The modified Rankin Scale (mRS), NIHSS and GCS at discharge and at 3, 6, and 9 months post surgery were used as outcome parameters. A functional outcome between mRS 0–3 was defined as favourable, while a functional outcome between mRS 4–6 was defined as poor.
Patients with GCS<4, coma with two dilated pupils and absent brainstem reflexes were excluded from the study. Patients with a history of disabling neurological disease, stroke, terminal illness, bleeding tendency or secondary parenchymal haemorrhage were excluded.
Surgical procedure
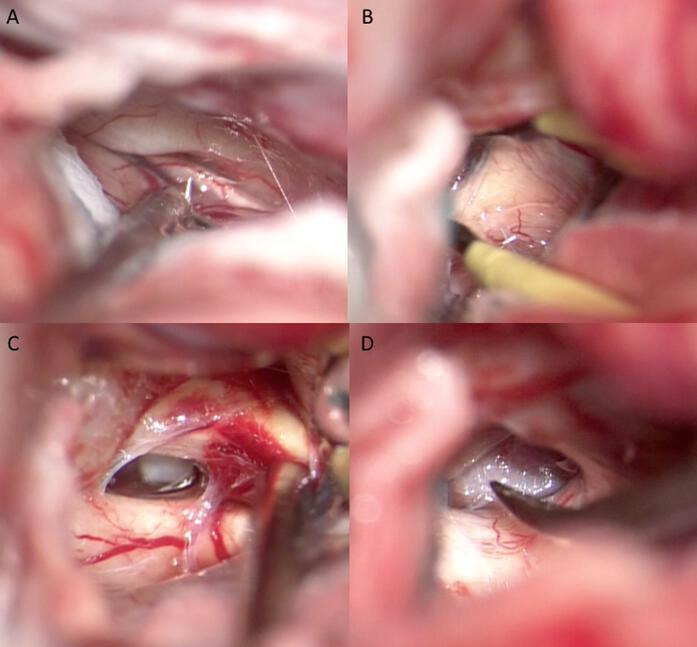
Under general anaesthesia, the patient is placed in supine position. The head, fixed with Mayfield three-pin head holder, is extended and rotated approximatively 30° to the contralateral side. A frontotemporal craniotomy was performed. The craniotomy is extended towards the skull base by the epidural drilling of the sphenoid ridge. Dura mater is opened in a curvilinear fashion close to the basal dura, to avoid precocious brain herniation. In all cases, by blunt dissection, the Silvian fissure was opened with microsurgical technique, until finding the olfactory cistern, which was fenestrated together with the optic-carotid and chiasmatic cisterns (figure 2). The subsequent opening of the contralateral cisterns allowed the communication of the CSF circulation of both sides. At this point, we observed a noticeable and progressive relaxation of the brain appearing detained and weakly pulsating. Dura is left open to facilitate the drainage of CSF from the cisterns to the subgaleal space where it can be absorbed until recovery of physiological CSF pathway. The bony operculum was repositioned with titanium screws and miniplates.
Figure 2.
Intraoperative images of cisternotomy: (A) opening of olfactory cistern, (B) and of chiasmatic cistern. (C) Opening of ipsilateral optic-carotid cistern, (D) and of controlateral optic-carotid cistern.
Results
The study included 16 patients, 10 males (62.5%) and 6 females (37.5%). The mean age at surgery was 60.1 years (range 19–73). On admission, patients underwent CT, CT angiography and MRI also with angiographic sequences (figure 3). All patients experienced MMCA infarction. Surgical procedure was performed in all patients. Two patients (12.5%), both male, died before discharge. The first patient had a GCS of 8 and an NIHSS score of 22 at admission which subsequently worsened (GCS: 5; NIHSS: 24). The second, on admission, had GCS of 9 and NIHSS score of 20. This patient also worsened (GCS: 5; NIHSS: 25), with the onset of anisocoria. Both patients died of pneumonia. On admission, the GCS for the remaining patients differed from 10 to 13 with a mean score of 11.2±1.38 while the NIHSS score varied from 10 to 19 with a mean score of 14.5±3.47. Preoperative clinical symptoms included disturbed consciousness, hemiparesis, facial nerve palsy and aphasia. Two patients (14.2%) subsequently presented anisocoria. A progressive worsening of the consciousness was observed in all patients. The preoperative GCS varied from 5 to 10 with a mean score of 8.21±1.31, while the NIHSS varied from 19 to 24 with a mean score of 22±1.41. The time interval between the progressive clinical deterioration and the surgical treatment varied between 6 and 8 hours. All the patients underwent surgical treatment in an interval of time that varied between 24–36 hours from admission. Table 1 show the GCS and the NIHSS score at admission and before surgical treatment. An immediate (within 90 min from surgical treatment) and 1 month postoperative brain CT as well as 1 month brain MRI also with angiographic sequences (figure 4) were performed in all patients, showing initial hypointensity of the basal cisterns both sides and a late recovery of the CSF circulation. In addition, we also proved a initial reduction of the midline shift in the first neuroradiological control, although to a variable extent, in each patient. A further reduction of the midline shift was highlighted in subsequent neuradiological examinations.
Figure 3.
MRI study with angiographic sequences demonstrates the lack of visualisation of the left middle cerebral artery.
Table 1.
Glasgow Coma Scale (GCS) and the National Institutes of Health Stroke Scale (NIHSS) score at admission and before surgical treatment; the GCS, the NIHSS score and the modified Rankin Scale (mRS) score recorded at discharge, and at 3, 6 and 9 months
| GCS | NIHSS | mRS | |
| Admission | 11.2±1.38 | 14.5±3.47 | – |
| Before surgery | 8.21±1.31 | 22±1.41 | – |
| Discharge | 9.14±0.94 | 19.2±1.72 | 4.35±0.47 |
| Three months | 10±1.07 | 16±2.01 | 4±0.53 |
| Six months | 10.5±1.22 | 15.4±1.84 | 3.78±0.55 |
| Nine months | 11.2±1.47 | 13.4±2.13 | 3.42±0.62 |
Figure 4.
The MRI study (axial view) with angiographic sequences 1 month after the surgical treatment demonstrates the recanalisation of the left MCA.
No further extended volume of the infarcted areas was demonstrated (figure 5). Neuroradiological control studies carried out showed any complications related to surgical treatment, in terms of haemorrhagic events, wound or site infection, fistula and hydrocephalus. Anisocoria progressively regressed in the two patients. Three patients (21.4%) needed tracheostomy. No patient presented seizures. A poor functional outcome (mRS 4–6) was observed in all patients after discharge, in 12 (85.7%) 1 month after discharge, in 10 (71.4%) 3 months after discharge, and in 5 (35.7%) patients 9 months after discharge. A functional outcome between mRS 0 and 3 defined as favourable was observed in no patient at discharge, in 2 (14.2%) patients 1 month after discharge, in 4 (28.5%) 3 months after discharge, and in 9 (64.2%) patients 9 months after discharge. The average hospitalisation was 35 days (range 21–58). Table 1 shows the GCS and the NIHSS score at admission and before surgical treatment; the GCS, the NIHSS score and the mRs score recorded at discharge, and at 3, 6 and 9 months.
Figure 5.
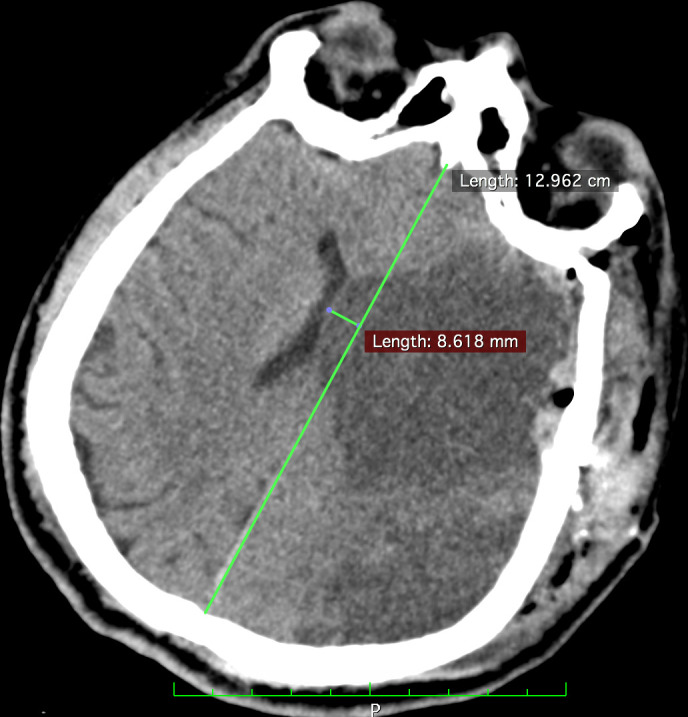
CT scan (axial view) performed in the immediate postoperative period shows a shift of more than 8 mm.
Discussion
In patients affected by MMCA infarction, surgical treatment may be necessary when medical therapy or endovascular thrombectomy is not feasible or ineffective. DC have been proposed to relieve the high intracranial pressure. DC is proven to be a life-saving procedure that increases the chances of survival from 29% to 78% in patient affected by MMCA infarction, but a significant improvement of patient’s functional outcome has not yet been reported.11 However, the results of surgical treatment of DC in patients with MMCA infarction are still debated and numerous trials are reported in literature. Recently, a meta-analysis12 correlated the results of some trials.13–18 Gulensoy et al states that a preoperative GCS score of 7 or less than 7 does not allow to obtain favourable results.19 Alam et al only reported a poor functional outcome (mRS >4) in patients undergoing DC with total mortality of 8.7%.20 In Kürten series, in a 3-month follow-up, 44.6% of surgically treated patients showed moderate–severe disability (mRS 4), while 32.6% suffered a poor outcome (mRS 5).21 In our series of 14 patients, affected by malignant MCA and underwent DC, a favourable mRS (mRs 0–3) at 9 months of follow-up was recorded in 5 patients (41.6%), while a poor functional outcome was evidenced in 7 patients (58.3%). Two patients died within 1 month of surgery (14.2%). The issue still opens is age of patients (it is not yet clear if patients aged >60 years would benefit from early DC) and the most appropriate timing of treatment. Usually patients affected by MMCA infarction; they should undergo DC within 48 hours from symptom onset.11 14 In any case, DC surgical treatment presents various complications that can arise both precociously and late. The incidence of any complication after DC is 50%–55%.22 In truth, general complications such as urinary tract infections, venous thrombosis and pneumonia seem to be more common than complications related to surgical treatment. However, in about 25% cases, haemorrhagic infarction, necrosis, haematomas and oedema at the site of craniectomy are considered complications.22 The delayed complications are CSF absorption disorders including subdural hygroma in 6%–21% and hydrocephalus in 10%–40% cases.22 In addition, patients undergoing DC must necessarily undergo cranioplasty surgery subsequently (after 6 weeks to 6 months). Cranioplasty is burdened by a high frequency of complications, the incidence of which can vary from 12% to 50% (23). The common complications after cranioplasty are infection, wound breakdown, intracranial haemorrhage, bone resorption and sunken cranioplasty.23
Recently, Cherian et al proposed a physiologic mechanism of cisternostomy in severe brain injury considering the key role of associated subarachnoid haemorrhage (SAH).24 This may cause an increasing of cisternal pressure and a rapid shift of CSF from the cisterns, through the VR spaces, into the brain parenchyma (CSF-shift oedema) resulting in an increased brain swelling.24 Cisternostomy opens the basal cisterns, resulting in a reverse CSF flow from brain parenchyma, through VR spaces, to cisterns, with reduction of intracranial pressure and reduced brain oedema (figure 6). This microsurgical procedure allows putting back the bone flap with reduction of further complications.
Figure 6.
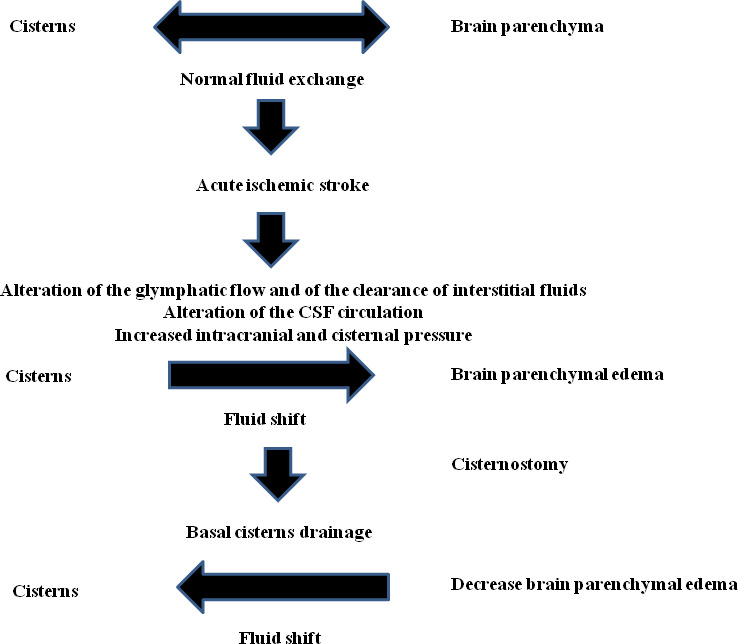
Schematic representation of the pathophysiology of brain oedema in acute ischaemic stroke and its decrease after cisternostomy.
Ischaemic brain oedema can be differentiated into two stages, an early cytotoxic oedema, developing few minutes following ischaemic injury and a later vasogenic oedema related to blood-brain barrier (BBB) breakdown after 4–6 hours. In the acute phase, cerebral ischaemia, lead to a subversion of the cell membrane architecture, with cessation of Na+/K+-ATPase and Na+-co-transporter. Osmotic water influx causes cellular swelling and a diminution of the extracellular compartment. These events occur within 30 min of onset of focal ischaemia. The tissue necrosis determines a loss of BBB integrity with altered endothelial tight junctions, and after 4–6 hours, also high molecular proteins begin to leak from the blood into the brain parenchyma. According to recent in vivo studies,25–27 it is believed that the glial cell-mediated lymphatic (glymphatic) system begin with CSF production by the choroid plexuses. Subarachnoid CSF must be able to enter the brain through VR spaces to renew ISF, and ISF and solute must be able to drain back to the CSF to achieve waste and solutes removal and brain homeostasis.28 Consequently, ventricular, subarachnoid, perivascular and interstitial compartments give rise to a fluid functional unit.
Decreased glymphatic flow has been observed secondary to SAH, multiple microinfarction and acute ischaemia.29 30 Contrast-enhanced MR in acute ischaemic stroke induced in rodent models, showed impaired CSF inflow in the ipsilateral cortex at 3 hours after MCA occlusion.29 Furthermore, cortical diffusion decrease in the ischaemic core, which could lead to neuronal damage in surrounding hypoxic tissue. This finding has also been implicated in impaired glymphatic system.31 Glymphatic altered flow could be also due to dysregulation of CSF-ISF water exchange in the astrocytes. Within this scenario, a primary role is represented by AQP4, that is a water channel predominantly expressed on the perivascular astrocytic end-foot processes. This peculiar localisation of AQP4 channels could decrease resistance to CSF-ISF exchange.7 It has been shown using contrast-enhanced MR that in acute ischaemic stroke there is a decreased paravascular CSF circulation with an impaired perfusion of glymphatic system.29 Moreover, acute ischaemic stroke lead to astrogliosis and altered expression of AQP4 in contrast to the normal AQP4 activity in the VR spaces, which may impede clearance of interstitial fluids with increased intracerebral pressure and altered CSF circulation with augmented cisternal pressure.32
Cisternostomy can help to a mechanic reduction of cisternal pressure that as result, improves glymphatic flow. Moreover, interstitial fluid and wastes such as ROS, proinflammatory cytokines and various solutes can be removed, also improving perfusion of ischaemic penumbra areas. Finally, intracerebral pressure is restored with decreased brain swelling. After cisternostomy, dura is left open to facilitate the drainage of CSF from the cisterns to the subgaleal space where it can be absorbed until recovery of physiological CSF pathway. Recently, Tartara et al proposed a surgical strokectomy associated to an extensive cisternal CSF drain for acute management of MMCA infarction.33 The authors performed a modified pterional approach to assess strokectomy, removing the ischaemic tissue until base of temporal fossa. Then, they open basal cisterns (ambiens, carotic) to determine an extensive CSF drainage. In our opinion, when a strokectomy is performed, the potential disruption of ischaemic penumbra areas is not taken into account.
In our opinion, cisternostomy can represent a safe surgical procedure in the treatment of patients affected by malignant MCA infarction. We believe that the fenestration of the basal cisterns allows to obtain a flow of CSF from the cerebral parenchyma, through VR spaces, towards the cisterns. In this way, it will be possible to obtain a reduction in intracranial pressure and cerebral oedema. CSF flows along a network of perivascular spaces surrounding blood vessels and communicates with interstitial fluid permeating brain tissue, favouring the removal of metabolites. After stroke attack, fluid accumulates in ischaemic tissue, and the brain becomes oedematous and begins to swell. The drainage of CSF from the cisterns is helpful because the lactate, tau and free radicals, which would have been present within the injured brain, are washed out, minimising the secondary damage. We highlighted the absence of postoperative seizures, hydrocephalus and no need to perform cranioplasty, although in this case series postoperative morbidity does not differ from the reported literature data. We are aware that low number of patients does not allow definitive conclusions and our proposed pathophysiological mechanisms are yet to be proven. It is mandatory to promote multicentric prospective studies to confirm our hypotheses in the treatment of this group of patients being able to compare data with a group of patients underwent DC.
Footnotes
Contributors: Concept and design: SMC, MC and GC. Acquisition of data: GS, NG, GG and GR. Analysis and interpretation of data: MC, GC, GS and AC. Acquisition of clinical data: GS, GR, GG and GR. Acquisition and interpretation of radiological data: SLV and FG. Drafting of the manuscript: MC, GC and SC. Critical revision of the manuscript: AG, MC, GC and VB. Guarantor: SMC. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Ethics Committee of Messina exempted this study. Participants gave informed consent to participate in the study before taking part.
References
- 1. Hacke W, Schwab S, Horn M. Middle cerebral infarction. Arch Neurol 1996;54:309–15. [DOI] [PubMed] [Google Scholar]
- 2. Godoy D, Piñero G, Cruz-Flores S. Malignant hemispheric infarction of the middle cerebral artery. Diagnostic consideration. Neurología 2016;31:332–43. [DOI] [PubMed] [Google Scholar]
- 3. Soinne L, Sundararajan S, Strbian D. Malignant hemispheric infarction: diagnosis and management by hemicraniectomy. Stroke 2014;45:185–7. [DOI] [PubMed] [Google Scholar]
- 4. Elsayed A, Elsayed A. Decompressive craniectomy in malignant hemispheric infarction: favorable outcome and disability. Egypt J Neurol Psychiatry Neurosurg 2019;55:25. 10.1186/s41983-019-0077-8 [DOI] [Google Scholar]
- 5. Robertson FC, Dasenbrock HH, Gormley WB. Decompressive hemicraniectomy for stroke in older adults: a review. J Neurol Neuromedicine 2017;2:1–7. 10.29245/2572.942X/2017/2.942X/2017/1.1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cherian I, Yi G, Munakomi S. Cisternostomy: replacing the age old decompressive hemicraniectomy? Asian J Neurosurg 2013;8:132–8. 10.4103/1793-5482.121684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 2012;4:147ra111. 10.1126/scitranslmed.3003748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cherian I, Beltran M, Kasper EM, et al. Exploring the Virchow-Robin spaces function: a unified theory of brain diseases. Surg Neurol Int 2016;7:711–4. 10.4103/2152-7806.192486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kobayashi A, Czlonkowska A, Ford GA, et al. European Academy of Neurology and European stroke organization consensus statement and practical guidance for pre-hospital management of stroke. Eur J Neurol 2018;25:425–33. 10.1111/ene.13539 [DOI] [PubMed] [Google Scholar]
- 10. Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 11. Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 2007;6:215–22. 10.1016/S1474-4422(07)70036-4 [DOI] [PubMed] [Google Scholar]
- 12. Pallesen L-P, Barlinn K, Puetz V. Role of decompressive craniectomy in ischemic stroke. Front Neurol 2018;9:1119. 10.3389/fneur.2018.01119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frank JI, Schumm LP, Wroblewski K, et al. Hemicraniectomy and durotomy upon deterioration from infarction-related swelling trial: randomized pilot clinical trial. Stroke 2014;45:781–7. 10.1161/STROKEAHA.113.003200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hofmeijer J, Kappelle LJ, Algra A, et al. Surgical decompression for space-occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life-threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurol 2009;8:326–33. 10.1016/S1474-4422(09)70047-X [DOI] [PubMed] [Google Scholar]
- 15. Jüttler E, Schwab S, Schmiedek P, et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (destiny): a randomized, controlled trial. Stroke 2007;38:2518–25. 10.1161/STROKEAHA.107.485649 [DOI] [PubMed] [Google Scholar]
- 16. Slezins J, Keris V, Bricis R, et al. Preliminary results of randomized controlled study on decompressive craniectomy in treatment of malignant middle cerebral artery stroke. Medicina 2012;48:76–4. 10.3390/medicina48100076 [DOI] [PubMed] [Google Scholar]
- 17. Vahedi K, Vicaut E, Mateo J, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke 2007;38:2506–17. 10.1161/STROKEAHA.107.485235 [DOI] [PubMed] [Google Scholar]
- 18. Zhao J, Su YY, Zhang Y, et al. Decompressive hemicraniectomy in malignant middle cerebral artery infarct: a randomized controlled trial enrolling patients up to 80 years old. Neurocrit Care 2012;17:161–71. 10.1007/s12028-012-9703-3 [DOI] [PubMed] [Google Scholar]
- 19. Gulensoy B, Karatay M, Erdem Y, et al. Decompressive hemicraniectomy for malignant middle cerebral artery infarct. Turk Neurosurg 2016;26:704–8. 10.5137/1019-5149.JTN.13241-14.1 [DOI] [PubMed] [Google Scholar]
- 20. Kamal Alam B, Bukhari AS, Assad S, et al. Functional outcome after decompressive craniectomy in patients with dominant or non-dominant malignant middle cerebral infarcts. Cureus 2017;9:e997. 10.7759/cureus.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kürten S, Munoz C, Beseoglu K, et al. Decompressive hemicraniectomy for malignant middle cerebral artery infarction including patients with additional involvement of the anterior and/or posterior cerebral artery territory-outcome analysis and definition of prognostic factors. Acta Neurochir 2018;160:83–9. 10.1007/s00701-017-3329-3 [DOI] [PubMed] [Google Scholar]
- 22. Kurland DB, Khaladj-Ghom A, Stokum JA, et al. Complications associated with decompressive craniectomy: a systematic review. Neurocrit Care 2015;23:292–304. 10.1007/s12028-015-0144-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piedra MP, Ragel BT, Dogan A, et al. Timing of cranioplasty after decompressive craniectomy for ischemic or hemorrhagic stroke. J Neurosurg 2013;118:109–14. 10.3171/2012.10.JNS121037 [DOI] [PubMed] [Google Scholar]
- 24. Cherian I, Grasso G, Bernardo A, et al. Anatomy and physiology of cisternostomy. Chin J Traumatol 2016;19:7–10. 10.1016/j.cjtee.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iliff JJ, Lee H, Yu M, et al. Brain-Wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 2013;123:1299–309. 10.1172/JCI67677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Plog BA, Nedergaard M. The Glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol 2018;13:379–94. 10.1146/annurev-pathol-051217-111018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verheggen ICM, Van Boxtel MPJ, Verhey FRJ, et al. Interaction between blood-brain barrier and glymphatic system in solute clearance. Neurosci Biobehav Rev 2018;90:26–33. 10.1016/j.neubiorev.2018.03.028 [DOI] [PubMed] [Google Scholar]
- 28. Ringstad G, Vatnehol SAS, Eide PK. Glymphatic MRI in idiopathic normal pressure hydrocephalus. Brain 2017;140:2691–705. 10.1093/brain/awx191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gaberel T, Gakuba C, Goulay R, et al. Impaired glymphatic perfusion after strokes revealed by contrast-enhanced MRI: a new target for fibrinolysis? Stroke 2014;45:3092–6. 10.1161/STROKEAHA.114.006617 [DOI] [PubMed] [Google Scholar]
- 30. Wang M, Ding F, Deng S, et al. Focal solute trapping and global glymphatic pathway impairment in a murine model of multiple microinfarcts. J Neurosci 2017;37:2870–7. 10.1523/JNEUROSCI.2112-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schain AJ, Melo-Carrillo A, Strassman AM, et al. Cortical spreading depression closes paravascular space and impairs glymphatic flow: implications for migraine headache. J Neurosci 2017;37:2904–15. 10.1523/JNEUROSCI.3390-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke 2013;44:S93–5. 10.1161/STROKEAHA.112.678698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tartara F, Colombo EV, Bongetta D, et al. Strokectomy and extensive cisternal CSF drain for acute management of malignant middle cerebral artery infarction: technical note and case series. Front Neurol 2019;10:1017. 10.3389/fneur.2019.01017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request.