Abstract
Introduction:
Little is known about the effects of long-term e-cigarette use, particularly the risks of relapse to cigarette smoking or increased dependence.
Methods:
In a 2012–2014 baseline online e-cigarette survey, 1,863 respondents consented to participate in future research. A follow-up online survey was conducted in 2017–2018 to assess changes in e-cigarette use behaviors and e-cigarette–related dependence. For both surveys, exclusive e-cigarette use was defined as only using e-cigarettes in the past 7 days, and poly use was defined as using both e-cigarettes and other tobacco or nicotine products in the past 7 days. The Penn State Electronic Cigarette Dependence Index (PSECDI) score was calculated for each study subject and was used to evaluate e-cigarette dependence. Paired t-tests or Pearson correlation coefficients were used to examine changes in e-cigarette use behaviors or PSECDI scores between baseline and follow-up. Baseline and follow-up survey data were analyzed in January 2019.
Results:
A total of 494 subjects provided complete data on both surveys. At baseline, 402 subjects (81.4%) were exclusive e-cigarette users, and 71 subjects (14.4%) were poly users. Among baseline exclusive e-cigarette users, the majority (88.3%) continued using e-cigarettes exclusively, but 37 users (9.2%) became poly users and 1 returned to cigarette smoking at follow-up. Among baseline poly users, 60.6% became exclusive e-cigarette users at follow-up. The mean PSECDI score remained similar over time (8.4 at baseline vs 8.3 at follow-up).
Conclusions:
Findings suggest that the risk of relapse to cigarette smoking is low, and e-cigarette-related dependence remains stable in long-term e-cigarette users.
INTRODUCTION
Cigarette smoking remains the leading cause of premature death in the U.S.1 Although most smokers desire to quit smoking, and pharmacologic interventions are available, smoking cessation is frequently unsuccessful owing to nicotine dependence.2 E-cigarettes deliver nicotine via an aerosol, and thus contain much lower concentrations of the known harmful toxicants found in combustible cigarettes.3–6 Newer e-cigarette devices that have larger batteries and manual controls can deliver nicotine levels comparable to those obtained by smoking cigarettes.7 Up to 35% of smokers have tried an e-cigarette with an intention to quit or reduce smoking.8 Although clinical trials to date do not support the use of e-cigarettes for smoking cessation, some adult smokers have either reduced their cigarette consumption or successfully transitioned from smoking cigarettes to using e-cigarettes exclusively.9–12 In a cross-sectional study conducted by Foulds et al.,13 current exclusive e-cigarette users who were former smokers were found to be less dependent on e-cigarettes than they were when they used cigarettes, implying a reduced dependence from e-cigarette use rather than cigarette smoking. Based on available evidence, the National Academies of Science, Engineering, and Medicine concluded that, “there is moderate evidence that risk and severity of dependence are lower for e-cigarettes than combustible tobacco cigarettes.”6 Therefore, e-cigarettes may be a potential tool to help adult smokers reduce or quit smoking.
Nonetheless, there have been ongoing debates about the effectiveness of e-cigarette use for smoking cessation, the public health benefits of switching from cigarette smoking to e-cigarettes, and the health risks of long-term e-cigarette use, including increased e-cigarette–related dependence, concurrent use with other tobacco products, and unforeseen health problems.3,6,14–25 Recent systematic reviews indicate that e-cigarette use in adults has rapidly increased in the U.S., with a prevalence of ever use being 6.2% in 2011 and 14.1% in 2014.18,26 Because most adult e-cigarette users are either current or former smokers, the concurrent use of e-cigarettes with other tobacco products—primarily cigarettes—raises concerns of possible relapse to cigarette smoking.16,27,28 In May 2016, the U.S. Food and Drug Administration (FDA) deemed that e-cigarettes meet the definition of “tobacco products” and are subject to FDA oversight and regulation.29 Because e-cigarettes are relatively new, there is a great need for longitudinal studies to understand the real-world evidence of e-cigarette use on smoking cessation and e-cigarette–related dependence. However, most of the previous studies that assessed e-cigarette use behaviors or dependence were either cross-sectional studies or longitudinal studies with short study periods and small sample sizes of exclusive e-cigarette users.30–37
In a previous online e-cigarette survey conducted in 2012–2014, a total of 1,863 people consented to participate in future research.13,38–40 These people were recontacted in 2017 for a follow-up online survey designed to assess how their e-cigarette use behaviors may have changed.41 Along with their existing baseline data, a large cohort of long-term e-cigarette users was established. The goals of this longitudinal study were to examine if e-cigarette users would maintain exclusive e-cigarette use or relapse to cigarette smoking and if e-cigarette–related dependence would increase over time.
METHODS
Study Population
The original study population in the baseline survey was ever e-cigarette users aged ≥18 years.13 Briefly, study subjects were recruited through various online sources and were invited to complete an online survey of e-cigarette use. About 7,000 people responded to that survey in 2012–2014, and 98.2% were ever cigarette smokers. A total of 1,863 study subjects consented to participate in future research and provided their contact information. Beginning in January 2017, these people were recontacted and invited to participate in an online follow-up e-cigarette survey. A unique link to the follow-up survey was sent to each individual via their e-mail address. Multiple strategies, including online notifications posted at the E-Cigarette Forum website, repeated e-mail invitations, or phone contacts to remind people to complete the follow-up survey, were utilized to recruit study subjects. The survey was administered using Penn State Research Electronic Data Capture.42 Implied consent was used for the follow-up survey, and no study compensation was provided. The Pennsylvania State University College of Medicine IRB approved this study (IRB #: STUDY00005398).
The follow-up survey was developed to assess the following domains: sociodemographics, past or current e-cigarette use behaviors (reasons for using e-cigarettes, length and frequency of e-cigarette use, device characterization, and e-cigarette liquids and flavor use), e-cigarette–related dependence, tobacco use history, and the use of other tobacco or nicotine products in the past 7 days. Identical questions and responses were asked in both surveys for sociodemographics, e-cigarette-related dependence, the use of other tobacco or nicotine products, and some e-cigarette use behaviors.13,38,41
Measures
To evaluate changes in e-cigarette use behaviors, repeated 7-day point prevalence measures were used to categorize study subjects as exclusive e-cigarette users, poly users, and ex-e-cigarette users at baseline and follow-up.43 Study subjects were classified into 5 groups according to their past-7-day use of e-cigarettes and other tobacco or nicotine products (including any cigarettes, chewing tobacco, snuff/dipping tobacco, snus, pipe tobacco, or other nicotine products such as patch, gum, lozenge, inhaler, or nasal spray). “Exclusive e-cigarette users” were subjects who had used only e-cigarettes, “poly users with other nicotine products” were subjects who used both e-cigarettes and any other nicotine product, “poly users with other tobacco products” were those who had used both e-cigarettes and any other tobacco product, “ex–e-cigarette users with cigarettes” were subjects who did not use an e-cigarette but consumed cigarettes, and “other ex–e-cigarette users” were subjects who did not use an e-cigarette or any cigarettes. The e-cigarette use behavior at baseline was compared with the follow-up behavior to evaluate if an individual’s e-cigarette use behavior persisted or changed over time.
To assess if e-cigarette–related dependence would increase, the Penn State Electronic Cigarette Dependence Index (PSECDI) scores at baseline and follow-up were calculated for each subject. PSECDI was developed according to individuals’ responses to 10 questions referring to their current use of e-cigarettes (or their peak use of e-cigarettes for ex–e-cigarette users).13 The PSECDI score, ranging from 0 to 20, indicated users’ dependence levels on their e-cigarettes: 0–3, no dependence; 4–8, low dependence; 9–12, medium dependence, and ≥13, high dependence.
Statistical Analysis
All variables used in the analyses were measured at both surveys. The means and percentages were presented to describe population characteristics at baseline and follow-up. Two-sided, paired t-tests for continuous variables, and McNemar’s tests or chi-square tests for categorical variables were used to assess statistical differences at p<0.05. The relationship between the baseline and follow-up PSECDI scores were examined using Pearson correlation coefficients. In this study, the existing baseline survey data and the follow-up survey data collected between January 2017 and May 2018 were analyzed in January 2019 using SAS, version 9.4.
RESULTS
A total of 742 study subjects answered the follow-up survey, forming the basis for this longitudinal study (crude retention rate, 39.8%). Subjects (n=248) with missing values in key variables or with inconsistent responses in age and sex were excluded. This left 494 subjects with a mean follow-up time of 3.7 years (SD=0.7; range, 2–6 years) for the analyses. The baseline characteristics between the current study population (n=494) and the target population (1,863 people from the baseline survey) were also compared. There were no differences in sociodemographics, but there was a higher proportion of baseline exclusive e-cigarette users in this study compared with the target population (81.4% vs 70.6%, p<0.0001).
As nearly all of the study subjects were ever smokers, 346 (70.2%) answered that they started e-cigarette use with an intention to quit smoking. However, at the follow-up, the top 2 reasons to use an e-cigarette were the perceived less-harmful health effects (36.4%) and using e-cigarettes to quit smoking or avoid relapsing (31.4%) (Appendix Table 1, available online). According to the reported past-7-day use of e-cigarettes and other products at follow-up, 412 (83.4%) subjects were categorized as exclusive e-cigarette users, 14 (2.8%) were poly users with other nicotine products, 59 (11.9%) were poly users with other tobacco products, and 9 (1.8%) were ex–e-cigarette users. For poly users with other tobacco products, the most frequently used tobacco product was cigarettes (n=52, 88.1%), with an average use of 3.7 days during the 7-day span and an average of 6.3 cigarettes per day. In all, 22 subjects smoked cigarettes every day in the past 7 days, with a mean consumption of 9.9 cigarettes per day. The use of chewing tobacco, snuff/dipping tobacco, snus, or pipe tobacco was not common (11.9%). Among the 9 ex–e-cigarette users who did not use an e-cigarette in the past 7 days at follow-up, 3 smoked cigarettes exclusively every day with an average of 13.3 cigarettes per day, and 6 did not use any product.
Table 1 presents comparisons of sociodemographics and e-cigarette use, at follow-up, between 412 exclusive e-cigarette users and 59 poly users with other tobacco products. Compared with poly users, exclusive e-cigarette users were older, predominately white, and had a higher income level. Regarding e-cigarette–use behaviors, exclusive e-cigarette users were more likely to report using e-cigarette to quit smoking or avoid relapsing (33.7% vs 17.0%) and had a higher proportion of daily e-cigarette use in the past 30 days (93.9% vs 66.1%) but were less likely to report that it was hard to keep from using e-cigarette (14.8% vs 25.4%) (all p<0.05). Changes in the characteristics between baseline and follow-up were also assessed in these 2 groups. Fewer poly users reported at follow-up that the top reason to use an e-cigarette was to quit smoking or avoid relapsing (30.5% vs 17.0%) compared with those at the baseline survey. Subjects in both groups had used 5 or more new devices since the baseline survey (all p<0.05). Although the mean PSECDI score remained similar over time in both groups, there were noticeable changes and wide variations in the 10 questions regarding e-cigarette–related dependence. At follow-up, exclusive e-cigarette users were less likely to feel that it was hard to quit e-cigarettes (32.4% at baseline vs 20.2% at follow-up, p<0.0001) but were more likely to report that it was hard to keep from using e-cigarettes (10.7% vs 14.8%, p=0.04). More poly users, however, reported strong cravings to use e-cigarettes (35.6% vs 55.9%, p=0.003) or that it was hard to keep from using e-cigarettes (15.3% vs 25.4%, p=0.04) at follow-up.
Table 1.
Characteristics Between Follow-Up Exclusive E-Cigarette Users and Poly Users
| Characteristics | Exclusive e-cigarette users at follow-up (n=412) |
Poly users (e-cigarette and other tobacco products) at follow-up (n=59) |
p-valuea | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | p-valueb | Baseline | Follow-up | p-valueb | ||
| Age (years), mean (SD) | 41.2 (11.9) | 44.9 (11.9) | — | 36.5 (11.9) | 40.1 (11.9) | — | 0.004 |
| Male, n (%) | 278 (67.5) | — | 38 (64.4) | — | 0.64 | ||
| White, n (%) | 378 (92.0) | — | 49 (86.0) | — | 0.03 | ||
| College or higher, n (%) | 189 (45.9) | 194 (47.1) | 0.46 | 28 (47.5) | 32 (54.2) | 0.16 | 0.30 |
| Full-time employed, n (%) | 290 (70.4) | 278 (67.5) | 0.17 | 30 (50.9) | 36 (61.0) | 0.18 | 0.33 |
| Income >$2,500/month, n (%) | NA | 262 (64.4) | — | NA | 29 (51.8) | — | 0.03 |
| E-cigarette–use behaviors | |||||||
| The most important reason to use an e-cigarette, n (%) | |||||||
| Less harmful to my health | 159 (38.7) | 149 (36.2) | 0.37 | 14 (23.7) | 21 (35.6) | 0.11 | 0.93 |
| To quit smoking or avoid relapsing | 126 (30.7) | 139 (33.7) | 0.27 | 18 (30.5) | 10 (17.0) | 0.03 | 0.01 |
| Mean number of devices used before the current device (SD) | 4.7 (5.7) | 10.6 (12.8) | <0.0001 | 3.4 (4.6) | 8.8 (13.9) | 0.003 | 0.18 |
| Mean amount of e-liquid used per day (in mL) (SD) | NA | 7.8 (7.1) | — | NA | 6.5 (5.6) | — | 0.23 |
| Daily e-cigarette use in the past 28 days or 30 days,c n (%) | 373 (90.5) | 387 (93.9) | 0.09 | 36 (61.0) | 39 (66.1) | 0.70 | 0.0001 |
| E-cigarette–related dependence | |||||||
| Mean PSECDI (SD) | 8.5 (3.4) | 8.4 (3.8) | 0.33 | 7.5 (3.8) | 8.0 (3.9) | 0.46 | 0.46 |
| Mean e-cigarette use times per day (SD) | 23.9 (24.7) | 21.8 (23.9) | 0.14 | 16.2 (14.6) | 15.9 (22.9) | 0.95 | 0.08 |
| Mean time to first e-cigarette use after waking (in minutes) (SD) | 44.5 (77.5) | 41.7 (73.3) | 0.54 | 64.9 (105.4) | 59.0 (109.3) | 0.75 | 0.12 |
| Awaken at night to use e-cigarette, n (%) | 29 (7.1) | 39 (9.5) | 0.10 | 6 (10.2) | 9 (15.3) | 0.32 | 0.17 |
| Mean number of nights per week awakened to use e-cigarette (SD) | 0.3 (1.2) | 0.4 (1.3) | 0.22 | 0.5 (1.5) | 0.5 (1.5) | 0.84 | 0.43 |
| Hard to quit e-cigarette, n (%) | 133 (32.4) | 83 (20.2) | <0.0001 | 20 (33.9) | 13 (22.0) | 0.14 | 0.74 |
| Have had strong cravings to use e-cigarette, n (%) | 176 (42.8) | 182 (44.3) | 0.60 | 21 (35.6) | 33 (55.9) | 0.003 | 0.09 |
| Strong urges to use e-cigarette, n (%) | 59 (14.3) | 59 (14.3) | 1.00 | 10 (17.0) | 10 (17.0) | 1.00 | 0.59 |
| Hard to keep from using e-cigarette, n (%) | 44 (10.7) | 61 (14.8) | 0.04 | 9 (15.3) | 15 (25.4) | 0.11 | 0.04 |
| Felt irritable if couldn’t use e-cigarette, n (%) | 131 (31.8) | 120 (29.1) | 0.34 | 20 (33.9) | 23 (39.0) | 0.47 | 0.12 |
| Felt nervous, restless, or anxious if couldn’t use e-cigarette, n (%) | 137 (33.3) | 130 (31.6) | 0.53 | 20 (33.9) | 26 (44.1) | 0.22 | 0.06 |
Note: Subjects with complete data on e-cigarette use and e-cigarette–related dependence were included. Boldface indicates statistical significance (p<0.05).
t-test or chi-square test p-values were used to examine the differences between exclusive e-cigarette users and poly users at follow-up.
Paired t-test or McNemar’s test p-values were used to examine changes in characteristics between baseline and follow-up.
In the baseline survey, the subjects were asked to report the number of e-cigarette use days in the past 28 days; in the follow-up survey, the subjects were asked to report the number of e-cigarette use days in the past 30 days.
NA, the questions were not comparable in the baseline survey; PSECDI, Penn State Electronic Cigarette Dependence Index.
At baseline, most of this study population was exclusive e-cigarette users (n=402, 81.4%), whereas 17 (3.4%) were poly users with other nicotine products, 71 (14.4%) were poly users with other tobacco products, and only 4 (0.8%) were ex–e-cigarette users (Figure 1). Among 402 baseline exclusive e-cigarette users, most (n=355, 88.3%) maintained exclusive e-cigarette use at follow-up and 5 (1.2%) switched to being a poly user with other nicotine products, but 37 (9.2%) became poly users with other tobacco products, primarily involving cigarettes. Furthermore, 1 baseline exclusive e-cigarette user relapsed to smoking cigarettes only, and 5 quit using any products. Most baseline poly users with other nicotine products switched to exclusive e-cigarette use (n=12, 70.6%) at follow-up. Among 71 baseline poly users with other tobacco products, 60.6% (n=43) switched to using e-cigarettes exclusively at follow-up, and 20 (28.2%) remained poly users; however, 2 (2.8%) returned to exclusive cigarette smoking. Two baseline ex–e-cigarette users became exclusive e-cigarette users at follow-up, 1 stopped using any products, and 1 became a poly user with other tobacco products.
Figure 1.
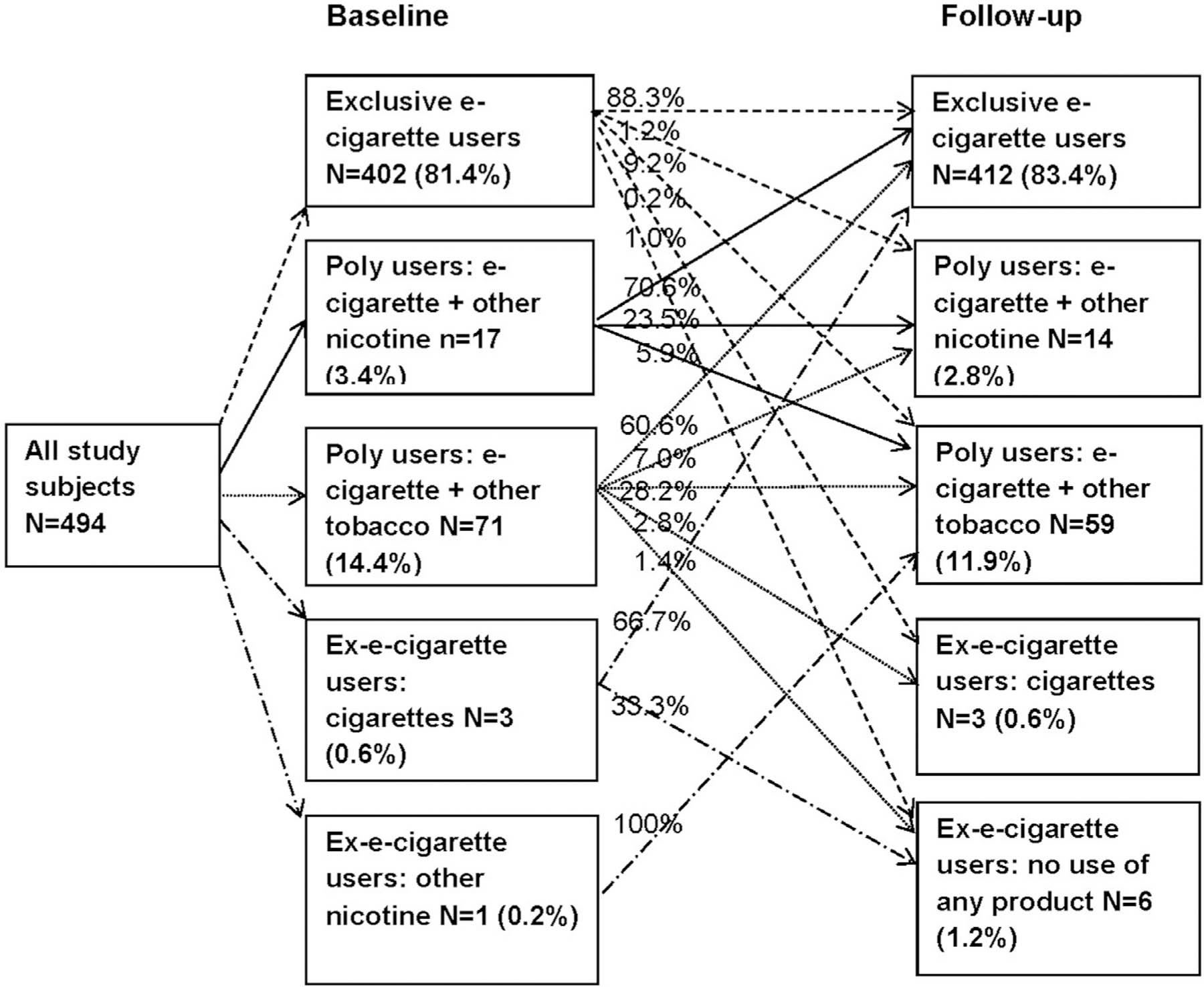
Changes in e-cigarette use behaviors over time.
To understand the factors that may contribute to the maintenance of exclusive e-cigarette use or a relapse to cigarette smoking, sociodemographics and e-cigarette–use behaviors were compared using the 2 surveys among 355 baseline exclusive e-cigarette users/follow-up exclusive e-cigarette users, 37 baseline exclusive e-cigarette users/follow-up poly users with other tobacco products, and 43 baseline poly users with other tobacco products/follow-up exclusive e-cigarette users (Table 2). The mean number of devices used between baseline and follow-up increased in all 3 groups (p<0.05). The mean number of e-cigarette use times per day was reduced among baseline exclusive e-cigarette users/follow-up exclusive e-cigarette users (24.5 vs 21.4). More baseline poly users/follow-up exclusive e-cigarette users reported daily e-cigarette use at follow-up (55.8% vs 95.3%). For baseline exclusive e-cigarette users/follow-up exclusive e-cigarette users and baseline poly users/follow-up exclusive e-cigarette users, compared with their baseline e-cigarette use behaviors, they were more likely to use advanced e-cigarette devices (with a button or battery voltage control, or a “mod” e-cigarette) at follow-up.
Table 2.
Characteristics Among Baseline Exclusive E-Cigarette Users/Follow-Up Exclusive E-Cigarette Users, Baseline Exclusive E-Cigarette Users/Follow-Up Poly Users,a and Baseline Poly Usersa/Follow-Up Exclusive E-Cigarette Users
| Characteristics | Baseline exclusive e-cigarette users/follow-up exclusive e-cigarette users (n=355) |
Baseline exclusive e-cigarette users/follow-up poly users (n=37) |
Baseline poly users/follow-up exclusive e-cigarette users (n=43) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-up | p-valueb | Baseline | Follow-up | p-valueb | Baseline | Follow-up | p-valueb | |
| Age (years), mean (SD) | 41.3 (11.9) | 45.0 (11.9) | — | 36.8 (11.4) | 40.6 (11.5) | — | 40.6 (11.5) | 44.3 (11.5) | — |
| College or higher, n (%) | 165 (46.5) | 167 (47.0) | 0.75 | 16 (43.2) | 20 (54.1) | 0.05 | 20 (46.5) | 22 (51.2) | 0.16 |
| Full-time employed, n (%) | 251 (70.7) | 241 (67.9) | 0.21 | 21 (56.8) | 24 (64.9) | 0.37 | 29 (67.4) | 28 (65.1) | 0.71 |
| Intended to quit smoking when starting e-cigarette use, n (%) | 263 (74.3) | 254 (71.8) | 0.25 | 24 (64.9) | 23 (62.2) | 0.76 | 33 (76.7) | 33 (76.7) | 1.00 |
| Mean number of devices used before the current device (SD) | 5.0 (6.0) | 11.0 (13.3) | 0.002 | 3.5 (4.9) | 9.9 (15.4) | 0.01 | 2.6 (2.6) | 8.9 (10.2) | <0.0001 |
| Mean e-cigarette use times per day (SD) | 24.5 (24.8) | 21.4 (22.9) | 0.03 | 15.7 (12.6) | 16.6 (24.1) | 0.84 | 21.7 (26.4) | 25.2 (27.3) | 0.50 |
| Daily e-cigarette use in the past 28 days or 30 days, n (%) | 338 (95.2) | 333 (93.8) | 0.51 | 29 (78.4) | 29 (78.4) | 1.0 | 24 (55.8) | 41 (95.3) | 0.0001 |
| Using a “ciglike” e-cigarette, n (%) | 35 (9.9) | 13 (3.7) | <0.0001 | 7 (18.9) | 6 (16.2) | 0.71 | 14 (32.6) | 7 (16.3) | 0.02 |
| Using an e-cigarette with a button that you can press before puffing, n (%) | 313 (88.7) | 337 (95.5) | <0.0001 | 30 (81.1) | 32 (86.5) | 0.32 | 28 (65.1) | 36 (83.7) | 0.01 |
| Using an e-cigarette that allows you to control the battery voltage, n (%) | 220 (62.2) | 258 (72.9) | 0.0008 | 22 (59.5) | 27 (73.0) | 0.13 | 17 (39.5) | 31 (72.1) | 0.001 |
| Using a “mod” e-cigarette, n (%) | 215 (60.6) | 244 (68.7) | 0.007 | 21 (56.8) | 23 (62.2) | 0.53 | 15 (34.9) | 22 (51.2) | 0.07 |
| Mean PSECDI (SD) | 8.6 (3.4) | 8.3 (3.8) | 0.25 | 7.4 (3.1) | 8.0 (3.4) | 0.39 | 8.3 (3.6) | 8.5 (3.7) | 0.82 |
Note: Boldface indicates statistical significance (p<0.05).
E-cigarette and other tobacco products.
Paired t-test or McNemar’s test p-values were used to examine changes in characteristics between baseline and follow-up.
PSECDI, Penn State Electronic Cigarette Dependence Index.
Overall, the mean PSECDI score remained unchanged over time (8.4 at baseline vs 8.3 at follow-up, p=0.49), indicating low to moderate dependence in these long-term e-cigarette users (Table 3). Study subjects were also classified according to changes in their e-cigarette–use behaviors, and there were no significant differences in the mean PSECDI scores across all groups. The mean PSECDI score was significantly correlated between baseline and follow-up (p<0.0001).
Table 3.
Changes in PSECDI Over Time by E-Cigarette Use Behaviors
| Baseline/follow-up | PSECDI |
p-valueb | |||
|---|---|---|---|---|---|
| Baseline, mean (SD) | Follow-up, mean (SD) | p-valuea | Pearson correlation coefficient | ||
| Exclusive e-cigarette users (n=402) | |||||
| Exclusive e-cigarette users (n=355) | 8.6 (3.4) | 8.3 (3.7) | 0.25 | 0.52 | <0.0001 |
| Poly users: e-cigarette and other nicotine (n=5) | 7.6 (3.0) | 6.4 (4.0) | 0.46 | 0.60 | 0.28 |
| Poly users: e-cigarette and other tobacco (n=37) | 7.4 (3.1) | 8.0 (3.4) | 0.39 | 0.33 | 0.045 |
| Ex–e-cigarette users (n=5) | 5.2 (4.2) | 5.4 (4.5) | 0.81 | 0.92 | 0.03 |
| Poly users (e-cigarette and other nicotine products) (n=17) | |||||
| Exclusive e-cigarette users (n=12) | 8.2 (4.0) | 8.8 (4.4) | 0.43 | 0.83 | 0.0008 |
| Poly users: e-cigarette and other nicotine (n=4) | 9.5 (4.2) | 8.8 (6.3) | 0.76 | NA | NA |
| Poly users: e-cigarette and other tobacco (n=1) | 14.0 | 11.0 | NA | NA | NA |
| Ex–e-cigarette users (n=0) | — | — | — | — | — |
| Poly users (e-cigarette and other tobacco products) (n=71) | |||||
| Exclusive e-cigarette users (n=43) | 8.3 (3.6) | 8.5 (3.7) | 0.82 | 0.36 | 0.02 |
| Poly users: e-cigarette and other nicotine (n=5) | 10.8 (5.9) | 8.4 (5.9) | 0.40 | 0.53 | 0.36 |
| Poly users: e-cigarette and other tobacco (n=20) | 7.5 (4.8) | 7.7 (4.8) | 0.86 | 0.14 | 0.56 |
| Ex–e-cigarette users (n=3) | 7.3 (4.7) | 12.0 (7.1) | 0.06 | NA | NA |
| Ex-e-cigarette users (n=4) | |||||
| Exclusive e-cigarette users (n=2) | 9.0 (0) | 6.0 (2.8) | 0.37 | NA | NA |
| Poly users: e-cigarette and other tobacco (n=1) | 5.0 | 10.0 | NA | NA | NA |
| Ex-e-cigarette users (n=1) | 4.0 | 0 | NA | NA | NA |
| Total study subjects (n=494) | 8.4 (3.5) | 8.3 (3.9) | 0.49 | 0.49 | <0.0001 |
Note: The PSECDI was calculated according to the individuals’ responses to 10 e-cigarette-related dependence questions. Boldface indicates statistical significance (p<0.05).
Paired t-test p-values were used to examine changes in the mean PSECDI between baseline and follow-up.
Pearson correlation coefficient p-values were used to assess the correlation between the baseline PSECDI and the follow-up PSECDI. Pearson correlation coefficients were not calculated for cells with <5 subjects.
NA, not applicable; PSECDI, Penn State Electronic Cigarette Dependence Index.
DISCUSSION
In this study of 494 e-cigarette users with about 4 years of follow-up, e-cigarette use behaviors appeared to be well established. Most exclusive e-cigarette users at baseline continued using e-cigarettes exclusively on a daily basis, and the risk of relapse to cigarette smoking was low. Consistent with previous findings that frequent e-cigarette use is associated with quitting smoking,11,30,31,35,36 this study also provides new evidence that once users have switched to e-cigarettes, many are able to maintain exclusive e-cigarette use over a long period (up to 6 years in this study). Because virtually all of the study subjects were ever smokers, and approximately 30% reported that they used e-cigarettes to support smoking abstinence, the findings suggest that e-cigarette use could facilitate smoking cessation in adult smokers.
At follow-up, only a small proportion (9.4%) of the baseline exclusive e-cigarette users reported using cigarettes (with or without e-cigarette use), but almost two thirds of the baseline poly users transitioned to exclusive e-cigarette users. To understand the factors affecting e-cigarette–use behaviors, multiple characteristics at baseline and follow-up were examined, and the results suggest that changes in e-cigarette devices may play an important role in supporting continuation of exclusive e-cigarette use.41 Advanced generations of e-cigarette devices yield a higher nicotine concentration that is comparable to combustible tobacco products and also allow users to have a better control of e-cigarette use.7,44 Thus, switching to advanced devices also may have helped poly users successfully quit other tobacco products and use e-cigarettes exclusively on a daily basis. A small proportion of study subjects (3.4% at baseline) used both e-cigarettes and other nicotine products (primarily over-the-counter products for nicotine-replacement therapy). As most (70.6%) of them switched to exclusive e-cigarette use at follow-up, poly use with other nicotine products may represent a transitioning stage to exclusive e-cigarette use as well.
An increased dependence level has been one of the primary concerns regarding the health risks of e-cigarettes.14,15,17,19,20,45 The PSECDI, a validated measure of e-cigarette–related dependence, was used in this study to evaluate if e-cigarette users would develop higher levels of dependence.13 Consistent with other studies,32,33,35,36,46 e-cigarette users reported only low to moderate dependence levels at follow-up. The study also showed that e-cigarette–related dependence remained stable over time, even among long-term exclusive e-cigarette users. Although there is no consensus on the methods to measure e-cigarette–related dependence,47,48 the PSECDI includes multiple domains of e-cigarette use (quantity and frequency of use, withdrawal symptoms, craving/urge to use, and impaired control) that are recommended by the Tobacco Center of Regulatory Science expert group.47 Several questions related to e-cigarette–related dependence, however, did show a wide range of responses and also suggested a possible increased dependence (e.g., hard to keep from using e-cigarettes) in some e-cigarette users. Therefore, further methodologic development is needed to better understand e-cigarette–related dependence.
Although this study indicates that e-cigarette use could result in smoking abstinence and that there is no increased e-cigarette–related dependence, e-cigarettes are not harmless, and the risks and benefits of e-cigarette use need to be carefully evaluated to inform public health efforts for tobacco control. In clinical practice, the FDA-approved smoking-cessation aids should still be the top choice for smokers.49 However, for adult smokers who are unable to quit, using e-cigarettes may lead to the reduction or cessation of cigarette smoking and sub-sequently less exposure to tobacco-related toxicants.5,6
Limitations
Several caveats need to be discussed. First, the study population consisted of e-cigarette users who volunteered to participate in both surveys and was not truly representative of general e-cigarette users. The study findings may be different from other cohort studies with different sampling frames. Moreover, the study’s retention rate was 39.8%, and it is possible that exclusive e-cigarette users were more likely to participate in the follow-up survey. Ex–e-cigarette users may also be excluded owing to missing data. Therefore, the number of poly users or ex–e-cigarette users who returned to cigarette smoking may be underestimated. Second, recall bias is a common challenge for survey research. With self-reported information, an accurate assessment of e-cigarette–use behaviors during a long follow-up period may not be possible. However, as the survey questions focused on use behaviors during the past 7 days, recall bias does not appear to be a major limitation. Third, repeated measures of the 7-day point prevalence were used to define exclusive e-cigarette use and poly use because the 7-day point prevalence was associated with prolonged measures of smoking abstinence.43 It is also unlikely that a poly user would remain abstinent for 7 days at both surveys. The high proportion of daily e-cigarette users (in the past 28 or 30 days) further supports using the 7-day point prevalence as a practical measure to assess stable e-cigarette–use behaviors. However, the 7-day point prevalence may not be a good measure for new or occasional e-cigarette users. A longer time frame would be ideal to capture fluctuations in e-cigarette or poly use behaviors and to define long-term smoking abstinence.50 Finally, biological samples or medical record data were not collected to validate smoking status or to evaluate clinical outcomes related to e-cigarette use. There is also a lack of data to examine how dependence level had changed after smokers switched to using e-cigarettes exclusively. Future cohort studies should include relevant biomarkers or conduct medical examinations to assess e-cigarette–related dependence, to measure the health effects of exposure to potentially harmful constituents of e-cigarettes, and to validate self-reported smoking abstinence.26,45,51 Despite these limitations, this study contributes to a better understanding of e-cigarette–use behaviors and dependence in a large sample of long-term e-cigarette users.
CONCLUSIONS
This study indicates that e-cigarette–use behaviors remain stable in long-term e-cigarette users and that the risk of relapse to cigarette smoking is low. Furthermore, there is no evidence of increased e-cigarette–related dependence. However, with the implementation of FDA regulations on e-cigarettes, more longitudinal studies are needed to evaluate smoking abstinence, e-cigarette–related dependence, and clinically relevant health outcomes.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute on Drug Abuse of NIH and the Center for Tobacco Products of the U.S. Food and Drug Administration (P50-DA-036107). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the U.S. Food and Drug Administration.
Jonathan Foulds has acted as a paid consultant for pharmaceutical companies involved in producing smoking-cessation medications, including GSK, Pfizer, Novartis, and J&J and received a research grant and study products from Pfizer Inc. No other financial disclosures were reported.
Footnotes
SUPPLEMENTAL MATERIAL
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2019.04.021.
REFERENCES
- 1.CDC. Tobacco-related mortality www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/tobacco_related_mortality/. Published August 18, 2015. Accessed November 25, 2015.
- 2.Agency for Healthcare Research and Quality. Treating Tobacco Use and Dependence: 2008 Update Rockville, MD: Agency for Health care Research and Quality, 2015. [Google Scholar]
- 3.Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf 2014;5(2):67–86. 10.1177/2042098614524430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nutt DJ, Phillips LD, Balfour D, et al. Estimating the harms of nicotine-containing products using the MCDA approach. Eur Addict Res 2014;20(5):218–225. 10.1159/000360220. [DOI] [PubMed] [Google Scholar]
- 5.McNeill A, Brose L, Calder R, et al. E-cigarettes: an evidence update London: Public Health England, 2015. PHE publications gateway number: 2015260. [Google Scholar]
- 6.National Academies of Sciences, Engineering, and Medicine. Public Health Consequences of e-Cigarettes Washington, DC: National Academies Press, 2018. [PubMed] [Google Scholar]
- 7.Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res 2015;17(2):150–157. 10.1093/ntr/ntu174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll Chapman SL, Wu LT. E-cigarette prevalence and correlates of use among adolescents versus adults: a review and comparison. J Psychiatr Res 2014;54:43–54. 10.1016/j.jpsychires.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farsalinos KE, Poulas K, Voudris V, Le Houezec J. Electronic cigarette use in the European Union: analysis of a representative sample of 27460 Europeans from 28 countries. Addiction 2016;111(11):2032–2040. 10.1111/add.13506. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 2016;9:CD010216. 10.1002/14651858.cd010216.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giovenco DP, Delnevo CD. Prevalence of population smoking cessation by electronic cigarette use status in a national sample of recent smokers. Addict Behav 2018;76:129–134. 10.1016/j.addbeh.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villanti AC, Feirman SP, Niaura RS, et al. How do we determine the impact of e-cigarettes on cigarette smoking cessation or reduction? Review and recommendations for answering the research question with scientific rigor. Addiction 2018;113(3):391–404. 10.1111/add.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foulds J, Veldheer S, Yingst J, et al. Development of a questionnaire for assessing dependence on electronic cigarettes among a large sample of ex-smoking e-cigarette users. Nicotine Tob Res 2015;17(2):186–192. 10.1093/ntr/ntu204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grana R, Benowitz N, Glantz SA. E-cigarettes: a scientific review. Circulation 2014;129(19):1972–1986. 10.1161/circulationaha.114.007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajek P, Etter JF, Benowitz N, Eissenberg T, McRobbie H. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction 2014;109(11):1801–1810. 10.1111/add.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulvers K, Hayes RB, Scheuermann TS, et al. Tobacco use, quitting behavior, and health characteristics among current electronic cigarette users in a national tri-ethnic adult stable smoker sample. Nicotine Tob Res 2015;17(9):1085–1095. 10.1093/ntr/ntu241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder MJ, Hoffman AC. Electronic cigarettes and nicotine clinical pharmacology. Tob Control 2014;23(suppl 2):ii30–ii35. 10.1136/tobaccocontrol-2013-051469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandon TH, Goniewicz ML, Hanna NH, et al. Electronic nicotine delivery systems: a policy statement from the American Association for Cancer Research and the American Society of Clinical Oncology. J Clin Oncol 2015;33(8):952–963. 10.1200/jco.2014.59.4465. [DOI] [PubMed] [Google Scholar]
- 19.Cobb CO, Hendricks PS, Eissenberg T. Electronic cigarettes and nicotine dependence: evolving products, evolving problems. BMC Med 2015;13:119. 10.1186/s12916-015-0355-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooke A, Fergeson J, Bulkhi A, Casale TB. The electronic cigarette: the good, the bad, and the ugly. J Allergy Clin Immunol Pract 2015;3 (4):498–505. 10.1016/j.jaip.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med 2016;4(2):116–128. 10.1016/s2213-2600(15)00521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu SH, Zhuang YL, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from U.S. current population surveys. BMJ 2017;358:j3262. 10.1136/bmj.j3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy DT, Borland R, Lindblom EN, et al. Potential deaths averted in USA by replacing cigarettes with e-cigarettes. Tob Control 2018;27 (1):18–25. 10.1136/tobaccocontrol-2017-053759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soneji SS, Sung HY, Primack BA, Pierce JP, Sargent JD. Quantifying population-level health benefits and harms of e-cigarette use in the United States. PLoS One 2018;13(3):e0193328. 10.1371/journal.pone.0193328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drope J, Cahn Z, Kennedy R, et al. Key issues surrounding the health impacts of electronic nicotine delivery systems (ENDS) and other sources of nicotine. CA Cancer J Clin 2017;67(6):449–471. 10.3322/caac.21413. [DOI] [PubMed] [Google Scholar]
- 26.Glasser AM, Collins L, Pearson JL, et al. Overview of electronic nicotine delivery systems: a systematic review. Am J Prev Med 2017;52(2): e33–e66. 10.1016/j.amepre.2016.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YO, Hebert CJ, Nonnemaker JM, Kim AE. Multiple tobacco product use among adults in the United States: cigarettes, cigars, electronic cigarettes, hookah, smokeless tobacco, and snus. Prev Med 2014;62:14–19. 10.1016/j.ypmed.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 28.Kasza KA, Ambrose BK, Conway KP, et al. Tobacco-product use by adults and youths in the United States in 2013 and 2014. N Engl J Med 2017;376(4):342–353. 10.1056/NEJMsa1607538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Food and Drug Administration, HHS. Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act; restrictions on the sale and distribution of tobacco products and required warning statements for tobacco products. Final rule. Fed Regist 2016;81(90):28973–29106. [PubMed] [Google Scholar]
- 30.Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addict Behav 2014;39(2):491–494. 10.1016/j.addbeh.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 31.Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res 2015;17 (2):127–133. 10.1093/ntr/ntu200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Etter JF. A longitudinal study of cotinine in long-term daily users of e-cigarettes. Drug Alcohol Depend 2016;160:218–221. 10.1016/j.drugalcdep.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Liu G, Wasserman E, Kong L, Foulds J. A comparison of nicotine dependence among exclusive e-cigarette and cigarette users in the PATH study. Prev Med 2017;104:86–91. 10.1016/j.ypmed.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasquereau A, Guignard R, Andler R, Nguyen-Thanh V. Electronic cigarettes, quit attempts and smoking cessation: a 6-month follow-up. Addiction 2017;112(9):1620–1628. 10.1111/add.13869. [DOI] [PubMed] [Google Scholar]
- 35.Buu A, Hu YH, Piper ME, Lin HC. The association between e-cigarette use characteristics and combustible cigarette consumption and dependence symptoms: results from a national longitudinal study. Addict Behav 2018;84:69–74. 10.1016/j.addbeh.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etter JF. Electronic cigarette: a longitudinal study of regular vapers. Nicotine Tob Res 2018;20(8):912–922. 10.1093/ntr/ntx132. [DOI] [PubMed] [Google Scholar]
- 37.Pulvers K, Emami AS, Nollen NL, et al. Tobacco consumption and toxicant exposure of cigarette smokers using electronic cigarettes. Nicotine Tob Res 2018;20(2):206–214. 10.1093/ntr/ntw333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yingst JM, Veldheer S, Hrabovsky S, Nichols TT, Wilson SJ, Foulds J. Factors associated with electronic cigarette users’ device preferences and transition from first generation to advanced generation devices. Nicotine Tob Res 2015;17(10):1242–1246. 10.1093/ntr/ntv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammett E, Veldheer S, Yingst J, Hrabovsky S, Foulds J. Characteristics, use patterns and perceptions of electronic cigarette users who were never traditional cigarette smokers. Addict Behav 2017;65:92–97. 10.1016/j.addbeh.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yingst JM, Veldheer S, Hammett E, Hrabovsky S, Foulds J. A method for classifying user-reported electronic cigarette liquid flavors. Nicotine Tob Res 2017;19(11):1381–1385. 10.1093/ntr/ntw383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yingst J, Foulds J, Veldheer S, Du P. Device characteristics of long term electronic cigarette users: a follow-up study. Addict Behav 2019;91:238–243. 10.1016/j.addbeh.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42(2):377–381. 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res 2003;5(1):13–25. 10.1093/ntr/5.1.13. [DOI] [PubMed] [Google Scholar]
- 44.Spindle TR, Breland AB, Karaoghlanian NV, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine Tob Res 2015;17(2):142–149. 10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dinakar C, O’Connor GT. The health effects of electronic cigarettes. N Engl J Med 2016;375(14):1372–1381. 10.1056/NEJMra1502466. [DOI] [PubMed] [Google Scholar]
- 46.Etter JF, Eissenberg T. Dependence levels in users of electronic cigarettes, nicotine gums and tobacco cigarettes. Drug Alcohol Depend 2015;147:68–75. 10.1016/j.drugalcdep.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bold KW, Sussman S, O’Malley SS, et al. Measuring e-cigarette dependence: initial guidance. Addict Behav 2018;79:213–218. 10.1016/j.addbeh.2017.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weaver SR, Kim H, Glasser AM, et al. Establishing consensus on survey measures for electronic nicotine and non-nicotine delivery system use: current challenges and considerations for researchers. Addict Behav 2018;79:203–212. 10.1016/j.addbeh.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rigotti NA. Balancing the benefits and harms of e-cigarettes: a National Academies of Science, Engineering, and Medicine Report. Ann Intern Med 2018;168(9):666–667. 10.7326/m18-0251. [DOI] [PubMed] [Google Scholar]
- 50.West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100 (3):299–303. 10.1111/j.1360-0443.2004.00995.x. [DOI] [PubMed] [Google Scholar]
- 51.Chang CM, Edwards SH, Arab A, Del Valle-Pinero AY, Yang L, Hatsukami DK. Biomarkers of tobacco exposure: summary of an FDA-sponsored public workshop. Cancer Epidemiol Biomark Prev 2017;26(3):291–302. 10.1158/1055-9965.epi-16-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


