Abstract
The immunohistochemical (IHC) evaluation of epidermal growth factor 2 (HER2) for the diagnosis of breast cancer is still qualitative with a high degree of inter-observer variability, and thus requires the incorporation of complementary techniques such as fluorescent in situ hybridization (FISH) to resolve the diagnosis. Implementing automatic algorithms to classify IHC biomarkers is crucial for typifying the tumor and deciding on therapy for each patient with better performance. The present study aims to demonstrate that, using an explainable Machine Learning (ML) model for the classification of HER2 photomicrographs, it is possible to determine criteria to improve the value of IHC analysis. We trained a logistic regression-based supervised ML model with 393 IHC microscopy images from 131 patients, to discriminate between upregulated and normal expression of the HER2 protein. Pathologists' diagnoses (IHC only) vs. the final diagnosis complemented with FISH (IHC + FISH) were used as training outputs. Basic performance metrics and receiver operating characteristic curve analysis were used together with an explainability algorithm based on Shapley Additive exPlanations (SHAP) values to understand training differences. The model could discriminate amplified IHC from normal expression with better performance when the training output was the IHC + FISH final diagnosis (IHC vs. IHC + FISH: area under the curve, 0.94 vs. 0.81). This may be explained by the increased analytical impact of the membrane distribution criteria over the global intensity of the signal, according to SHAP value interpretation. The classification model improved its performance when the training input was the final diagnosis, downplaying the weighting of the intensity of the IHC signal, suggesting that to improve pathological diagnosis before FISH consultation, it is necessary to emphasize subcellular patterns of staining.
Keywords: breast cancer, HER2, IHC, ML, SHAP
Introduction
Breast cancer is the most common malignancy in women worldwide and despite advances in research and therapy, it remains a highly lethal malignant disease that constitutes a heavy burden to public health in >154 countries, with an ~600,000 per year (1,2). In Chile, it has been the leading cause of cancer-associated death since 2009 with 11.8% of deaths from cancer in women per year and a high level of mortality in elder patients. The average number of patients diagnosed with this pathology has improved considerably in recent years; several symptoms are prevalent, especially in the population with inadequate access to national health systems (3,4). The recommended treatment according to the molecular subtypes agreed upon in the St. Gallen consensus has shown favorable effects on patient survival (5,6). According to the St. Gallen protocol, the histopathological aspects of the mammary tumor are defined in a standardized manner, and recommendations for adjuvant therapies are decided based on observation of tumor biopsies under the microscope (5). In this context, the diagnosis of tumors using a minimal panel of molecular techniques, where immunodetection of hormonal receptors [estrogen receptor (ER) and progesterone receptor (PR)], Ki67 antigen, and epidermal growth factor receptor 2 (HER2), is central to pathological analyses due to their fundamental role in appropriately establishing the therapeutic strategy and prognosis for each patient, and for ultimately reducing breast cancer-associated deaths (5,7,8).
Specifically, HER2 protein is overexpressed in 10–25% of invasive breast cancer cases (2,7). Detection of HER2 in tumor cells through histological analysis is an important measure of prognostic and predictive outcomes in invasive tumors (2,9,10). The detection of HER2 by microscopy is more challenging than for the other antigens due to the complex pattern of HER2 expression within cells, which often requires the use of complementary techniques such as fluorescent in situ hybridization (FISH) to detect the gene encoding ERBB2 (8,11,12). The American Society for Clinical Oncology (ASCO) has proposed a specific qualitative algorithm for HER2 using immunohistochemistry (IHC), based on the intensity of the signal, the completeness of the pattern of expression in the cell membrane, and the approximate minimum percentage of expression (8). Following this approach, invasive tumor samples can be categorized as HER2 0, 1+, 2+, or 3+, where the last category corresponds to upregulated expression protein and represents cases that should be treated with anti-HER2 therapy (12,13). Being able to adequately differentiate the HER2-IHC 2+ category (equivocal) from a false positive, or 3+, by traditional microscopy is crucial from several points of view, including appropriate referral to the oncology service and subsequent suitable adjuvant treatment with trastuzumab in this pathology and in gastric cancer (14–16). However, due to the analytical complexity of this biomarker, the HER2 category represents an important obstacle when it comes to determining the histopathological diagnosis under the St. Gallen protocol, with unfavorable consequences on the diagnostic procedure, treatment strategy, and public health (17).
Analyzing the intensity and distribution of the IHC signal in situ provides important information for the diagnosis and prognosis of cancer. Visual scoring of markers is currently employed, using light microscopy with medium magnification capacity (×20 or ×40 objective lenses) (15,18). The tumor can be scored as 0/1+ (negative), 2+ (equivocal), or 3+ (positive) according to official guidelines such as those proposed by ASCO (8,12); however, the microscopic observation of the IHC performed by the pathologist is highly time-consuming and suffers from high inter- and intra-observer variability, with the results being conditioned by subjective factors (19,20). In contrast, analysis performed by computational tools shortens the time required and decreases inter-operator variation in the evaluation of staining levels (20,21). Used for the first time for immunostaining in 1980, computerized image analysis has since been applied in several studies, in combination with innovations in laboratory techniques for protein and gene detection; some examples include AQUA Technology (22), HERmark (23), Mammaprint (24), Oncotype Dx (25), and PAM50 Breast Cancer Intrinsic Classifier (26). Unfortunately, most assays that provide quantitative data work with reference centers and laboratories that do not fit the needs and capabilities of public health systems in Latin America (27). This strengthens the importance of developing low-cost technologies for high-precision analysis and generating digital references of existing material. In addition to this problem, the existing computerized analysis techniques of immunohistochemical signals only integrate 1–2 image parameters to qualify the diagnosis through defined thresholds based on controls (15,21,23). Due to these limitations, innovative techniques are needed to integrate a large number of quantitative parameters to build a mathematical classification model that could be optimized by data training itself (28,29).
Machine learning (ML) and observation with computational tools have been used in a wide variety of clinical tasks ranging from the segmentation of medical images to generation, classification, and clinical prediction (30–33). Different research centers and biotechnology corporations have been exploring the use of artificial intelligence (AI) and ML in key clinical areas (30,34–36). Specifically, ML classified as Supervised Learning allows for the resolution of classification problems where the machine must learn to be able to predict discrete values. This means that the machine can predict the most likely category, class, or label for new examples, or solve regression problems to predict the value of a continuous variable (28,37). In certain problems, the response variable is not normally distributed, for example, a coin toss can only have two outcomes: Positive or negative. In these cases, the use of logistic regression as a classification model is relatively popular and has been used regularly in both industry and medicine (38). The performance of this response is shaped thanks to a mathematical optimization procedure; the use of the cost function in conjunction with the downward gradient that derives from the maximum likelihood makes it possible to find weight coefficients for each input parameter that maximize the performance of the classifier (39).
Despite the promising properties described for the ML classification, the constant increase in the complexity of learning algorithms has led to the frequent appearance of ‘black box’ models, where the interpretation of their internal functioning is very difficult with traditional analytical methods (40). These models are increasingly used in decision-making in important contexts, such as in clinical development, implying that the demand for transparency is essential when decisions based on AI are unjustifiable in real life (41,42). In this context, the concept of explainability makes it possible to detect and correct the training bias, provides robustness by detecting disturbances in the prediction, and establishes arguments that allow us to understand changes in the performance of the classification tasks. Different systems that are coupled to ML models allow opening the black box, such as decision trees, visualization of inputs and outputs through graphs, and Shapley Additive exPlanation (SHAP) values, where the latter stands out primarily for being applicable to any classification model, with easily understandable graphic interpretation and allowance of both explanation and advice (41,43).
The primary question of this study is whether it is possible to obtain a classification as good as pathologists' using ML and photomicrographs features. Additionally, we attempt to understand how the classifier is modified when including information provided by FISH.
Material and methods
Type of study
The present study was a retrospective diagnostic assessment performed to analyze images of anonymized microscopic slides of paraffin-embedded tissues with a histological diagnosis of breast cancer.
Collection of images
Histological slides of IHC-stained breast cancer tissues from 2019 were randomly collected for the lab technician team of Carlos Van Buren Hospital Pathology Service (Valparaíso, Chile) with prior authorization from the Chief pathologist. Only those classed as HER2-positive and a corresponding pathologist's diagnosis reported by IHC and IHC + FISH were included. Data from 141 samples were tabulated from the local database, and anonymized using the internal code of each sample together with its IHC-HER2 diagnosis as 0, 1, 2, or 3 and its FISH diagnosis as 0 or 1. These two classifications were considered for the ML classification to be compared. The histological slides were imaged using a Leica microscope (DM750) with a CCD ICC50-W digital camera integrated into the Las EZ software (Leica Microsystems). Three images were obtained at an ×400 magnification with 3 different areas imaged for each tumor sample, with staining in at least 10% of the tumor area. We used automatic parameters for light exposure, hue, saturation, and γ, calibrated by the factory with in vitro diagnostics standard (https://www.leica-microsystems.com/products/light-microscopes/p/leica-dm2000/). The pathologist provided coded and tabulated data in a password-protected file. The use of digital image samples and anonymized data was approved by the Carlos van Buren Hospital directive (Valparaiso, Chile) and by the V Region, Valparaíso-San Antonio, Scientific Ethics Committee SSVSA (approval no.: 1765-07.10.2021).
Automated image quantification
Using ImageJ 1.53 (National Institutes of Health), automatic batch analysis was performed to extract four features per image, and these were used to differentiate the expression levels of HER2 according to the ASCO algorithm with the following protocol (Fig. 1): i) Resize images to 1,000×750 pixels with bilinear interpolation; ii) separate colors based on an 8-bit channel red/green/blue (RGB) color set; iii) determine the region of interest associated with cell membranes, based on a pixel-by-pixel analysis of RGB information: The retained pixels are denoted as ‘signal’; iv) Obtain the average intensity value of the total signal (feature 1: 8 bit mean gray value, named as MGV); v) convert the 8-bit signal into binary; vi) invert the binary code to transform the fully enclosed empty spaces delimited by the surrounding signal into black particles of a similar size to cells; vii) determine the individual particles meeting two criteria: Area ranging from 250-2,500 pixles2 with a circularity between 0.3–1.0 (feature 2: Circ count, or cells that meet the criterion of fully stained membrane, named as COUNT), quantification of the total percentage area (feature 3: % Area circ, named as %AREA) and average area of the previous counts (feature 4: Mean size, named as M.SIZE). The data (4 features) obtained from 423 photographs (131 patient samples and 10 controls) were tabulated in Excel 365 (Microsoft Corporation). The data corresponding to the patient cohort was subsequently imported into the classifier's input data. The controls (30 samples) corresponded to reference cases (both healthy and non-healthy) and were used to illustrate controlled cases.
Figure 1.
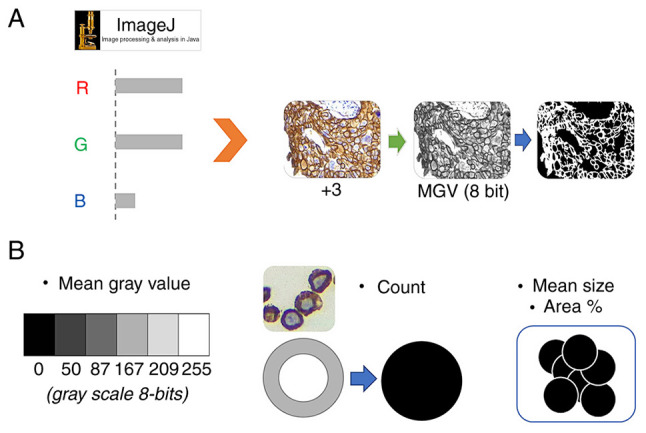
Automated analysis procedure in ImageJ. (A) Pattern extraction procedure from the IHC signal. By means of RGB deconvolution, it was possible to convert the brown signal into an 8-bit scale with which the binary mask for counting cells with a completely stained membrane was generated. (B) From the 8-bit image and the binary mask, the four features obtained where MGV corresponds to feature 1, the count to feature 2. and the average size plus the total area of the positive cells corresponds to features 3 and 4, respectively. MGV, mean gray value; count, count of cells with completely stained membrane; IHC, immunohistochemistry; RGB, red/green/blue.
Training and testing of logistic regression classifiers
ANACONDA open-source toolkit (https://www.anaconda.com) was used to train, implement, and test a logistic amplified (+3) vs. non-amplified (0, +1, or +2) signal classifier, and the Spyder 4 IDE (https://www.spyder-ide.org/) to program the instruction set in Python 3.7, using Scikit-learn packages for machine learning (https://scikit-learn.org/stable/index.html). The previously stored data was first divided into: i) Set of input data, corresponding to the 4 features in each image, to represent the variables of the X axis of the 4-dimensional distribution; ii) output data set 1 (first Y variable), corresponding to the IHC diagnoses of each image, where the scores were grouped according to whether it is an amplified signal or not, such that 0, 1+, and 2+ were merged into a unique output 0 (HER2 normal expression) and 3+ corresponded to output 1 (HER2 overexpression); and iii) output data set 2 (second Y variable), corresponding to the IHC diagnoses corrected by FISH. This converted all the IHC diagnoses that had negative FISH (0) to 0 if they were declared as 1 and vice versa.
Subsequently, the previous data sets were divided into training_set and test_set with a ratio of 0.65:0.35 respectively using the Scikit tool ‘train_test_split’. Next, we implemented a logistic regression model with the default parameters and the model coefficients were automatically adjusted to the training_set. Finally, we used the adjusted model to predict the diagnoses in the test_set and compared them with the original diagnoses to obtain the prediction probabilities and build a confusion matrix, calculate the efficiency parameters of the model, and perform receiver operating characteristic (ROC) curve analysis between the IHC diagnoses and IHC + FISH corrected data.
The whole procedure was tested 20 times to obtain the best performance model.
Model performance explainability using SHapley Additive exPlanations (SHAP) values analysis
To obtain an overview of the most important features for the model output, and how their analytic priority order affected the HER2 diagnostic performance, we plotted the SHAP values of each feature for every sample and mean absolute SHAP values for all features, using SHAP libraries for python (https://shap.readthedocs.io/en/latest/index.html) coupled with the classifier code. SHAP values can further show positive and negative relationships of the predictor, and by plotting all dots in the training data with a blue-to-red color gradient, the following information can be obtained: i) Single value impact: The horizontal location shows whether the effect of that value is associated with higher or lower predictive performance; ii) original value: Color shows whether that variable is high (in red) or low (in blue) for that observation; iii) Mean feature importance: Using mean absolute values, data can be summarized in a simplified bar plot to show the overall impact of each feature on model output. Variables were ranked in descending order.
Classifier performance evaluation
To assess the performance of the model, a confusion matrix was constructed with the ML-predicted diagnoses vs. the actual diagnoses to define true positives and negatives (TP/TN) and false positives and negatives (FP/FN). With this, matrix, precision, recall, specificity, accuracy, and FPR were calculated to perform ROC analysis to determine, using the area under the curve (AUC), the set of diagnoses that allowed building a better predictor (44). ROC analysis was performed using binary prediction probabilities of positive class over test_dataset to calculate false rates (FPR) and true positive rates (TPR) with Sklearn metrics libraries. Next, the FPR and TPR were plotted to calculate the AUC score, and the 95% confidence intervals were determined by bootstrapped scores (n_bootstraps=1,000, random state=42).
Statistical analysis
Bar graph results are presented as the mean ± standard deviation from at least three independent evaluations for each experimental condition. Data were analyzed with Origin Pro 9.0 (OriginLab Corporation Northampton). Differences between multiple groups were compared using a Fisher's least significant difference test and ANOVA followed by a Bonferroni posthoc test in Statgraphics Plus version 5.0 (GraphPad Software, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Automated image analysis
The first approach was to test if the distribution values of the four features, MGV, COUNT, %AREA and M.SIZE, were statically different between the control samples, as references in the laboratory.
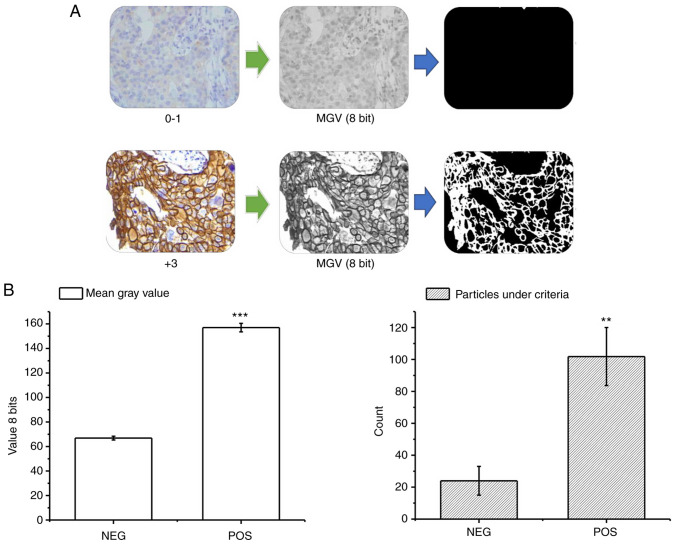
Through the previously described procedure, it was possible to differentiate the samples according to their immunohistochemical expression levels through the average intensity of the signal and the parameters derived from the particle count in the binary results. The negative control samples (corresponding to known diagnoses 0/1+) presented a low overall signal intensity with an average of 65 (±2) 8 bit-grayscale, and a particle count under the positivity criterion of 21 (±9) by visual field (Fig. 2A). Conversely, in the positive control samples (corresponding to known diagnoses 3+) the global intensity and the particle count under the criterion were significantly higher (Fig. 2A), with values of 155 (±3) and 101 (±20), respectively (Fig. 2B). With this procedure, the MGV, COUNT, %AREA determined by this count, and the M.SIZE were obtained for the cohort samples, resulting in a data matrix for the training with dimensions: (3,934).
Figure 2.
Results of automated image analysis in control cases. (A) Representative images of the results of the automatic analytical procedure in the negative controls (top, 0–1) and positive controls (bottom, +3). The original image was decomposed into the total signal and the binary mask of the circular spaces, which then showed the substantial differences between the two groups. (B) Graphs of the quantification in the controls (positive and negative) of the MGV (left) and the particle count of interest (right). Data are presented as the mean ± SD. **P<0.01, ***P<0.001. n=30. MGV, mean grey value.
Classifier performance evaluation
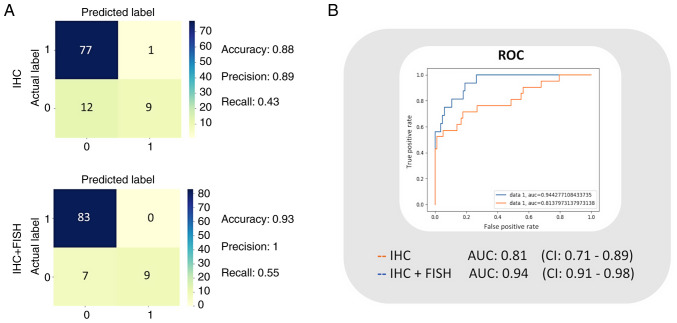
The logistic model was trained with the training data set, and the classifier performance was evaluated with the data intended for testing (n=99). In this manner, a confusion matrix for training based on the diagnoses reported with HER2 IHC, and another matrix for the trained classifier based on confirmed diagnoses after FISH (IHC + FISH) were obtained to calculate the performance indicators. The best accuracy, precision, and recall calculated for the IHC model were 0.88, 0.89, and 0.43 respectively, while for the IHC + FISH classifier they were 0.93, 1.00, and 0.55, respectively (Fig. 3A). Subsequently, to illustrate the sensitivity vs. specificity using the compared ROC curves, we calculated the TPR and FPR. The resulting AUC value for the IHC classifier was 0.81 (0.71–0.89), and for the IHC + FISH model, it was 0.94 (0.92–0.98). Confidence intervals are based on 20 samples (Fig. 3B).
Figure 3.
Classification model of the performance metrics. (A) Confusion matrices for the IHC vs. IHC + FISH classifier models and the resulting best accuracy, precision, and recall. (B) ROC analysis and graphical representation of the AUC for the two models. The intervals are adjusted to 95% confidence. n=20. IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; ROC, receiver operating characteristic; AUC, area under the curve.
Model explanation by SHAP values
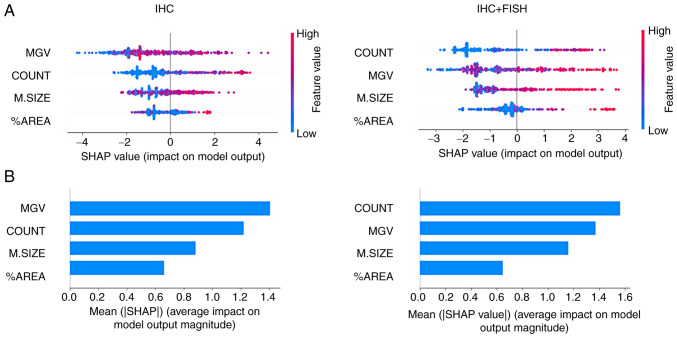
Differences in classifier performance trained based on the IHC diagnosis provided by the pathologist vs. the training based on IHC + FISH can be explained primarily by changes in the order of the analytical priority of the features. As the graphs in Fig. 4A show, the MGV primarily influenced the negative output of the logistic classifier (appearance of 0), while in the case of the COUNT value, at higher values the impact was positive (appearance of 1) and vice versa. Meanwhile, M.SIZE and %AREA values showed a more diffuse impact pattern on the binary output; however, it was possible to observe how the distribution of the points became more evident towards the binary output with IHC + FISH training. The summary of this effect is shown in Fig. 4B, where it can be seen that MGV and COUNT had a greater absolute average impact than the other two variables; however, the first parameter loses priority below the count of cells that meet the membrane completeness criteria when the training is based on IHC + FISH.
Figure 4.
Model classification differences explained by the SHAP values. (A) A plot was used to sort the features based on the sum of SHAP value magnitudes over all samples and show the distribution of the impact that each feature had on the model output. The color represents the feature value (red high, blue low) and the x-axis represents the impact score according to binary output (HER2- or +). (B) The mean absolute value of the SHAP values for each feature to get a standard bar plot showed the average impact on global model output. SHAP, Shapley Additive exPlanations.
Discussion
The complex expression pattern of HER2 within cells presents a challenge for pathologists and the high inter-observer variability highlights the necessity of tools that allow for improved predictive results to better tailor treatment strategies. Deep learning automatic-assisted diagnosis is the most suitable and potentially accurate solution. Automatic quantification of images allows for the factorization of two key points highlighted in the HER2 classification algorithm recommended by ASCO. First, the visual field signal intensity in the low, medium, and high categories, a continuous variable of 256 dimensions (8-bit depth), is internationally used in other immune detection techniques in brightfield and fluorescence formats (45,46). The second parameter corresponds to the completeness of the staining around the cell membrane, whose quantitative estimation still represents a significant challenge for the pathologist. In the present study, the signal was transformed into an inverted binary, where the surrounding blanks by the nuclear signal were converted into particles with a size ranging between 250 and 2,500 pixels2 and medium circularity when working with images with a standardized resolution of 1,000 pixels in width (47). This strategy allows for the generation of statistically significant differences when comparing amplified vs. normal HER2 expression samples (set of positive vs. negative controls), based on a reduced analytical complexity in comparison with current analytical methods of membrane signals from other research groups that use a skeletonization technique or cell segmentation through the specialized filters (48,49). Moreover, our automated process also used a low complexity method to extract the IHC signal by decomposing the colors into RGB data matrices pixel by pixel, where the combinatorial intensity in red, green, and blue allowed the pixels to be separated. A promising approach to refine the signal could be to use the technique proposed by Fu et al (18), with a filter detection complex for brown color generated by the DAB chromogen typically used in immunohistochemistry.
With the parameters obtained, it was possible to train a logistic regression ML model for classification and explainability, and we were able to show significant changes in the classification performance when the training was performed based on the diagnosis supplemented with FISH results: When evaluating the basic performance parameters, the classifier showed high precision and accuracy in the two trained models (IHC: 0.84 and 0.72 vs. IHC + FISH: 0.97 and 0.89, respectively), highlighting that both values increased when the FISH criterion was added. This obtained data increases the performance and seems to be primarily explained by reducing the output impact of the parameter associated with the global intensity of IHC MGV and increasing the weight of the subcellular location of the protein, in this case towards the plasma membrane through the positive cells, the criterion of completeness (COUNT). In this regard, although IHC takes advantage of the high affinity of the antigen-antibody reaction that allows identifying the expression of biomarkers or proteins, its results, known as immunoexpression, immunostaining, and/or chromogenic signal intensity, are influenced by multiple factors from the pre-analytical to the post-analytical phase, and depending on the performance of these stages, several results can be obtained even using the same antibody, affecting clinical interpretation (50,51). If each stage of the preanalytical phase of histopathology is analyzed in detail, the first step of taking a sample for biopsy and fixing the tissue in formaldehyde solution is one of the most important steps, as the result of the IHC is highly dependent on the time required to fix a sample after extraction: The cold ischemic time must be as low as possible, since a prolonged cold ischemic time leads to tissue acidosis, enzymatic degradation, and altered immunoreactivity, resulting in artifactual lower antigenic expression (50,51). Conversely, over-fixing may occur if fixing times exceed 72 h in small samples such as core breast biopsies. This increases variability and can generate false negatives/positives at the time of interpretation when qualitatively assessing the staining intensity (50). Finally, another preanalytical factor is the histological paraffin section technique. In IHC, the optimal section thickness is 3 µm, when these sections are thicker (>5 µm), they can generate an overgrowth of the staining intensity during the IHC-reveal stage, due to overlapping of the cell layers, which saturates the reaction and may produce an more intense signal, inappropriately augmenting intensity instead of the completeness of the cell membrane, which can generate a false diagnosis of +3 HER2, translating into a completely different treatment and prognosis (52–54).
Based on the performance improvements obtained by including the FISH results as an extra dimension in the reference diagnosis, it is hypothesized that the addition of more features to the ML algorithm training, that include the construction of a training supervision matrix based on unsupervised learning will improve predictive ability. This complementary information may include the basic histological subtype of the tumor, the expression pattern in lymph node metastases, the response to treatment, and patient survival, to better study the predictive variables of each tumor (2,55–57). With this increase in the dimension of the known output variables, the performance of the test may potentially improve, specifically in the recall values, which were low in this study (IHC: 0.43 and IHC + FISH: 0.55), this may assist in determining the proportion of actual positives that were correctly identified (58). Low false positive detection is very attractive in practice regarding the implications of a diagnosis of breast cancer and referring the patient to the oncology service, with tremendous burden in terms of complexity of health services, costs, and impact to the patient in vain (2,59). In addition, the general performance of the test through the ROC analysis, showed that this HER2 classifier had AUC values of 0.81 and 0.94 for training with the IHC and IHC + FISH sets respectively, a very positive scenario since values between 0.8–0.9 are considered very good for complex and high-throughput detection, such as in blood biochemical profiling (60,61).
Finally, the classification model presented here can potentially be trained with a larger number of samples to generate a robust digital pathological reference, through a free access interoperability platform for public health services, just as pathology laboratories do in developed countries, applying data management systems currently available on the market, but at a high cost (62–64). Furthermore, the methods and technology proposed here have been developed motivated by previous research in this field, to test the performance of different classification algorithms for breast cancer, through the unitary evaluation of multiple molecular markers in existing protocols such as Ki67, ER, and PR (6,65,66), or in proposals under development for molecular classification including new markers such as PDL1, Claudin, MCL-1, and TRPV1 (67–70). The ability of ML classifiers to decrease the inter-observer variability improves the accuracy of breast cancer diagnoses, which may have important implications since treatment is based on the subtype of tumor diagnosed (5,54,71). This approach has the potential to reduce breast cancer mortality with a significant impact on health systems and affected populations. Our approach however has some limitations. The model presented here is only limited to diagnosis and does not provide analytical evidence to improve tumor prognosis, as it does not integrate other relevant immunohistochemical biomarkers such as Ki67, or hormone receptors to subclassify the tumor according to the risk and the treatment corresponding to the standard categories (6). Additionally, the model requires an image preprocessing phase, which increases the level of complexity and time required for analysis. Therefore, the implementation of deep learning models based on convolutional neural networks that allow working directly with the images and integrating other biomarkers in a 4-dimensional hyperimage (32,35), could be explored in the future to propose an easy-to-use procedure to be assessed in clinical trials.
In conclusion, our current findings suggest that ML classifiers may be an important contribution to pathology services and pathologists as, despite their limitations, they can assist in obtaining a more accurate diagnosis of HER2 immunohistochemical expression as a tool to predict future potential patient outcomes.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- IHC
immunohistochemistry
- ML
machine learning
- AI
artificial intelligence
- HER2
human epidermal growth factor 2
- FISH
fluorescence in situ hybridization
- SHAP
Shapley Additive exPlanations
Funding Statement
This study was funded by Regular FONDECYT, Chile (grant no. 1201311), a national doctoral scholarship from the “Agencia Nacional de Investigación y Desarrollo ANID”, Chile (grant no. 21220332), a PhD Program in Health Sciences and Engineering, Universidad de Valparaíso, Chile, and Andrés Bello National University (Viña del Mar, Chile), Los Andes University (Santiago, Chile) and the Carlos Van Buren Hospital (Valparaiso, Chile) basic operational funds.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
CC, PO, and JGM conceived and designed the study. CL, PG and IM collected, prepared, and anonymized the histological samples. CC and JGM designed and executed the automated image analysis. CC, RO, and RM implemented and tested the explainable machine learning model. CC, WGA and PO analyzed the data and performed the statistical tests. CC, WGA, PO, PG, and IM wrote the manuscript. CC, JGM, CL, IM and PO confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The use of digital image samples and anonymized data was approved by the Carlos van Buren Hospital directive and by the V Region, Valparaíso-San Antonio, Scientific Ethics Committee SSVSA (approval no.: 1765-07.10.2021).
Patient consent for publication
The need for informed consent was waived with authorization from V Region, Valparaíso-San Antonio, Scientific Ethics Committee SSVSA (approval no.: 1765-07.10.2021).
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, editors. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Waks AG, Winer EP. Breast cancer treatment: A review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 3.Banegas MP, Püschel K, Martínez-Gutiérrez J, Anderson JC, Thompson B. Perceived and objective breast cancer risk assessment in Chilean women living in an underserved area. Cancer Epidemiol Biomarkers Prev. 2012;21:1716–1721. doi: 10.1158/1055-9965.EPI-12-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Icaza G, Núñez L, Bugueño H. Epidemiological analysis of breast cancer mortality in women in Chile. Rev Med Chil. 2017;145:106–114. doi: 10.4067/S0034-98872017000100014. (in Spanish) [DOI] [PubMed] [Google Scholar]
- 5.Thomssen C, Balic M, Harbeck N, Gnant M. St. Gallen/Vienna 2021: A brief summary of the consensus discussion on customizing therapies for women with early breast cancer. Breast Care (Basel) 2021;16:135–143. doi: 10.1159/000516114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balic M, Thomssen C, Würstlein R, Gnant M, Harbeck N. St. Gallen/Vienna 2019: A brief summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care (Basel) 2019;14:103–110. doi: 10.1159/000499931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li C, Bian X, Liu Z, Wang X, Song X, Zhao W, Liu Y, Yu Z. Effectiveness and safety of pyrotinib-based therapy in patients with HER2-positive metastatic breast cancer: A real-world retrospective study. Cancer Med. 2021;10:8352–8364. doi: 10.1002/cam4.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang B, Ding W, Sun K, Wang X, Xu L, Teng X. Impact of the 2018 ASCO/CAP guidelines on HER2 fluorescence in situ hybridization interpretation in invasive breast cancers with immunohistochemically equivocal results. Sci Rep. 2019;9:16726. doi: 10.1038/s41598-019-53003-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masuda N, Lee SJ, Ohtani S, Im YH, Lee ES, Yokota I, Kuroi K, Im SA, Park BW, Kim SB, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 10.Slomski A. Adjuvant therapy for HER2-positive breast cancer. JAMA. 2019;322:1134. doi: 10.1001/jama.2019.14754. [DOI] [PubMed] [Google Scholar]
- 11.Gown AM. Current issues in ER and HER2 testing by IHC in breast cancer. Mod Pathol. 2008;21((Suppl 2)):S8–S15. doi: 10.1038/modpathol.2008.34. [DOI] [PubMed] [Google Scholar]
- 12.Press MF, Seoane JA, Curtis C, Quinaux E, Guzman R, Sauter G, Eiermann W, Mackey JR, Robert N, Pienkowski T, et al. Assessment of ERBB2/HER2 status in HER2-equivocal breast cancers by FISH and 2013/2014 ASCO-CAP guidelines. JAMA Oncol. 2019;5:366–375. doi: 10.1001/jamaoncol.2018.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Neumeister V, McGuire J, Song YS, Acs B, Ho K, Weidler J, Wong W, Rhees B, Bates M, et al. Quantitative assessments and clinical outcomes in HER2 equivocal 2018 ASCO/CAP ISH group 4 breast cancer. NPJ Breast Cancer. 2019;5:28. doi: 10.1038/s41523-019-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díaz-Serrano A, Angulo B, Dominguez C, Pazo-Cid R, Salud A, Jiménez-Fonseca P, Leon A, Galan MC, Alsina M, Rivera F, et al. Genomic profiling of HER2-positive gastric cancer: PI3K/Akt/mTOR pathway as predictor of outcomes in HER2-positive advanced gastric cancer treated with trastuzumab. Oncologist. 2018;23:1092–1102. doi: 10.1634/theoncologist.2017-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jensen K, Krusenstjerna-Hafstrøm R, Lohse J, Petersen KH, Derand H. A novel quantitative immunohistochemistry method for precise protein measurements directly in formalin-fixed, paraffin-embedded specimens: Analytical performance measuring HER2. Mod Pathol. 2017;30:180–193. doi: 10.1038/modpathol.2016.176. [DOI] [PubMed] [Google Scholar]
- 16.Goddard KAB, Weinmann S, Richert-Boe K, Chen C, Bulkley J, Wax C. HER2 evaluation and its impact on breast cancer treatment decisions. Public Health Genomics. 2011;15:1–10. doi: 10.1159/000325746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of American pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241–256. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu R, Ma X, Bian Z, Ma J. Digital separation of diaminobenzidine-stained tissues via an automatic color-filtering for immunohistochemical quantification. Biomed Opt Express. 2015;6:544–558. doi: 10.1364/BOE.6.000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morelli P, Porazzi E, Ruspini M, Banfi G. Analysis of errors in histology by root cause analysis: A pilot study. J Prev Med Hyg. 2013;54:90–96. [PMC free article] [PubMed] [Google Scholar]
- 20.Qaiser T, Mukherjee A, Reddy Pb C, Munugoti SD, Tallam V, Pitkäaho T, Lehtimäki T, Naughton T, Berseth M, Pedraza A, et al. HER2 challenge contest: A detailed assessment of automated HER2 scoring algorithms in whole slide images of breast cancer tissues. Histopathology. 2018;72:227–238. doi: 10.1111/his.13333. [DOI] [PubMed] [Google Scholar]
- 21.Varghese F, Bukhari AB, Malhotra R, De A. IHC profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS One. 2014;9:e96801. doi: 10.1371/journal.pone.0096801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCabe A, Dolled-Filhart M, Camp RL, Rimm DL. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. J Natl Cancer Inst. 2005;97:1808–1815. doi: 10.1093/jnci/dji427. [DOI] [PubMed] [Google Scholar]
- 23.Larson JS, Goodman LJ, Tan Y, Defazio-Eli L, Paquet AC, Cook JW, Rivera A, Frankson K, Bose J, Chen L, et al. Analytical validation of a highly quantitative, sensitive, accurate, and reproducible assay (HERmark) for the measurement of HER2 total protein and HER2 homodimers in FFPE breast cancer tumor specimens. Patholog Res Int. 2010;2010:814176. doi: 10.4061/2010/814176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zanconati F, Cusumano P, Tinterri C, Di Napoli A, Lutke Holzik MF, Poulet B, Dekker L, Sapino A. P205 The 70-gene expression profile, Mammaprint, for breast cancer patients in mainly European hospitals. Breast. 2011;20:S45. doi: 10.1016/S0960-9776(11)70146-1. [DOI] [Google Scholar]
- 25.Cronin M, Sangli C, Liu ML, Pho M, Dutta D, Nguyen A, Jeong J, Wu J, Langone KC, Watson D. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem. 2007;53:1084–1091. doi: 10.1373/clinchem.2006.076497. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen TO, Parker JS, Leung S, Voduc D, Ebbert M, Vickery T, Davies SR, Snider J, Stijleman IJ, Reed J, et al. A comparison of PAM50 intrinsic subtyping with immunohistochemistry and clinical prognostic factors in tamoxifen-treated estrogen receptor-positive breast cancer. Clin Cancer Res. 2010;16:5222–5232. doi: 10.1158/1078-0432.CCR-10-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Economic Comission for Latin America and the Caribbean, corp-author. United Nations Publication; 2021. Plan for self-sufficiency in health matters in Latin America and the Caribbean: Lines of action and proposals (LC/TS.2021/115) [cited 2022 Jun 7]. Available from: [Google Scholar]
- 28.Hey T, Butler K, Jackson S, Thiyagalingam J. Machine learning and big scientific data. Philos Trans A Math Phys Eng Sci. 2020;378:20190054. doi: 10.1098/rsta.2019.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larmuseau M, Sluydts M, Theuwissen K, Duprezd L, Dhaenec T, Cottenier S. Race against the machine: Can deep learning recognize microstructures as well as the trained human eye? Scr Mater. 2021;193:33–37. doi: 10.1016/j.scriptamat.2020.10.026. [DOI] [Google Scholar]
- 30.Shah P, Kendall F, Khozin S, Goosen R, Hu J, Laramie J, Ringel M, Schork N. Artificial intelligence and machine learning in clinical development: A translational perspective. NPJ Digit Med. 2019;2:69. doi: 10.1038/s41746-019-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong J, Dong J. A 19-miRNA support vector machine classifier and a 6-miRNA risk score system designed for ovarian cancer patients. Oncol Rep. 2019;41:3233–3243. doi: 10.3892/or.2019.7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esteva A, Kuprel B, Novoa RA, Ko J, Swetter SM, Blau HM, Thrun S. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115–118. doi: 10.1038/nature21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elsharawy KA, Gerds TA, Rakha EA, Dalton LW. Artificial intelligence grading of breast cancer: A promising method to refine prognostic classification for management precision. Histopathology. 2021;79:187–199. doi: 10.1111/his.14354. [DOI] [PubMed] [Google Scholar]
- 34.Trivizakis E, Ioannidis GS, Melissianos VD, Papadakis GZ, Tsatsakis A, Spandidos DA, Marias K. A novel deep learning architecture outperforming ‘off-the-shelf’ transfer learning and feature-based methods in the automated assessment of mammographic breast density. Oncol Rep. 2019;42:2009–2015. doi: 10.3892/or.2019.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rajkomar A, Oren E, Chen K, Dai AM, Hajaj N, Hardt M, Liu PJ, Liu X, Marcus J, Sun M, et al. Scalable and accurate deep learning with electronic health records. NPJ Digit Med. 2018;1:18. doi: 10.1038/s41746-018-0029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilbur DC, Smith ML, Cornell LD, Andryushkin A, Pettus JR. Automated identification of glomeruli and synchronised review of special stains in renal biopsies by machine learning and slide registration: A cross-institutional study. Histopathology. 2021;79:499–508. doi: 10.1111/his.14376. [DOI] [PubMed] [Google Scholar]
- 37.Burrell J. How the machine ‘thinks’: Understanding opacity in machine learning algorithms. Big Data Soc. 2016;3:1–12. doi: 10.1177/2053951715622512. [DOI] [Google Scholar]
- 38.Rashidi HH, Tran NK, Betts EV, Howell LP, Green R. Artificial intelligence and machine learning in pathology: The present landscape of supervised methods. Acad Pathol. 2019;6:2374289519873088. doi: 10.1177/2374289519873088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmad A, Quegan S. Analysis of maximum likelihood classification on multispectral data. Appl Math Sci. 2012;6:6425–6436. [Google Scholar]
- 40.Adadi A, Berrada M. Peeking inside the black-box: A survey on explainable artificial intelligence (XAI) IEEE Access. 2018;6:52138–52160. doi: 10.1109/ACCESS.2018.2870052. [DOI] [Google Scholar]
- 41.Arrieta AB, Díaz-Rodríguez N, Del Ser J, Bennetot A, Tabik S, Barbado A, Garciag S, Gil-Lopez S, Molina D, Benjamins R, et al. Explainable artificial intelligence (XAI): Concepts, taxonomies, opportunities and challenges toward responsible AI. Inf Fusion. 2020;58:82–115. doi: 10.1016/j.inffus.2019.12.012. [DOI] [Google Scholar]
- 42.Tosun AB, Pullara F, Becich MJ, Taylor DL, Fine JL, Chennubhotla SC. Explainable AI (xAI) for anatomic pathology. Adv Anat Pathol. 2020;27:241–250. doi: 10.1097/PAP.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 43.Goodman B, Flaxman S. European union regulations on algorithmic decision-making and a ‘right to explanation’. AI Mag. 2017;38:50–57. [Google Scholar]
- 44.Huang G, Huang GB, Song S, You K. Trends in extreme learning machines: A review. Neural Netw. 2015;61:32–48. doi: 10.1016/j.neunet.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Zinchuk V, Zinchuk O. Quantitative colocalization analysis of confocal fluorescence microscopy images. Curr Protoc Cell Biol. 2008 doi: 10.1002/0471143030.cb0419s39. Chapter 4: Unit 4.19. [DOI] [PubMed] [Google Scholar]
- 46.Fereidouni F, Bader AN, Gerritsen HC. Spectral phasor analysis allows rapid and reliable unmixing of fluorescence microscopy spectral images. Opt Express. 2012;20:12729–12741. doi: 10.1364/OE.20.012729. [DOI] [PubMed] [Google Scholar]
- 47.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. molecular Biology of the cell. 4th edition. New York: Garland Science; 2002. Looking at the structure of cells in the microscope. [Google Scholar]
- 48.Moulisová V, Jiřík M, Schindler C, Červenková L, Pálek R, Rosendorf J, Arlt J, Bolek L, Šůsová S, Nietzsche S, et al. Novel morphological multi-scale evaluation system for quality assessment of decellularized liver scaffolds. J Tissue Eng. 2020;11:2041731420921121. doi: 10.1177/2041731420921121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aguilera A, Pezoa R, Rodríguez-Delherbe A. A novel ensemble feature selection method for pixel-level segmentation of HER2 overexpression. Complex Intell Syst. 2022;8:5489–5510. doi: 10.1007/s40747-022-00774-x. [DOI] [Google Scholar]
- 50.Taylor CR, Rudbeck L, editors. Sixth Edition. Agilent Technologies; Santa Clara, CA: 2021. Immunohistochemical staining methods; pp. 22–76. [Google Scholar]
- 51.Dabbs DJ, editor. Sixth Edition. Elsevier; Amsterdam: 2021. Diagnostic immunohistochemistry: Theranostic and genomic applications; pp. 15–54. [Google Scholar]
- 52.Lin F, Prichard J, editors. Second Edition. Springer; New York, NY: 2015. Handbook of Practical Immunohistochemistry; pp. 220–224. [Google Scholar]
- 53.Untch M, Harbeck N, Huober J, von Minckwitz G, Gerber B, Kreipe HH, Liedtke C, Marschner N, Möbus V, Scheithauer H, et al. Primary therapy of patients with early breast cancer: Evidence, controversies, consensus. Geburtshilfe Frauenheilkd. 2015;75:556–565. doi: 10.1055/s-0035-1546120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Breast cancer. Nat Rev Dis Primers. 2019;5:66. doi: 10.1038/s41572-019-0111-2. [DOI] [PubMed] [Google Scholar]
- 55.Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, Fulton LL, Dooling DJ, Ding L, Mardis ER, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hariri N, Roma AA, Hasteh F, Walavalkar V, Fadare O. Phenotypic alterations in breast cancer associated with neoadjuvant chemotherapy: A comparison with baseline rates of change. Ann Diagn Pathol. 2017;31:14–19. doi: 10.1016/j.anndiagpath.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Brasó-Maristany F, Griguolo G, Pascual T, Paré L, Nuciforo P, Llombart-Cussac A, Bermejo B, Oliveira M, Morales S, Martínez N, et al. Phenotypic changes of HER2-positive breast cancer during and after dual HER2 blockade. Nat Commun. 2020;11:385. doi: 10.1038/s41467-019-14111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Powers DMW. Evaluation: From precision, recall and F-measure to ROC, informedness, markedness and correlation. arXiv: 2020;2010:16061. [Google Scholar]
- 59.Sharma D, Kumar S, Narasimhan B. Estrogen alpha receptor antagonists for the treatment of breast cancer: A review. Chem Cent J. 2018;12:107. doi: 10.1186/s13065-018-0472-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Favretto D, Cosmi E, Ragazzi E, Visentin S, Tucci M, Fais P, Cecchetto G, Zanardo V, Viel G, Ferrara SD. Cord blood metabolomic profiling in intrauterine growth restriction. Anal Bioanal Chem. 2012;402:1109–1121. doi: 10.1007/s00216-011-5540-z. [DOI] [PubMed] [Google Scholar]
- 61.Lokhov PG, Dashtiev MI, Moshkovskii SA, Archakov AI. Metabolite profiling of blood plasma of patients with prostate cancer. Metabolomics. 2010;6:156–163. doi: 10.1007/s11306-009-0187-x. [DOI] [Google Scholar]
- 62.Ellin J, Haskvitz A, Premraj P, Shields K, Smith M, Stratman C, Wrenn M. Madison: Digital Pathology Association; 2011. Interoperability between anatomic pathology laboratory information systems and digital pathology systems; pp. 1–10. [Google Scholar]
- 63.Pathology and Tissue Imaging | MetaSystems [Internet] https://metasystems-international.com/us/applications/patho/ [cited 2020 Aug 10]. Available from. [Google Scholar]
- 64.Patología digital, corp-author. Leica Biosystems [Internet] https://www.leicabiosystems.com/es/patologia-digital/ [cited 2020 Aug 10]. Available from: [Google Scholar]
- 65.Dunbier AK, Anderson H, Ghazoui Z, Salter J, Parker JS, Perou CM, Smith IE, Dowsett M. Association between breast cancer subtypes and response to neoadjuvant anastrozole. Steroids. 2011;76:736–740. doi: 10.1016/j.steroids.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 66.Becker S. A historic and scientific review of breast cancer: The next global healthcare challenge. Int J Gynecol Obstet. 2015;131((Suppl 1)):S36–S39. doi: 10.1016/j.ijgo.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 67.Planes-Laine G, Rochigneux P, Bertucci F, Chrétien AS, Viens P, Sabatier R, Gonçalves A. PD-1/PD-l1 targeting in breast cancer: The first clinical evidences are emerging. A literature review. Cancers (Basel) 2019;11:1033. doi: 10.3390/cancers11071033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lozano C, Córdova C, Marchant I, Zúñiga R, Ochova P, Ramírez-Barrantes R, González-Arriagada WA, Rodriguez B, Olivero P. Intracellular aggregated TRPV1 is associated with lower survival in breast cancer patients. Breast Cancer (Dove Med Press) 2018;10:161–168. doi: 10.2147/BCTT.S170208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Campbell KJ, Dhayade S, Ferrari N, Sims AH, Johnson E, Mason SM, Dickson A, Ryan KM, Kalna G, Edwards J, et al. MCL-1 is a prognostic indicator and drug target in breast cancer. Cell Death Dis. 2018;9:19. doi: 10.1038/s41419-017-0035-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Zheng A, Lu H, Jin Z, Peng Z, Jin F. The expression and prognostic significance of claudin-8 and androgen receptor in breast cancer. Onco Targets Ther. 2020;13:3437–3448. doi: 10.2147/OTT.S242406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, Chien AJ, Forero-Torres A, Ellis E, Han H, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: An analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6:676–684. doi: 10.1001/jamaoncol.2019.6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.