Abstract
Microplastics (MPs) have become increasingly serious global problems due to their wide distribution and complicated impacts on living organisms. To obtain a comprehensive overview of the latest research progress on MPs, we conducted a bibliometric analysis combined with a literature review. The results showed that the number of studies on MPs has grown exponentially since 2010. Recently, the hotspot on MPs has shifted to terrestrial ecosystems and biological health risks, including human health risks. In addition, the toxic effects, identification and quantification of MPs are relatively new research hotspots. We subsequently provide a review of MPs studies related to health risks to terrestrial higher mammals and, in particular, to humans, including detection methods and potential toxicities based on current studies. Currently, MPs have been found existing in human feces, blood, colon, placenta and lung, but it is still unclear whether this is associated with related systemic diseases. In vivo and in vitro studies have demonstrated that MPs cause intestinal toxicity, metabolic disruption, reproductive toxicity, neurotoxicity, immunotoxicity through oxidative stress, apoptosis and specific pathways, etc. Notably, in terms of combined effects with pollutants and neurotoxicity, the effects of MPs are still controversial. Future attention should be paid to the detection and quantification of MPs in human tissues, exploring the combined effects and related mechanisms of MPs with other pollutants and clarifying the association between MPs and the development of pre-existing diseases. Our work enhances further understanding of the potential health risks of MPs to terrestrial higher mammals.
Graphical abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s10653-022-01458-8.
Keywords: Microplastics, Bibliometrics, Research trend, Terrestrial higher mammals, Detection methods, Toxicity
Introduction
Since the mass production and use of microplastics (MPs) in the 1950s, they have been widely used in packaging, construction building, electric power, industrial machinery and other industries (Geyer et al., 2017). Global plastic production is growing exponentially due to low manufacturing costs and a wide range of applications. In 2017, the cumulative production all worldwide of plastics was approximately 8.3 billion tons and is expected to increase to 34 billion tons in 2050 (Petersen & Hubbart, 2021). Plastic waste entering the environment can fragment into MPs after weathering, photo-oxidation, crushing and biological decomposition (van Wezel et al., 2016). Alternatively, MPs are manufactured specially in a small size range for various commercial uses (Banerjee & Shelver, 2021). In general, MPs are plastic fibers, particles and films with particle size < 5 mm, including nanoplastics (NPs) with diameter < 0.1 µm (Banerjee & Shelver, 2021; Hartmann et al., 2019). The chemical composition of MPs mainly includes polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polyamide (PA), polyester polyethylene terephthalate (PET) and polystyrene (PS) (Alimi et al., 2018; Browne et al., 2011). MPs mostly originate from plastic beads used for exfoliation or cleaning in cosmetics and personal care products, breakage of plastic pellets in factories and during transportation, tire wear and loss of fibers during textile washing (He et al., 2019; Khalid et al., 2021). In addition, studies have proven that landfills are also an important source of MPs, which have also been identified in the leachates from both active and closed landfills (He et al., 2019). MPs can be transferred over long distances through rivers and wind due to their chemical stability, small particle size and light density, and are widely detected in air, aquatic and terrestrial ecosystems (Amato-Lourenco et al., 2020; Auta et al., 2017; Horton et al., 2017; Shan et al., 2018).
At present, MPs pollution is an emerging environmental problem that not only causes environmental pollution but also poses a great threat to living organisms. MPs provide a potential transfer pathway for contaminants, plastic additives, and microorganisms to enter living organisms, thereby causing health threats to organisms. MPs in the environment can be ingested by organisms through the food chain, thereby causing toxic effects, such as intestinal damage, neurotoxicity, immunotoxicity, reproductive toxicity and cardiotoxicity (da Costa Araujo & Malafaia, 2021; Deng et al., 2017; Hou et al., 2021a, b; Lim et al., 2021; Wei et al., 2021; Zheng et al., 2021). MPs can be enriched in organisms and produce biological amplification. MPs have been detected in food products, such as seafood, salt, honey, sugar, tap water, bottled water, beer, etc. (Banerjee & Shelver, 2021). As the top of the food chain, humans are inevitably exposed to health threats from MPs. Notably, MPs have been detected in human feces, colonic tissue, lung and placenta, which indicates a potential impact of MPs on human health (Ibrahim et al., 2021; Ragusa et al., 2021; Schwabl et al., 2019; Yan et al., 2020b). Currently, MPs are gradually becoming a research hotspot and have been extensively studied by researchers. However, current studies mostly focus on the biogenic risks of MPs to aquatic organisms, and there is an urgent need to provide a review of studies related to health risks to terrestrial higher mammals and, in particular, to humans.
Bibliometrics are interdisciplinary discipline that use mathematical and statistical methods to quantitatively analyze all knowledge carriers and can effectively analyze trends and patterns in academic literature (Zou et al., 2018). In this study, we use bibliometric methods to analyze the literature related to MPs from 2010 to 2021 to obtain more insight into the research progress and trends of MPs. More importantly, we further summarize the techniques for the characterization of MPs in human tissues and terrestrial higher mammalian tissues, and in vivo and in vitro MPs toxicology studies performed using mice/rats and human-derived cells. Our study helps people understand the current research progress on the health hazards of MPs and toxicological mechanisms, and provides a scientific reference for further exploring the potential health risks of MPs on terrestrial higher mammals, even on humankind.
Methodology
Data source and search criteria
The papers in this study were retrieved from Web of Science Core Collection (Classic) on September 13, 2021. All searches were completed within the same day to avoid bias caused by database updates. Several associated terms were selected to retrieve as many papers related to MPs research. The following string was used as a subject line to obtain literature related to MPs research: microplastic* or Plastic Microparticles* or Microparticle, Plastic* or Microparticles, Plastic* or Plastic Microparticle*. An Asterisk was added behind the word to expand the search. The publication year was set as “2010–2021”. Then the initial selection of the publications obtained from the search was conducted. Articles occupied the bulk of the searched papers, which can well reflect the research progress and trend of MPs, so we chose articles and excluded review, proceedings paper, editorial material, meeting abstract and other types of paper. In addition, English was chosen as the language of reported literature. The flow chart of paper selection and research framework is shown in Fig. 1 (Zhang et al., 2020b).
Fig. 1.
Research framework of document selection and analysis
Analysis method
Data on annual publications, source journals, authors, co-cited authors, co-cited references, countries, institutions, research fields, and keywords of MPs-related papers from 2010 to 2021 were obtained during the search. Microsoft Excel 2016 (Microsoft Corporation, Santa Rosa, California, USA) was conducted to analyze the data and draw related figures. VOSviewer (version 1.6.6, van Eck and Waltman, Leiden, Netherlands) software was used to visualize the data to form social network maps, in which the nodes represent the number or frequency, and the lines between the nodes indicate associations (Gao et al., 2019). CiteSpace software (version 5.8.R2) was used to obtain research frontiers. In the visual graphs obtained by VOSviewer, “co-occurring keywords” are an important way to reveal the hotspots of a certain research field. To better understand the research on MPs, additional analysis of MPs research hotspots was conducted.
Characteristics, sources, research hotspots and trends of the papers
Temporal trend of publications
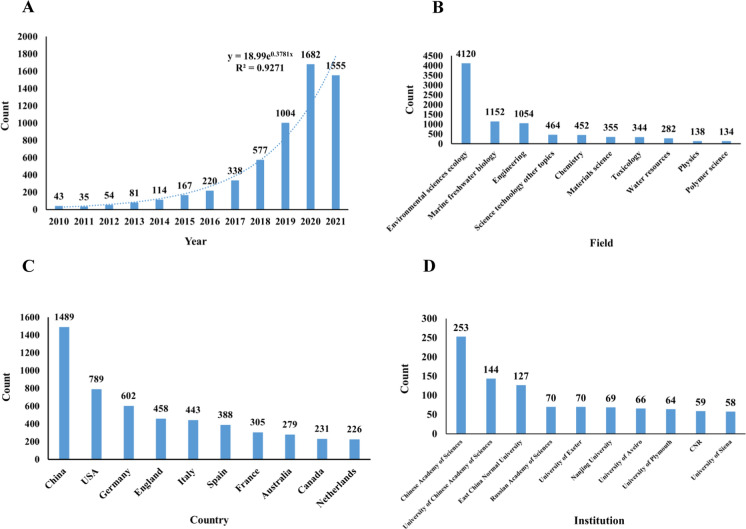
Search results on Web of Science showed that the first article related to MPs were published in the 1960s. There were few publications on MPs since then. We noted that the number of articles on MPs research in 2019 has reached more than 1000. Therefore, we selected publications from 2010 to 2021 to analyze the research progress of MPs in the last decade. Among them, the year 2021 includes articles from January 1 to September 13. According to the above method, a total of 5869 papers on MPs were included in this study (Fig. 1). Figure 2A shows the annual publications regarding the topic of MPs from 2010 to 2021. The number of annual publications has gradually increased except for 2011, when the number of publications decreased slightly compared to 2010. In general, the number of publications has been on the rise over time during the last decade. Then a polynomial fitting curve was made to describe the relationship between publication year and the number of publications, as shown in Fig. 2A. However, the model did not show a good correlation between the publication year and the number of publications, which may be related to the search time. In this study, only publications from January 1 to September 13 were included in 2021. It is difficult to estimate the number of publications in the future precisely according to the curve fitting, but it can be expected to increase.
Fig. 2.
A The temporal trend of publication on MPs research. B Distribution of the top 10 popular fields of MPs research. C Distribution of the top 10 productive countries on MPs research. D Distribution of the top 10 productive institutions on MPs research
Most productive authors, co-cited authors and co-cited references
We found a total of 17,908 authors working on MPs, while 12,810 authors published only 1 article on MPs, accounting for 71.53%. This shows that most of the authors did not continue their research in the field of MPs. Supplement Table 2 lists the top 10 most productive authors. The top ten productive authors published 388 articles, accounting for 6.61% of the total number of articles recorded. Of these 10 authors, Shi, HH from China had the highest number of publications, followed by Koelmans, AA from Netherlands and Thompson, RC from England. The h-index is a more accurate reflection of a researcher’s academic achievements. Shi, HH from China had the highest number of publications (65), with an h-index of 33, ranking first. Koelmans, AA from Netherlands and Thompson, RC from England ranked second and third, respectively, in terms of the number of articles published, and both had a higher h-index of 32, ranking second. This indicates that they paid attention to the quantity of papers as well as the quality. Moreover, the findings remind other prolific authors that more attention should be paid to the quality of research.
Table 1 lists the top 10 co-cited authors, which are ranked by citations. Cole, MA had the highest citations (3045), followed by Browne, MA with 2853 citations and Andrady, AL with 2391 citations. A co-citation relationship is formed when two authors are cited by another (or more) paper at the same time (Gao et al., 2019). Two prolific authors, Thompson, RC and Rochman, CM, were also included in the top 10 co-cited authors. Browne, MA had a relatively higher citations of 2853, ranking second, while he published only 6 articles on MPs. This indicates that his articles were highly influential, and attracted the attention of MPs researchers. Thompson, RC can be considered as the pioneer in this field. He first proposed the term “microplastics” in an article published in Science in 2004. He ranked second in the number of publications on MPs and fifth in citations. In addition, he has collaborated with a number of top 10 co-cited authors, including Rochman, CM from Canada, Browne, MA from Australia, Cole, MA from England, Wright, SL from England, Galloway TS from England and Lusher, AL from Norway. It is also worth noting that among the top 10 co-cited authors, there were no authors from developing countries, suggesting that authors from developing countries should seek more collaborations with international authors to develop their research in this area.
Table 1.
The top 10 co-cited authors and co-cited references on MPs research
| Rank | Author | Country | Citations | Reference | Citations |
|---|---|---|---|---|---|
| 1 | Cole, MA | England | 3045 | Andrady AL, 2011. Microplastics in the marine environment. Marine Pollution Bulletin, V62, P1596 | 1576 |
| 2 | Browne, MA | Australia | 2853 | Jambeck JR, 2015. Plastic waste inputs from land into the ocean. Science, V347, P768 | 1315 |
| 3 | Andrady, AL | USA | 2391 | Cole, M., 2011. Microplastics as contaminants in the marine environment: A review. Marine Pollution Bulletin, V62, P2588 | 1294 |
| 4 | Rochman, CM | Canada | 2347 | Thompson RC, 2004. Lost at sea: Where is all the plastic? Science, V304, P838 | 1171 |
| 5 | Thompson, RC | England | 1812 | Hidalgo-Ruz, V, Microplastics in the marine Environment: a review of the methods used for identification and quantification. Environment Science & Technology, V46, P3060 | 1156 |
| 6 | Wright, SL | England | 1802 | Browne MA, 2011. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environment Science and Technology, V45, P9175 | 1116 |
| 7 | Lusher, AL | Norway | 1772 | Barnes D.K.A., 2009. Accumulation and fragmentation of plastic debris in global environments. Philosophical Transactions of the Royal Society B-Biological Sciences, V364, P1985 | 1111 |
| 8 | Eriksen, M | USA | 1591 | Wright SL, 2013. The physical impacts of microplastics on marine organisms: A review. Environmental Pollution, V178, P483 | 1064 |
| 9 | Vancauwenberghe, L | Belgium | 1468 | Eriksen M, 2014. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. Plos One, V9 | 868 |
| 10 | Barnes, David K. A | England | 1361 | Geyer, R, 2017. Production, use, and fate of all plastics ever made. Science Advances, V3 | 699 |
The most cited literature reflects the problems that researchers are generally concerned about, and provides a foundation for future research. Therefore, we listed the top 10 co-cited references (Table 1). Co-citation reflects the concern of researchers. In addition, the high number of citations of a paper indicates that it has produced a profound impact on the development and trend in a specific field and provides a basis for future research (Eugene Garfield, 1987). The most cited article, “Microplastics in the marine environment”, was published in the Marine Pollution Bulletin in 2011. It reviews the generation mechanisms and potential impacts of MPs in the marine environment. In addition, MPs might concentrate persistent organic pollutants (POPs), which produce toxic effects on organisms, and toxins might be delivered across nutrient levels. This study provides a basis for future MPs research (Andrady, 2011). Similarly, the article “Microplastics as contaminants in the marine environment: A review” summarized the properties and sources of MPs and how MPs enter the marine environment. It also evaluated detection methods and spatial and temporal trends of MPs in marine environment (Cole et al., 2011). The paper “lost at Sea: Where Is All the Plastic?” written by Thompson RC, published in Science in 2004, first proposed the term “microplastics”. It identified the composition of the less dense particles of sediments in beaches, estuaries and subtidal around Plymouth, England and checked 17 beaches to initially assess the degree of MPs pollution (Thompson et al., 2004). These three articles provided the basis for subsequent research on MPs.
The top 10 most cited references were mainly published between 2009 and 2015. This indicates that this time period might be identified as the prime time for MPs research. In terms of journals, the two most cited papers are from the journal Marine Pollution Bulletin (IF = 5.553). In addition, 2 papers were published in Environment Science & Technology and 2 in Science. There is no necessary connection between co-cited references and the impact factors of the journals they published in. Generally, literature reviews are more easily cited than other publications.
Sources of papers
Figure 2B illustrates the top 10 fields of MPs research. The field of Environmental Sciences Ecology was the dominant category, accounting for 70.20% of the total articles. The following main fields included Marine Freshwater Biology (1152, 19.63%), Engineering (1054, 17.96%). This indicates that research on MPs mainly focuses on Environmental Sciences Ecology, Marine Freshwater Biology and Engineering. Toxicology, Water Resources, Physics and Polymer Science are relatively cold research areas, which may become research hotspots in the future.
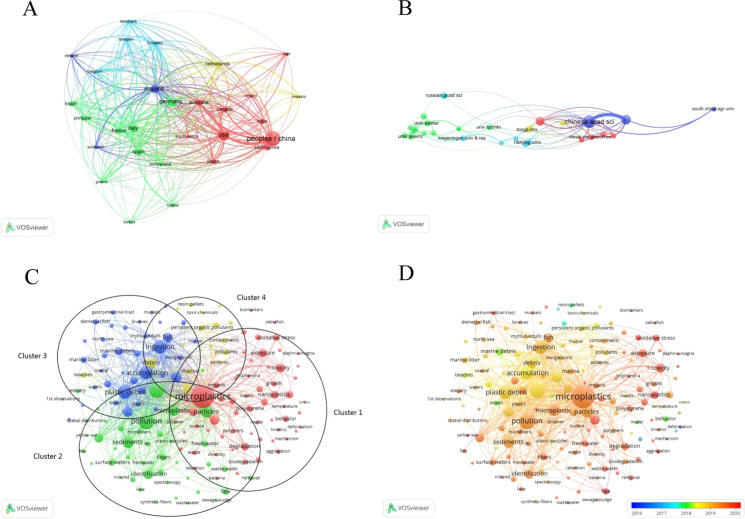
The top 10 most productive countries are listed in Fig. 2C. It shows that China is the most prominent country, with 1489 articles (25.37%) related to MPs research, followed by the USA (789, 13.44%), Germany (602, 10.26%), England (458, 7.80%) and Italy (443, 7.55%). In addition, we present the top 10 countries in Supplement Table 3, based on the total publications, citations, average citations and h-index. China had the highest number of publications (1544, 26.31%), contributing more than 1/5 of the total published papers, showing its huge role in this field. However, there were few highly productive authors in China. Among the top 10 active authors, there were only 2 Chinese authors. There were no Chinese authors among the top ten co-cited authors. Overall, China still needs to work hard in terms of the level and quality of papers. In addition, China was the only developing country among the top 10 most productive countries. This shows the lack of research on MPs in developing countries. The reason may be that developing countries are lagging behind developed countries and do not pay enough attention to MPs research. With the development of the economy, they may pay more attention to research in this field. In short, further global research is needed. The network visualization map shows that extensive cooperation existed between China, the United States, the United Kingdom, Germany, Australia and Italy (Fig. 3A).
Fig. 3.
The network visualization map of A countries collaboration and B institutions collaboration on MPs research. The network C and overlay D visualization map of keywords occurrences on MPs research
Figure 2D illustrates the top 10 contributing institutions. The Chinese Academy of Sciences ranked first, with 253 publications (4.31%). It is followed by the University of Chinese Academy of Sciences (144, 2.45%) and East China Normal University (127, 2.16%). Network maps can provide information on influential countries/institutions and potential collaborations. Network visualization maps of countries/institutions collaboration on MPs research are listed in Fig. 3 to illustrate the cooperation between countries and institutions, which facilitates researchers in choosing institutions that are best suited for exchanges, learning, and promoting cooperation between institutions. The network visualization shows that three of the top five institutions with high citations maintained close cooperation, including University Plymouth, University Exeter, and IFREMER (French Research Institute for exploitation of the Sea). Some institutions in China, such as the Chinese Academy of Sciences, East China Normal University and TongJi University, have launched international cooperation with foreign organizations.
To better understand the publication status of MPs-related papers, the main journals and fields on this topic were analyzed. A total of 5869 publications were found in 779 journals. However, the results showed that 85.36% of the journals published fewer than 5 articles related to MPs, and 53.57% of the journals published only one paper on this topic, which indicates that only a few journals have focused on the research progress of MPs. The top 10 journals contributing to MPs are listed in Supplement Table 1. Among them, three journals, Marine Pollution Bulletin (14.01%), Science of the Total Environment (11.11%), and Environmental Pollution (10.21%), have made significant contributions to MPs research, all with publications over 500. The Marine Pollution Bulletin from England published the most articles, but it had a lower IF of 5.553, ranking seventh. Water Research had fewer publications on MPs research, ranking eighth, but it had a higher IF, ranking first at 11.236. In addition, eight of the top 10 journals had an IF of 5 or more, with the number of papers accounting for (3037/5869) 51.75% of the total. Moreover, all the top 10 journals have impact factors of 4 or more. This indicates a better research trend for MPs, which is favored by journals with high impact factors (defined as greater than 4.00). Four of the top ten journals are from England, accounting for 30.29% (1778 papers) of the total papers, with two of them ranking first and third respectively. This indicates that UK journals include more articles on MPs research and pay more attention to the research on MPs. This may be related to the fact that MPs were first discovered in the UK, which increased the attention of UK journals to the problem of MPs pollution (E.J. et al., 1972). However, Chinese journals are not included in the top 10 journals, which need to continue their efforts in this area.
Co-occurrence keywords and burst keywords
Keywords are an important part of the literature. Research hotspots and research trends in a certain field can be obtained by analyzing co-occurrence keywords. VOSviewer software was used to obtain the visualization map of keywords. In the network visualization map, the size of the node represents the frequency of keywords appearing. The larger the node is, the more frequently the keyword appears. As shown in Fig. 3C, the most frequent word is “microplastics”. There are some high-frequency keywords, including intake, marine-environment, pollution, plastic debris, ingestion, accumulation, particles, sediments, etc. The proximity of the nodes indicates the correlation between these research topics. The terms, marine environment, pollution, ingestion and accumulation, are closely related to MPs, which indicates that MPs pollution in the marine environment and the ingestion of MPs by organisms are topics that researchers are concerned about in the field of MPs research. In addition, the keywords were divided into 4 clusters, which are represented by different colors. Terms with higher frequency in cluster 1 included toxicity, additives, bisphenol-A, metals, oxidative stress, bioaccumulation, bioavailability, etc. This shows that cluster 1 focused on the combined toxic effects of MPs adsorbing harmful chemicals and the toxic effects of MPs on organisms. In Cluster 2, various freshwater environments were included, such as surface water, rivers, lakes and water treatment plants. The high co-occurrence of fresh-water, identification and quantification in Cluster 2 showed that the identification and quantification of MPs in aquatic environment was one of the most concerning issues in this field. Cluster 3 mainly concentrated on the accumulation of MPs in marine environment and the uptake of MPs by aquatic organisms. Cluster 4 concerned the transport of different MPs and the release of additives from MPs, as well as the adsorption of various harmful chemicals, which are considered persistent organic pollutants.
The overlay visualization map in Fig. 3D illustrates the time distribution of keywords. The keywords in blue appeared earlier, and the keywords in red appeared recently. There are some keywords in the early stage, including microparticles, deformation, mechanical-properties, mechanical properties, behavior, etc. Keywords related to refresh-water, toxicity, oxidative stress and adsorption appeared recently. As can be seen from the overlay visualization map, most of the keywords appeared in the last three years. In addition, cluster 3 and cluster 4 were relatively early and mature research hotspots. Words related to marine, accumulation, abundance, uptake, and mechanical properties appeared earlier. Cluster 1 and cluster 2 were relatively new research hotspots.
Keywords with the strongest citation bursts refer to words that appear frequently in a certain period of time. By analyzing them, one can understand the research frontiers and hotspots in a certain field. As shown in Fig. 4, the red line is the hot bar, which represents the time period when the keywords were found to have a burst (Shi et al., 2020). The focus in 2010 was to study the properties of MPs in the laboratory, such as mechanical properties and deformation behavior. From 2012 to 2013, the accumulation of MPs and their sorption and transfer of chemical pollutants became hotspots. MPs in the aquatic environment (Pacific Ocean, Mediterranean sea, great lake) and their impact on organisms (zooplankton) were hot topics from 2014 to 2016. The main concern from 2017 to 2018 was human consumption, a major pathway through which MPs pose a threat to human health. The hot topic of MPs research has shifted from the initial study of MPs properties to the impact of MPs on marine environmental and marine organisms. Referring to the overlay visualization map, the hotspot of MPs has shifted from the aquatic environment to human consumption, meaning that the focus of MPs research is shifting to terrestrial ecosystems and the potential deleterious effect on terrestrial lives, including health risks to humans.
Fig. 4.
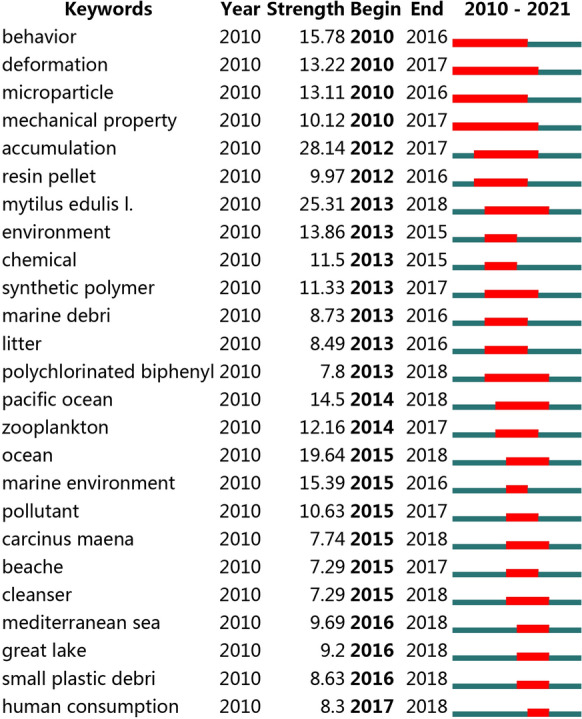
The keywords with the strongest citation bursts. (The red line represents the time period when the keywords were found to have a burst)
We noted that some of the results differed from those of Zhang et al. (Zhang et al., 2020b) who also previously conducted a bibliometrics to analyze characteristics of microplastics-related literature in Web of Science Core Collection (WoSCC) database based on the data from 1986 to September 21, 2019. The reason may be that there are differences in the search time and search terms. We searched the microplastics-related literature published 2010 to September 13, 2019, a period in which the number of publications increased dramatically, providing the updated data.
The present study shows that MPs have been extensively studied. In particular, MPs in the marine environment and their ecotoxicity are relatively early and mature research hotspots. In recent years, the research hotspot of MPs has shifted to terrestrial ecosystems, potential deleterious effect on terrestrial lives, identification and quantification. In addition, MPs have been detected in food, such as mussels, commercial fish, salt, sugar, and bottled water (Banerjee & Shelver, 2021). Human are inevitably exposed to MPs through food consumption.
Therefore, we further summarize the techniques for the characterization of MPs in human and terrestrial higher mammalian biological samples and toxic effects based on mice/rats and human-derived cells, to provide new evidences and insights for assessing the potential health risks of MPs to terrestrial higher mammals and even to humans.
Detection of microplastics in human and terrestrial higher mammalian biological samples
Studies have shown that environmental MPs can enter terrestrial higher mammals and even humans through air inhalation, water ingestion and consumption of food contaminated by MPs. However, the distribution and characteristics of MPs in terrestrial higher mammalian tissues are unclear. Here, we summarize the presence of MPs in human and terrestrial higher mammalian tissues and related detection methods.
Existence of microplastics in human and terrestrial higher mammalian biological samples
Compared with environmental samples, biological samples bring certain difficulties to the detection of MPs due to their uniqueness and complexity. To provide a technical reference for further studies exploring the distribution characteristics of MPs in human and terrestrial higher mammalian biological samples, we provide an overview and analysis of the current detection methods. As shown in Tables 2 and 3, the types of MPs detected in human and terrestrial higher mammalian biological samples, the methods used, and their advantages and limitations are summarized.
Table 2.
Summary of the detection of microplastics in human biological samples
| Human biological samples | Sample weight (volume) | MPs | Detection | Pre-processing | Identification | Limit | Advantage | Reference |
|---|---|---|---|---|---|---|---|---|
| Citrated blood | 2 mL | PA | Detection rate: 76% | (1) Digestion; (2) Filtered with Durapore® membrane; (3) Rinsed with warm nitric acid solution; (4) Ultrasound burst; (5) Filtered with alumina membrane and dried at 40 °C overnight | μFT-IR spectrometer combined with light microscope | (1) The detection limit of μFT-IR is approximately 20 μm; (2) Aluminum filter affects the identification | (1) The characteristics of plastics did not change significantly after pretreatment; (2) The combination of spectroscopy and microscope can simultaneously determine the type, size and location of plastics | Monteleone et al., (2019) |
| Human stool (n = 8) | 7 g | PE, PP, PVC, PS, PET, PA, PU, PC, POM | 20 pieces per 10 g stool (size: 50–500 µm) | (1) Shaked for 12 h; (2) Digested over 2 weeks; (3) Saponified fatty stool compounds; (4) Dissolved biological fibers;(5) Filtered with 50 µm metal sieve; (6) Filtered vacuously with inorganic filter membrane and dried at 50 °C | μFT-IR | (1) Chemical pretreatment may degrade MPs; (2) Remaining solids may mask plastic particles; (3) Particles smaller than 50 μm were not investigated | The pretreatment method has less damage to the plastic fragments | Schwabl et al., (2019) |
| Human feces (n = 10) | Wet weight: 1–5 g | PBT and PVB | Occurrence of MPs: 40% | (1) Vacuum freeze-drying; (2) Digested with Fenton reagent; (3) Vacuum filtration;(4) Digested with 65% nitric acid; (5) Filtration; (6) Rinsed with absolute ethyl alcohol, followed by ultrasonic treatment; (7) Vacuum filtration | Raman spectra | The particle size detection range is > 1 μm | (1) Higher recovery rate, less intermediate steps and less damage; (2) High digestion efficiency total recovery rate;(3) No significant change in the characteristics of MPs after digestion | Yan et al. (2020b) |
| Human feces (n = 24) | Wet weight: 3 g ± 0.1 g | PP, PET, PS, PE, PA, PC, PVC, PU | 1–36 particles/g (size: 20–800 μm) | (1) Digested with H2O2 for 20 days; (2) Freeze-drying; (3) Remove particles larger than 5 mm; (4) Filtered through a polycarbonate microporous membrane with negative pressure | μFT-IR microspectroscopy | (1) μFT-IR spectroscopy has limitations in size; (2) Misjudgment risk to differentiate PVC and PVDC | MPs in human feces have been determined quantitatively and qualitatively through μFT-IR | Zhang et al. (2021) |
| Human colectomy specimens (n = 11) | 13.4 ± 4.5 g | PC, PA, PP | 28.1 ± 15.4 particles/g colon tissue | (1) Chemically digested with potassium hydroxide at 60℃ for 7–10 h; (2) Filtered using 0.45 μm cellulose membrane paper, and then dried at room temperature over 2 days | Stereo dissecting microscope; scanning electron microscope; μFT-IR microscope | (1) Size limitations of μFT-IR; (2) Only a small number of filaments and a limited number of polymer types have been studied | NP | Ibrahim et al. (2021) |
| Human placenta (n = 6) | 23.3 ± 5.7 g | Stained MPs: PP and paint/coating/dye | 12 MP fragments were detected | (1) Incubated with 10% KOH solution for 7 days at room temperature; (2) Filtered through 1.6 µm pore size filter membrane; (3) Dried at room temperature | Light microscope; Raman spectroscopy | (1) Size limitations of Raman spectroscopy; (2) 10% KOH solution may destroy the properties of microplastics | Adopted plastic-free solution throughout the experiment, including in the delivery room | Ragusa et al. (2021) |
| Human lung tissue (n = 20) | Mean weight: 3.28 g | PP (35.1%); PE (24.3%); cotton (16.2%); PVC and cellulose acetate (5.4%); PA, PE PS, and PU (2.7%), etc | 32 MPs particles were detected (mean size: 3.44 μm) | (1) Frozen at − 20℃; (2) digested with enzymatic mixture at 60℃ for 12 h; (3) Added ZnCl2, and stirred for 15 min and held for 45 min; (4) Filtered with 0.45 µm pore size silver membrane. (5) Oven-dried at 60℃ for 24 h | Raman spectroscopy | The maximum spatial resolution of Raman spectroscopy setup is 0.5 µm | Silver membrane are efficient in identifying polymeric materials using Raman spectrometry | Amato-Lourenco et al. (2021) |
|
Human blood (n = 22) |
1 mL | PET, PE and polymers of styrene | The mean of the sum quantifiable concentration: 1.6 µg/ml | (1) 1. Denatured proteins at 60°C for 1 h; (2) Added Proteinase K and CaCl2, incubating for 2 h at 50°C; (3) Filtered over glass fiber filter and dried the filter; (4) Treated the dried filters with tetramethylammonium hydroxide reagent and dried the filters again | Py-GC/MS | The low spike experiment recoveries were lower | Quantified thermal degradation products of the plastic particles present in the samples | Leslie et al., (2022) |
| Human liver tissue (n=11) | 1-2 cm3 (0.7-7.1g) | PS, PVC, PET, PMMA, POM and PP | 3.2 particles/g tissue | (1) Digested at 40 °C for 72 h (KOH/NaOCl=2:1); (2) Filtered via silver membrane; (3) Digested secondly with hydrogen peroxide and acetone; (4) Filtered secondly and transferred the filter to beakers with ethanol (30 ml) | Raman spectroscopy | (1) The position where MPs accumulated in the liver cannot be determined exactly. (2) The lower MP detection limit is 4 μm; (3) The sample size was small | MPs in human feces have been determined quantitatively and qualitatively through Raman spectroscopy | (Horvatits et al., 2022) |
| Placentas (n=18) and meconiu m (n=12) | NP | PA, PU, PE, PVC, PTFE, PET | 18 particles/g in the placenta; 54 particles/g in the meconium | (1) Digested with concentrated nitric acid at 95 °C for 3 h; (2) Filtered with stainless steel membrane (13 μm-pore-size) and then rinsed; (3) Ultrasonic at 40 kHz for at least 30 min; (4) Filtered and repeated step 3 | Agilent 8700 laser infrared imaging spectrometer (LDIR) | (1) The sample size is small; (2) Exogenous microplastic contamination cannot be ruled out | High diverse types of MPs were found | (Liu et al., 2022) |
| Human breastmilk (n=34) | 4.16 ± 1.73 g | PE, PVC, PVOH, PP, PVOH, PEVA, PEMA, PES and PC | NP | (1) Digested with KOH at 40℃ for 48 h; (2) Filtered with 1.6 μm pore-size filter membrane; (3) Dried the filter membrane at room temperature and stored in glass Petri dishes | Raman microspectroscopy | (1) The maximum spatial resolution of Raman spectroscopy setup is 0.5 μm; (2) The concentration of microplastics in the sample was not provided | Strict quality control | (Ragusa et al., 2022) |
| Human sputum (n=22) | 4-36 mL | 21 types, mainly including PU, PES, chlorinated polyethylen e, and alkyd varnish | The median level is 39.5 particles/10 mL | (1) Digested with concentrated nitric acid, NaOH and ZnCl2; (2) Filtered with 0.45 μm pore silver membrane; (3) Rinsed the membrane and dried in a clean Petri dish | Agilent 8700 laser infrared imaging spectrometer and μFTIR | (1) The sample size is small; (2) Particle size smaller than 20 μm cannot be detected | Analyze the factors associated with the presence of microplastics in the respiratory tract | (Huang et al., 2022) |
| Human placenta (n=17) | NP | Mainly including PVC, PP, PBS | Average abundance of 2.70±2.65 particles/g | (1) Digested and shaked constantly for 3 days; (2) Filtered through stainless-steel membrane; (3) After ultrasonic treatment, filtered with stainless-steel membrane; (4) Concentrated to 0.5 mL | Laser direct infrared (LD-IR) spectroscopy | Particle size smaller than 20 μm cannot be detected | More microplastics were detected | (Zhu et al., 2022) |
| Human enclosed body fluids (n=104) | 500 μL | PP, PS, PTFE, PVB, PA, LDPE, PEAA, PVA. | NP | (1) Body fluid sample (except blood) was digested with KOH and NaClO, and dried at 50 °C for 48h, and then filtered with quartz membrane; (2) Blood samples (500 μL) were digested with trypsin and NaClO and filtered with glass fiber filter membrane | Raman microspectroscopy | The maximum spatial resolution of Raman spectroscopy setup is 0.5 μm | NP | (Guan et al., 2023) |
| Human lung tissue (n=13) | 4.26 ± 3.87g | Mainly include PP, PET, resin, and PE | 0.69 ± 0.84 MP/g of tissue | (1) Digested with H2O2 and shaked incubator at 55 °C, 65 rpm for approximately 11 days; (2) Filter with aluminium oxide filters (0.02 μm) | μFTIR | The detection limit of μFT-IR is approximately 20 μm | A limit of detection (LOD) and limit of quantification (LOQ) technique was used to account for background contamination | (Jenner et al., 2022) |
PA polyamide, PE polyethylene, PP polypropylene, PVC polyvinyl chloride, PS polystyrene, PET polyethylene terephthalate, PU polyurethane, PC polycarbonate, POM polyoxymethylene, PBT polybutylene terephthalate, PVB poly (vinyl alcoholco-vinyl butyral), μFT-IR Fourier-transform infrared microspectroscopy, PVDC polyvinylidene chloride. NP means not provide
Table 3.
Summary of the detection of microplastics in mouse tissues
| Mouse biological samples | Tissue weight | MPs type | Concentration | Pre-processing | Test and identification | Reference |
|---|---|---|---|---|---|---|
| The liver, kidney and gut of mice | Dry tissues (0.1 g) | PS (5 μm and 20 μm) | 0.01 mg/day, 0.1 mg/day, 0.5 mg/day | (1) Lyophilization; (2) Digested in nitric acid at 70 ℃ for 2 h and in hydrogen peroxide at 85 ℃ for 2 h; (3) Diluted with deionized water; (4) Fixed in 10% formalin, embedded in paraffin wax, sectioned at 4 μm thickness, and stained with hematoxylin and eosin (H and E) for final observation | Light-field and dark-field images of the tissue were obtained by microscopy and epifluorescence microscopy, and the two sets of images were overlaid together using AxioVision Rel. 4.7 software. Then the standard curve was used for quantification | Deng et al. (2017) |
| The gut of mice | NP | PS (1 μm, 4 μm and 10 μm) | 1 μm (4.55×107 particles), 4 μ (4.55×107 particles) and 10 μm (1.49×106 particles) MPs in CMC at a volume of 10 mL/kg body weight, | (1) The small intestine were fixed in 4% (w/v) paraformaldehyde and the large intestine were fixed in Mirsky’s fixative; (2) Tissues were fixed and stored at 4 ℃, and then transferred into 30% (w/v) sucrose for 24 h; (3) Embedding was carried out in Shandon M-1Embedding Matrix in a dry ice–isopentane bath; (4) All sections were cut at 10 μm thickness | Fluorescence microscopy was used to detect MPs in the intestinal wall | Stock et al., (2019) |
| The gut of mice | NP | PS (5 μm) | 100 μg/L and 1000 μg/L | (1) Cut into small pieces and fixed with 10% (vol/vol) formaldehyde; (2) Embedded in paraffin wax, cut into 5 μm-thick sections | High resolution confocal microscope was used to observe fluorescently labelled PS MPs in the intestine of mice | Jin et al. (2019) |
| The liver of mice | NP | PE (35.46 ± 18.17 μm) | Tadpoles: 60 mg/L; Tambatinga fish: 3.18 g of tadpoles, twice a day for seven days; Mice: 2 mL (0.53 g) of crushed tambatinga/day | (1) Digested with acid; (2) Filtered in vacuum; (3) Washed membrane in purified water; (4) Evaporated water from hot plate; (5) Stained with Nile red dye | The 10 × magnification under-fluorescence microscopy was ued to photograph filtered membranes. Then the ImageJ software was used to obtain morphometric featuring of PE MPs for further quantification | da Costa Araujo & Malafaia, (2021) |
| Small intestines of mice | NP | PE (45–53 μm) | 100 mg/kg/day (approximately 5.25 × 104 particles/day) | (1) Fixed in 10% formalin; (2) Embedded in paraffin wax, sectioned at 4 μm thickness, and stained with H and E | Polarized light microscopy was used to locate MPs in small intestine tissues | Deng et al., (2020) |
| Livers and guts of mice | NP | PS and PE (0.5–1.0 μm) | 2 mg/L | (1) Fixed in 10% formalin; (2) Embedded in paraffin wax, sectioned at 4 μm thickness, and stained with H and E | MPs were observed in mice liver and gut using Polarized-light microscopy | Deng et al., (2018) |
| Ovarian tissue (granulosa cells) of rats | 1 mm3 | PS (0.5 μm) | 0.015 mg/kg/d, 0.15 mg/kg/d, 1.5 mg/kg/d | (1) Fixed in 2.5% glutaraldehyde; (2) Dehydrated and embedded in epoxy resin; (3) Cut into 50 nm thick sections | The transmission electron telescope (TEM) was used to observe PS-MPs in granulosa cells of ovarian | (Hou et al., 2021b) |
| Testis of mice | NP | Fluorescent PS (0.5 μm, 4 μm and 10 μm) | 100 μL (10 mg/mL) once a day | Collected gastric tissue and testis, and gastric tissue was used as a positive control | Biofluorescence imaging (BFI) system equipped with cooled slow scan CCD camera was used to detect the accumulation of fluorescent PS-MPs in testis tissues | Jin et al., (2021) |
| The fetal liver, lung, kidney, heart, and brain of rats | NP | Rhodamine-labelled PS (20 nm) | 300 μL (2.64 × 1014 particles) | (1) Fetal tissues were fixed with formalin; (2) embedded in paraffin and sectioned to 4 μm to identify nano-PS particles deposition within the fetal tissues | Bruker In-Vivo Multispectral (MS) FX PRO Imager was used to obtain optical images of maternal and fetal tissues; then nano-PS particle deposition within the fetal tissues were identified using enhanced hyperspectral microscopy | Fournier et al., (2020) |
| Ovary of rats | NP | PS (0.5 μm) | 0.015, 0.15, and 1.5 mg/d | (1) The rat ovaries (0.15 mg/d group) were fixed in 2.5% glutaraldehyde, dehydrated, embedded and sectioned at 50 nm-ultrathin slices; (2) stained the slices | TEM was used to observe PS-MPs in ovary of rats | An et al., (2021) |
| Testis of mice | NP | PE (0.4–5 μm) | MPs + L-DEHP (0.2 g/L MPs and 5 μg/L DEHP), MPs + H-DEHP (0.2 g/L MPs and 50 μg/L DEHP), MPs + L-MIX (0.2 g/L MPs and 5 μg/L PAE mixture), MPs + H-MIX (0.2 g/L MPs and 50 μg/L PAE mixture) | (1) Testes were fixed using 2.0% paraformaldehyde and 2.5% glutaraldehyde, and then kept in 1% osmium tetroxide for 1 h; (2) rinsed with distilled water and stained with 1% aqueous uranyl acetate for 1 h; (3) dehydrated in ethanol and embedded in epoxy resin and polymerized at 70℃ overnight; (4) cut at 90 nm thick, stained with uranyl acetate and lead citrate | Images of MPs in mouse testis were observed by JEOL 1200 EX transmission electron microscope | Deng et al., (2021) |
| Sheep feces | 5g | NP | NP | (1) Place dried sample into distilled water and shacked at 150 rpm for 30 min; (2) Centrifuged and filtered the supernatant with a Whatman No. 42 filter paper; (3) Repeat step 1 and step 2; (4) The filters were air dried for 24 h | Stereo microscope (ZEISS Stemi 508) equipped with digital camera (CMEX-18 PRO) | (Beriot et al., 2021) |
| Fecal samples of European hedgehog, wood mouse, field vole, brown rat | Polyester, Polynorbornene, Polyethylene | NP | (1) The fecal sample were dried at 40 °C; (2) All samples except the insectivores’ were digested with Fenton's reagent; samples of insectivores were digested with KOH, HCl and Fenton's reagent. (3) The samples were filtered with 1.2 μm glass filter and dried at 40 °C overnight | Dissecting microscope and μFTIR | (Thrift et al., 2022) |
NP means not provided
Existence of microplastics in human biological samples
We noticed that some researchers have detected MPs in human biological samples, including blood, breastmilk, sputum, enclosed body fluids, feces, colon tissues, liver, lung and placenta and meconium. A recent study performed by Leslie et al. detected and quantified four types of plastic particles in human blood samples, showing that plastic particles can be absorbed into human blood (Leslie et al., 2022). In addition, Monteleone et al. developed a method by mixing MPs with human blood and then extracting them (Monteleone et al., 2019). Although this method has not been validated in blood samples by other researchers, it provides a way for future studies. MPs have also been observed in breast milk, tantalum, pelvic cyst fluid and effusions (Guan et al., 2023; Huang et al., 2022; Ragusa et al., 2022). Due to ethical and technical limitations, it is difficult to directly quantify MPs in the human gastrointestinal tract. Feces, as the residual fraction resulting from unabsorbed food, provide direct evidence of human ingestion of MPs. Yan et al. detected two types of MPs, polybutylene terephthalate (PBT) and poly (vinyl alcohol-co-vinyl butyral) (PVB) particles, in stool samples of 10 patients with inflammatory bowel disease collected from the hospital, with an incidence rate of 40% (Yan et al. 2020b). In addition, to precisely assess the burden of the human gastrointestinal tract on MPs, the abundance of MPs in human fecal samples was investigated in another two studies. In a study containing 8 stool samples from healthy volunteers, the median number of plastic particles in 10 g stool was 20 MPs particles (Schwabl et al., 2019). Zhang et al. found that the abundance of MPs in 23 fecal samples collected from young male students ranged from 1 to 36 particles/g feces (Zhang et al., 2021). Apart from the three major types of MPs, PP, PET and PS, PE, polyamide (PA), polycarbonate (PC), PVC, polyurethane (PU), and polyoxymethylene (POM) were also found in human feces(Schwabl et al., 2019; Yan et al. 2020b; Zhang et al., 2021). Since the digestive tract is the main route for MPs to enter the body, the levels of MPs in intestinal tissues were also explored. Ibrahim et al. detected three types of MPs (PC, PA, PP) with an abundance of 28.1 ± 15.4 particles/g tissue in human colectomy specimens (Ibrahim et al., 2021). MPs detected in feces and colon may be inadvertently ingested by a person from the mouth. In addition, MPs have been proven to be present in the air, and are highly likely to be inhaled (Liu et al., 2019; Wright et al., 2020). In the study of Amato-Lourenco et al., 31 synthetic polymer particles and fibers were observed in 20 cases of human lung tissues obtained at coroner autopsies (Amato-Lourenco et al., 2021). Moreover, MPs were also found existing in placenta, which is an important organ for the exchange of substances between fetal and maternal. Ragusa et al. identified dyed MPs (PP) and paint/coating/dye MPs in human placentas. These compounds are widely used in paints, coatings, adhesives, cosmetics and personal care products. The particle size of the MPs (≈10 μm and ≈5 μm) detected in the placenta is compatible with blood transport (Ragusa et al., 2021). It is speculated that MPs may enter the bloodstream and reach the placenta through the maternal respiratory system and gastrointestinal tract (GIT). Therefore, MPs may affect fetal development through the material exchange process of the placenta. Moreover, whether MPs invade further into other tissues needs to be studied.
Existence of microplastics in terrestrial higher mammalian biological samples
(1) Studies conducted in laboratory samples
Except for human tissues, accumulating laboratory studies have shown that MPs were detected in the tissues of mice/rats exposed to MPs, which provides a scientific basis for the potential health risks of MPs. Table 3 summarizes the methods of MPs detection in mouse tissues, including pretreatment and identification of MPs. Two types of MPs, PE and PS (favored), were mostly chosen in these studies. Orally ingested MPs can be detected in the mouse intestine, liver, and kidney (Deng et al., 2017, 2018, 2020; Jin et al., 2019; Stock et al., 2019). MPs can also enter the reproductive system. Studies have found that nano-scale MPs accumulate in mouse testes and rat ovaries (An et al., 2021; Deng et al., 2021; Hou et al., 2021b). Jin et al. found that 4 μm and 10 μm PS-MPs can be detected in testicular tissue after oral exposure for 24 h (Jin et al., 2021). Even without direct contact, MPs can be deposited from mother to fetus through the placenta or be transferred to upper trophic levels through the food chain. A rat inhalation experiment showed that maternal exposure to 20 nm PS nanoparticles in the lungs during late pregnancy led to the detection of such particles not only in the maternal lung, heart and spleen but also in the placenta and fetal liver, lung, heart, kidney and brain (Fournier et al., 2020). da Costa Araújo et al. studied the migration of MPs from water to terrestrial trophic levels. They quantified MPs in mouse livers and found that MPs can be transferred to upper trophic levels through the food chain (tadpoles- fish-mice) (da Costa Araujo & Malafaia, 2021).
Current researches have focused more on the effects of size on tissue accumulation of MPs. Existing studies suggest that MPs smaller than 10 µm can be transferred from the gut to the lymphatic and circulatory systems and accumulate in tissues including the liver, kidney and brain (Yong et al., 2020). MPs smaller than 0.1 µm may be able to pass through cell membranes, the placenta and the brain, etc. (Gruber et al., 2020; Prust et al., 2020). However, whether there is a dose-dependent accumulation of MPs in tissues is still unknown. Although some researchers have set different concentration in their experiments, this has not been explored.
(2) Studies conducted on field samples
Studies conducted on field samples showed that MPs also accumulated in fecal samples of terrestrial wild higher mammals, including sheep, European hedgehog, wood mouse, field vole and brown rat. Nicolas Beriot et al. collected sheep feces from farmland to assess the intake of microplastics by sheep and reported a level of MPs in sheep feces of approximately 103 particles kg−1, but they did not identify the chemical composition of the MPs (Beriot et al., 2021). Emily Thrift et al. found (polyester, polynorbornene, polyethylene) MPs in the feces of European hedgehogs, wood mice, voles, brown rat (Thrift et al., 2022). The exposure features and health risks of wildlife to MPs is also an important issue in terrestrial systems, and further research are required to clarify it.
Pre-treatment and cautions for microplastics detection in human and terrestrial higher mammalian biological samples
For the identification of MPs in biological samples, pretreatment is a key step and researchers usually conduct several key procedures. Firstly, chemical digestion is performed, then the membrane is selected for filtration and separation, and the last step is identifying MPs on the membrane or filter. Poor digestion of samples leads to the remaining organic matter wrapping around the surface of MPs, which affects further characterization of plastic particles. Conversely, excessive digestion may destroy the properties of MPs, which will affect the spectroscopy results and cause the levels of MPs to be underestimated. KOH, NaOH, and H2O2 are usually chosen as chemical abatement agents. However, these reagents may alter the characteristics of MPs, which may further affect MPs identification (Kumar et al., 2020). When extracting different kinds of MPs from chicken and human feces, Yan et al. carefully optimized the pretreatment method in terms of digestion reagents (Fenton’s reagent, nitric acid and absolute ethyl alcohol) and temperature (70 °C). This method is proven to be an efficient way with 97.00% digestion efficiency and 97.78% recovery rate (Yan et al. 2020b). However, digestibility and recovery rate were not tested in other pretreatment methods, which could affect the accuracy of the results. Therefore, the influences of different digestion and pretreatment methods should be examined in future studies, which will facilitate further identification and quantification of MPs. In addition, Monteleone et al. found that aluminum oxide membranes used for filtration have fluorescent properties, which affect the identification of MPs. Thus, the autofluorescence of filters should be noticed and tested. Besides, in the detection of human biological samples, sample pollution is also a problem worthy of attention. Monteleone et al. found that plastic PS not used in the experiment was also detected, indicating that samples are susceptible to external MPs contamination due to environmental and improper handling (Jenner et al., 2022; Monteleone et al., 2019). Besides, MPs were also detected in laboratory blanks in the study by Jenner et al. (2022). We suggest that more efforts should be made to avoid MPs pollution in future research in this area. There are several measures that researchers can consider to reduce MPs contamination from non-sample sources, such as keeping the laboratory environment clean, using a positive pressure laminar flow hood, wearing cotton lab coats and gloves, and avoiding the use of plastic materials. In addition, blank samples should be used during the experiment to monitor potential contamination. Kutralam-Muniasamy et al., summarized the measures taken to avoid contamination in laboratory experiments and during sample collection process in detail, which provide an excellent reference (Kutralam-Muniasamy et al., 2022).
Identification methods of microplastics in human and terrestrial higher mammalian biological samples
Currently, spectroscopy and microscopy are the main published methods for identifying MPs in human biological samples, including Fourier transform infrared microspectroscopy (μFT–IR), Raman spectroscopy, light microscope, stereo dissecting microscope, scanning electron microscope and energy-dispersive X-ray. μFT-IR and Raman spectroscopy are the most commonly used methods for MPs identification. μFT-IR determines the molecular structure of particles by using infrared wavelengths in the range of 400–4000 cm−1, and it can be used to measure MPs more than 20 µm (Zhang et al., 2020b). For example, μFT-IR was used to detect nine types MPs (PE, PP, PVC, PS, PET, PA, PU, PC, POM, ranging from 20 to 800 µm in size) in human fecal samples (Schwabl et al., 2019; Zhang et al., 2021). Although Raman spectroscopy is a relatively new technique for the study of MPs, it has a larger detectable range than μFT-IR, and can detect particles down to 1 µm in size. Compared with μFT-IR, Raman spectroscopy is more suitable for the analysis of wet samples. However, Raman spectra are more susceptible to interference from sample fluorescence (Lee & Chae, 2021). Yan Z. et al. identified two types of MPs, PBT and PVB using Raman spectroscopy in human feces (Yan Z. et al., 2020b). Besides, Ragusa et al. detected microplastic fragments (5–10 µm in size) identified as PP and pigments in human placentas using Raman microspectrometer (Ragusa et al., 2021).
Different visualization techniques are selected for the identification of MPs in terrestrial higher mammalian biological samples depending on the characteristics of MPs and tissues, such as light microscopy and electron microscopy. Optical microscopes are used for image analysis of MPs with smaller sizes that are difficult to observe with the naked eye, including polarized light microscopy, high resolution confocal microscope and hyperspectral microscopy. For example, Deng et al. used polarized light microscopy to localize MPs in the intestine and liver (Deng et al., 2018, 2020). Jin et al. observed fluorescent signals from fluorescently labelled PS MPs in mouse intestines using high resolution confocal microscope (Jin et al., 2019). Fournier et al. observed PS nanoparticles in the placenta, fetal liver, lung, heart, kidney, and brain using enhanced hyperspectral microscopy, suggesting the presence of nanoparticle translocation in maternal lung-to-fetal tissue after PS exposure during late gestation (Fournier et al., 2020). Besides, high resolution confocal microscope and hyperspectral microscopy are convenient and do not require tissue staining.
Fluorescence microscope, another type of optical microscope, uses ultraviolet light as a light source to irradiate the object to be examined with a suitable filter or laser excitation wavelength, so that it emits fluorescence, and then the shape of the object and its location can be observed under the microscope (Lichtman & Conchello, 2005). Fluorescence microscope is used, firstly, to facilitate direct observation of the distribution of MPs in body tissues and, secondly, for quantitative detection and to obtain information on the accumulation of MPs in the body. Typically, fluorescent dye-labelled MPs are chosen when detecting MPs in tissues or organisms. Stock et al. observed a small amount of fluorescently labeled MPs in the intestinal wall of mice with the aid of fluorescence microscope (Stock et al., 2019). Deng et al. found fluorescent PS-MPs accumulating in mouse liver, kidney and intestine by epifluorescence microscope, and quantified the concentration of MPs in mouse tissues with an external standard calibration curve based on fluorescence spectroscopy. Meanwhile, the concentrations of MPs in these three tissues were found to reach steady state within 14 days after exposure and the maximum concentrations of different MPs sizes in these three tissues were given (Deng et al., 2017).
In general, electron microscope has a higher resolution and is able to observe cellular structures such as membranes, mitochondria and other organelles. The electron microscope can see fine structures smaller than 0.2 μm that cannot be seen under an optical microscope, and it can reach a resolution of 0.1–0.2 nm. Electron microscope is often used to observe the distribution of MPs in the tissues and specific cells of model animals in in vivo toxicology experiments. Certain studies have observed the accumulation of MPs in granulosa cells (GCs) of rat ovary and Sertoli cells of mouse testis with the help of transmission electron microscopy (TEM) (An et al., 2021; Deng et al., 2021; J. Hou et al., 2021a, 2021b). Notably, when using TEM for detection, due to the weak penetration of the electron beam, tissue specimens need to be made into ultrathin sections with a thickness of approximately 50 nm, which is different from the thick tissue sections of approximately 5 μm required by optical microscopy.
Visual identification is a convenient and fast method for classifying an enormous variety of MPs, but it has its own limitations with a high misclassification rate up to 20–70% (Kumar et al., 2020). In most cases, a combination of identification techniques was chosen. The combination of spectroscopy and microscope can simultaneously determine the type, size and location of plastics. Ibrahim et al. combined microscopy with μFT-IR to detect MPs in human colon tissue and revealed that three types of MPs (PC, PA, PP) were present in human colon tissue with an average size of 1.1 ± 0.3 mm. In their work, firstly, a stereo dissecting microscope was used to determine the abundance and physical characteristics (length, shape, and color) of the MPs. Afterwards, the surface morphology and elemental composition of MPs were obtained using a scanning electron microscope with energy-dispersive X-ray, and the polymer type was identified with μFT-IR (Ibrahim et al., 2021).
In addition, μFT-IR and Raman spectroscopy were chosen to identify the types of microplastics in these studies, which has some limitations in the particle size of microplastics. The chemical composition of microplastics can be further characterized by combining multiple techniques in future studies to make the analytical results more accurate. For example, thermochemical (Py-GCMS, TD) analysis methods offer the possibility of identifying microplastics smaller than 1 µm (Zhang et al., 2020a). Thermal analysis utilizes the thermal decomposition of the polymer at high temperatures (500 °C) and further characterizes the resulting gases using mass spectrometry. It offers the possibility of identifying microplastics smaller than 1 µm (Lee & Chae, 2021; Yulan Zhang et al., 2020a). It is independent of the particle size and shape of the microplastic, and does not require pre-treatment of the sample, and its analysis time is relatively short (Fischer & Scholz-Bottcher, 2017; Penalver et al., 2020). Thermal analysis methods include pyrolysis–gas chromatography–mass spectroscopy (Py-GC-MS) and thermal extraction desorption gas chromatography-mass spectrometry (TED-GC-MS). Py-GC-MS is suitable for characterizing complex structures, such as microplastics containing organic contaminants. Py-GC-MS has been applied to the quantification of PE, PP, PS and the characterization of microplastics in the marine environment (Fries et al., 2013; Steinmetz et al., 2020). TED-GC-MS has the advantage of reducing the analysis time for large sample sizes (up to 100 mg), compared to Py-GC-MS (Lee & Chae, 2021). Dumichen et al. used TED-GC-MS to identify PP, PE, and PS from a biogas plant and rivers (Dumichen et al., 2017). In addition, Gao et al. successfully used Py-GC-MS to identify microplastics in oyster and mice tissues(Gao et al., 2021). Thermal analysis can be used as a complement to other identification methods.
To sum up, MPs have been proven to accumulate in human and terrestrial higher mammalian tissues. Currently, studies have been conducted to detect MPs in biological samples, especially in human biological samples, and to distinguish MPs between different types. In addition, they have made explorations to optimize the pre-treatment as well as to avoid contamination. However, there are still some problems that need to be considered and resolved in further research. For example, there are no standardized methods available for MPs extraction from biological samples. Besides, technological limits for the detection of MPs smaller than 1 μm are issues that need to be solved in the future. Finally, quantitative detection of MPs is essential for the accurate assessment of their toxic effects and the toxicological mechanisms research. However, few studies have been conducted to quantify the MPs in biological samples, which requires attention.
Potential health hazards of microplastics to human and terrestrial higher mammals
In recent years, the health risks arising from MPs to terrestrial higher mammals have attracted much attention. Although there is no direct population-based research evidence, a growing number of in vitro and in vivo studies suggest the association of MPs pollution with various diseases. Mice and rats are the most heavily used and most exhaustively studied mammalian laboratory animals, and have been commonly used as model species for the health risk assessment of environmental pollutants, including MPs (Deng et al., 2018; Xie et al., 2021). Here, we reviewed and summarized the biological effects and related mechanisms of MPs on mice/rats and human-derived cells to provide new evidences and insights for assessing the potential health risks of MPs to humans and terrestrial higher mammals.
Research based on population
Studies based on population have found that MPs have lung effects, liver effects and immune effects on people (Zarus et al., 2021). A case study have found that exposure to polyethylene flock and polypropylene flock caused clinical changes in the respiratory system of factory workers, reduced lung function, reduced lung volume, dyspnea, cough, etc. (Atis et al., 2005; Barroso et al., 2002). Vobecky et al. conduct a case control study and revealed that synthetic fiber caused an increased risk of colorectal cancer (Vobecky et al., 1984). PVC causes an increased risk of liver cancer in workers (Gennaro et al., 2008). In addition, particles generated by wear and corrosion of joint replacement prostheses can disseminate to liver, spleen, and abdominal para-aortic lymph nodes, even leading to a visceral granulomatous reaction and hepatosplenomegaly (Urban et al., 2000).
Research based on laboratory
Given that MPs can enter terrestrial higher mammals and humans through various pathways and then accumulate to exert health hazards, we reviewed the relevant literature and summarized the toxic effects and related mechanisms of MPs on terrestrial higher mammals in Supplement Table 4. As is shown, the toxic effects of MPs mainly include intestinal toxicity, metabolic disruption, reproductive toxicity, neurotoxicity, immunotoxicity, cardiotoxicity, and impaired pulmonary function.
Gastrointestinal tract, liver and metabolism Oral ingestion is the main route of MPs exposure in organisms (Mallik et al., 2021). MPs have been found in various foods, such as mussels, commercial fish, salt, sugar, and bottled water (Prata et al., 2020). The intake of MPs has been estimated to be 39,000–52,000 particles/person/year based on the consumption of food (Cox et al., 2019). As a result, the gastrointestinal tract of organisms has a high burden of microplastic exposure. Studies have reported that MPs disturb gut microbiota in mice, cause intestinal inflammation and affect liver energy metabolism and lipid metabolism. In vivo experiments in mice have shown that MPs reduce intestinal mucus secretion, disrupt intestinal barrier function, and modify the gut microbiota composition (Jin et al., 2019; Li et al., 2020; Lu et al., 2018). High concentration of PE- MPs (600 μg/day/mouse, diameter 10–150 µm) might induce intestinal inflammation through the activation of TLR4/AP-1 and TLR4/IRF5 signaling (Li et al., 2020). In addition, histopathology showed a small amount of mucosal hypertrophy (or hyperplasia) in the mucosal layer (chief cells, parietal cells and other cell types) around the glandular stomach of PE treated mice (Park et al., 2020). When passing through the gastrointestinal tract, MPs with small size may be absorbed by the intestinal lining, and then enter the bloodstream and shift (Mallik et al., 2021). Deng et al. found that MPs accumulate in the liver, kidney and gut of orally ingested mice, and their distribution in tissues strongly depends on the particle size. Histopathology showed that lipid droplets and inflammation were observed in the liver. Besides, ingested MPs changed lactate dehydrogenase (LDH) activity and the levels of ATP, total cholesterol (TCH) and triglycerides (TGs) in the liver and induced disturbance of energy and lipid metabolism (Deng et al., 2017). Lu et al. found that the transcription levels of hepatic PPARγ and genes involved in TG synthesis were significantly down-regulated in epididymal fat due to mouse MPs exposure, and they speculated that MPs might affect liver lipid metabolism through gut microbiota dysbiosis (Lu et al., 2018). MPs also increased the level of total bile acid (TBA) in the liver and changed the process of bile acid metabolism in mice. Besides, ingested MPs also modulated serum pyruvate (PYR) levels, TG levels, total cholesterol (TCH) levels, and arginine (ARG) levels, leading to abnormalities in glycolipid and amino acid metabolism (Jin et al., 2019). Notably, MPs have been reported to aggravate gastrointestinal diseases. For example, microplastic ingestion aggravated intestinal permeability damage and liver damage, increased the level of liver lipid peroxidation, and promoted abnormal lipid metabolism and inflammation in mice with acute colitis (Zheng et al., 2021).. Given the fact that MPs have been detected in human colectomy, it is necessary to conduct more experiments to verify the association between MPs and various digestive system diseases based on population studies.
-
Reproductive toxicity Studies have found that MPs can reduce sperm quantity, quality, and activity, and reduce ovarian reserve capacity, thereby affecting reproductive function. Histopathological analysis of testes showed that male mice exposed to MPs showed atrophy and detachment of sperm cells, abscission and disorganization of spermatogenic cells, and appearance of multinucleated gonocytes in the seminiferous tubules. MPs also cause testicular inflammation, disruption of the blood-testis barrier, and accumulation in testicular tissue (Hou et al., 2021a, 2021b; Jin et al., 2021). The activities of LDH and succinate dehydrogenase (SDH), specific enzymes involved in sperm energy metabolism, were reduced in male mice due to MPs ingestion, which leads to interference with spermatogenesis (Xie et al., 2020). Meanwhile, reproductive toxicity induced by MPs was also found in female rats. Ingestion of MPs has been reported to induce granulosa cell apoptosis and fibrosis in rat ovaries and reduce ovarian reserve capacity, causing reproductive damage (An et al., 2021; J. Hou et al., 2021a, 2021b). Induction of oxidative stress is the main mechanism of the toxic effects of PS-MPs on the reproductive system (An et al., 2021). It was found that MPs lead to reproductive toxicity through oxidative stress and activation of p38 MAPK signaling pathway, Wnt/β-Catenin signaling pathway, NLRP3/Caspase-1 signaling pathway and aberrant expression of Nrf2/HO-1/NF-κB pathway (An et al., 2021; Hou et al., 2021a, 2021b; Xie et al., 2020).
Furthermore, exposure to MPs also affects offspring, including the number, weight, and metabolism of offspring. Oral exposure of mice to PE-MPs resulted in a reduction in the number of live births and body weight of the next generation, as well as changes in the sex ratio (Park et al., 2020). Moreover, MPs exposure also causes metabolic disorders in offspring. Although the offspring were not directly exposed to MPs, maternal mice exposure to MPs during gestation caused metabolic disorders in the offspring (42 days after delivery), including amino acid metabolism and fatty acid metabolism. The serum metabolites of F1 offspring, including amino acids and acylcarnitine, were changed. In addition, serum TG, TCH, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) levels and liver TG, TCH levels were altered in F1 offspring, and were different between female mice and male mice of F1 offspring (Luo et al., 2019a, b). Luo et al. pointed out that maternal exposure to MPs during pregnancy and lactation also affected the liver glycolysis and glucose transport of F1 offspring, which may be related to the poor nutrient absorption and health conditions observed in the maternal mice due to the destruction of intestinal function, dysregulation of gut microbiota and glycolipid metabolism (Luo et al., 2019a, b). Moreover, maternal exposure to MPs during gestation and lactation induces long-term effects on offspring. Lipid accumulation in the liver of F1 female offspring and alterations in genes related to lipid metabolism in F2 offspring were observed at postnatal day 280 and postnatal day 42, respectively (Luo et al., 2019a, b). In addition, lymphocyte subpopulations in the spleen of the offspring were also affected (Park et al., 2020). However, the specific mechanisms of reproductive toxicity are less studied, and need to be further elucidated.
Neurotoxicity There are relatively few studies on the neurotoxicity caused by MPs, and the findings are inconsistent. Rafiee et al. orally exposed male Wistar rats to 25 and 50 nm PS-NPs for five weeks (1, 3, 6, and 10 mg/kg per day), and no significant change in behavior was found (Rafiee et al., 2018). Conversely, another study revealed that mice orally ingested PE-MPs for 7 days (35.46 ± 18.17 μm, 60 mg/L) had directly reduced locomotor behavior, including shorter movement distance, slower movement speed, and higher anxiety rate. In addition, this study indicated that nutritional transfer of MPs through the food chain induced neurobehavioral disorders in mice. Mice consuming fish feeding on tadpoles exposed to MPs showed significant differences in latent and aggregation behaviors when confronted with predators compared to controls (da Costa Araujo & Malafaia, 2021). The large differences in the size, dose, and type of the MPs and even the difference between model animals used in the above articles may contribute to the inconsistent conclusions. Additionally, different indexes applied to evaluate the behavior of model animals may also have an impact on the results. In the meantime, it is worth noting that neither of them detected MPs in the brain tissue of model animals. However, other studies have shown that MPs were detected in the brain tissue of MPs-exposed rodents, which provides direct evidence for the possibility of neurotoxicity from MPs. For instance, MPs were detected in the fetal brain in rats exposed to nano-PS via intratracheal instillation (2.64 × 1014 particles) in late gestation (Fournier et al., 2020). In addition, PS were detected to accumulate in the brains of mice with intraperitoneal injection of PS at a dose of 14.6 ng/kg body weight (bw) for 3 days (Estrela et al., 2021). Therefore, more studies are required to clarify whether MPs are neurotoxic to higher terrestrial mammals, and to explore related influencing factors and mechanisms.
Other impacts In addition to above-mentioned toxic effects, MPs also cause cardiotoxicity, immunotoxicity, and impaired pulmonary function. MPs could accumulate in cardiomyocytes of rats and induce cardiac tissue damage and apoptosis of myocardial cells (Wei et al., 2021). Besides, immune organs are potential target organs for the effects of microplastics. A study showed that MPs could accumulate in lymph nodes surrounding joint replacements, thereby inducing impacts on the immune system (Wright & Kelly, 2017). MPs could cause a significant increase in blood IgA concentrations and interleukin-1 (IL-1α) levels in serum and alter the lymphocyte subsets in the spleen (Li et al., 2020; Park et al., 2020). Inhalation is also a major route for MPs to enter the body. MPs concentrations in the air were estimated to be 0.3–1.5 particles/m3 outdoor and 0.4–56.5 particles/m3 indoors (Dris et al., 2017). Individual inhalation has been estimated to be 26–130 airborne microparticles/day (Prata, 2018). MPs may induce certain toxicity to the respiratory system. In a study by Fournier et al., PS particles were detected in rat lungs after intratracheal instillation of nano-PS suspension (Fournier et al., 2020). Long-term exposure to MPs may lead to airway-related respiratory symptoms and inflammation (Prata et al., 2020). Furthermore, an inhalation experiment showed that MPs caused significant changes in biochemical indicators in rats, including blood (aminotransferase)AST levels, white blood cell (WBC) levels, lymphocyte (LYM) levels and the eosinophil (EO) levels, and had an effect on the function of the lung (Lim et al., 2021). In general, there are relatively few studies on the cardiotoxicity, immunotoxicity and effects on the respiratory system caused by MPs, which can be used as directions in future research. Moreover, in regard to respiratory toxicity, studies should be performed in a simulated air environment for a more realistic understanding of the association between microplastic exposure and respiratory toxicity.
Joint toxicity In the environment, MPs usually coexist with multiple pollutants, and the toxicity caused by joint exposure to MPs and chemical pollutants can better reflect the effects in the real environment. On the one hand, MPs themselves can release plastic additives, including dissolved organic carbon (DOC), trihalomethanes (THMs), and other substances(Ateia et al., 2020). On the other hand, MPs adsorb multiple chemical pollutants from the environment and induce combined toxic effects on organisms, mainly including hydrophobic organic pollutants (such as polychlorinated biphenyls [PCBs], polycyclic aromatic hydrocarbons [PAHs] and personal care products) and heavy metals (Hg, Cr, Cd, Au, Cu, Pb, Zn and Ag). Thus, MPs act as carriers to transfer absorbed chemical contaminants and release additives into the tissues of organisms, inducing combined effects, and may further affect humans through the food chain (Huang et al., 2021; Jiang et al., 2020). The interaction between MPs and chemical pollutants is affected by the hydrophobicity of chemical pollutants, the type, color, degree of aging, particle size of MPs, and environmental salinity, pH and temperature (Shan et al., 2018). In addition, microorganisms, such as Vibrio spp. and human coronaviruses, may colonize the surface of MPs and affect the organisms as they migrate (Kampf et al., 2020; Prata et al., 2020).
Researchers have explored the combined effects of MPs and chemical pollutants under laboratory conditions. Several studies have indicated that co-exposure to MPs and other chemicals exacerbates existing toxicity. Deng et al. showed that compared with single organophosphorus flame retardants (OPFRs) exposure, co-exposure to MPs (0.5–1.0 μm, 3.7 × 108 items/L) and OPFRs caused a significant increase in superoxide dismutase (SOD), catalase (CAT) and malonaldehyde (MDA) activities in the liver and a decrease in AChE activity in the brain, leading to increased oxidative stress and neurotoxicity. The combined effect also resulted in significant disruption of amino acid metabolism and energy metabolism in mice (Deng et al., 2018). Another study indicated that PE-MPs (45–53 μm, approximately 5.25 × 104 particles/day) increased the accumulation of di- (2-ethylhexyl) phthalate (DEHP) in the intestine of mice. Compared with virgin MPs and H-DEHP groups, DEHP-contaminated MPs induced a significant increase in d-lactate (d-Lac) levels and a decrease in diamine oxidase (DAO) activity in mouse intestines, and altered the expression of genes related to oxidative responses, lipid metabolism, immune responses and hormone metabolism (Deng et al., 2020). Deng et al. revealed that compared with MPs and PAEs (Phthalate esters) alone, PAE-contaminated MPs (0.4–5 µm, 100 mg/kg bw) increased PAE accumulation in the gut and liver of mice, enhanced testicular transcriptomic alteration effects, and caused enhanced reproductive toxicity in mice (Deng et al., 2021).
However, a study showed that combined exposure to MPs and pollutants weakened or inhibited toxic effects caused by relevant individual contaminants. For example, the combined exposure of zinc oxide and PS at a dose of 14.6 ng/kg bw for 3 days resulted in a lower level of PS in the brain compared with the single PS group. Moreover, compared with exposure to zinc oxide and MPs separately, binary combination exposure has inhibitory effects on new-object recognition rates and oxidative stress responses in mice (Estrela et al., 2021).
From these studies, combined exposure to MPs and chemical pollutants causes a bidirectional effect. This may depend on the characteristics of the MPs (type, degree of ageing, particle size, etc.), the type of chemical contaminant and the exposure conditions (pH, ambient salinity, temperature, etc.). Future attention should be paid to the effects of combined exposure to MPs and heavy metals. In addition, as MPs are mainly ingested orally, there is an urgent need to determine the combined effects of MPs and pathogenic bacteria.
It is worth noting that the current studies on the toxic effects of MPs on terrestrial higher mammals are based on mice and rats. In fact, MPs have been found to be ingested by other terrestrial mammals, such as sheep, hedgehogs and voles. Therefore, the effects of MPs on other terrestrial higher mammals need to be further investigated.
Research based on human-derived cells
Presently, although MPs have been detected in human tissues, data on the toxicity of MPs in humans are not yet available due to ethical considerations, and the effects of MPs on human health are unknown. Fortunately, accumulating studies on the effects of MPs on human cells have been conducted in vitro, which may provide some insights into this issue. As shown in Supplement Table 5, current MPs cytotoxicity studies have been conducted in gastrointestinal tract cells, lung cells, immune cells, and other human-derived cells, such as early human expanded blastocysts (EBls) and human induced pluripotent stem cells (hiPSCs), hinting at a potential risk that MPs induce gastrointestinal toxicity, respiratory toxicity and immunotoxicity in human.
-
Gastrointestinal tract cells The toxicity of MPs toward cells in the gastrointestinal tract has been extensively studied. The normal epithelium of the stomach are columnar cells, including gastric mucosa covered epithelial cells and fundic gland cells. Oral absorption occurs primarily in the intestine, but the MPs ingested orally come into contact with the gastric cells first (Banerjee & Shelver, 2021). An in vitro experiment based on gastric cancer cells (AGS) showed that high concentration (600 mg/L) PS-NPs (60 nm and 500 nm) could reduce cell viability, stimulate oxidative stress and apoptosis, and cause DNA damage after 24 h exposure. Moreover, 60 nm PS-NPs caused more serious cytotoxicity than 500 nm PS-MPs (X. ).
The intestine is an important absorption organ of the body, supplying the body with nutrients. The intestinal epithelium mainly consists of intestinal epithelial cells, cup cells, and M cells. Caco-2 cells are the most commonly used cell model for simulating intestinal transit experiments in vivo and have been applied to the study of MPs effects via the oral exposure route. Studies have shown that MPs increase the level of reactive oxygen species (ROS) in Caco-2 cells, cause mitochondrial depolarization, and affect the gene expression profile of Caco-2 cells. Su Liu et al. found that 120 mg/ml PS-MPs induced a significant decrease in Caco-2 cell viability and a significant increase in intracellular ROS levels (Wang et al., 2020). PS of 0.1 μm and 5 μm could cause mitochondrial depolarization and inhibit plasma membrane ATP-binding cassette (ABC) transporter activity (Wu et al., 2019). In addition, the gene expression profile of Caco-2 cells was affected after exposed to PS-MPs at a concentration of 12.5 mg/L or 50.0 mg/L for 24 h, including proliferation-associated genes and inflammation-associated genes (Wang et al., 2020).
However, several studies found that MPs treatment did not cause toxicity or significant toxicity to Caco-2 cells (Cortés et al., 2020; Hesler et al., 2019; Lehner et al., 2020; Magri et al., 2018; Stock et al., 2019). In a study, Caco-2 cells were exposed to 1, 5, 15 and 30 μg/mL PET nano-plastics (100 nm), but no toxic effects were observed (Magri et al., 2018). The toxic effects of MPs are related to many factors, such as, size, dose, polymer type, exposure time, chemical additives, adsorbed contaminants, etc. This may explain difference in the result of different experiments, which need to be explored in the future.
As the body's first line of defense against intestinal exogenous agents, intestinal epithelial cells can initiate an immune response in a short period of time. Thus, the Caco-2 cell and immune cell co-culture system facilitates the understanding of the integrated response of MPs exposure to the gastrointestinal tract. Stock et al., explored particle uptake and transport of PS in co-cultures of Caco-2, Raji B and HT29-MTX and observed higher particle uptake rates of 1 μm and 4 μm particles in the co-cultures, compared to the Caco-2 monoculture (Stock et al., 2019). Besides, Hesler et al. used in vitro co-culture models (Caco-2 and HT29-MTX-E12) to study the acute toxicity of COOH-modified PS to the human intestinal barrier, and only minor acute toxic effects were observed, which may be related to the fact that the PS particles used in the study are functionalized by carboxyl groups (COOH) (Hesler et al., 2019). Particles with different properties are required in future studies to better understand the potential role of MPs in in vitro intestinal co-culture models.
MPs internalized by intestinal cells may reach the liver via the body circulation and cause damage, and the liver has been reported to be one of the major organs for the metastasis and accumulation of MPs. Many researchers have conducted studies on hepatocytes. Research indicated that PS-NPs (approximately 50 nm) treatment induces oxidative damage in HepG2 cells (He et al., 2020). Decreased cell viability, increased apoptosis and lipid peroxidation were observed in SMMC-7721 cells treated with PS-MPs (500 nm) (Wang et al., 2021).
Overall, MPs can be absorbed by cells in the gastrointestinal tract and produce toxic effects. Oral ingestion is the main source of human exposure to MPs, therefore it is of significance to explore the toxicity of MPs in cells of the gastrointestinal tract. However, there are still relatively few studies on gastrointestinal cells and more research is needed in other gastric cells. Furthermore, when designing doses for in vitro studies, there is less consideration of the oral MPs exposure dose in humans.
Respiratory cells Inhalation of airborne MPs through breathing is another important route of human exposure to MPs. Based on current studies, MPs can induce respiratory cytotoxic effects, such as a decrease in cell viability, inflammatory effects, oxidative stress, and damage to cell membrane integrity. PS particles are the main type of polymer studied in respiratory cells and can be transported over long distances through the atmosphere. Dong et al. used normal human lung epithelial BEAS-2B cells to determine the potential risk of PS-MPs causing adverse pulmonary effects. High concentrations of PS-MPs (1000 μg/cm2) or prolonged (48 h) exposure were found to cause cytotoxic and inflammatory effects in BEAS-2B cells by inducing ROS formation, which may further lead to COPD (chronic obstructive pulmonary disease), asthma and respiratory allergy. This suggests that MPs exposure may lead to the development of occupational diseases in specific jobs, such as plastic production workers, textile workers, tire manufacturing workers, sanitation workers, etc. Furthermore, a key pathological feature in the development of COPD is the loss of airway epithelial barrier integrity. Studies have shown that both low levels (10 μg/cm2) and high levels (1000 μg/cm2) of PS-MPs might disrupt the protective pulmonary barrier by reducing the expression of tight junction (TJ) proteins (Dong et al., 2020). Xu et al. found that both PS-NP25 (25 nm diameter) and PS-NP70 (70 nm diameter) accumulated in the cytoplasm of A549 cells in a time-dependent manner, reaching a peak value after co-incubation for 30 min to 1 h. And such PS-NPs significantly affected cell viability, caused cell cycle S-phase arrest, activated inflammatory gene transcription and upregulated the expression levels of pro-apoptosis-related proteins (Xu et al., 2019). Overall, MPs/NPs can be internalized by respiratory cells and trigger toxic effects. In future studies, more types and longer exposure times need to be tested to comprehensively assess the impact of MPs/NPs on the respiratory system.
Immune cells Immune cells are cells involved in or associated with the immune response, including innate lymphocytes, various phagocytes, and lymphocytes. Current studies have shown that MPs reduce immune cell activity, stimulate immune cells, cause inflammation and induce intracellular oxidative stress, hemolysis, and DNA damage (Ballesteros et al., 2020; Choi et al., 2020, 2021; Hwang et al., 2019, 2020; Rubio et al., 2020). Different immune cells internalize MPs to different degrees, and the resulting adverse reactions vary. For PS-related toxicity in vitro, a few kinds of immune cell types were tested, including HDFs, HMC-1, PBMCs, THP-1, Hs27 cell line and other types. Rubio et al. used three types of human leukocytic cell lines, Raji-B (B-lymphocytes), TK6 (lymphoblasts) and THP-1 (monocytes) to determine the toxicity of PS (500 nm) at concentrations of 5, 10, 25 and 50 μg/mL. They found that although monocytic THP-1 cells showed the highest ability to internalize particles, no significant cytotoxic effects were observed in THP-1 cells. However, mild toxicity, ROS production and genotoxicity were detected in lymphocytes Raji-B and TK6 with less particle uptake (Rubio et al., 2020). Ballesteros et al. found that PS-NPs induced a significant increase in DNA damage in monocytes at high concentrations (100 μg/mL for 24 h) but not in lymphocytes and polymorphonuclear cells (Ballesteros et al., 2020). PS particles were used to investigate biological effects on immune cells in majority of these studies. Studies using different plastic polymers are needed to conduct to clearly understand effects of various plastic types on immune cells.
Other human-derived cells MPs may reach other sub-organs via the body circulation and have potentially harmful effects on other cells. The toxicity of microplastic particles has also been explored in other human-derived cells, including human embryonic stem cells, brain cells, and breast cancer cells. Bojic et al. found that MPs influenced early human development and caused changes in pluripotency genes and transforming growth factor-beta genes related to eye and atrioventricular heart valve development in EBls and hiPSCs. (Bojic et al., 2020). In a study reported by Schirinzi et al., PS induced higher ROS generation in T98G and HeLa cells (Schirinzi et al., 2017). In addition, PS nanoparticles have a synergistic effect with parabens, increasing the proliferation of parabensy-induced breast cancer cells and greatly promoting the development of breast cancer, which may be related to the translocation and adsorption properties of nanoplastics (Roje et al., 2019).
Conclusions and future perspectives
We firstly conducted bibliometrics to analyze the literature related to MPs in the WoS database over the last decade. It enables the visualization of publications, journals, authors, countries, institutions to obtain a comprehensive overview of the latest research progress on MPs. Considering the large number of publications of 2019–2021, this study suggests that MPs are a relatively new research topic that is receiving increasing attention. The data shows that Shi, HH, Koelmans, AA, Thompson, RC are the main authors with a high number of publications. China, the USA, Germany are the top three most productive countries. In addition, the data shows that there is a lack of research on microplastics in developing countries, indicating a relatively promising area for conducting research on MPs in developing countries. Besides, the major research institutions and inter-institutional collaboration were obtained in the study, which facilitates researchers in choosing institutions that are best suited for exchanges and learning. Papers are published in journals with high impact factors, including Marine Pollution Bulletin, Science of the Total Environment, and Environmental Pollution, indicating a relevant scientific interest of the theme. Finally, we further summarize the techniques for MPs characterization in human tissues and terrestrial higher mammalian tissues, and toxic effects based on mice/rats and human-derived cells exposed to MPs.
Overall, the evidence from in vitro, animal and human studies is rapidly increasing, suggesting a health risk to the public induced by MPs. However, there are limitations in the perception of the health risks of MPs. Therefore, the following high-priority should be explored in the direction of current and future research on the health risks and toxicity of MPs. Firstly, there are numerous types of MPs, however, only a few toxicity studies have been performed on individual types of MPs. Additionally, there is a lack of comparative toxicity studies on different types of MPs. Secondly, the metering methods of MPs dose used in certain toxicity studies are different, which leads to poor comparability of results. Thirdly, most toxicological tests have been conducted with higher concentrations of MPs than environmentally relevant concentrations.
Hence, we propose that researchers in the future should focus on the following aspects: (1) In future toxicological tests, research on different types of MPs should be strengthened, and environmentally relevant concentrations of MPs should be used. (2) Increasing monitoring of MPs in the environment (including soil, freshwater environment, and atmosphere) to better understand MPs contamination in the environment. (3) Proposing international authorities to establish standard procedures for pre-treatment, identification and quantification that can be recognized by all.. (4) Focusing on the association between MPs and pre-existing diseases, such as people with pre-existed gastrointestinal diseases, who are more likely to be harmed by MPs. Animal models should be used to study the susceptibility of children, the elderly and pregnant women to MPs.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
JYL, FX, KX, YQP, JWH, contributed to the collection and assembly of data, and data analysis. MML contributed to the collection and assembly of data, data analysis and interpretation, and manuscript writing and editing. YPP, JZ and KPC reviewed the manuscript. RLS conceived and designed the project, provided final approval of the manuscript, and acquired funding. The final version of the manuscript was read and approved by all authors.
Funding
This research was supported by the Fundamental Research Funds for the Central University (2242018K40019, 2242018K3DN25), the Support Project of SEU (2242019R40050), and National Undergraduate Innovation and Entrepreneurship Training Program innovative Training Project (202110286153).
Data availability
The data and materials that support the findings of this study are openly available.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rongli Sun, Email: 101012172@seu.edu.cn.
Keping Cheng, Email: chengkeping@sina.com.
References
- Alimi OS, Farner Budarz J, Hernandez LM, Tufenkji N. Microplastics and nanoplastics in aquatic environments: Aggregation, deposition, and enhanced contaminant transport. Environmental Science and Technology. 2018;52(4):1704–1724. doi: 10.1021/acs.est.7b05559. [DOI] [PubMed] [Google Scholar]
- Amato-Lourenco LF, Dos Santos Galvao L, de Weger LA, Hiemstra PS, Vijver MG, Mauad T. An emerging class of air pollutants: Potential effects of microplastics to respiratory human health? Science of the Total Environment. 2020;749:141676. doi: 10.1016/j.scitotenv.2020.141676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato-Lourenco LF, Carvalho-Oliveira R, Junior GR, Dos Santos Galvao L, Ando RA, Mauad T. Presence of airborne microplastics in human lung tissue. Journal of Hazardous Materials. 2021;416:126124. doi: 10.1016/j.jhazmat.2021.126124. [DOI] [PubMed] [Google Scholar]
- An R, Wang X, Yang L, Zhang J, Wang N, Xu F, Hou Y, Zhang H, Zhang L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology. 2021;449:152665. doi: 10.1016/j.tox.2020.152665. [DOI] [PubMed] [Google Scholar]
- Andrady AL. Microplastics in the marine environment. Marine Pollution Bulletin. 2011;62(8):1596–1605. doi: 10.1016/j.marpolbul.2011.05.030. [DOI] [PubMed] [Google Scholar]
- Ateia M, Kanan A, Karanfil T. Microplastics release precursors of chlorinated and brominated disinfection byproducts in water. Chemosphere. 2020;251:126452. doi: 10.1016/j.chemosphere.2020.126452. [DOI] [PubMed] [Google Scholar]
- Atis S, Tutluoglu B, Levent E, Ozturk C, Tunaci A, Sahin K, Saral A, Oktay I, Kanik A, Nemery B. The respiratory effects of occupational polypropylene flock exposure. European Respiratory Journal. 2005;25(1):110–117. doi: 10.1183/09031936.04.00138403. [DOI] [PubMed] [Google Scholar]
- Auta HS, Emenike CU, Fauziah SH. Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environment International. 2017;102:165–176. doi: 10.1016/j.envint.2017.02.013. [DOI] [PubMed] [Google Scholar]
- Ballesteros S, Domenech J, Barguilla I, Cortés C, Marcos R, Hernández A. Genotoxic and immunomodulatory effects in human white blood cells after ex vivo exposure to polystyrene nanoplastics. Environmental Science Nano. 2020;7(11):3431–3446. doi: 10.1039/d0en00748j. [DOI] [Google Scholar]
- Banerjee A, Shelver WL. Micro- and nanoplastic induced cellular toxicity in mammals: A review. Science of the Total Environment. 2021;755(Pt 2):142518. doi: 10.1016/j.scitotenv.2020.142518. [DOI] [PubMed] [Google Scholar]
- Barroso E, Ibanez MD, Aranda FI, Romero S. Polyethylene flock-associated interstitial lung disease in a Spanish female. European Respiratory Journal. 2002;20(6):1610–1612. doi: 10.1183/09031936.02.00030102. [DOI] [PubMed] [Google Scholar]
- Beriot N, Peek J, Zornoza R, Geissen V, Huerta Lwanga E. Low density-microplastics detected in sheep faeces and soil: A case study from the intensive vegetable farming in Southeast Spain. Science of the Total Environment. 2021;755(Pt 1):142653. doi: 10.1016/j.scitotenv.2020.142653. [DOI] [PubMed] [Google Scholar]
- Bojic S, Falco MM, Stojkovic P, Ljujic B, Gazdic Jankovic M, Armstrong L, Markovic N, Dopazo J, Lako M, Bauer R, Stojkovic M. Platform to study intracellular polystyrene nanoplastic pollution and clinical outcomes. Stem Cells. 2020;38(10):1321–1325. doi: 10.1002/stem.3244. [DOI] [PubMed] [Google Scholar]
- Browne MA, Crump P, Niven SJ, Teuten E, Tonkin A, Galloway T, Thompson R. Accumulation of microplastic on shorelines woldwide: Sources and sinks. Environmental Science and Technology. 2011;45(21):9175–9179. doi: 10.1021/es201811s. [DOI] [PubMed] [Google Scholar]
- Choi D, Bang J, Kim T, Oh Y, Hwang Y, Hong J. In vitro chemical and physical toxicities of polystyrene microfragments in human-derived cells. Journal of Hazardous Materials. 2020;400:123308. doi: 10.1016/j.jhazmat.2020.123308. [DOI] [PubMed] [Google Scholar]
- Choi D, Hwang J, Bang J, Han S, Kim T, Oh Y, Hwang Y, Choi J, Hong J. In vitro toxicity from a physical perspective of polyethylene microplastics based on statistical curvature change analysis. Science of the Total Environment. 2021;752:142242. doi: 10.1016/j.scitotenv.2020.142242. [DOI] [PubMed] [Google Scholar]
- Cole M, Lindeque P, Halsband C, Galloway TS. Microplastics as contaminants in the marine environment: A review. Marine Pollution Bulletin. 2011;62(12):2588–2597. doi: 10.1016/j.marpolbul.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Cortés C, Domenech J, Salazar M, Pastor S, Marcos R, Hernández A. Nanoplastics as a potential environmental health factor: Effects of polystyrene nanoparticles on human intestinal epithelial Caco-2 cells. Environmental Science Nano. 2020;7(1):272–285. doi: 10.1039/c9en00523d. [DOI] [Google Scholar]
- Cox KD, Covernton GA, Davies HL, Dower JF, Juanes F, Dudas SE. Human Consumption of Microplastics. Environmental Science and Technology. 2019;53(12):7068–7074. doi: 10.1021/acs.est.9b01517. [DOI] [PubMed] [Google Scholar]
- da Costa Araujo AP, Malafaia G. Microplastic ingestion induces behavioral disorders in mice: A preliminary study on the trophic transfer effects via tadpoles and fish. Journal of Hazardous Materials. 2021;401:123263. doi: 10.1016/j.jhazmat.2020.123263. [DOI] [PubMed] [Google Scholar]
- Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Science and Reports. 2017;7:46687. doi: 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Zhang Y, Qiao R, Bonilla MM, Yang X, Ren H, Lemos B. Evidence that microplastics aggravate the toxicity of organophosphorus flame retardants in mice (Mus musculus) Journal of Hazardous Materials. 2018;357:348–354. doi: 10.1016/j.jhazmat.2018.06.017. [DOI] [PubMed] [Google Scholar]
- Deng Y, Yan Z, Shen R, Wang M, Huang Y, Ren H, Zhang Y, Lemos B. Microplastics release phthalate esters and cause aggravated adverse effects in the mouse gut. Environment International. 2020;143:105916. doi: 10.1016/j.envint.2020.105916. [DOI] [PubMed] [Google Scholar]
- Deng Y, Yan Z, Shen R, Huang Y, Ren H, Zhang Y. Enhanced reproductive toxicities induced by phthalates contaminated microplastics in male mice (Mus musculus) Journal of Hazardous Materials. 2021;406:124644. doi: 10.1016/j.jhazmat.2020.124644. [DOI] [PubMed] [Google Scholar]
- Dong CD, Chen CW, Chen YC, Chen HH, Lee JS, Lin CH. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. Journal of Hazardous Materials. 2020;385:121575. doi: 10.1016/j.jhazmat.2019.121575. [DOI] [PubMed] [Google Scholar]
- Dris R, Gasperi J, Mirande C, Mandin C, Guerrouache M, Langlois V, Tassin B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environmental Pollution. 2017;221:453–458. doi: 10.1016/j.envpol.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Dumichen E, Eisentraut P, Bannick CG, Barthel AK, Senz R, Braun U. Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere. 2017;174:572–584. doi: 10.1016/j.chemosphere.2017.02.010. [DOI] [PubMed] [Google Scholar]
- E.J., C., S.J., A., H.P., M., B.B., P., & G.R., H. (1972). Polystyrene spherules in coastal waters. Science, 178, 749-750. Doi: 10.1126/science.178.4062.749 [DOI] [PubMed]
- Estrela FN, Guimaraes ATB, Araujo A, Silva FG, Luz TMD, Silva AM, Pereira PS, Malafaia G. Toxicity of polystyrene nanoplastics and zinc oxide to mice. Chemosphere. 2021;271:129476. doi: 10.1016/j.chemosphere.2020.129476. [DOI] [PubMed] [Google Scholar]
- Eugene Garfield P. 100 Citation classics from the Journal of the American Medical Association. JAMA. 1987;257:52–59. doi: 10.1001/jama.1987.03390010056028. [DOI] [PubMed] [Google Scholar]
- Fischer M, Scholz-Bottcher BM. Simultaneous trace identification and quantification of common types of microplastics in environmental samples by pyrolysis-gas chromatography-mass spectrometry. Environmental Science and Technology. 2017;51(9):5052–5060. doi: 10.1021/acs.est.6b06362. [DOI] [PubMed] [Google Scholar]
- Fournier SB, D'Errico JN, Adler DS, Kollontzi S, Goedken MJ, Fabris L, Yurkow EJ, Stapleton PA. Nanopolystyrene translocation and fetal deposition after acute lung exposure during late-stage pregnancy. Particle and Fibre Toxicology. 2020;17(1):55. doi: 10.1186/s12989-020-00385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E, Dekiff JH, Willmeyer J, Nuelle MT, Ebert M, Remy D. Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environmental Science. Processes and Impacts. 2013;15(10):1949–1956. doi: 10.1039/c3em00214d. [DOI] [PubMed] [Google Scholar]
- Gao Y, Ge L, Shi S, Sun Y, Liu M, Wang B, Shang Y, Wu J, Tian J. Global trends and future prospects of e-waste research: A bibliometric analysis. Environmental Science and Pollution Research International. 2019;26(17):17809–17820. doi: 10.1007/s11356-019-05071-8. [DOI] [PubMed] [Google Scholar]
- Gao H, Lin Y, Wei J, Zhang Y, Pan H, Ren M, Li J, Huang L, Zhang X, Huang Q, Shen H. A novel extraction protocol of nano-polystyrene from biological samples. Science of the Total Environment. 2021;790:148085. doi: 10.1016/j.scitotenv.2021.148085. [DOI] [PubMed] [Google Scholar]
- Gennaro V, Ceppi M, Crosignani P, Montanaro F. Reanalysis of updated mortality among vinyl and polyvinyl chloride workers: Confirmation of historical evidence and new findings. BMC Public Health. 2008;8:21. doi: 10.1186/1471-2458-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer R, Jambeck JR, Law KL. Production, use, and fate of all plastics ever made. Science Advances. 2017;3:e1700782. doi: 10.1126/sciadv.1700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber MM, Hirschmugl B, Berger N, Holter M, Radulovic S, Leitinger G, Liesinger L, Berghold A, Roblegg E, Birner-Gruenberger R, Bjelic-Radisic V, Wadsack C. Plasma proteins facilitates placental transfer of polystyrene particles. J Nanobiotechnology. 2020;18(1):128. doi: 10.1186/s12951-020-00676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Q, Jiang J, Huang Y, Wang Q, Liu Z, Ma X, Yang X, Li Y, Wang S, Cui W, Tang J, Wan H, Xu Q, Tu Y, Wu D, Xia Y. The landscape of micron-scale particles including microplastics in human enclosed body fluids. Journal of Hazardous Materials. 2023;442:130138. doi: 10.1016/j.jhazmat.2022.130138. [DOI] [PubMed] [Google Scholar]
- Hartmann NB, Huffer T, Thompson RC, Hassellov M, Verschoor A, Daugaard AE, Rist S, Karlsson T, Brennholt N, Cole M, Herrling MP, Hess MC, Ivleva NP, Lusher AL, Wagner M. Are we speaking the same language? Recommendations for a definition and categorization framework for plastic debris. Environmental Science and Technology. 2019;53(3):1039–1047. doi: 10.1021/acs.est.8b05297. [DOI] [PubMed] [Google Scholar]
- He P, Chen L, Shao L, Zhang H, Lu F. Municipal solid waste (MSW) landfill: A source of microplastics? -Evidence of microplastics in landfill leachate. Water Research. 2019;159:38–45. doi: 10.1016/j.watres.2019.04.060. [DOI] [PubMed] [Google Scholar]
- He Y, Li J, Chen J, Miao X, Li G, He Q, Xu H, Li H, Wei Y. Cytotoxic effects of polystyrene nanoplastics with different surface functionalization on human HepG2 cells. Science of the Total Environment. 2020;723:138180. doi: 10.1016/j.scitotenv.2020.138180. [DOI] [PubMed] [Google Scholar]
- Hesler M, Aengenheister L, Ellinger B, Drexel R, Straskraba S, Jost C, Wagner S, Meier F, von Briesen H, Buchel C, Wick P, Buerki-Thurnherr T, Kohl Y. Multi-endpoint toxicological assessment of polystyrene nano- and microparticles in different biological models in vitro. Toxicology in Vitro. 2019;61:104610. doi: 10.1016/j.tiv.2019.104610. [DOI] [PubMed] [Google Scholar]
- Horton AA, Walton A, Spurgeon DJ, Lahive E, Svendsen C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Science of the Total Environment. 2017;586:127–141. doi: 10.1016/j.scitotenv.2017.01.190. [DOI] [PubMed] [Google Scholar]
- Horvatits T, Tamminga M, Liu B, Sebode M, Carambia A, Fischer L, Puschel K, Huber S, Fischer EK. Microplastics detected in cirrhotic liver tissue. eBioMedicine. 2022;82:104147. doi: 10.1016/j.ebiom.2022.104147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou B, Wang F, Liu T, Wang Z. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. Journal of Hazardous Materials. 2021;405:124028. doi: 10.1016/j.jhazmat.2020.124028. [DOI] [PubMed] [Google Scholar]
- Hou J, Lei Z, Cui L, Hou Y, Yang L, An R, Wang Q, Li S, Zhang H, Zhang L. Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicology and Environmental Safety. 2021;212:112012. doi: 10.1016/j.ecoenv.2021.112012. [DOI] [PubMed] [Google Scholar]
- Huang W, Song B, Liang J, Niu Q, Zeng G, Shen M, Deng J, Luo Y, Wen X, Zhang Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. Journal of Hazardous Materials. 2021;405:124187. doi: 10.1016/j.jhazmat.2020.124187. [DOI] [PubMed] [Google Scholar]
- Huang S, Huang X, Bi R, Guo Q, Yu X, Zeng Q, Huang Z, Liu T, Wu H, Chen Y, Xu J, Wu Y, Guo P. Detection and analysis of microplastics in human sputum. Environmental Science and Technology. 2022;56(4):2476–2486. doi: 10.1021/acs.est.1c03859. [DOI] [PubMed] [Google Scholar]
- Hwang J, Choi D, Han S, Choi J, Hong J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Science of the Total Environment. 2019;684:657–669. doi: 10.1016/j.scitotenv.2019.05.071. [DOI] [PubMed] [Google Scholar]
- Hwang J, Choi D, Han S, Jung SY, Choi J, Hong J. Potential toxicity of polystyrene microplastic particles. Science and Reports. 2020;10(1):7391. doi: 10.1038/s41598-020-64464-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim YS, Tuan AS, Azmi AA, Wan MKWMA, Lehata S, Hamzah SR, Ismail D, Ma ZF, Dzulkarnaen A, Zakaria Z, Mustaffa N, Tuan SSE, Lee YY. Detection of microplastics in human colectomy specimens. JGH Open. 2021;5(1):116–121. doi: 10.1002/jgh3.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner LC, Rotchell JM, Bennett RT, Cowen M, Tentzeris V, Sadofsky LR. Detection of microplastics in human lung tissue using muFTIR spectroscopy. Science of the Total Environment. 2022;831:154907. doi: 10.1016/j.scitotenv.2022.154907. [DOI] [PubMed] [Google Scholar]
- Jiang B, Kauffman AE, Li L, McFee W, Cai B, Weinstein J, Lead JR, Chatterjee S, Scott GI, Xiao S. Health impacts of environmental contamination of micro- and nanoplastics: A review. Environmental Health and Preventive Medicine. 2020;25(1):29. doi: 10.1186/s12199-020-00870-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Lu L, Tu W, Luo T, Fu Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Science of the Total Environment. 2019;649:308–317. doi: 10.1016/j.scitotenv.2018.08.353. [DOI] [PubMed] [Google Scholar]
- Jin H, Ma T, Sha X, Liu Z, Zhou Y, Meng X, Chen Y, Han X, Ding J. Polystyrene microplastics induced male reproductive toxicity in mice. Journal of Hazardous Materials. 2021;401:123430. doi: 10.1016/j.jhazmat.2020.123430. [DOI] [PubMed] [Google Scholar]
- Kampf G, Todt D, Pfaender S, Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. Journal of Hospital Infection. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid N, Aqeel M, Noman A, Hashem M, Mostafa YS, Alhaithloul HAS, Alghanem SM. Linking effects of microplastics to ecological impacts in marine environments. Chemosphere. 2021;264(Pt 2):128541. doi: 10.1016/j.chemosphere.2020.128541. [DOI] [PubMed] [Google Scholar]
- Kumar M, Xiong X, He M, Tsang DCW, Gupta J, Khan E, Harrad S, Hou D, Ok YS, Bolan NS. Microplastics as pollutants in agricultural soils. Environmental Pollution. 2020;265(Pt A):114980. doi: 10.1016/j.envpol.2020.114980. [DOI] [PubMed] [Google Scholar]
- Kutralam-Muniasamy G, Shruti VC, Perez-Guevara F, Roy PD. Microplastic diagnostics in humans: "The 3Ps" Progress, problems, and prospects. Science of the Total Environment. 2022;856(Pt 2):159164. doi: 10.1016/j.scitotenv.2022.159164. [DOI] [PubMed] [Google Scholar]
- Lee J, Chae KJ. A systematic protocol of microplastics analysis from their identification to quantification in water environment: A comprehensive review. Journal of Hazardous Materials. 2021;403:124049. doi: 10.1016/j.jhazmat.2020.124049. [DOI] [PubMed] [Google Scholar]
- Lehner R, Wohlleben W, Septiadi D, Landsiedel R, Petri-Fink A, Rothen-Rutishauser B. A novel 3D intestine barrier model to study the immune response upon exposure to microplastics. Archives of Toxicology. 2020;94(7):2463–2479. doi: 10.1007/s00204-020-02750-1. [DOI] [PubMed] [Google Scholar]
- Leslie HA, van Velzen MJM, Brandsma SH, Vethaak AD, Garcia-Vallejo JJ, Lamoree MH. Discovery and quantification of plastic particle pollution in human blood. Environment International. 2022;163:107199. doi: 10.1016/j.envint.2022.107199. [DOI] [PubMed] [Google Scholar]
- Li B, Ding Y, Cheng X, Sheng D, Xu Z, Rong Q, Wu Y, Zhao H, Ji X, Zhang Y. Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere. 2020;244:125492. doi: 10.1016/j.chemosphere.2019.125492. [DOI] [PubMed] [Google Scholar]
- Lichtman JW, Conchello JA. Fluorescence microscopy. Nature Methods. 2005;2(12):910–919. doi: 10.1038/nmeth817. [DOI] [PubMed] [Google Scholar]
- Lim D, Jeong J, Song KS, Sung JH, Oh SM, Choi J. Inhalation toxicity of polystyrene micro(nano)plastics using modified OECD TG 412. Chemosphere. 2021;262:128330. doi: 10.1016/j.chemosphere.2020.128330. [DOI] [PubMed] [Google Scholar]
- Liu K, Wang X, Fang T, Xu P, Zhu L, Li D. Source and potential risk assessment of suspended atmospheric microplastics in Shanghai. Science of the Total Environment. 2019;675:462–471. doi: 10.1016/j.scitotenv.2019.04.110. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu X, Guo J, Yang R, Wang H, Sun Y, Chen B, Dong R. The association between microplastics and microbiota in placentas and meconium: The first evidence in humans. Environmental Science and Technology. 2022 doi: 10.1021/acs.est.2c04706. [DOI] [PubMed] [Google Scholar]
- Lu L, Wan Z, Luo T, Fu Z, Jin Y. Polystyrene microplastics induce gut microbiota dysbiosis and hepatic lipid metabolism disorder in mice. Science of the Total Environment. 2018;631–632:449–458. doi: 10.1016/j.scitotenv.2018.03.051. [DOI] [PubMed] [Google Scholar]
- Luo T, Wang C, Pan Z, Jin C, Fu Z, Jin Y. Maternal polystyrene microplastic exposure during gestation and lactation altered metabolic homeostasis in the dams and their F1 and F2 offspring. Environmental Science and Technology. 2019;53(18):10978–10992. doi: 10.1021/acs.est.9b03191. [DOI] [PubMed] [Google Scholar]
- Luo T, Zhang Y, Wang C, Wang X, Zhou J, Shen M, Zhao Y, Fu Z, Jin Y. Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environmental Pollution. 2019;255(Pt 1):113122. doi: 10.1016/j.envpol.2019.113122. [DOI] [PubMed] [Google Scholar]
- Magri D, Sanchez-Moreno P, Caputo G, Gatto F, Veronesi M, Bardi G, Catelani T, Guarnieri D, Athanassiou A, Pompa PP, Fragouli D. Laser ablation as a versatile tool to mimic polyethylene terephthalate nanoplastic pollutants: Characterization and toxicology assessment. ACS Nano. 2018;12(8):7690–7700. doi: 10.1021/acsnano.8b01331. [DOI] [PubMed] [Google Scholar]
- Mallik A, Xavier KAM, Naidu BC, Nayak BB. Ecotoxicological and physiological risks of microplastics on fish and their possible mitigation measures. Science of the Total Environment. 2021;779:146433. doi: 10.1016/j.scitotenv.2021.146433. [DOI] [PubMed] [Google Scholar]
- Monteleone A, Schary W, Fath A, Wenzel F. Validation of an extraction method for microplastics from human materials. Clinical Hemorheology and Microcirculation. 2019;73(1):203–217. doi: 10.3233/CH-199209. [DOI] [PubMed] [Google Scholar]
- Park EJ, Han JS, Park EJ, Seong E, Lee GH, Kim DW, Son HY, Han HY, Lee BS. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicology Letters. 2020;324:75–85. doi: 10.1016/j.toxlet.2020.01.008. [DOI] [PubMed] [Google Scholar]
- Penalver R, Arroyo-Manzanares N, Lopez-Garcia I, Hernandez-Cordoba M. An overview of microplastics characterization by thermal analysis. Chemosphere. 2020;242:125170. doi: 10.1016/j.chemosphere.2019.125170. [DOI] [PubMed] [Google Scholar]
- Petersen F, Hubbart JA. The occurrence and transport of microplastics: The state of the science. Science of the Total Environment. 2021;758:143936. doi: 10.1016/j.scitotenv.2020.143936. [DOI] [PubMed] [Google Scholar]
- Prata JC. Airborne microplastics: Consequences to human health? Environmental Pollution. 2018;234:115–126. doi: 10.1016/j.envpol.2017.11.043. [DOI] [PubMed] [Google Scholar]
- Prata JC, da Costa JP, Lopes I, Duarte AC, Rocha-Santos T. Environmental exposure to microplastics: An overview on possible human health effects. Science of the Total Environment. 2020;702:134455. doi: 10.1016/j.scitotenv.2019.134455. [DOI] [PubMed] [Google Scholar]
- Prust M, Meijer J, Westerink RHS. The plastic brain: Neurotoxicity of micro- and nanoplastics. Particle and Fibre Toxicology. 2020;17(1):24. doi: 10.1186/s12989-020-00358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafiee M, Dargahi L, Eslami A, Beirami E, Jahangiri-Rad M, Sabour S, Amereh F. Neurobehavioral assessment of rats exposed to pristine polystyrene nanoplastics upon oral exposure. Chemosphere. 2018;193:745–753. doi: 10.1016/j.chemosphere.2017.11.076. [DOI] [PubMed] [Google Scholar]
- Ragusa A, Svelato A, Santacroce C, Catalano P, Notarstefano V, Carnevali O, Papa F, Rongioletti MCA, Baiocco F, Draghi S, D'Amore E, Rinaldo D, Matta M, Giorgini E. Plasticenta: First evidence of microplastics in human placenta. Environment International. 2021;146:106274. doi: 10.1016/j.envint.2020.106274. [DOI] [PubMed] [Google Scholar]
- Ragusa A, Notarstefano V, Svelato A, Belloni A, Gioacchini G, Blondeel C, Zucchelli E, De Luca C, D'Avino S, Gulotta A, Carnevali O, Giorgini E. Raman microspectroscopy detection and characterisation of microplastics in human breastmilk. Polymers (Basel) 2022 doi: 10.3390/polym14132700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roje Z, Ilic K, Galic E, Pavicic I, Turcic P, Stanec Z, Vrcek IV. Synergistic effects of parabens and plastic nanoparticles on proliferation of human breast cancer cells. Arhiv Za Higijenu Rada i Toksikologiju. 2019;70(4):310–314. doi: 10.2478/aiht-2019-70-3372. [DOI] [PubMed] [Google Scholar]
- Rubio L, Barguilla I, Domenech J, Marcos R, Hernandez A. Biological effects, including oxidative stress and genotoxic damage, of polystyrene nanoparticles in different human hematopoietic cell lines. Journal of Hazardous Materials. 2020;398:122900. doi: 10.1016/j.jhazmat.2020.122900. [DOI] [PubMed] [Google Scholar]
- Schirinzi GF, Perez-Pomeda I, Sanchis J, Rossini C, Farre M, Barcelo D. Cytotoxic effects of commonly used nanomaterials and microplastics on cerebral and epithelial human cells. Environmental Research. 2017;159:579–587. doi: 10.1016/j.envres.2017.08.043. [DOI] [PubMed] [Google Scholar]
- Schwabl P, Koppel S, Konigshofer P, Bucsics T, Trauner M, Reiberger T, Liebmann B. Detection of various microplastics in human stool: A prospective case series. Annals of Internal Medicine. 2019;171(7):453–457. doi: 10.7326/M19-0618. [DOI] [PubMed] [Google Scholar]
- Shan J, Zhao J, Liu L, Zhang Y, Wang X, Wu F. A novel way to rapidly monitor microplastics in soil by hyperspectral imaging technology and chemometrics. Environmental Pollution. 2018;238:121–129. doi: 10.1016/j.envpol.2018.03.026. [DOI] [PubMed] [Google Scholar]
- Shi J, Shi S, Shi S, Jia Q, Yuan G, Chu Y, Wang H, Hu Y, Cui H. Bibliometric analysis of potassium channel research. Channels (austin, Tex.) 2020;14(1):18–27. doi: 10.1080/19336950.2019.1705055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz Z, Kintzi A, Muñoz K, Schaumann GE. A simple method for the selective quantification of polyethylene, polypropylene, and polystyrene plastic debris in soil by pyrolysis-gas chromatography/mass spectrometry. Journal of Analytical and Applied Pyrolysis. 2020 doi: 10.1016/j.jaap.2020.104803. [DOI] [Google Scholar]
- Stock V, Bohmert L, Lisicki E, Block R, Cara-Carmona J, Pack LK, Selb R, Lichtenstein D, Voss L, Henderson CJ, Zabinsky E, Sieg H, Braeuning A, Lampen A. Uptake and effects of orally ingested polystyrene microplastic particles in vitro and in vivo. Archives of Toxicology. 2019;93(7):1817–1833. doi: 10.1007/s00204-019-02478-7. [DOI] [PubMed] [Google Scholar]
- Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AW, McGonigle D, Russell AE. Lost at sea: Where is all the plastic? Science. 2004;304(5672):838. doi: 10.1126/science.1094559. [DOI] [PubMed] [Google Scholar]
- Thrift E, Porter A, Galloway TS, Coomber FG, Mathews F. Ingestion of plastics by terrestrial small mammals. Science of the Total Environment. 2022;842:156679. doi: 10.1016/j.scitotenv.2022.156679. [DOI] [PubMed] [Google Scholar]
- Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc'h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. Journal of Bone and Joint Surgery. American Volume. 2000;82(4):457–476. doi: 10.2106/00004623-200004000-00002. [DOI] [PubMed] [Google Scholar]
- van Wezel A, Caris I, Kools SA. Release of primary microplastics from consumer products to wastewater in the Netherlands. Environmental Toxicology and Chemistry. 2016;35(7):1627–1631. doi: 10.1002/etc.3316. [DOI] [PubMed] [Google Scholar]
- Vobecky J, Devroede G, Caro J. Risk of large-bowel cancer in synthetic fiber manufacture. Cancer. 1984;54(11):2537–2542. doi: 10.1002/1097-0142(19841201)54:11<2537::AID-CNCR2820541138>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Wang Q, Bai J, Ning B, Fan L, Sun T, Fang Y, Wu J, Li S, Duan C, Zhang Y, Liang J, Gao Z. Effects of bisphenol A and nanoscale and microscale polystyrene plastic exposure on particle uptake and toxicity in human Caco-2 cells. Chemosphere. 2020;254:126788. doi: 10.1016/j.chemosphere.2020.126788. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang Y, Xu M, Ma J, Zhang S, Liu S, Wang K, Tian H, Cui J. Enhanced hepatic cytotoxicity of chemically transformed polystyrene microplastics by simulated gastric fluid. Journal of Hazardous Materials. 2021;410:124536. doi: 10.1016/j.jhazmat.2020.124536. [DOI] [PubMed] [Google Scholar]
- Wei J, Wang X, Liu Q, Zhou N, Zhu S, Li Z, Li X, Yao J, Zhang L. The impact of polystyrene microplastics on cardiomyocytes pyroptosis through NLRP3/Caspase-1 signaling pathway and oxidative stress in Wistar rats. Environmental Toxicology. 2021;36(5):935–944. doi: 10.1002/tox.23095. [DOI] [PubMed] [Google Scholar]
- Wright SL, Kelly FJ. Plastic and human health: A micro issue? Environmental Science and Technology. 2017;51(12):6634–6647. doi: 10.1021/acs.est.7b00423. [DOI] [PubMed] [Google Scholar]
- Wright SL, Ulke J, Font A, Chan KLA, Kelly FJ. Atmospheric microplastic deposition in an urban environment and an evaluation of transport. Environment International. 2020;136:105411. doi: 10.1016/j.envint.2019.105411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Wu X, Liu S, Wang Z, Chen L. Size-dependent effects of polystyrene microplastics on cytotoxicity and efflux pump inhibition in human Caco-2cells. Chemosphere. 2019;221:333–341. doi: 10.1016/j.chemosphere.2019.01.056. [DOI] [PubMed] [Google Scholar]
- Xie X, Deng T, Duan J, Xie J, Yuan J, Chen M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicology and Environmental Safety. 2020;190:110133. doi: 10.1016/j.ecoenv.2019.110133. [DOI] [PubMed] [Google Scholar]
- Xie WQ, He M, Yu DJ, Wu YX, Wang XH, Lv S, Xiao WF, Li YS. Mouse models of sarcopenia: Classification and evaluation. Journal of Cachexia, Sarcopenia and Muscle. 2021;12(3):538–554. doi: 10.1002/jcsm.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Halimu G, Zhang Q, Song Y, Fu X, Li Y, Li Y, Zhang H. Internalization and toxicity: A preliminary study of effects of nanoplastic particles on human lung epithelial cell. Science of the Total Environment. 2019;694:133794. doi: 10.1016/j.scitotenv.2019.133794. [DOI] [PubMed] [Google Scholar]
- Yan Z, Zhao H, Zhao Y, Zhu Q, Qiao R, Ren H, Zhang Y. An efficient method for extracting microplastics from feces of different species. Journal of Hazardous Materials. 2020;384:121489. doi: 10.1016/j.jhazmat.2019.121489. [DOI] [PubMed] [Google Scholar]
- Yan X, Zhang Y, Lu Y, He L, Qu J, Zhou C, Hong P, Sun S, Zhao H, Liang Y, Ren L, Zhang Y, Chen J, Li C. The complex toxicity of tetracycline with polystyrene spheres on gastric cancer cells. International Journal of Environmental Research and Public Health. 2020 doi: 10.3390/ijerph17082808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong CQY, Valiyaveettil S, Tang BL. Toxicity of microplastics and nanoplastics in mammalian systems. International Journal of Environmental Research and Public Health. 2020 doi: 10.3390/ijerph17051509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Kang S, Allen S, Allen D, Gao T, Sillanpää M. Atmospheric microplastics: A review on current status and perspectives. Earth-Science Reviews. 2020 doi: 10.1016/j.earscirev.2020.103118. [DOI] [Google Scholar]
- Zhang Y, Pu S, Lv X, Gao Y, Ge L. Global trends and prospects in microplastics research: A bibliometric analysis. Journal of Hazardous Materials. 2020;400:123110. doi: 10.1016/j.jhazmat.2020.123110. [DOI] [PubMed] [Google Scholar]
- Zhang N, Li YB, He HR, Zhang JF, Ma GS. You are what you eat: Microplastics in the feces of young men living in Beijing. Science of the Total Environment. 2021;767:144345. doi: 10.1016/j.scitotenv.2020.144345. [DOI] [PubMed] [Google Scholar]
- Zheng H, Wang J, Wei X, Chang L, Liu S. Proinflammatory properties and lipid disturbance of polystyrene microplastics in the livers of mice with acute colitis. Science of the Total Environment. 2021;750:143085. doi: 10.1016/j.scitotenv.2020.143085. [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhu J, Zuo R, Xu Q, Qian Y, An L. Identification of microplastics in human placenta using laser direct infrared spectroscopy. Science of the Total Environment. 2022;856(Pt 1):159060. doi: 10.1016/j.scitotenv.2022.159060. [DOI] [PubMed] [Google Scholar]
- Zou X, Yue WL, Vu HL. Visualization and analysis of mapping knowledge domain of road safety studies. Accident Analysis and Prevention. 2018;118:131–145. doi: 10.1016/j.aap.2018.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and materials that support the findings of this study are openly available.