Abstract
Immunohistochemistry is a valuable technique that provides information on protein localization and interactions in tissues. Mosquito salivary gland immunohistochemistry requires the meticulous dissection of a delicate tissue. The integrity of the salivary glands must be closely monitored throughout the entire process to prevent structural damage and loss of saliva. This protocol describes a series of simple steps to perform salivary gland immunohistochemistry including tissue dissection, permeabilization, immunostaining, mounting, and imaging by confocal microscopy.
MATERIALS
It is essential that you consult the appropriate Material Safety Data Sheets and your institution’s Environmental Health and Safety Office for proper handling of equipment and hazardous materials used in this protocol.
RECIPES: Please see the end of this protocol for recipes indicated by <R>. Additional recipes can be found online at http://cshprotocols.cshlp.org/site/recipes.
Reagents
Alexa Fluor 488 phalloidin (2-μg/mL; Thermo Fisher Scientific A12379)
CO2 for anesthetizing mosquitoes (see Step 1)
-
Custom primary antibodies raised in mouse against salivary proteins of interest such as maltases, D7 long form proteins, or CqDVP proteins
These are available from the authors upon request.
DAPI (1-mg/mL; Sigma-Aldrich D9542)
Ethanol (70%)
Immunohistochemistry blocking buffer <R>
Immunohistochemistry washing buffer <R>
-
Mosquitoes (5- to 7-d-old females, raised as described in Ote and Kanuka 2018)
The protocol can be used for both female and male mosquitoes. However, female mosquitoes are the only ones that transmit diseases, so only salivary glands from females have been described here.
Paraformaldehyde (Electron Microscopy Science 15710), diluted to 4% (v/v) in distilled H2O
Phosphate buffered saline (PBS), pH 7.4 (Gibco 10010-023)
ProLong Gold antifade reagent with DAPI mounting medium (Invitrogen P36935)
Texas Red-X goat anti-mouse IgG (H + L) fluorescent secondary antibody (Thermo Fisher T6390)
Equipment
Aluminum sealing foil
Computer with Imaris software version 9.2.1
Corning PYREX 9 Depression Glass Spot Plates, 85 × 100-mm, with well dimensions of 22-mm (outer diameter) × 7-mm (deep) (Thomas Scientific 7812G17)
Entomological tweezers (Dumont No. 5)
Glass concave well slides
-
Homemade dissection needles
To prepare these needles, insert and glue entomological BioQuip minutien stainless steel short pins, number 20, with a 0.20-mm diameter (EntoSPHINX manufactured for BioQuip 1208SB) to a wooden applicator (Puritan 807) using regular superglue (e.g., Loctite).
Leica SP8 Microscope with a 20× oil immersion objective and a photomultiplier tube/hybrid detector
Microscope coverslips
Microscope glass slides
Mosquito aspirator, electric (PN 419, John W. Hock Company)
Petri dishes
Stereo microscope (Stemi 508, Zeiss)
METHOD
Salivary Gland Dissection
See Coleman et al. (2007) and Fig. 1 for details on dissection.
FIGURE 1.

Immunohistochemistry of mosquito salivary glands. (A) Dissection station. (B) Mosquito aspiration. (C) Fine needles and concave well slide for dissections (Step 2/3). (D) Aedes aegypti female mosquito dissection (Step 3). (E) Stereomicroscope setup. (F) While dissecting, salivary glands are deposited into a glass concave well plate and maintained on ice (Step 6). (G,H) Concave well plate with dissected salivary glands. (I) Ae. aegypti salivary glands surrounded by fat body tissue (Step 5). (J–L) Clean Ae. aegypti, Anopheles gambiae, and Culex quinquefasciatus salivary glands, respectively. (M) Washing step monitored under stereoscope. (N) Salivary glands are transferred to a drop of PBS onto a slide (Step 17). (O) Prolong Gold mounting media is added to a coverslip (Step 19). (P) Leica SP8 confocal microscope (Step 22).
-
1
Pick up approximately 20 mosquitoes with an electronic aspirator. Anesthetize the mosquitoes by applying CO2 for 20–30 sec to the aspirator. Immediately transfer the anesthetized mosquitoes to a Petri dish set on ice.
-
2
Using tweezers, transfer one mosquito to a concave well slide containing three to four drops of cold PBS (Fig 1C).
-
3
Under the stereomicroscope, separate the head from the thorax and body using fine homemade needles (Fig 1D).
-
4
Gently press down on the thorax with one needle while scooping the salivary glands out of the thorax with the other needle.
For salivary gland recognition, see Coleman et al. (2007) and Figure 1I–L.
-
5
Using a needle, transfer the glands to a new drop of cold PBS within the same concave well to separate and remove the fat bodies, which are visualized as diffuse white tissue surrounding the salivary glands (Fig 1I).
See Troubleshooting.
-
6
Transfer the glands to a nine-depression glass spot plate containing cold PBS. Place the plate on ice (Fig 1E–H).
The optimal number of glands is five to 10 glands/well.
Salivary Gland Immunostaining
Perform all buffer exchanges under the microscope to monitor that no glands are lost while pipetting (Fig. 1M). Use incubation volumes of 500 μL unless stated otherwise.
-
7
Remove PBS carefully and fix the tissues with 4% paraformaldehyde for 30 min at room temperature.
No agitation is required.
See Troubleshooting.
-
8
Remove the paraformaldehyde solution and wash the glands three times with PBS for 10 min each wash at room temperature.
The fixed tissues can be stored in PBS at 4°C until further processing (up to 1 yr).
See Troubleshooting.
-
9
Remove PBS and incubate the tissues with blocking buffer for 30 min at room temperature.
The Triton X-100 in the blocking buffer permeabilizes the cell membrane, thus facilitating antibody access into the tissue.
See Troubleshooting.
-
10
Remove blocking buffer by washing three times for 10 min each with washing buffer at room temperature to remove traces of Triton X-100 that might interfere with the antibody binding.
See Troubleshooting.
-
11
Using a needle, separate glands into wells labeled with the appropriate primary antibody name, replace the washing buffer with the primary antibody diluted in washing buffer, and incubate overnight at 4°C.
Most primary antibodies usually work at a concentration of 1–10 μL/mL, although the optimal antibody concentration for each primary antibody should be determined empirically.
Five salivary gland pairs per well is good number with which to start.
See Troubleshooting.
-
12
On the following day, remove the primary antibody solution and wash with PBS three times for 10 min each at room temperature. Incubate for 1 h at room temperature with the appropriate fluorescent secondary antibody diluted in washing buffer.
From this step on, keep concave well dishes protected from the light by covering them with aluminum foil.
See Troubleshooting.
-
13
Remove the secondary antibody solution and wash tissues three times for 10 min each with washing buffer at room temperature.
-
14
Stain DNA and actin, respectively, with 1 μg/mL DAPI and 0.04 μg/mL phalloidin Alexa 488 diluted in PBS for 20 min at room temperature.
-
15
Remove the solution with DAPI and phalloidin and wash tissues three times for 10 min each with washing buffer at room temperature.
Stained salivary glands can be stored in PBS for at least 6 mo at 4°C.
Salivary Gland Mounting
-
16
Clean microscope slides with 70% ethanol, and label them with preparation date and sample information.
-
17
Place three to four individual drops of ~5 μL of PBS onto the slide within a range that would fit under the coverslip (Fig. 1N).
-
18
Carefully, using fine needles, transfer one salivary gland per drop of PBS. Make sure the lobes are well-separated and the medial lobe, which is shorter in length, is located between the two lateral lobes (see Fig. 1 in Introduction: Performing Immunohistochemistry in Mosquito Salivary Glands [Martin-Martin et al. 2022]).
-
19
Once there are three to four glands on the slide, pipette 20–25 μL of ProLong Gold antifade mounting medium onto a coverslip (Fig. 1O). Remove any resulting air bubbles in the mounting medium using the pipette tip.
-
20
To dry the glands and make sure the glands remain well-positioned, remove the remaining PBS by pipetting. Avoid overdrying by immediately covering the glands entirely with the coverslip.
-
21
Leave the slides to dry at room temperature overnight and then preserve at 4°C until imaging.
Imaging
See Figures 2 and 3 for examples of acquired images of salivary glands.
FIGURE 2.
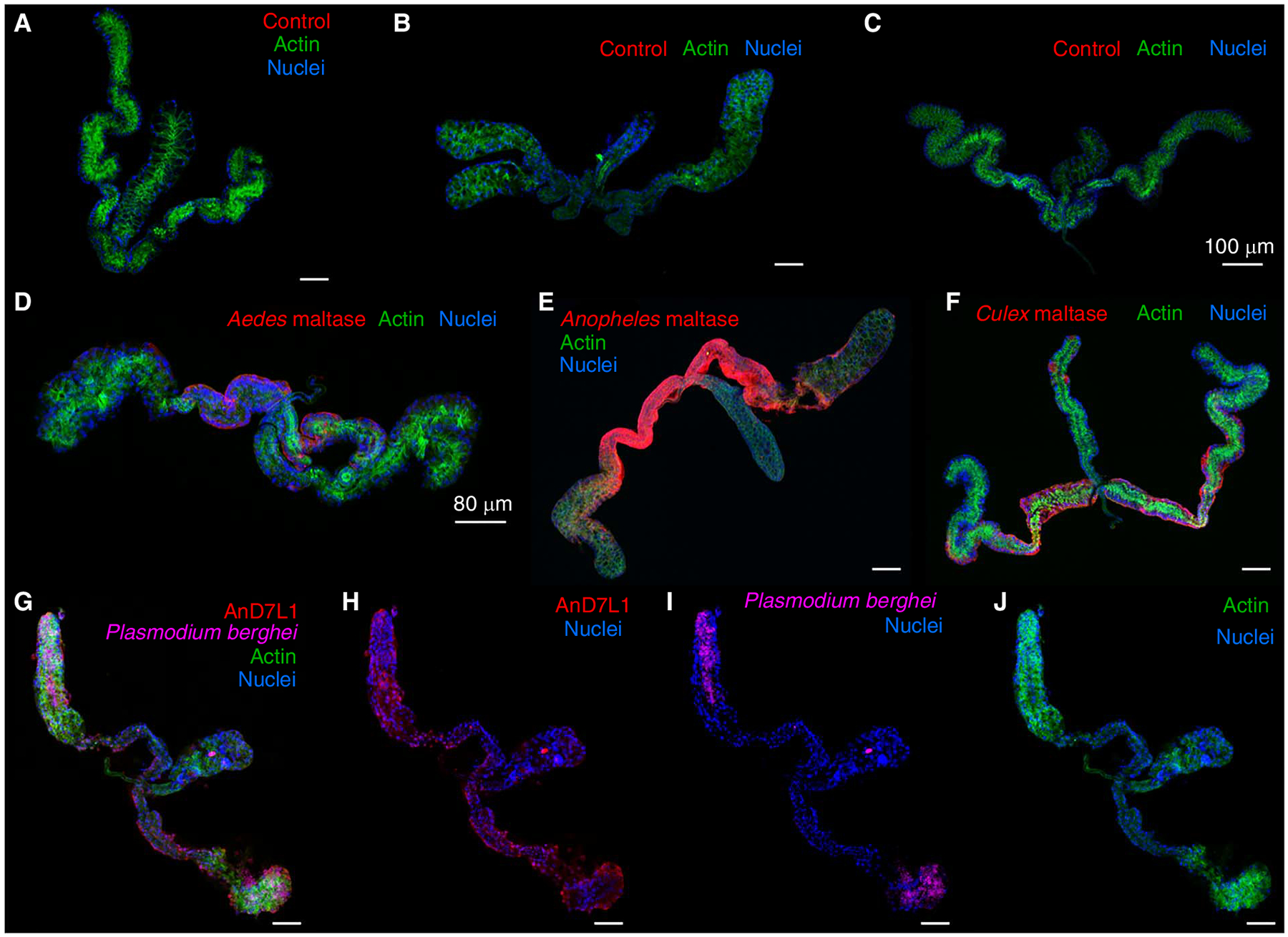
Confocal microscopy images of mosquito salivary glands. Salivary glands of Aedes aegypti (A,D), Anopheles gambiae (B,E), and Culex quinquefasciatus (C,F) stained with mouse antibodies raised against adjuvant only (A–C) or Aedes maltase (AAEL009524) (D), Anopheles maltase (AGAP002102) (E), and Culex maltase (CPIJ016362) (F). Maltases are localized in the proximal lateral lobes. (G–J) An. gambiae salivary gland infected with Plasmodium berghei-green fluorescent protein (GFP). Salivary glands were dissected 24 d after parasite infection and stained with Alexa Fluor 647 mouse anti-GFP fluorescent antibody. (G) An. gambiae salivary D7 long form protein 1 (AGAP008278) showed an ubiquitous localization, whereas P. berghei invaded the distal lateral lobes, as previously described (Wells and Andrew 2019) (H). (I) Merged image showing 498 E and 405 channels. Texas Red-X anti-mouse IgG; actin, stained with Phalloidin 488; and nuclei, stained with DAPI, are shown in red, green, and blue, respectively. Scale bars, 50 μm, unless otherwise indicated in the images.
FIGURE 3.
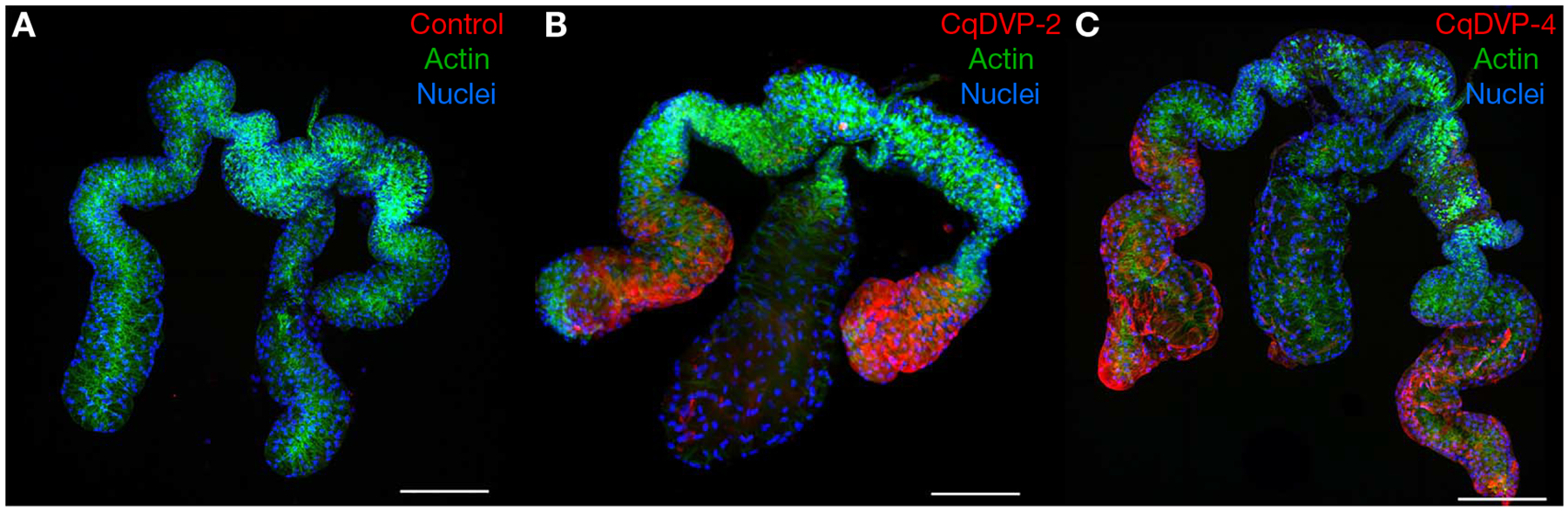
Salivary CqDVP-2 and CqDVP-4 localize to the distal lateral lobes of Culex quinquefasciatus salivary glands. Glands incubated with sera from mice immunized against only adjuvant, CqDVP-2, and CqDVP-4 are shown in A–C, respectively. Texas Red-X anti-mouse IgG; actin, stained with Phalloidin 488; and nuclei, stained with DAPI, are shown in red, green, and blue, respectively. Scale bars, 100 μm. (Modified from Kern et al. 2021.)
-
22
Capture fluorescent images with a Leica SP8 Microscope using a 20× oil immersion objective (using zoom factor 2 or 3; numerical aperture 1.25) equipped with a photomultiplier tube/hybrid detector (Fig. 1P).
See Troubleshooting.
-
23
Visualize salivary glands using a 405 laser (for DAPI) and white light laser. Set peaks of excitation for each fluorophore at 498, 595, and 665 nm when Alexa 488, Texas red, and Alexa 647, respectively, are used as the fluorophores conjugated to the secondary antibodies (Figs. 2 and 3).
-
24
Acquire images using sequential mode and variable z-steps.
TROUBLESHOOTING
Problem (Step 5): The salivary glands have too much fat tissue attached.
Solution: Certain mosquito species have a higher content of thoracic fat body tissues surrounding the salivary glands that must be removed as follows before initiating the immunohistochemistry protocol.
Using the highest magnification of the stereomicroscope, remove fat body tissues with fine needles. It might take practice, as salivary gland lobes might break during this process.
Alternatively, fat body tissues can be washed away. While the salivary glands are in PBS in the well, gently pipette the salivary gland in and out several times with a 10-μL pipette tip.
Problem (Steps 7–12): Salivary glands are lost during the washing steps.
Solution: Always perform the washing steps under the stereomicroscope to monitor that no glands are accidentally removed during washings. Remove air bubbles due to traces of Triton X-100 or BSA that may entrap salivary glands, thus making it difficult to visualize them. Working with a small number of glands per well will minimize this issue; the optimal number is five to 10 glands/well.
Problem (Step 22): Staining is impaired as a result of the attachment of the salivary glands to the well surface during immunostaining.
Solution: During Steps 7–12, use fine needles to separate the glands from the walls under the stereoscope at high magnification (40×).
Problem (Step 22): There is high background during imaging.
Solution: Make sure that glands are well-washed and ensure the following.
Remove all buffer contents in the well before adding the washing buffer.
Change tips between different sample washings.
Optimize the antibody concentration.
RECIPES
Immunohistochemistry Blocking Buffer
| Reagents | Concentration |
|---|---|
| Bovine serum albumin (BSA) (Sigma-Aldrich A7409) | 2% |
| Triton X-100 (Sigma-Aldrich T8787) | 0.5% |
Prepare in phosphate buffered saline (PBS) with a pH of 7.4 (Gibco 10010-023). Store for up to 6 mo at 4°C.
Immunohistochemistry Washing Buffer
| Reagent | Concentration |
|---|---|
| Bovine serum albumin (BSA) (Sigma-Aldrich A7409) | 2% |
Prepare in phosphate buffered saline (PBS) with a pH of 7.4 (Gibco 10010-023). Store for up to 6 mo at 4°C.
ACKNOWLEDGMENTS
The authors thank Brian Bonilla for excellent salivary gland dissections and photos and Olivia Kern and Sundar Ganesan for their input in the protocol optimization. We also thank Leticia B. Smith for providing antibodies against Anopheles gambiae D7L1.
REFERENCES
- Coleman J, Juhn J, James AA. 2007. Dissection of midgut and salivary glands from Ae. aegypti mosquitoes. J Vis Exp 5: 228. doi: 10.3791/228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern O, Valenzuela Leon PC, Gittis AG, Bonilla B, Cruz P, Chagas AC, Ganesan S, Ribeiro JMC, Garboczi DN, Martin-Martin I, et al. 2021. The structures of two salivary proteins from the West Nile vector Culex quinquefasciatus reveal a β-trefoil fold with putative sugar binding properties. Curr Res Struct Biol 3: 95–105. doi: 10.1016/j.crstbi.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Martin I, Alves E Silva TL, Williams AE, Vega-Rodriguez J, Calvo E. 2022. Performing immunohistochemistry in mosquito salivary glands. Cold Spring Harb Protoc. doi: 10.1101/pdb.top107699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ote M, Kanuka H. 2018. A highly secure method for rearing Aedes aegypti mosquitoes. Trop Med Health 46: 16. doi: 10.1186/s41182-018-0098-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MB, Andrew DJ. 2019. Anopheles salivary gland architecture shapes Plasmodium sporozoite availability for transmission. mBio 10: e01238–19. doi: 10.1128/mBio.01238-19 [DOI] [PMC free article] [PubMed] [Google Scholar]


