Abstract
Studying protein localization in mosquito salivary glands provides novel insights on the function and physiological relevance of salivary proteins and also provides an avenue to study interactions between mosquitoes and pathogens. Salivary proteins display compartmentalization. For example, proteins involved in blood feeding are stored in the medial and distal lateral lobes, whereas proteins related to sugar metabolism localize to the proximal portion of the lateral lobes. Immunohistochemistry assays use antibodies raised against recombinant salivary proteins to reveal the protein localization and interactions within the tissue. In this assay, permeabilization of the salivary glands allows the antibodies to enter the cells and bind their target proteins. The primary antibody–antigen complexes are later marked with fluorescently labeled secondary antibodies. Antibodies that recognize pathogen-specific proteins can also be incorporated in these assays, providing information about pathogen localization within the salivary glands or pathogen interactions with mosquito salivary proteins. Here, we introduce immunohistochemistry assays for use in mosquito salivary glands.
MOSQUITO SALIVARY GLANDS
Salivary glands are a paired organ located in the anterior portion of the thoracic hemocele (Jobling 1978). Each gland contains three cylindrical lobes: one medial lobe and two lateral lobes divided into proximal and distal regions. Salivary proteins are secreted from gland cells to the cell secretory cavity and into the salivary duct that connects both glands and carries the saliva through the length of the proboscis. Saliva is injected into the host skin during mosquito probing, and salivary proteins facilitate blood feeding by preventing hemostasis (Ribeiro and Arca 2009).
COMPARTMENTALIZATION OF SALIVARY PROTEINS
Mosquito salivary proteins are produced by the salivary gland epithelial cells (Sanchez-Vargas et al. 2021). Compartmentalization of salivary proteins within the salivary glands has been observed in many studies (Ribeiro 1992; Juhn et al. 2011; Chagas et al. 2014; Wells and Andrew 2015; Martin-Martin et al. 2020; Kern et al. 2021). Salivary proteins that facilitate blood feeding are produced and stored in the medial and distal lateral lobes, whereas salivary proteins involved in sugar metabolism localize to the proximal portion of the lateral lobes. Neuron innervation varies among the salivary gland lobes, leading to specific responses to different stimuli. For example, mosquito feeding on blood or sugar triggers different responses that release neurotransmitters to activate specific sets of neurons (Soohoo-Hui et al. 2021). These mechanisms are poorly understood, and salivary protein compartmentalization remains a mystery.
The localization of salivary transcripts and proteins within the glands has been investigated using RNA in situ hybridization (James et al. 1991; Juhn et al. 2011) and immunohistochemistry, respectively (Ribeiro 1992; Chagas et al. 2014; Wells and Andrew 2015; Islam et al. 2019; O’Brochta et al. 2019; Martin-Martin et al. 2020). Juhn et al. (2011) reported the in situ hybridization patterns of 30 transcripts expressed in the salivary glands of adult Aedes aegypti females, providing the first catalog for salivary transcript localization in Aedes mosquitoes. Immunohistochemistry has been used to study the localization of specific proteins in the salivary glands of Ae. aegypti (James et al. 1991; Ribeiro 1992; Chagas et al. 2014; Wells and Andrew 2015; Islam et al. 2019; O’Brochta et al. 2019; Martin-Martin et al. 2020; Soohoo-Hui et al. 2021), Anopheles spp. (Wells and Andrew 2015; Islam et al. 2019; O’Brochta et al. 2019; Nozaki et al. 2020), and the less well-studied Culex quinquefasciatus (Martin-Martin et al. 2020; Kern et al. 2021). Comparing transcript and protein localization among different species could provide insights on translational regulation or protein transportation. Figure 1 illustrates the morphology of Ae. aegypti salivary glands.
FIGURE 1.
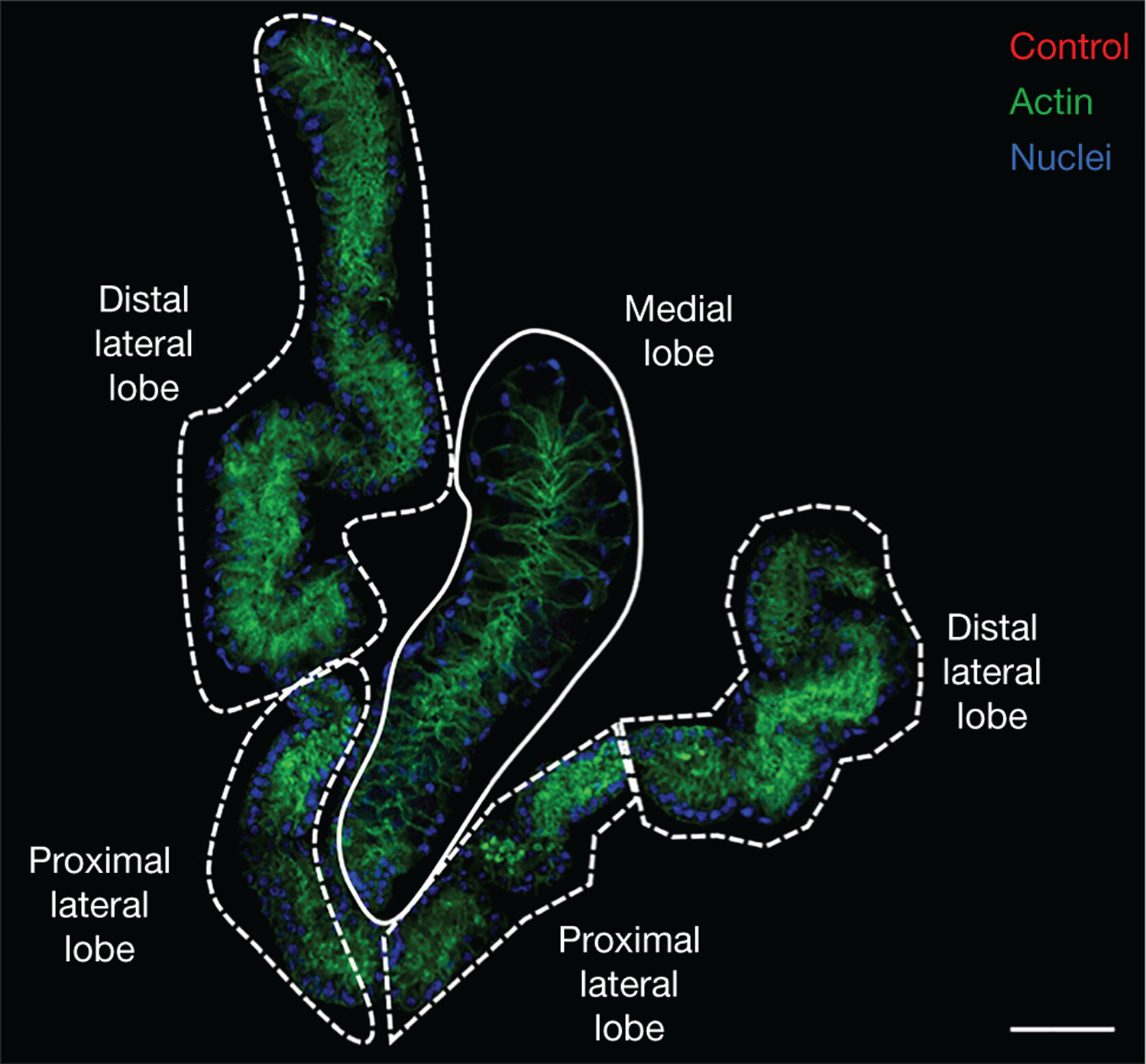
Anatomy of salivary glands. Salivary glands of Aedes aegypti stained with mouse antibodies raised against adjuvant only. Distal and proximal lateral lobes are indicated with dashed white lines. The medial lobe is surrounded by a solid white line. Actin, stained with Phalloidin 488, and nuclei, stained with DAPI, are shown in green and blue, respectively. Scale bar, 50 μm.
MORPHOLOGY AND CELLULAR ORGANIZATION
Confocal microscopy following immunohistochemistry using antibodies against well-conserved structural and organellar markers can be used to define the morphology and cellular organization of the salivary glands. Immunohistochemistry has validated published electron microscopy data showing morphological details of mosquito salivary glands, including the specific localization of proteins and organelles. Each salivary gland lobe consists of a basal lamina surrounding a single layer of acinar cells that are distributed around a central salivary duct. Each cell is basally compressed and contains a cup-like apical cavity for saliva storage (Sánchez-Vargas et al. 2021). Wells and colleagues described the morphology and development of An. stephensi and An. gambiae female and male salivary glands showing a high degree of sex-specific morphological variation. They extensively described the architecture of the salivary duct, which is the passageway for salivary proteins and mosquito-borne pathogens from the salivary glands to the host (Wells and Andrew 2015; Wells et al. 2017).
MOSQUITO-BORNE PATHOGENS
Salivary glands are critical organs for mosquito-borne pathogen transmission. There are several tissue barriers that, together with mosquito genetic factors, affect the ability of a mosquito to acquire, maintain, and transmit a pathogen, therefore reducing vector competence (Sánchez-Vargas et al. 2021). Immunofluorescence studies in infected mosquito vectors have facilitated the characterization of salivary gland tissue barriers. Barriers in the salivary glands include factors that prevent arbovirus invasion or replication in the salivary gland epithelial cells, and factors that prevent arbovirus release from the salivary glands into the saliva (Franz et al. 2015). Salivary gland barriers against other medically relevant viruses including dengue, Chikungunya, and Venezuelan equine encephalomyelitis viruses in Ae. aegypti have been characterized (Gaidamovich et al. 1973; Salazar et al. 2007; Le Coupanec et al. 2017; Raquin and Lambrechts 2017). The insect-specific Sindbis virus has also been imaged in the salivary glands of Ae. aegypti and Ae. albopictus (Gaidamovich et al. 1973; Bowers et al. 1995; Bowers et al. 2003). Different viruses preferentially replicate in specific lobes or regions of the salivary glands (Salazar et al. 2007). For example, dengue and Chikungunya viruses infect the medial and lateral lobes of Ae. aegypti (Tchankouo-Nguetcheu et al. 2012), whereas Sindbis virus does not infect the medial lobes of Aedes spp. (Bowers et al. 2003; Ciano et al. 2014).
Immunofluorescence confocal microscopy has provided new insights into the biology of Plasmodium sporozoite invasion of Anopheles salivary glands. Immunohistochemistry showed that Plasmodium berghei sporozoites form clusters within the secretory cavities of An. stephensi salivary gland cells. Sporozoites were most often detected in the distal lateral lobes, although they occasionally invaded the medial and proximal lateral lobes (Wells and Andrew 2019).
Immunohistochemistry, coupled with technologies like fluorescence lifetime imaging microscopy (FLIM), can also be used to characterize protein–protein interactions. FLIM is an imaging technique based on the differences in the exponential decay rate of the photon emission of a fluorophore. As the fluorescence lifetime does not depend on concentration, absorption by the sample, sample thickness, photobleaching, and/or excitation intensity, it is more robust than intensity-based methods (Datta et al. 2020). The production of specific antibodies against salivary recombinant proteins coupled with the wide availability of commercial antibodies against mosquito-borne pathogens, such as Plasmodium parasites and dengue and Zika viruses, make the study of host–pathogen interactions possible.
IMMUNOHISTOCHEMISTRY TECHNIQUE
Immunohistochemistry of mosquito salivary glands is a technique that requires meticulous attention to detail. The most critical step is the dissection and isolation of intact salivary glands that are free of other mosquito tissues, like the fat body. Salivary glands are localized within the thorax and are surrounded by fat bodies. Dissection of intact and fat body–free salivary glands requires expertise and practice. For salivary gland dissection protocols, see Coleman et al. (2007) and Schmid et al. (2017). Salivary glands are small and delicate tissues that must be manipulated with care under a stereoscope to avoid lost or broken glands. Thus, removing fat bodies surrounding salivary glands, antibody washing, and slide-mounting steps require extra care. Our accompanying protocol (see Protocol: A Simple Method for Immunohistochemistry and Imaging of Mosquito Salivary Glands [Martin-Martin et al. 2022]) describes in detail the methodology for successful immunohistochemistry of mosquito salivary glands.
In conclusion, immunohistochemistry provides valuable information on the overall structure of mosquito salivary glands as well as the cellular and subcellular localization of mosquito- and pathogen-specific proteins within the salivary glands. These studies improve our understanding of salivary gland protein function and show how saliva impacts vector competence and pathogen transmission.
ACKNOWLEDGMENTS
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) (AI001246).
REFERENCES
- Bowers DF, Abell BA, Brown DT. 1995. Replication and tissue tropism of the alphavirus Sindbis in the mosquito Aedes albopictus. Virology 212: 1–12. doi: 10.1006/viro.1995.1447 [DOI] [PubMed] [Google Scholar]
- Bowers DF, Coleman CG, Brown DT. 2003. Sindbis virus-associated pathology in Aedes albopictus (Diptera: Culicidae). J Med Entomol 40: 698–705. doi: 10.1603/0022-2585-40.5.698 [DOI] [PubMed] [Google Scholar]
- Chagas AC, Ramirez JL, Jasinskiene N, James AA, Ribeiro JM, Marinotti O, Calvo E. 2014. Collagen-binding protein, Aegyptin, regulates probing time and blood feeding success in the dengue vector mosquito, Aedes aegypti. Proc Natl Acad Sci 111: 6946–6951. doi: 10.1073/pnas.1404179111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciano KA, Saredy JJ, Bowers DF. 2014. Heparan sulfate proteoglycan: an arbovirus attachment factor integral to mosquito salivary gland ducts. Viruses 6: 5182–5197. doi: 10.3390/v6125182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J, Juhn J, James AA. 2007. Dissection of midgut and salivary glands from Ae. aegypti mosquitoes. J Vis Exp 2007: 228. doi: 10.3791/228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta R, Heaster TM, Sharick JT, Gillette AA, Skala MC. 2020. Fluorescence lifetime imaging microscopy: fundamentals and advances in instrumentation, analysis, and applications. J Biomed Opt 25: 071203. doi: 10.1117/1.JBO.25.7.071203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz AWE, Kantor AM, Passarelli AL, Clem RJ. 2015. Tissue barriers to arbovirus infection in mosquitoes. Viruses 7: 3741–3767. doi: 10.3390/v7072795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaidamovich SY, Khutoretskaya NV, Lvova AI, Sveshnikova NA. 1973. Immunofluorescent staining study of the salivary glands of mosquitoes infected with group A arboviruses. Intervirology 1: 193–200. doi: 10.1159/000148846 [DOI] [PubMed] [Google Scholar]
- Islam A, Emran TB, Yamamoto DS, Iyori M, Amelia F, Yusuf Y, Yamaguchi R, Alam MS, Silveira H, Yoshida S. 2019. Anopheline antiplatelet protein from mosquito saliva regulates blood feeding behavior. Sci Rep 9: 3129. doi: 10.1038/s41598-019-39960-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AA, Blackmer K, Marinotti O, Ghosn CR, Racioppi JV. 1991. Isolation and characterization of the gene expressing the major salivary gland protein of the female mosquito, Aedes aegypti. Mol Biochem Parasitol 44: 245–253. doi: 10.1016/0166-6851(91)90010-4 [DOI] [PubMed] [Google Scholar]
- Jobling B 1978. Anatomical drawings of biting flies. British Museum (Natural History) and Wellcome Trust, London. [Google Scholar]
- Juhn J, Naeem-Ullah U, Maciel Guedes BA, Majid A, Coleman J, Paolucci Pimenta PF, Akram W, James AA, Marinotti O. 2011. Spatial mapping of gene expression in the salivary glands of the dengue vector mosquito, Aedes aegypti. Parasit Vectors 4: 1. doi: 10.1186/1756-3305-4-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern O, Valenzuela Leon PC, Gittis AG, Bonilla B, Cruz P, Chagas AC, Ganesan S, Ribeiro JMC, Garboczi DN, Martin-Martin I, et al. 2021. The structures of two salivary proteins from the West Nile vector Culex quinquefasciatus reveal a β-trefoil fold with putative sugar binding properties. Curr Res Struc Biol 3: 95–105. doi: 10.1016/j.crstbi.2021.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Coupanec A, Tchankouo-Nguetcheu S, Roux P, Khun H, Huerre M, Morales-Vargas R, Enguehard M, Lavillette D, Missé D, Choumet V. 2017. Co-infection of mosquitoes with chikungunya and dengue viruses reveals modulation of the replication of both viruses in midguts and salivary glands of Aedes aegypti mosquitoes. Int J Mol Sci 18: 1708. doi: 10.3390/ijms18081708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Martin I, Paige A, Valenzuela Leon PC, Gittis AG, Kern O, Bonilla B, Chagas AC, Ganesan S, Smith LB, Garboczi DN, et al. 2020. ADP binding by the Culex quinquefasciatus mosquito D7 salivary protein enhances blood feeding on mammals. Nat Commun 11: 2911. doi: 10.1038/s41467-020-16665-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Martin I, Alves E Silva TL, Williams AE, Vega-Rodriguez J, Calvo E. 2022. A simple method for immunohistochemistry and imaging of mosquito salivary glands. Cold Spring Harb Protoc doi: 10.1101/pdb.prot107990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki M, Baba M, Tachibana M, Tokunaga N, Torii M, Ishino T. 2020. Detection of the rhoptry neck protein complex in Plasmodium sporozoites and its contribution to sporozoite invasion of salivary glands. mSphere 5: e00325–20. doi: 10.1128/mSphere.00325-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brochta DA, Alford R, Harrell R, Aluvihare C, Eappen AG, Li T, Chakravarty S, Sim BKL, Hoffman SL, Billingsley PF. 2019. Is Saglin a mosquito salivary gland receptor for Plasmodium falciparum? Malar J 18: 2. doi: 10.1186/s12936-018-2634-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raquin V, Lambrechts L. 2017. Dengue virus replicates and accumulates in Aedes aegypti salivary glands. Virology 507: 75–81. doi: 10.1016/j.virol.2017.04.009 [DOI] [PubMed] [Google Scholar]
- Ribeiro JM. 1992. Characterization of a vasodilator from the salivary glands of the yellow fever mosquito Aedes aegypti. J Exp Bio 165: 61–71. doi: 10.1242/jeb.165.1.61 [DOI] [PubMed] [Google Scholar]
- Ribeiro JMC, Arca B. 2009. From sialomes to the sialoverse: an insight into salivary potion of blood-feeding insects. Adv In Insect Phys 37: 59–118. doi: 10.1016/S0065-2806(09)37002-2 [DOI] [Google Scholar]
- Salazar MI, Richardson JH, Sánchez-Vargas I, Olson KE, Beaty BJ. 2007. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol 7: 9. doi: 10.1186/1471-2180-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Vargas I, Olson KE, Black WC. 2021. The genetic basis for salivary gland barriers to arboviral transmission. Insects 12: 73. doi: 10.3390/insects12010073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid MA, Kauffman E, Payne A, Harris E, Kramer LD. 2017. Preparation of mosquito salivary gland extract and intradermal inoculation of mice. Bio Protoc 7: e2407. doi: 10.21769/BioProtoc.2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soohoo-Hui A, Li Z, Maldonado-Ruiz LP, Zhang G, Swale DR. 2021. Neurochemical regulation of Aedes aegypti salivary gland function. J Insect Physiol 129: 104193. doi: 10.1016/j.jinsphys.2021.104193 [DOI] [PubMed] [Google Scholar]
- Tchankouo-Nguetcheu S, Bourguet E, Lenormand P, Rousselle J-C, Namane A, Choumet V. 2012. Infection by chikungunya virus modulates the expression of several proteins in Aedes aegypti salivary glands. Parasit Vectors 5: 264. doi: 10.1186/1756-3305-5-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MB, Andrew DJ. 2015. Salivary gland cellular architecture in the Asian malaria vector mosquito Anopheles stephensi. Parasit Vectors 8: 617. doi: 10.1186/s13071-015-1229-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MB, Andrew DJ. 2019. Anopheles salivary gland architecture shapes Plasmodium sporozoite availability for transmission. mBio 10: e01238–19. doi: 10.1128/mBio.01238-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells MB, Villamor J, Andrew DJ. 2017. Salivary gland maturation and duct formation in the African malaria mosquito Anopheles gambiae. Sci Rep 7: 601. doi: 10.1038/s41598-017-00672-0 [DOI] [PMC free article] [PubMed] [Google Scholar]


