Abstract
Objective
To investigate the effects of completed pregnancy with childbirth and incomplete pregnancy without childbirth on the late-life cognition and the risk of Alzheimer disease (AD) in women.
Methods
Using the pooled data of 3,549 women provided by 2 population-based cohort studies, we conducted logistic regression analyses to examine retrospectively the associations of completed and incomplete pregnancy with the risks of mild cognitive impairment and AD. For women without dementia, we also conducted analyses of covariance to examine the associations of completed and incomplete pregnancy with Mini-Mental State Examination (MMSE) score.
Results
Grand multiparous women who experienced ≥5 completed pregnancies showed an ≈1.7-fold higher risk of AD than those who experienced 1 to 4 completed pregnancies (odds ratio [OR] 1.68, 95% confidence interval [CI] 1.04–2.72), while those who had incomplete pregnancies showed half the level of AD risk compared with those who never experienced an incomplete pregnancy (OR 0.43, 95% CI 0.24–0.76 for 1 incomplete pregnancy; OR 0.56, 95% CI 0.34–0.92 for ≥2 incomplete pregnancies). In women without dementia, the grand multiparous had worse MMSE scores than those with 1 to 4 completed pregnancies (p < 0.001), while those who experienced ≥1 incomplete pregnancies had better MMSE scores than those who never experienced an incomplete pregnancy (p = 0.008).
Conclusions
Grand multiparity was associated with high risk of AD, while incomplete pregnancy was associated with low risk of AD in late life.
Alzheimer disease (AD) is more prevalent in women than men,1 and pregnancy is a distinctive experience of women. The number of pregnancies or childbirths has been associated with the risk of AD,2–4 and it may be attributable to various pregnancy-associated factors such as hormones, health, employment, and lifestyle during or after pregnancy. In particular, estrogen levels change considerably during pregnancy and can be either neuroprotective or neurotoxic, according to the concentration.5,6 Serum estradiol begins to increase from the sixth to eighth week, reaching in the third trimester up to 40-fold higher than its peak level during natural menstrual cycles, and then decreases to the average level during natural menstrual cycles within just 4 days after delivery.7,8 Therefore, the effect of pregnancy on the risk of AD may be different according to whether pregnancy is completed with childbirth (CPREG) or incomplete without childbirth (IPREG) because pregnancy is mostly aborted within the first trimester.3,9–11 However, the effect on AD risk associated with IPREG has been seldom investigated separately from that of CPREG. Furthermore, many previous studies did not comprehensively adjust for the effects of other reproductive experiences9–11 or psychosocial and medical conditions, were limited in sample size,2,3,9–11 and had participants with relatively low numbers of pregnancies.3,11
In this study, we investigated the differential association of CPREG and IPREG with the risk of AD after controlling for potential effects of other reproductive experiences and psychosocial and medical conditions comprehensively in 2 large community-based cohorts.
Methods
Study population
Two member studies of the Cohort Studies of Memory in an International Consortium (COSMIC),12 the Korean Longitudinal Study on Cognitive Aging and Dementia (KLOSCAD)13 and the Hellenic Longitudinal Investigation of Aging and Diet (HELIAD),14 provided data for the current analysis. KLOSCAD and HELIAD are population-based prospective cohort studies conducted independently in Korea and Greece, respectively. In KLOSCAD, 30 villages or towns across Korea were randomly sampled. From their residential rosters, 10% (in urban areas) or 20% (in rural areas) of residents ≥60 years of age were randomly selected from the each village or town. In all, 12,694 elderly individuals were sampled, and 6,818 (53.7%) participated in the baseline assessment conducted between November 2010 and September 2012. Among them, 3,574 were women, and 3,278 of these provided full data on reproductive history and completed diagnostic assessments. A total of 2,737 women were included in the current analysis, after the exclusion of 541 who took hormone replacement therapy (HRT) at the time of assessment (n = 69) or had a history of oophorectomy (n = 216) or hysterectomy (n = 435). In HELIAD, ≈6% of community-dwelling elderly individuals were randomly selected from the residents of Larissa and Marousi, Greece (n = 14,000), and 1,814 (13.0%) completed the baseline assessment conducted between November 2009 and October 2015. Among them, 1,074 were women ≥60 years of age; 957 of these provided full data on reproductive history and completed diagnostic assessments. A total of 812 women were included in the current analysis after the exclusion of 145 who took HRT at the time of assessment (n = 3) or had a history of oophorectomy (n = 5) or hysterectomy (n = 141). Overall 3,549 women from both studies were included in the current analysis.
Assessments
In both cohorts, research clinicians or nurses obtained a comprehensive history of reproductive experiences that included the numbers of pregnancies, childbirths, stillbirths, and aborted pregnancies, including both spontaneous and induced abortion; the ages at the first and last childbirths; and the ages at menarche and menopause. History of breastfeeding and previous HRT were evaluated in KLOSCAD only. Geriatric psychiatrists or neurologists administered a standardized diagnostic interview that included a detailed medical history, physical and neurologic examinations, and laboratory tests. Trained neuropsychologists or research nurses conducted comprehensive neuropsychological assessments, including the Mini-Mental State Examination (MMSE) and the Geriatric Depression Scale Short Form.15 Both cohorts evaluated comorbid medical illnesses. However, only KLOSCAD quantified the cumulative burden of comorbid medical illnesses using the Cumulative Illness Rating Scale (CIRS). Both studies conducted brain CT or MRI in the patients who were diagnosed as having dementia. Both studies diagnosed AD according to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association criteria,16 and mild cognitive impairment (MCI) according to the consensus criteria from the International Working Group on Mild Cognitive Impairment.17
Standard protocol approvals, registrations, and patient consents
KLOSCAD was approved by the Institutional Ethics Review Board of the Seoul National University Bundang Hospital, and HELIAD was approved by the Institutional Ethics Review Board of the University of Thessaly. Written informed consent was obtained from the participants and/or their legal guardians in both studies.
Statistical analysis
We processed, harmonized when necessary, and pooled the data provided by KLOSCAD and HELIAD. We conducted multinomial logistic regression analyses to investigate the associations of CPREG and IPREG with the risks of MCI and AD. In these analyses, we categorized CPREG into 3 strata—no CPREG (nullipara), 1 to 4 CPREGs, and ≥5 CPREGs (grand multipara)18—because both grand multipara and nullipara have been associated with an increased risk of medical conditions such as diabetes mellitus and coronary heart disease.19,20 We also categorized IPREG into 3 strata: no IPREG, 1 IPREG, and ≥2 IPREGs. As covariates, we controlled for cohort (KLOSCAD and HELIAD), other reproductive experiences that may influence sex hormone levels (length of reproductive period and age at menopause), and demographic and clinical characteristics that may influence the risk of AD. Two regression models were constructed. In model 1, we estimated the effects of the number of CPREGs and IPREGs on the risks of MCI and AD after adjusting for the aforementioned reproductive experiences and demographic and clinical variables that were evaluated in both cohorts (age; total years of education; socioeconomic status; employment; history of diabetes mellitus, hypertension, and hyperlipidemia; and Geriatric Depression Scale Short Form score). In model 2, using data obtained in KLOSCAD only, we also adjusted for the history of breastfeeding and history of HRT and CIRS.
Because the distributions of CPREGs and IPREGs were different between KLOSCAD and HELIAD, we also analyzed the associations of CPREGs and IPREGs with the risk of MCI and AD using multinomial logistic regression analyses (model 1) separately in each cohort. In these analyses, we categorized CPREGs into 4 strata in KLOSCAD (no, 1, 2–4, and ≥5 CPREGs), but with only 3 grand multiparous women, we did not specify a ≥5 CPREGs strata in HELIAD (no, 1, and ≥2 CPREGs). We categorized IPREGs into 3 strata (no, 1, and ≥2 IPREGs) in both KLOSCAD and HELIAD.
We investigated the effects of CPREG and IPREG on MMSE scores in the women without dementia using 2-way analysis of covariance. In this analysis, we also constructed 2 models (models 1 and 2) that adjusted for the same covariates as in the multinomial logistic regressions models 1 and 2, respectively. All data were analyzed with the Statistical Package for Social Sciences, version 18 (SPSS Inc, Chicago, IL).
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Results
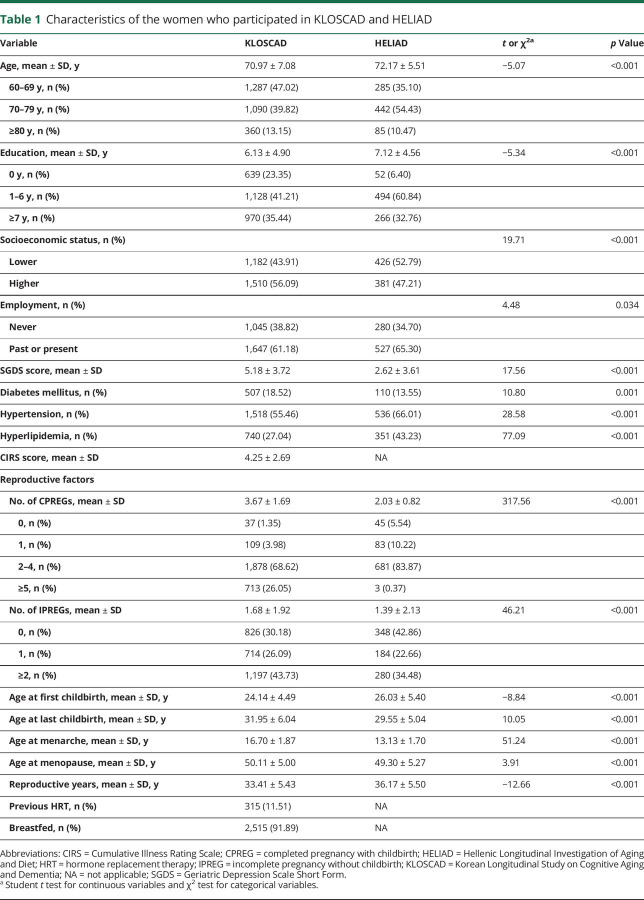
The KLOSCAD participants were slightly younger and less educated than the HELIAD participants (p < 0.001). Hypertension and hyperlipidemia were more prevalent in HELIAD than in KLOSCAD (p < 0.001), whereas diabetes mellitus was more prevalent in KLOSCAD than in HELIAD (p = 0.001). The KLOSCAD participants experienced more CPREGs (p < 0.001) and IPREGs (p < 0.001) than the HELIAD participants. The KLOSCAD participants experienced their menarche and menopause later (p < 0.001) but had a shorter mean reproductive period (p < 0.001) than the HELIAD participants (table 1).
Table 1.
Characteristics of the women who participated in KLOSCAD and HELIAD
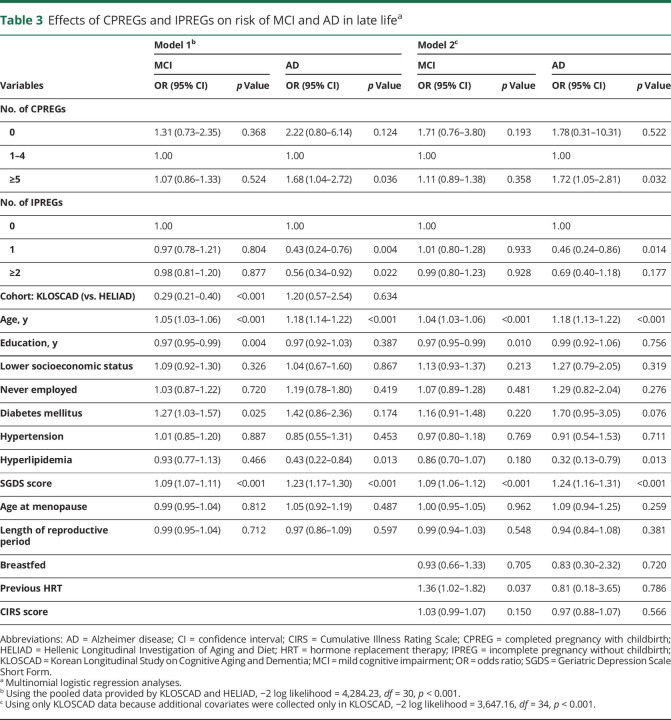
In the pooled KLOSCAD and HELIAD dataset, 896 and 118 participants were diagnosed as having MCI and AD, respectively. The distributions of CPREGs and IPREGs in the pooled dataset are summarized in table 2. Regression model 1 showed that CPREGs and IPREGs were associated with AD risk in opposite directions (table 3). Specifically, grand multiparous women who experienced ≥5 CPREGs showed an ≈1.7-fold higher risk of AD than those who experienced 1 to 4 CPREGs, and women who experienced IPREGs showed half the level of AD risk compared to those who never experienced an IPREG. Regression model 2 revealed that ≥5 CPREGs and IPREG remained differentially associated with AD risk after controlling for CIRS, history of HRT, and breastfeeding. The length of the reproductive period and age at menopause were not associated with AD risk. The numbers of CPREGs and IPREGs were not associated with the risk of MCI.
Table 2.
Distribution of CPREGs and IPREGs
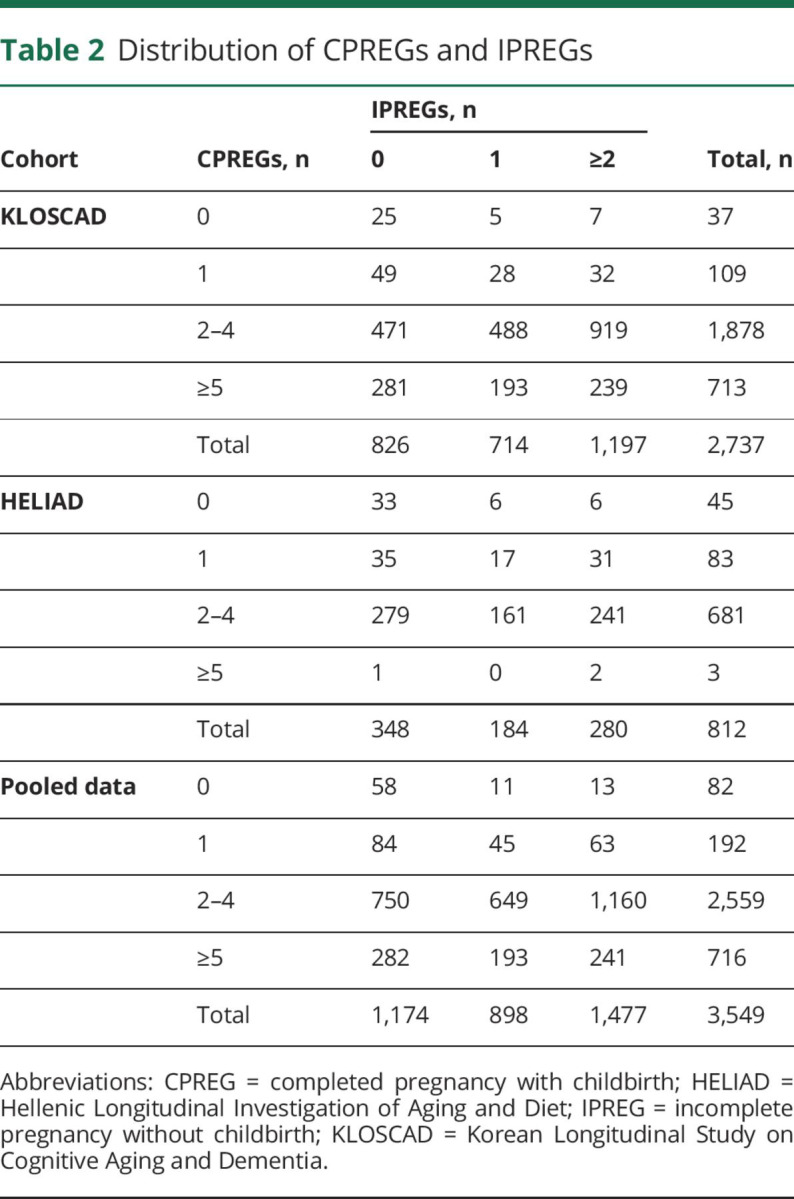
Table 3.
Effects of CPREGs and IPREGs on risk of MCI and AD in late lifea
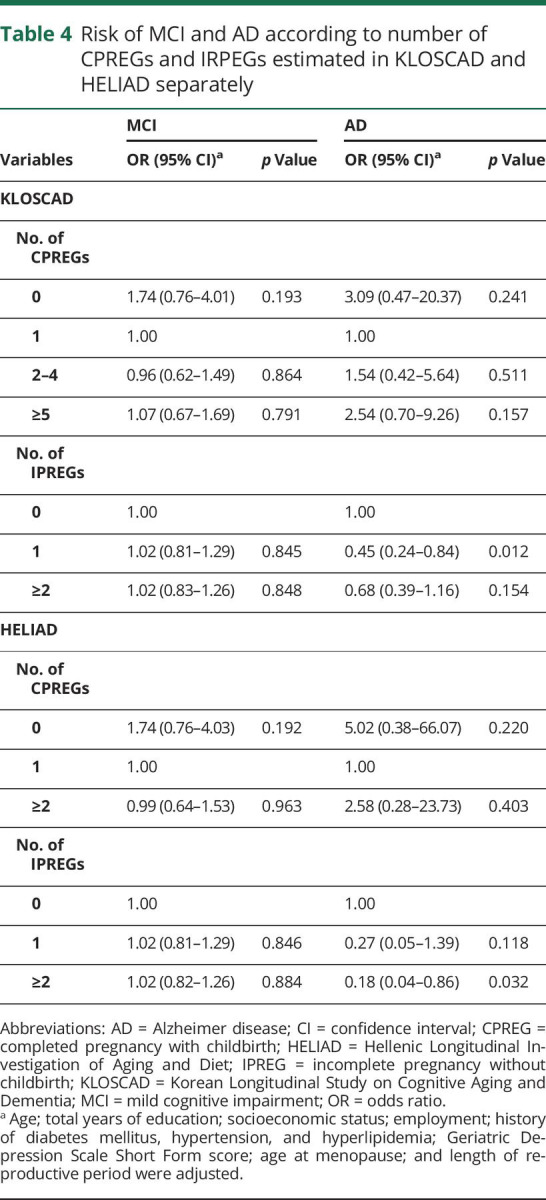
When we analyzed the KLOSCAD and HELIAD samples separately (table 4), the effects of CPREGs and IPREGs on the AD risk were in the same direction as the results of pooled data. However, the effects on the AD risk were significant only in the women who experienced 1 IPREG in KLOSCAD and ≥2 IPREGs in HELIAD. Although the effects were not significant, nulliparous women (no CPREG) showed an ≈3-fold higher risk of AD in KLOSCAD and an ≈5-fold higher risk of AD in HELIAD compared to the primiparous women (1 CPREG). Compared to the primiparous women, multiparous women also showed higher risk of AD in both cohorts.
Table 4.
Risk of MCI and AD according to number of CPREGs and IRPEGs estimated in KLOSCAD and HELIAD separately
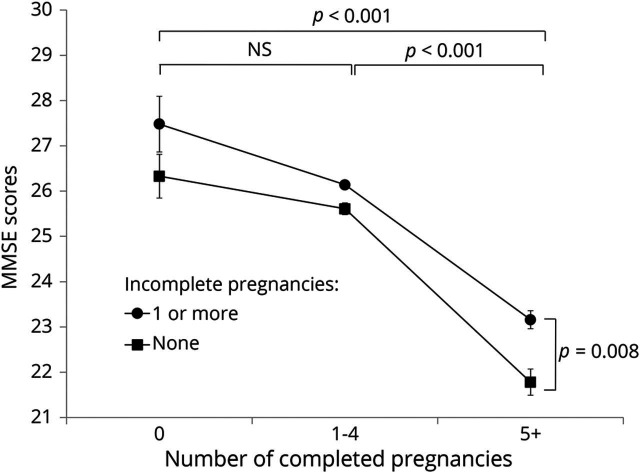
As shown in the figure, the numbers of both CPREGs and IPREGs were associated with MMSE scores for the women without dementia (p < 0.001 for CPREGs and p = 0.008 for IPREGs). The interaction between the numbers of IPREGs and CPREGs was not significant (p = 0.29). Post hoc comparisons revealed that women with ≥5 CPREGs had lower MMSE scores than those with either no CPREG (p < 0.001) or 1 to 4 CPREGs (p < 0.001). MMSE scores were comparable between the women with no CPREGs and those with 1 to 4 CPREGs. The women who never experienced an IPREG had lower MMSE scores than those who experienced ≥1 IPREGs (p = 0.008). When CIRS, history of HRT, and breastfeeding were additionally adjusted for, the effect of CPREGs on MMSE scores remained statistically significant (p < 0.001), while that of IPREGs became marginally significant (p = 0.067).
Figure. Effects of completed and incomplete pregnancies on MMSE scores of women without dementia in late life.
Analysis of variance with the Tukey post hoc comparisons adjusted for cohort; age; level of education; socioeconomic status; employment; history of diabetes mellitus, hypertension, and hyperlipidemia; Geriatric Depression Scale Short Form score; years of reproductive period; and age at menopause. Error bars indicate standard error. MMSE = Mini-Mental State Examination.
Discussion
In this study, the association between pregnancy and AD risk differed between CPREG and IPREG. Grand multiparous women (≥5 CPREGs) showed an ≈1.7-fold higher AD risk in late life compared to women with 1 to 4 CPREGs. In contrast, women who had experienced IPREGs showed about half the level of AD risk in late life compared to those with no IPREGs. Consistent with these findings, among women without dementia, those with a greater number of CPREGs showed lower MMSE scores in late life, although this effect was offset to some degree by having also experienced IPREGs.
Because pregnancy induces considerable changes in various hormones other than estrogen, the differential association of CPREGs and IPREGs with AD risk might not be explained simply by pregnancy-induced estrogen changes. However, compared to other hormones, estrogen shows a greater change in levels during pregnancy,8 and its effects on cognition and the risk of AD have been more extensively investigated.21 Although progesterone was found to be neuroprotective in spinal cord injury, stroke, and amyotrophic lateral sclerosis,22 its effect on AD risk has seldom been studied, and how it interacts with estrogen in modulating AD risk is unclear. Although human chorionic gonadotropin was associated with β-amyloid levels in the rat brain,23 associations between either IPREGs or CPREGs and risk of AD are unlikely to be mediated by pregnancy-induced human chorionic gonadotropin. The reason is that human chorionic gonadotropin is upregulated mostly in the first trimester of pregnancy in humans and thus should have similar effects on the risk of AD associated with IPREGs and CPREGs, which this study found to be in opposite directions.
Because pregnancy and childbirth may induce considerable changes in lifestyle and health, the differential association of CPREGs and IPREGs with AD risk might not be explained simply by the hormonal changes during pregnancy. One study reported that women who breastfed had lower risk of AD than women who did not.9 Women with ≥5 live births reportedly had high risk of chronic medical illnesses such as hypertension,24 coronary heart disease,19 and diabetes mellitus,20,24 which are associated with AD risk. Lower socioeconomic status has been associated with both higher parity and AD risk.25 However, associations of CPREGs and IPREGs with AD risk were statistically significant even after controlling these potential confounders in the current study.
In separate analyses of KLOSCAD and HELIAD, we subdivided the participants according to the number of CPREGs. Although the associations of CPREG with AD risk became nonsignificant, ≥2 CPREGs increased AD risk compared to 1 CPREG in both KLOSCAD and HELIAD, and the AD risk of ≥5 CPREGs was higher than that of 2 to 4 CPREGs in KLOSCAD. These results also support our hypothesis that multiple CPREGs may increase AD risk.
Multiple CPREGs may increase AD risk in several ways. First, in rats, estrogen upregulated neurogenesis and facilitated cognitive function at optimal levels but was ineffective at low levels and even harmful at high levels.5,6 Also in rats, estradiol was found to be harmful to cognitive function when serum levels increased to >4 times the peak level reached during the menstrual cycle.26 Serum estradiol levels are maintained at >10 times peak menstrual cycle levels for 7 months in pregnant women,27 and multiple CPREGs may thus have a harmful effect on late-life cognition by inducing repeated extreme upregulation of estradiol. Second, there are reports of estrogen withdrawal after a hormone-simulated pregnancy suppressing hippocampal cell proliferation21,28 and glucocorticoid upregulation inducing neuronal loss in the hippocampus.21,29 Because delivery induces an abrupt postpartum withdrawal of estrogen and upregulation of cortisol, multiple CPREGs may have compounding effects that decrease brain and cognitive reserves in women. Finally, compared to nulliparous women, women having given childbirth within the past 3 years were found to have 22% lower levels of a urinary estradiol metabolite that is correlated with levels of serum estradiol.30,31 In addition, postmenopausal serum free estradiol decreases as the number of childbirths increases.32 Because the neuroprotective effect of estrogen was inverted U shaped5,6 and low serum bioavailable estradiol was associated with the risk of cognitive impairment,33 multiple CPREGs may be harmful to cognition in late life by exposing women to reduced serum estrogen for life.
We did not evaluate the trimester in which pregnancy was aborted in the present study, although we assume that IPREGs exposed our participants to only modestly upregulated estrogen because >80% of spontaneous abortions and >90% of induced abortions occur within the first trimester.8 Serum estrogen levels remain within the range of the normal menstrual cycle until the fifth week of pregnancy and become modestly upregulated to ≈2-fold compared to peak levels of the normal menstrual cycle at the eighth week.27 If this modestly upregulated level of estrogen is within the optimal range for neurogenesis and cognition, IPREGs may directly reduce AD risk. This may also be why IPREGs offset the AD risk associated with CPREGs. Only 1 previous study has considered the separate effect of IPREG from CPREG, and it found no association between IPREG and AD risk.3 However, compared to our study, their numbers of IPREGs (0.3 ± 0.7) and pregnancies (2.0 ± 1.3) were relatively low.3
In recent clinical trials, HRT in postmenopausal women ≥65 years of age did not reduce the risk of MCI and increased the risk of dementia.34 However estradiol-based oral contraceptives appear beneficial to the cognitive function of women in their premenopausal and postmenopausal periods,35 and HRT reduced AD risk by ≈30% when provided within 5 years of menopause.36 The results from previous studies thus seem to suggest that premenopausal or perimenopausal exposures to a modest increase in estrogen may reduce AD risk in women. Mean estradiol levels obtained during conventional HRT in postmenopausal women are reportedly not raised to even the lowest levels associated with normal menstrual cycles.37 It would be worth exploring whether modifying HRT to induce serum estrogen levels to somewhere between the peak and 2 times the peak level of the normal menstrual cycle makes it more effective in reducing AD risk than conventional HRT. Merely several months of HRT modified as such may be sufficient for this effect.
Our study has several limitations. First, it was subject to recall biases because we evaluated reproductive experiences retrospectively by self-reports. Although pregnancy and childbirth are life events not easily forgotten, the participants with MCI or AD may be subject to recall biases for these events. However, any effects of recall bias associated with MCI or AD are likely to be minimal because the differential associations of CPREG and IPREG with the risk of AD were supported by similar differential associations of CPREG and IPREG with MMSE score in the cognitively normal participants. Second, the numbers of IPREGs may have been underestimated, either deliberately underreported because induced abortion may be associated with shame in some cultures or because spontaneous IPREGs may not have always been recognized as such. Third, we did not evaluate the exact time and cause of incomplete pregnancy. Fourth, we did not measure postmenopausal serum estrogen levels at the time of diagnosis. Fifth, we did not adjust for the influence of APOE genotype because it was assessed in only some of the participants. Sixth, changes in lifestyle and behaviors that may be induced by CPREGs or IPREGs could be associated with the risk of AD and may have affected the results of the current study despite our efforts to control the influence of such factors.
We found that grand multiparity was associated with high risk of AD, while incomplete pregnancy was associated with low risk of AD in late life. Replicating our results in other populations may lead to the development of hormone-based preventive strategies aimed at reducing AD risk in women, particularly those who are grand multipara. This could be of great relevance for the >20% of women ≥60 years of age in Asia who have experienced ≥5 childbirths38 and younger generations in sub-Saharan Africa who currently experience >5 childbirths on average.39
Author contributions
K.W. Kim had full access to all the data in the study and was responsible for the decision to submit for publication. H. Jang and J.B. Bae are joint first authors. K.W. Kim and N. Scarmeas are the principal investigators of KLOSCAD and HELIAD, respectively. Conception and design of the study: K.W. Kim, H. Jang, and J.B. Bae. Acquisition of data: K.W. Kim, J.W. Han, T.H. Kim, K.P. Kwak, B.J. Kim, S.G. Kim, J.L. Kim, S.W. Moon, J.H. Park, S.-H. Ryu, J.C. Youn, D.Y. Lee, D.W. Lee, S.B. Lee, J.J. Lee, J.H. Jhoo, E. Dardiotis, N. Scarmeas, M. Yannakoulia, M.H. Kosmidis, G.M. Hadjigeorgiou, and P. Sakka. Analysis of data and statistical analysis: K.W. Kim, H. Jang, and J.B. Bae. Drafting of the manuscript, figure, and tables: K.W. Kim, H. Jang, and J.B. Bae. Critical revision of the manuscript: all authors.contributions
Study funding
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (grant HI09C1379 [A092077]), IIRG-09-133014 from the Alzheimer's Association, 189 10276/8/9/2011 from the National Strategic Reference Framework - European Union (NSRF-EU) program: Excellence (ARISTEIA), which is cofunded by the European Social Fund and Greek National resources, ΔΥ2β/οικ.51657/14.4.2009 from the Ministry for Health and Social Solidarity of Greece, and a National Health and Medical Research Council of Australia Program grant (ID 568969; P.S.S.).
Acknowledgment
The authors thank the patients and their families for their participation in the study and the research clinicians, nurses, and neuropsychologists for their contributions in gathering the data.
Glossary
- AD
Alzheimer disease
- CIRS
Cumulative Illness Rating Scale
- COSMIC
Cohort Studies of Memory in an International Consortium
- CPREG
completed pregnancy with childbirth
- HELIAD
Hellenic Longitudinal Investigation of Aging and Diet
- HRT
hormone replacement therapy
- IPREG
incomplete pregnancy without childbirth
- KLOSCAD
Korean Longitudinal Study on Cognitive Aging and Dementia
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
Footnotes
CME Course: NPub.org/cmelist
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol 2014;6:37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ptok U, Barkow K, Heun R. Fertility and number of children in patients with Alzheimer's disease. Arch Womens Ment Health 2002;5:83–86. [DOI] [PubMed] [Google Scholar]
- 3.Colucci M, Cammarata S, Assini A, et al. The number of pregnancies is a risk factor for Alzheimer's disease. Eur J Neurol 2006;13:1374–1377. [DOI] [PubMed] [Google Scholar]
- 4.Beeri MS, Rapp M, Schmeidler J, et al. Number of children is associated with neuropathology of Alzheimer's disease in women. Neurobiol Aging 2009;30:1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav 2010;58:415–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barha CK, Galea LA. Influence of different estrogens on neuroplasticity and cognition in the hippocampus. Biochim Biophys Acta 2010;1800:1056–1067. [DOI] [PubMed] [Google Scholar]
- 7.Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. Am J Obstet Gynecol 1972;112:1095–1100. [DOI] [PubMed] [Google Scholar]
- 8.Cunningham FG. Williams Obstetrics, 24th ed. New York: McGraw-Hill Education/Medical; 2014. [Google Scholar]
- 9.Fox M, Berzuini C, Knapp LA. Maternal breastfeeding history and Alzheimer's disease risk. J Alzheimers Dis 2013;37:809–821. [DOI] [PubMed] [Google Scholar]
- 10.Fox M, Berzuini C, Knapp LA. Cumulative estrogen exposure, number of menstrual cycles, and Alzheimer's risk in a cohort of British women. Psychoneuroendocrinology 2013;38:2973–2982. [DOI] [PubMed] [Google Scholar]
- 11.Zucchella C, Sinforiani E, Citterio A, Giarracca V, Bono G, Mauri M. Reproductive life events and Alzheimer's disease in Italian women: a retrospective study. Neuropsychiatr Dis Treat 2012;8:555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachdev PS, Lipnicki DM, Kochan NA, et al. COSMIC (Cohort Studies of Memory in an International Consortium): an international consortium to identify risk and protective factors and biomarkers of cognitive ageing and dementia in diverse ethnic and sociocultural groups. BMC Neurol 2013;13:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JH, Lim S, Lim J, et al. An overview of the Korean Longitudinal Study on Health and Aging. Psychiatry Invest 2007;4:84. [Google Scholar]
- 14.Dardiotis E, Kosmidis MH, Yannakoulia M, Hadjigeorgiou GM, Scarmeas N. The Hellenic Longitudinal Investigation of Aging and Diet (HELIAD): rationale, study design, and cohort description. Neuroepidemiology 2014;43:9–14. [DOI] [PubMed] [Google Scholar]
- 15.Yesavage JA, Brink T, Rose TL, et al. Development and validation of a Geriatric Depression Screening Scale: a preliminary report. J Psychiatr Res 1983;17:37–49. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939. [DOI] [PubMed] [Google Scholar]
- 17.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 18.Babinszki A, Kerenyi T, Torok O, Grazi V, Lapinski RH, Berkowitz RL. Perinatal outcome in grand and great-grand multiparity: effects of parity on obstetric risk factors. Am J Obstet Gynecol 1999;181:669–674. [DOI] [PubMed] [Google Scholar]
- 19.Lawlor DA. Is the association between parity and coronary heart disease due to biological effects of pregnancy or adverse lifestyle risk factors associated with child-rearing? Findings from the British Women's Heart and Health Study and the British Regional Heart Study. Circulation 2003;107:1260–1264. [DOI] [PubMed] [Google Scholar]
- 20.Nicholson WK, Asao K, Brancati F, Coresh J, Pankow JS, Powe NR. Parity and risk of type 2 diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care 2006;29:2349–2354. [DOI] [PubMed] [Google Scholar]
- 21.Workman JL, Barha CK, Galea LAM. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci 2012;126:54–72. [DOI] [PubMed] [Google Scholar]
- 22.De Nicola AF, Gonzalez Deniselle MC, Garay L, et al. Progesterone protective effects in neurodegeneration and neuroinflammation. J Neuroendocrinol 2013;25:1095–1103. [DOI] [PubMed] [Google Scholar]
- 23.Berry A, Tomidokoro Y, Ghiso J, Thornton J. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-beta levels in female rats. Horm Behav 2008;54:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu B, Chen Y, Xiong J, Lu N, Tan X. Association of female reproductive factors with hypertension, diabetes and LQTc in Chinese women. Scientific Rep 2017;7:42803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marden JR, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM. Contribution of socioeconomic status at three lifecourse periods to late life memory function and decline: early and late predictors of dementia risk. Am J Epidemiol 2017;186:805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wide JK, Hanratty K, Ting J, Galea LA. High level estradiol impairs and low level estradiol facilitates non-spatial working memory. Behav Brain Res 2004;155:45–53. [DOI] [PubMed] [Google Scholar]
- 27.Loriaux DL, Ruder H, Knab D, Lipsett M. Estrone sulfate, estrone, estradiol and estriol plasma levels in human pregnancy. J Clin Endocrinol Metab 1972;35:887–891. [DOI] [PubMed] [Google Scholar]
- 28.Green AD, Galea LA. Adult hippocampal cell proliferation is suppressed with estrogen withdrawal after a hormone-simulated pregnancy. Horm Behav 2008;54:203–211. [DOI] [PubMed] [Google Scholar]
- 29.Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol 1999;34:721–732. [DOI] [PubMed] [Google Scholar]
- 30.Munro CJ, Stabenfeldt G, Cragun J, Addiego L, Overstreet J, Lasley B. Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay. Clin Chem 1991;37:838–844. [PubMed] [Google Scholar]
- 31.Barrett ES, Parlett LE, Windham GC, Swan SH. Differences in ovarian hormones in relation to parity and time since last birth. Fertil Steril 2014;101:1773–1780.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavez-MacGregor M, van Gils CH, van der Schouw YT, Monninkhof E, van Noord PA, Peeters PH. Lifetime cumulative number of menstrual cycles and serum sex hormone levels in postmenopausal women. Breast Cancer Res Treat 2008;108:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yaffe K, Lui L-Y, Grady D, Cauley J, Kramer J, Cummings SR. Cognitive decline in women in relation to non-protein-bound oestradiol concentrations. Lancet 2000;356:708–712. [DOI] [PubMed] [Google Scholar]
- 34.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2651–2662. [DOI] [PubMed] [Google Scholar]
- 35.Mordecai KL, Rubin LH, Maki PM. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Horm Behav 2008;54:286–293. [DOI] [PubMed] [Google Scholar]
- 36.Shao H, Breitner JC, Whitmer RA, et al. Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology 2012;79:1846–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edlefsen KL, Jackson RD, Prentice RL, et al. The effects of postmenopausal hormone therapy on serum estrogen, progesterone, and sex hormone-binding globulin levels in healthy postmenopausal women. Menopause 2010;17:622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li FD, He F, Chen TR, et al. Reproductive history and risk of cognitive impairment in elderly women: a cross-sectional study in Eastern China. J Alzheimers Dis 2016;49:139–147. [DOI] [PubMed] [Google Scholar]
- 39.Shapiro D, Gebreselassie T. Fertility transition in sub-Saharan Africa: falling and stalling. Afr Popul Stud 2013:23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.