Abstract
Background:
Type 2 Diabetes Mellitus (T2DM) is the prominent public health issue. Pharmacotherapy and diet modification should be integrated into T2DM management.
Aims:
To investigate the effects of vegetables consumption before carbohydrates on blood glucose and GLP-1 levels in T2DM patients.
Methods:
A non-randomized quasi experimental study was conducted to recruit T2DM patients who attended at the Gatot Soebroto Central Army Hospital, Jakarta, Indonesia from April to May 2016. The Lemeshow's formula was used to determine sample size. A total of 12 non-diabetic and 24 diabetic patients were participated in our study. Glucose levels were measured using a routine hexokinase method while serum GLP-1 levels were determined using the ELISA. The student t-test was used to compare two groups with parametric data. The significant difference was at P < 0.05.
Results:
Our data showed that T2DM patients who consumed vegetables before carbohydrates, had relatively stable glucose levels at 0, 60 and 120 mins (164.25 ± 86.89 vs 183.5 ± 55.96 vs 167.83 ± 65.53, P = 0.163) and stay lowered within the normal range compared to T2DM patients who consumed vegetables after carbohydrates (165.08 ± 67.89 vs 241.92 ± 68.03 vs 204.92 ± 81.76, P = 0.022). Additionally, GLP-1 levels remained stable after 60 and 120 min at day 1 (P = 0.816) and day 3 (P = 0.955).
Conclusions:
Vegetables consumption before carbohydrate is a promising and simple method of diabetes diet for maintaining blood glucose and GLP-1 levels and preventing from vascular complication.
Keywords: Blood glucose level, glucagon-like peptide 1, type 2 diabetes mellitus, vegetable consumption
Introduction
Type 2 Diabetes Mellitus (T2DM) is one of major public health problems with an approximately 9.3% prevalence or 463 million of worldwide population in 2019. It has been estimated that this number will increase significantly over time period and will rise into 578 million or 10.2% of worldwide population in 2030. Indonesia occupies the seventh position in the world for the highest number of population accounting to 10.7 million and is projected to increase to 13.7 million in 2030.[1]
Blood glucose levels are routinely used for monitoring the clinical progress of T2DM patients who undertake lifestyle modification and or pharmacotherapy.[2] In Indonesian community and clinical settings, fasting and two-hours postprandial glucose tests are commonly used to diagnose T2DM instead of testing glycated hemoglobin (HbA1C). In addition, the post prandial blood glucose level in T2DM patients has a stronger correlation with HbA1C level than the fasting blood glucose level to predict micro and macro-vascular complications.[3,4] Additionally, a recent study also reported that elevated one-hour glucose level was correlated to increased risk of diabetic complications and mortality.[5]
Glucagon-like peptide-1 (GLP-1) is a class of incretin hormones which exhibits insulinotropic activity and plays its essential role in diabetes pathophysiology. The impaired GLP-1 secretion was often correlated to T2DM[6] and its biological activity is counterbalanced by the presence of dipeptidyl peptidase (DPP)-4.[7] Such GLP-1 receptor agonist and DPP-4 inhibitor are currently available for the second line of diabetes treatment.[8,9]
Recent studies have reported that vegetables consumption before carbohydrate can decrease the postprandial glucose levels among diabetic patients in USA and Japan.[10,11,12] Growing evidence also indicates that the consumption of vegetables before carbohydrate decreases post prandial glucose and insulin levels and up-regulates GLP-1 secretion in healthy people,[13] pre diabetes patients[14] and T2DM patients.[10] Therefore, these findings become a strategic and promising method for diet modification in T2DM patients.
Indonesian dietary pattern differs from western or Japanese dietary pattern, which was used aforementioned. Indonesian people tend to consume a very high proportion of rice in their daily meals (≥ 60% total energy) as a staple food.[15] A recent study reported that some popular Indonesian rice has high glycemic index (GI).[16,17] We assumed that different vegetables which are commonly consumed in Indonesia and high proportion of rice might result in a different blood glucose, insulin and GLP-1 levels in T2DM patients. Therefore, this present study aimed to investigate the effects of vegetables consumption before carbohydrates on blood glucose and GLP-1 levels in T2DM patients.
Methods
Study design
A non-randomized quasi experimental study was conducted in the Gatot Soebroto Central Army Hospital, Jakarta, Indonesia from April to May 2016. Patients with T2DM who attended at the Department of Internal Medicine were recruited as research participants and selected using the following criteria: aged 18-60 years old, BMI >23 kg/m2, performed mild and moderate physical activities, and taking oral anti glycemic drugs whereas the control group consisted of healthy people who matched with the research participant criteria. Pregnant women and T2DM patients who were taking DPP-4 inhibitors such as sitagliptin and vildagliptin and suffered chronic diseases such as heart, renal and hepatic diseases were excluded. We used the Lemeshow's formula[18] to calculate the sample size to estimate the different mean of fasting blood glucose levels between control and treatment groups. Therefore, we got a total number of 36 subjects who were divided into 12 subjects/group. This study was approved by the Ethical Committee of the Faculty of Medicine, Universitas Sebelas Maret/Dr. Moewardi Hospital, Surakarta (No. 252/IV/HREC/2016). Informed consents for all selected research participants were proposed in order to draw venous blood and to recall their daily food consumptions using a 24 hours questionnaire. Basic characteristics of research participants were obtained from data collection using an open questionnaire.
Study protocol
Selected research participants received the equal amount of their breakfast which consisted of 333.8 kcal energy, 42.4 g carbohydrate, 16.3 g protein, 11.9 g fat and 5.2 g dietary fibers representing 20% of daily energy need per person. The meal order was classified into two categories: vegetable consumption before carbohydrates and vegetables consumption after carbohydrate which were simply referred as G1 and G2 in this study. In addition, the control group consisted of healthy participants who consumed vegetables before carbohydrates.
The food composition of their breakfast for three days intervention was prepared as follows:
-
1st and 3rd days
We provided cooked vegetables that contained the mixture of chinese cabbage, carrots, and snaps. The carbohydrate source used rice which was consumed along with fried fish as a side dish.
-
2nd day
Research participants consumed cooked vegetables that consisted of the mixture of chinese cabbage, carrots, cauliflowers, and chayote. The carbohydrate source used rice which was consumed along with fried egg as a side dish.
Anthropometric measurement
The body weight (kg) was measured without shoes and after the participants emptying the bladder. The height (cm) was performed by using a stadiometer and measured the distance between the top of head (vertex) to the bottom of the foot (without shoes). The body mass index was calculated by dividing the weight with the square of height (m) and the cut off points of BMI of research participants were determined using World Health Organization (WHO) classification for Asian population.[19] All procedures were done by a professional paramedic in the Department of Internal Medicine, Gatot Soebroto Central Army Hospital.
Nutritional intake
We measured daily intake of macro and micro-nutrients after 24 hours including quantity, size, food type, composition and food processing. Nutrient data were collected using a 24 h food recall questionnaire for three times and were converted into daily macronutrient intake using a free nutrisurvey software (www.nutrisurvey.de) which has been translated in Indonesian language.
Blood glucose level
Blood glucose levels were measured on the 1st and 3rd days of intervention. The blood samples were obtained from all selected research participants who were fasting for 8 hours before, as well as one and two hours after breakfast. Glucose levels in venous blood were measured using a routine hexokinase method in the Clinical Laboratory of Gatot Soebroto Central Army Hospital.
Enzyme-linked Immunosorbent Assay (ELISA)
Blood serum samples from research participants as mentioned above were used to measure GLP-1 concentration using the ELISA kit (Elabscience, Wuhan, China). All steps of GLP-1 measurement were based on the manufacturer's protocol and was carried out at the Biomedical Laboratory, Faculty of Medicine, UNS, Surakarta, Indonesia.
Statistical analysis
Numerical data were presented as mean ± standard deviation (SD) and frequencies were for categorical data. Normality and homogeneity of numerical data were verified through the Shapiro-Wilk and Levene tests prior to analyze the significant difference among groups. The student t-test was used to compare two groups with parametric data whilst the Mann Whitney test was used if the data did not meet criteria for the parametric test. Differences in categorical data were tested using the Chi-square test. All statistical analyses were performed using SPSS 20.0 for Windows (SPSS, Inc., Chicago, IL, USA) and the significant difference was set up at P < 0.05.
Results
Characteristics of research participants and socio-demographic profile
Out of 36 research participants, 12 participants were not treated (the control group) whilst 14 participants took metformin alone [Table 1]. Other 8 participants took metformin and glimepirid or acarbose or diamicron and one participant took a combination of metformin, glimepirid and acarbose. The two other participants took glimepirid and acarbose, and one participant was glucobain alone respectively.
Table 1.
The basic characterictis of research participants
| Characteristics | Control (n=12) | G1 (n=12) | G2 (n=12) | P |
|---|---|---|---|---|
| Age (years) | 38.08±12.49 | 51.33±9.40 | 51.33±10.07 | 0.006 |
| Duration of T2DM | - | 7.75±4.13 | 7.41±5.43 | 0.104* |
| Sex: | ||||
| Male | 2 (16.7%) | 2 (16.7%) | 5 (41.7%) | 0.163 |
| Female | 10 (83.3%) | 10 (83.3%) | 7 (58.3%) | |
| Anthropometric parameters: | ||||
| Height (cm) | 158.47±5.42 | 155.57±8.78 | 157.58±7.30 | 0.941 |
| Weight (kg) | 72.00±5.42 | 70.00±12.32 | 72.45±16.65 | 0.613 |
| BMI (kg/m2) | 28.68±4.06 | 29.04±3.54 | 29.00±5.13 | 0.974 |
| BMI categories | ||||
| Overweight | 7 (58.3%) | 8 (66.7%) | 2 (16.7%) | 0.523 |
| Obesity | 5 (41.7%) | 4 (33.3%) | 10 (83.3%) | |
| Waist circumference (cm) | ||||
| Male | 104.25±8.13 | 97.85±0.64 | 98.93±9.91 | 0.718 |
| Female | 92.91±7.74 | 90.97±5.50 | 94.93±11.28 | 0.605 |
This present study enrolled 36 participants in which 24 subjects were diagnosed with T2DM. G1 and G2 referred to diabetic participants who consumed vegetable before carbohydrates and vegetables after carbohydrate, respectively. There were no significant differences in terms of duration of T2DM between two groups, gender, BMI and waist circumference. However, the average of age was significantly younger in the control group
Jakarta is the Capital City of Indonesia where at least 10 million population resides. The Jakarta's population indicates multi ethnic groups which originally come from almost entire parts of Indonesia. Mostly, Jakarta people work in public service and industrial sectors. In addition, The Gatot Soebroto Central Army Hospital is located in the Central Jakarta administrative city and thus our study participants were reflecting the urban population.
The daily intake of energy and macronutrients was higher in control than two other groups
In general, the control group had higher daily intake of energy and macronutrients, compared to two other groups except dietary fiber [Table 2]. Even though none of them fulfilled the minimal amount of energy and macronutrient intake as recommended, higher daily intake of fat was observed in all groups. Compared to daily intake of energy and macronutrients in the control, only the G1 reached significant differences. Furthermore, the highest fiber intake was in the G1 and significantly higher than the fiber intake in the G2 (P = 0.012). The similar significant difference was found in control vs G2 groups with P = 0.038.
Table 2.
Daily intake of macronutrients and its comparison with Recommended Daily Allowance (RDA)
| Daily intake of macronutrients | RDA | Control (n=12) | G1 (n=12) | G2 (n=12) | Control vs G1 | Control vs G2 | G1 vs G2 |
|---|---|---|---|---|---|---|---|
| Energy (kcal/day) | 1,700 | 1675.14±211.60 | 1406.00±64.36 | 1514.16±23.10 | P=0.013 | n.s. | n.s. |
| Carbohydrates (g/day) | 255.00 | 217.19±27.61 | 187.03±53.80 | 196.19±45.73 | P=0.038 | n.s | n.s. |
| Protein (g/day) | 63.75 | 61.29±7.12 | 51.96±12.78 | 51.98±13.12 | P=0.024 | n.s. | n.s. |
| Fat (g/day) | 47.22 | 64.80±14.39 | 53.70±7.54 | 58.71±17.30 | P=0.015 | n.s. | n.s. |
| Dietary fiber (g/day) | 25.00 | 13.45±1.40 | 15.43±4.91 | 11.23±2.75 | n.s. | P=0.038 | P=0.012 |
n.s. non significant
Glucose level was relatively stable after three days consumption of vegetables before carbohydrates
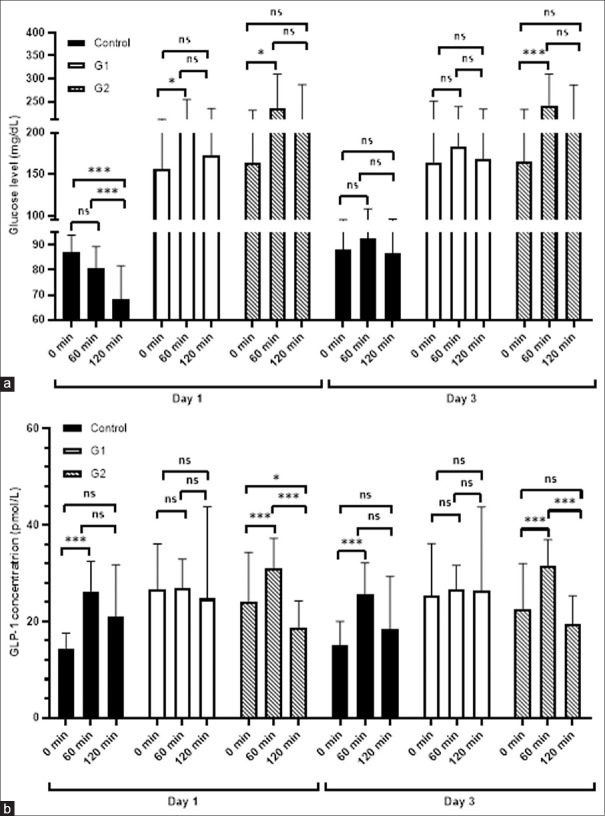
The glucose level of G1 after three days were relatively stable between three different time points (164.25 ± 86.89 vs 183.5 ± 55.96 vs 167.83 ± 65.53 mg/dL, Kruskall Wallis P = 0.163) in comparison to G2 (165.08 ± 67.89 vs 241.92 ± 68.03 vs 204.92 ± 81.76 mg/dL, Kruskall Wallis P = 0.022). A significant increase of glucose level in the G2 still occurred from 0 min to 60 min in day 3. This pattern was the same as the glucose levels at the day 1 intervention whilst this pattern was not found in the G1.
The G1 trend of glucose level alteration in day 3 was similar to its non-diabetic control counterparts with implementing the same diet protocol. The pattern of glucose level in non-diabetic control changed dramatically in which there was a decrease of glucose level at respective time points in day 1 as expected whereas in day 3, the glucose level remained stable.
Vegetables consumption before carbohydrates significantly lowered postprandial glucose level at 60 min after three days of consumption
At day 1, the glucose level of control was significantly lower in comparison with two other groups at three different time points. However, we found no significant differences between G1 and G2 groups at day 1. A significant lower level of glucose was observed in G1 compared to G2 (183.5 ± 55.96 vs 241.92 ± 68.03 mg/dL, P = 0.018) at 60 min at day 3 (data not shown).
GLP-1 levels of G1 were sustained after vegetables consumption before carbohydrate
From day 1 to day 3, there were significant variations of serum GLP-1 levels between time points in control and G2 groups compared to G1. At day 1, the GLP-1 levels of G2 increased significantly from 0 min to 60 min (23.97 ± 10.39 vs 30.95 ± 6.32 pmol/L, P = 0.05, but then it also decreased significantly and even lower than the basal level at 0 min (30.95 ± 6.32 vs 18.70 ± 5.59 pmol/L, P = 0.028). Similar to G2, the trend of control group was same from 0 to 60 min in which GLP-1 increased significantly (14.44 ± 3.09 vs 26.29 ± 6.18 pmol/L, P = 0.004) but it remained stable until 120 min (26.29 ± 6.18 vs 21.08 ± 10.69 pmol/L, P = 0.6). The similar trends of both control and G2 groups were also observed at day 3. On the contrary, the GLP-1 levels in G1 were remained constant over three different time points at day 1 (26.65 ± 9.41 vs 27.64 ± 5.97 pmol/L, P = 0.887 and 27.64 ± 5.97 vs 23.34 ± 19.07 pmol/L, P = 0.816) and day 3 (25.33 ± 10.85 vs 26.98 ± 5.02 pmol/L, P = 0.746 and 26.98 ± 5.02 vs 26.46 ± 17.29 pmol/L, P = 0.955) compared to its non-diabetic control and G2.
Discussion
In this present study, we have documented that vegetables consumption before carbohydrate has a significant impact on both glucose and GLP-1 levels among T2DM patients in Jakarta, Indonesia. This diet pattern was able to stabilize glucose and GLP-1 levels compared to its non-diabetic control counterparts and the other group with vegetables consumption after carbohydrate. In addition, a significant lowered glucose level especially at 60 min postprandial time was found in the group with vegetables consumption before carbohydrate. Daily nutrient intake significantly reduced in the G1 group compared to the control group whereas daily fiber intake significantly increased in the G1 group compared to the G2 group.
Lowering fasting and postprandial glucose levels into a normal range is the main goal of diabetes management through either diet, physical exercise, pharmacotherapy or combination of them. Patients with T2DM should follow the rules of mealtime, food composition and amount of caloric consumption.[2] To achieve near normal glucose levels, some studies have recommended to eat carbohydrates with low-moderate GI.[20,21,22,23] However, it is difficult to modify the diabetes diet for most Indonesian people since they are used to eating rice as the main carbohydrate source.[15] Additionally, the most common rice products, which are consumed by Indonesians exhibit a high GI.[16,17] Thus, short-term food order modification in our study may not completely regulate the postprandial glucose itself. Another evidence from a mouse model has proven that long-term consumption of high energy foods (3 months) can increase the glucose level.[24] Therefore, it might be the reason why in our study we did not observe any significant difference between G1 and G2 groups especially at day 1 as research participants consumed rice for a long term without considering differences of rice GI, processing, cooking and serving.
Our study was different from a previous study which reported that GLP-1 was significantly higher from 90 to 150 min in a research group that consumed carbohydrates later than consumed carbohydrates first.[10] The result discrepancy might be the different food composition used in our study. GLP-1 level can be modulated by the presence of several compounds such as glucose[25] and polyphenol.[26] A Recent study has reported that curry consumption with high content of polyphenols increases GLP-1 level in young Chinese men.[27] Our study participants mainly consumed chinese cabbage and carrots whilst Shukla's study[10] used lettuce, tomatoes and cucumber. Those vegetables differently contain polyphenols in which fresh tomatoes has lower polyphenols (62 mg/100g) than fresh carrots (156 mg/100 g).[28] Moreover, white rice consumption among our participants might contribute to GLP-1 modulation due to its content of monosaccharides and polyphenols.[29,30]
Another study also reported that sustained GLP-1 levels might be required to inhibit progression of β-cell failure in a diabetes animal model.[31] The result of our study showed that vegetables consumption before carbohydrate kept GLP-1 levels remained stable from 0 to 120 mins [Figure 1]. These results suggest that vegetables consumption before carbohydrate may prevent the defect of pancreatic β-cells, resulting in insulin deficiency and diabetes progression. Thus, further studies are needed to elucidate the mechanism of action underlying that beneficial effect of vegetables consumption.
Figure 1.
Comparison of glucose and GLP-1 levels in control and treatment groups at day 1 and day 3. (a) The blood glucose levels of three groups at three different time points and (b) The serum GLP-1 levels of three groups at three time points. Data were presented as mean ± SD and each bar represented 12 research participants.
We realized that vegetables consumption before carbohydrates did not only regulate glucose level through GLP-1 but also other gut hormones. One study in Japan reported that white rice consumption also regulated glucose-dependent insulinotropic polypeptide level.[32] Meanwhile, natural phenolic compounds derived from vegetables and fruits demonstrated the DPP-4 inhibition in dose-dependent manner.[33] Altogether, it indicates that natural phenolic compounds could lower glucose levels by several mechanisms.
Reduction of blood glucose levels below the upper limit is an important strategy to inhibit diabetes progression[2] and can decrease the risk of cardiovascular-related death.[34] Our results showed that vegetables consumption before carbohydrate can maintain postprandial glucose levels below the upper limit in 3 days after intervention [Figure 1]. In addition, one-hour post prandial glucose level was reduced in the G1 group at day 3. This finding probably supports previous studies that reduction of elevated one-hour post prandial glucose level decreases the cardiovascular risk.[35,36] Other studies also reported that polyphenols-rich vegetables play an important role in cardiovascular risk reduction.[37,38] Therefore, vegetables consumption before carbohydrate is a promising and simple method of diabetes diet for maintaining poor diabetes progression and preventing from vascular complication.
Our study has a very limited time to follow up research participants and thus does not represent the long-term food modification in diabetes management. Furthermore, our research participants originally came from Jakarta urban area, where cannot generally consider to rural population area. In near future, further studies are required to give us better understanding of the role of food modification on diabetes management, based on different population areas and previous diets.
Our results do not only support growing evidence on how important of food order modification on diabetes management but also give a new insight of sustained glucose and GLP-1 levels in diabetes food modification. Finally, we concluded that vegetables consumption before carbohydrate is a promising and simple method of diabetes diet for maintaining blood glucose and GLP-1 levels and preventing from vascular complication.
Financial support and sponsorship
This study was funded by the Postgraduate Research Grant from Universitas Sebelas Maret (UNS).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin Diabetes. 2015;33:97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ceriello A, Barakat M, Bahendeka S, Colagiuri S, Gerich J, Hanefeld M, et al. Guideline for management of postmeal glucose in diabetes. Diabetes Res Clin Pract. 2014;103:256–68. doi: 10.1016/j.diabres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Avignon A, Radauceanu A, Monnier L. Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care. 1997;20:1822–6. doi: 10.2337/diacare.20.12.1822. [DOI] [PubMed] [Google Scholar]
- 5.Pareek M, Bhatt DL, Nielsen ML, Jagannathan R, Eriksson KF, Nilsson PM, et al. Enhanced predictive capability of a 1-hour oral glucose tolerance test: A prospective population-based cohort study. Diabetes Care. 2018;41:171–7. doi: 10.2337/dc17-1351. [DOI] [PubMed] [Google Scholar]
- 6.Holst JJ, Gromada J. Role of incretin hormones in the regulation of insulin secretion in diabetic and nondiabetic humans. Am J Physiol Endocrinol Metab. 2004;287:E199–206. doi: 10.1152/ajpendo.00545.2003. [DOI] [PubMed] [Google Scholar]
- 7.Ahrén B. The dynamic incretin adaptation and type 2 diabetes. J Clin Endocrinol Metab. 2011;96:620–2. doi: 10.1210/jc.2011-0299. [DOI] [PubMed] [Google Scholar]
- 8.Boyle JG, Livingstone R, Petrie JR. Cardiovascular benefits of GLP-1 agonists in type 2 diabetes: A comparative review. Clin Sci (Lond) 2018;132:1699–709. doi: 10.1042/CS20171299. [DOI] [PubMed] [Google Scholar]
- 9.Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2018;41:2669–2701. doi: 10.2337/dci18-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shukla AP, Andono J, Touhamy SH, Casper A, Iliescu RG, Mauer E, et al. Carbohydrate-last meal pattern lowers postprandial glucose and insulin excursions in type 2 diabetes. BMJ Open Diabetes Res Care. 2017;5:e000440. doi: 10.1136/bmjdrc-2017-000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imai S, Fukui M, Kajiyama S. Effect of eating vegetables before carbohydrates on glucose excursions in patients with type 2 diabetes. J Clin Biochem Nutr. 2014;54:7–11. doi: 10.3164/jcbn.13-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai S, Fukui M, Ozasa N, Ozeki T, Kurokawa M, Komatsu M, et al. Eating vegetables before carbohydrates improves postprandial glucose excursions. Diabet Med. 2013;30:370–2. doi: 10.1111/dme.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishino K, Sakurai M, Takeshita Y, Takamura T. Consuming carbohydrates after meat or vegetables lowers postprandial excursions of glucose and insulin in nondiabetic subjects. J Nutr Sci Vitaminol (Tokyo) 2018;64:316–20. doi: 10.3177/jnsv.64.316. [DOI] [PubMed] [Google Scholar]
- 14.Shukla AP, Dickison M, Coughlin N, Karan A, Mauer E, Truong W, et al. The impact of food order on postprandial glycaemic excursions in prediabetes. Diabetes Obes Metab. 2019;21:377–81. doi: 10.1111/dom.13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ickowitz A, Rowland D, Powell B, Salim MA, Sunderland T. Forests, Trees, and Micronutrient-Rich Food Consumption in Indonesia. PLoS One. 2016;11:e0154139. doi: 10.1371/journal.pone.0154139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusmiyati F, Lukiwati DR, Kristanto BA, Herwibawa B. Glycemic index of ten commercially Indonesian rice cultivars. IOP Conf Ser Earth Environ Sci. 2019;250:012028. [Google Scholar]
- 17.Widowati S, Astawan M, Muchtadi D, Wresdiyati T. Hypoglycemic activity of some Indonesian rice varieties and their physicochemical properties. Indones J Agric Sci. 2006;7:57–66. [Google Scholar]
- 18.Lwanga SK, Lemeshow S. Sample Size Determination in Health Studies: A Practical Manual. Geneva, Switzerland: World Health Organization; 1991. [Google Scholar]
- 19.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 20.Hu ZG, Tan RS, Jin D, Li W, Zhou XY. A low glycemic index staple diet reduces postprandial glucose values in Asian women with gestational diabetes mellitus. J Investig Med. 2014;62:975–9. doi: 10.1097/JIM.0000000000000108. [DOI] [PubMed] [Google Scholar]
- 21.Rovner AJ, Nansel TR, Gellar L. The effect of a low-glycemic diet vs a standard diet on blood glucose levels and macronutrient intake in children with type 1 diabetes. J Am Diet Assoc. 2009;109:303–7. doi: 10.1016/j.jada.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riccardi G, Rivellese AA, Giacco R. Role of glycemic index and glycemic load in the healthy state, in prediabetes, and in diabetes. Am J Clin Nutr. 2008;87:269S–74S. doi: 10.1093/ajcn/87.1.269S. [DOI] [PubMed] [Google Scholar]
- 23.Sheard NF, Clark NG, Brand-Miller JC, Franz MJ, Pi-Sunyer FX, Mayer-Davis E, et al. Dietary carbohydrate (amount and type) in the prevention and management of diabetes: A statement by the american diabetes association. Diabetes Care. 2004;27:2266–71. doi: 10.2337/diacare.27.9.2266. [DOI] [PubMed] [Google Scholar]
- 24.Pang J, Xi C, Huang X, Cui J, Gong H, Zhang T. Effects of excess energy intake on glucose and lipid metabolism in C57BL/6 Mice. PLoS One. 2016;11:e0146675. doi: 10.1371/journal.pone.0146675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun EW, de Fontgalland D, Rabbitt P, Hollington P, Sposato L, Due SL, et al. Mechanisms controlling glucose-induced glp-1 secretion in human small intestine. Diabetes. 2017;66:2144–9. doi: 10.2337/db17-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Domínguez Avila JA, Rodrigo García J, González Aguilar GA, de la Rosa LA. The antidiabetic mechanisms of polyphenols related to increased Glucagon-Like Peptide-1 (GLP1) and insulin signaling. Molecules. 2017;22:903. doi: 10.3390/molecules22060903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haldar S, Chia SC, Henry CJ. Polyphenol-rich curry made with mixed spices and vegetables increases postprandial plasma GLP-1 concentration in a dose-dependent manner. Eur J Clin Nutr. 2018;72:297–300. doi: 10.1038/s41430-017-0069-7. [DOI] [PubMed] [Google Scholar]
- 28.Cieslik E, Greda A, Adamus W. Contents of polyphenols in fruit and vegetables. Food Chem. 2006;94:135–42. [Google Scholar]
- 29.Tian S, Nakamura K, Kayahara H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J Agric Food Chem. 2004;52:4808–13. doi: 10.1021/jf049446f. [DOI] [PubMed] [Google Scholar]
- 30.Bodnaruc AM, Prud’homme D, Blanchet R, Giroux I. Nutritional modulation of endogenous glucagon-like peptide-1 secretion: A review. Nutr Metab (Lond) 2016;13:92. doi: 10.1186/s12986-016-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubo F, Miyatsuka T, Sasaki S, Takahara M, Yamamoto Y, Shimo N, et al. Sustained expression of GLP-1 receptor differentially modulates β-cell functions in diabetic and nondiabetic mice. Biochem Biophys Res Commun. 2016;471:68–74. doi: 10.1016/j.bbrc.2016.01.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kameyama N, Maruyama C, Matsui S, Araki R, Yamada Y, Maruyama T. Effects of consumption of main and side dishes with white rice on postprandial glucose, insulin, glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 responses in healthy Japanese men. Br J Nutr. 2014;111:1632–40. doi: 10.1017/S0007114513004194. [DOI] [PubMed] [Google Scholar]
- 33.Fan J, Johnson MH, Lila MA, Yousef G, de Mejia EG. Berry and citrus phenolic compounds inhibit dipeptidyl peptidase IV: Implications in diabetes management? Evid Based Complement Alternat Med 2013. 2013:479505. doi: 10.1155/2013/479505. doi: 10.1155/2013/479505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ACCORD Study Group. Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 2016;39:701–8. doi: 10.2337/dc15-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavalot F, Pagliarino A, Valle M, Di Martino L, Massucco P, Anfossi G, et al. Postprandial blood glucose predicts cardiovascular events and all-cause mortality in type 2 diabetes in a 14-year follow-up: Lessons from the San Luigi Gonzaga Diabetes Study. Diabetes Care. 2011;34:2237–43. doi: 10.2337/dc10-2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Succurro E, Marini MA, Arturi F, Grembiale A, Lugarà M, Andreozzi F, et al. Elevated one-hour post-load plasma glucose levels identifies subjects with normal glucose tolerance but early carotid atherosclerosis. Atherosclerosis. 2009;207:245–9. doi: 10.1016/j.atherosclerosis.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Vitale M, Vaccaro O, Masulli M, Bonora E, Prato SD, Giorda CB, et al. Polyphenol intake and cardiovascular risk factors in a population with type 2 diabetes: The TOSCA. IT study. Clin Nutr. 2017;36:1686–92. doi: 10.1016/j.clnu.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 38.Tangney CC, Rasmussen HE. Polyphenols, inflammation, and cardiovascular disease. Curr Atheroscler Rep. 2013;15:324. doi: 10.1007/s11883-013-0324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]