Abstract
In the current issue of Cell Stem Cell, Bogeska et al. demonstrate that repeated exposures to inflammation cause indelible and specific functional compromise and accelerated aging of long-term hematopoietic stem cells (LT-HSCs). This study proposes the notion that the cumulative inflammatory events over the course of an organism’s lifespan may irreversibly damage LT-HSCs.
As primitive hematopoietic stem cells (HSCs), long-term hematopoietic stem cells (LT-HSCs) give rise to all of the mature hematopoietic cell types and undergo self-renewal to maintain a reservoir of stem cells (Mann et al., 2018). With aging, LT-HSCs exhibit reduced self-renewal capacity and myeloid-skewed differentiation (Mann et al., 2018). Often elevated to chronic, subacute levels during aging, inflammation has been implicated in the pathogenesis of age-associated hematological diseases, including transformation of the preleukemic condition clonal hematopoiesis of indeterminate potential (CHIP) to malignancy (Jaiswal et al., 2014). While many clinical sources of inflammation are experienced throughout the lifetime of an individual, current studies focus on the role of inflammation in active hematological diseases rather than its ability to predispose individuals to these diseases (Pietras et al., 2016; Trowbridge and Starczynowski, 2021). Consequently, it has been unknown whether the functional impairment of HSCs is long-lasting and whether inflammation encountered earlier in life can contribute to hematological disease later in life.
To investigate the cumulative effects of multiple inflammatory experiences on the hematopoietic system, Bogeska et al. (2022) challenged wild-type mice with repeated exposures to polyinosinic:polycytidylic acid (pI:pC), which mimics an infectious stimulus. In impressive time-course experiments, they then utilized competitive transplant and complex labeling approaches to determine the consequences of these inflammatory events on stem-cell function over time. Remarkably, they showed that progressive inflammatory insults hindered the ability of LT-HSCs to self-renew. The greater susceptibility of LT-HSCs to these inflammatory exposures, compared to more differentiated progenitors, implies a unique relationship between HSCs and inflammation, further supporting a direct role for HSCs in the inflammatory response (Cai et al., 2018). It also highlights the possibility that inflammation may damage HSCs without immediate effects on mature hematopoietic cell types, which are commonly measured clinically for indicators of disease, revealing a potential need for better diagnostic tools to detect damage due to inflammation. While previous studies have shown that chronic inflammation exhausts hematopoietic stem and progenitor cells (HSPCs), the authors demonstrate that the functional suppression of LT-HSCs persisted even after the absence of the source of inflammation and that HSPCs failed to regenerate up to even 1 year after challenge (Pietras et al., 2016). Furthermore, the damage to LT-HSCs led to an accelerated aging phenotype, suggesting that successive inflammatory experiences can impair self-renewal by prematurely driving the biological clock to transform younger LT-HSCs into older LT-HSCs regardless of their chronological age.
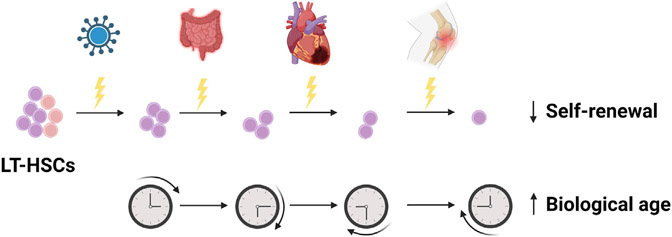
Through these studies, Bogeska et al. (2022) redefine the paradigm of inflammation in aging by proposing that the inflammatory events throughout an individual’s lifetime can have a lasting and permanent impact on the functionality of HSCs. As illustrated in Figure 1, these experiences may originate from a variety of clinical sources, including infection, aging, arthritis, cardiovascular disease, cancer and its treatment, and ulcerative colitis. This concept has very significant clinical implications, especially as recent studies are linking these conditions to CHIP, which is characterized by the age-associated acquisition of preleukemic mutations in HSCs (Jaiswal et al., 2017; Savola et al., 2018; Zhang et al., 2019). The recapitulation of the effects on LT-HSCs using Mycobacterium avium infection, which is observed in immunocompromised patients, underscores the clinical relevance of these findings. Using a multicolored genetic labeling system, the authors reveal that successive inflammatory exposure reduces clonal complexity, indicating that previous inflammatory events can bias the expansion of specific clones. Similar experiments using HSCs carrying preleukemic mutations would be informative. It will also be important to determine the threshold of inflammation that leads to long-term damage of LT-HSCs. They allude to the potential existence of such a threshold by demonstrating that single exposure to pI:pC activates a subset of the LT-HSC compartment, whereas multiple exposures exhaust LT-HSCs. Consistently, specific LT-HSC subpopulations can be stimulated by inflammation (Mann et al., 2018). It will also be critical to better understand the consequences of strong inflammatory events versus low-level chronic inflammation. The effects of inflammatory exposures may help to explain disease heterogeneity. For example, they may elucidate why some patients carrying CHIP-associated mutations develop disease while others do not or why certain patients with hematological malignancies exhibit worse outcomes to specific treatments. The notion that inflammatory events may exert irreversible damage on LT-HSCs will have key implications for the treatment of inflammation and raises the question of whether prevention of inflammation can protect against damage to LT-HSCs.
Figure 1. Successive inflammatory experiences via multiple clinical forms may impair the ability of LT-HSCs to self-renew and may accelerate their biological age.
Bogeska et al. demonstrated that repeated exposure to inflammation hinders LT-HSC self-renewal and increases the biological age of these cells. This finding has implications for clinical sources of inflammation experienced throughout life, such as infection, ulcerative colitis, cardiovascular disease, and arthritis. This figure was created using BioRender.com.
In future studies, it will be important to determine the mechanisms by which inflammation permanently impairs LT-HSCs and the characteristics that make these cells uniquely susceptible to inflammation. Several inflammatory signaling pathways have been implicated in the pathogenesis of CHIP (Trowbridge and Starczynowski, 2021). While significant changes in gene expression in LT-HSCs were not observed, hypomethylation of the promoters regulating interferon and inflammatory response genes was detected, indicating a potential role for epigenetic mechanisms. This observation is further supported by the fact that several of the most commonly mutated genes in CHIP are epigenetic regulators (Jaiswal et al., 2014). As both cell autonomous and non-cell autonomous mechanisms have been proposed in the contexts of aging and inflammation, the contributions of these factors in these processes remain controversial (SanMiguel et al., 2020). Notably, the ability of wild-type HSCs to engraft in pI:pC-treated mice suggests that the bone marrow microenvironment (BME) is not irreversibly damaged by inflammation and highlights potential stem-cell intrinsic effects. The converse experiment may bolster this possibility. The authors imply that while the BME may be involved in the initial process of damaging LT-HSCs, the long-lasting damage due to inflammation is specific to LT-HSCs. Additional studies are needed to clarify these mechanisms. Repeated inflammatory exposures led to cytopenias and bone marrow (BM) cellularity reminiscent of that seen in CHIP and myelodysplastic syndrome. While previous studies indicate that inflammation can induce genomic instability, it is not yet known whether these inflammatory insults can induce transformation to hematological malignancy or if additional factors are required (Pietras et al., 2016). A more thorough understanding of these processes will have critical consequences for assessing the clinical risks of successive inflammatory experiences. Collectively, by shifting the focus on inflammation’s pathogenic role toward the cumulative impact of lifetime inflammatory experiences, this study offers a significant change in the perspective of the contributions of inflammation to hematological disease and will have valuable clinical implications.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Bogeska R, Mikecin A-M, Kaschutnig P, Fawaz M, Büchler-Schäff M, Le D, Ganuza M, Vollmer A, Paffenholz SV, Asada N, et al. (2022). Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell 29 1273–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Kotzin JJ, Ramdas B, Chen S, Nelanuthala S, Palam LR, Pandey R, Mali RS, Liu Y, Kelley MR, et al. (2018). Inhibition of inflammatory signaling in Tet2 mutant preleukemic cells Mitigates Stress-Induced Abnormalities and clonal hematopoiesis. Cell Stem Cell 23 833–849.e5. 10.1016/j.stem.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, et al. (2014). Age-related clonal hematopoiesis associated with adverse outcomes. N. Engl. J. Med 371 2488–2498. 10.1056/nejmoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, et al. (2017). Clonal hematopoiesis and risk of Atherosclerotic cardiovascular disease. N. Engl. J. Med 377, 111–121. 10.1056/nejmoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann M, Mehta A, de Boer CG, Kowalczyk MS, Lee K, Haldeman P, Rogel N, Knecht AR, Farouq D, Regev A, and Baltimore D (2018). Heterogeneous responses of hematopoietic stem cells to inflammatory Stimuli are Altered with age. Cell Rep. 25, 2992–3005.e5. 10.1016/j.celrep.2018.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang S, Lakshminarasimhan R, Chin CP, Techner JM, Will B, et al. (2016). Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat. Cell Biol 18, 607–618. 10.1038/ncb3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SanMiguel JM, Young K, and Trowbridge JJ (2020). Hand in hand: intrinsic and extrinsic drivers of aging and clonal hematopoiesis. Exp. Hematol 91, 1–9. 10.1016/j.exphem.2020.09.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savola P, Lundgren S, Keränen MAI, Almusa H, Ellonen P, Leirisalo-Repo M, Kelkka T, and Mustjoki S (2018). Clonal hematopoiesis in patients with rheumatoid arthritis. Blood Cancer J. 8, 69. 10.1038/s41408-018-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge JJ, and Starczynowski DT (2021). Innate immune pathways and inflammation in hematopoietic aging, clonal hematopoiesis, and MDS. J. Exp. Med 218, e20201544. 10.1084/jem.20201544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CR, Nix D, Gregory M, Ciorba MA, Ostrander EL, Newberry RD, Spencer DH, and Challen GA (2019). Inflammatory cytokines promote clonal hematopoiesis with specific mutations in ulcerative colitis patients. Exp. Hematol 80, 36–41.e3. 10.1016/j.exphem.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]